Abstract
Background & objectives:
Type 2 diabetes mellitus (T2DM) is considered to be a protective factor against development of osteoporosis. But oral hypoglycaemic agents (OHA) are likely to increase the risk of osteoporosis. This study was carried out to evaluate the effect of various OHA on bone mineral density (BMD) in patients with T2DM.
Methods:
Forty one patients (study group) with T2DM (mean age 51.9±5.5 yr; 31 females) receiving treatment with oral hypoglycaemic agents (OHA) [thiazolidinediones alone (n=14) or in combination with other OHA (n=27)] for a period of at least three consecutive years and 41 age- and gender-matched healthy controls (mean age 51.4±5.1 yr) were included in the study. A detailed clinical history was taken and all were subjected to physical examination and recording of anthropometric data. BMD was assessed for both patients and controls.
Results:
The mean body mass index (kg/m2) (26.5±4.90 vs 27.3 ±5.33) and median [inter-quartile range (IQR)] duration of menopause (yr) among women [6(2-12) vs 6(1-13)] were comparable between both groups. The bone mineral density (BMD; g/cm2) at the level of neck of femur (NOF) (0.761±0.112 vs 0.762±0.110), lumbar spine antero-posterior view (LSAP) (0.849±0.127 vs 0.854±0.135); median Z-score NOF {0.100[(-0.850)-(0.550)] vs -0.200[(-0.800)-(0.600)]}, LSAP {-1.200[(-1.700)-(-0.200)] vs -1.300 [(-1.85)-(-0.400)]} were also similar in study and control groups. Presence of normal BMD (9/41 vs 8/41), osteopenia (16/41 vs 18/41) and osteoporosis (16/41 vs 15/41) were comparable between the study and control groups. No significant difference was observed in the BMD, T-scores and Z-scores at NOF and LSAP among T2DM patients treated with thiazolidinediones; those treated with other OHA and controls.
Interpretation & conclusions:
The present findings show that the use of OHA for a period of three years or more does not significantly affect the BMD in patients with T2DM.
Keywords: Bone mineral density, oral hypoglycaemic agents, osteoporosis, thiazolidinediones, type 2 diabetes mellitus
Type 2 diabetes mellitus (T2DM) has emerged as one of the important public health problems in South-East Asia, especially India1. Osteoporosis is another important public health problem in India, especially, among elderly individuals. Screening for osteoporosis has been recommended in western population in their sixth decade of life2. However, given the lower bone mass and density in Indian subjects and the tendency for the occurrence of an early menopause in Indian women3, earlier screening for osteoporosis appears beneficial. Patients with T2DM tend to have more frequent falls in the elderly age compared to their healthy counterparts which have been ascribed to presence of peripheral and autonomic neuropathy, underlying friable bony architecture in spite of having normal bone mineral density (BMD), underlying chronic kidney disease (CKD), and poor healing properties in these subjects after sustaining a fall4.
Data have emerged in the past decade about the deleterious effects of the OHA belonging to the class thiazolidinediones (TZD) on the bone metabolism5. The published data do not provide concrete evidence regarding the exact relationship between these agents and the development of osteoporosis. Some studies5,6 have documented the tendency of TZD to cause osteoporosis. Limited data are available on the effects of other OHA such as metformin and sulphonylureas on BMD7. Given the complex interplay of factors viz. high BMI in patients with T2DM which is supposed to be a protective factor for osteoporosis, high risk of fractures independent of BMD7,8, effect of various OHA on BMD in patients with T2DM, and lack of evidence in studies conducted in human subjects on causal relationship between osteoporosis and OHA prompted the present study. We therefore, studied the occurrence of osteoporosis and the effect of various OHA (especially TZD) treatment on BMD in patients with T2DM in south India.
Material & Methods
Patients attending the out-patient and in-patient services of Medicine and Endocrinology departments of the Sri Venkateswara Institute of Medical Sciences (SVIMS), Tirupati, Andhra Pradesh, a tertiary care teaching hospital in south India, during the period February 2010 to June 2011 were enrolled in this cross-sectional study.
The sample size for the present study was calculated based on the following assumptions: Prevalence of osteoporosis in Indian, adult population = 35.1 per cent9. While decreased bone mineral density (BMD) has consistently been observed in type 1 diabetes mellitus patients, in patients with type 2 diabetes mellitus, an elevated, decreased and normal BMD has been observed7,8. Given this background, we assumed the prevalence of osteoporosis in patients with T2DM was 2-fold than that observed in general population, α = 5%, power of the study (1-β) = 80%. The sample size thus calculated was 62 (31 subjects in each group).
The study protocol was approved by the Institutional Ethical Committee and patients were enrolled into the study after obtaining a written informed consent.
Cases and controls: Forty one patients with T2DM aged between 40 and 60 yr who were receiving treatment with OHA for a period of at least three consecutive years or more were included. Exclusion criteria were patients with T2DM on diet control alone, patients with T2DM on insulin (with or without concomitant OHA), on treatment with OHA for a period less than three years, using medications that are known to interfere with the calcium metabolism, such as, women who used oral contraceptive pills for a period more than one year prior to entering the study, women receiving hormone replacement therapy, history of fracture at sites, such as, hip, spine or radius following trivial trauma, seriously ill patients requiring bed rest, hospital admission for more than one month in the preceding six months prior to enrollment into the study, patients with known co-morbid medical conditions, such as, chronic inflammatory diseases (tuberculosis, sarcoidosis), CKD, decompensated heart disease, chronic liver disease, rheumatologic diseases, and long standing endocrine disorders, among others. Furthermore, patients with a BMI ≤ 18.5 kg/m2, current and past tobacco smokers, patients with a history of alcohol consumption and those unwilling to participate in the study were also excluded. Forty one age- and gender-matched apparently healthy individuals chosen from hospital staff and employees were included as controls.
A detailed clinical history, extent of glycaemic control, details regarding the duration of OHA use and their dosage were meticulously documented. All the participants were subjected to a detailed physical examination including recording of anthropometric data.
T2DM was diagnosed by the American Diabetes Association criteria10. In all the patients in the study group, the following laboratory investigations were also carried out: complete haemogram, serum biochemistry, glycosylated haemoglobin (HbA1c) estimation and urinalysis. HbA1c levels were estimated by the Bio-Rad D10 Hemoglobin Testing System® (Bio-Rad, Hercules, CA, USA) functioning on high performance liquid chromatography based ion exchange chromatography.
Bone mineral density assessment: BMD was assessed in the study group and control subjects at the lumbar spine [L1-L4 anteroposterior (LSAP)] and left proximal femur using dual energy X-ray absorptiometry (DEXA) (Hologic, Prodigee, Walthm, MA, USA). The BMD values were analyzed separately for femoral neck (NOF) and LSAP. The BMD was recorded in terms of absolute mineral content (g/cm2) at both the sites. As race specific standards are not available for patients in India, Caucasian normative data provided by the manufacturer were used as reference range for T-scores.
Throughout the study, the same machine was used to assess the BMD. The same trained technician carried out all scans and analysis as per the manufacturer's instructions. The scanner was subjected to calibration daily and its performance was monitored as per the quality assurance protocol prescribed by the manufacturer. During the study period no sign of scanner drift was observed. The coefficient of variation of BMD measurement at both the regions studied was one per cent or less throughout the period of study. Patients in the study group and control subjects were categorized as per the World Health Organization (WHO) definition11,12 to have normal BMD, osteoporosis and osteopenia. The individual T-scores and Z-scores were also recorded.
Statistical analysis: Data were recorded on a predesigned proforma and managed using Microsoft Excel 2007 (Microsoft Corp, Redmond, WA). Descriptive statistics for the categorical variables were performed by computing the frequencies (percentages) in each category. For the quantitative variables, approximate normality of the distribution was assessed. Variables following normal distribution were summarized by mean and standard deviation; the remaining variables were summarized as median [inter-quartile range (IQR)]. The association between two categorical variables was evaluated by Chi-square test or Fisher's exact test as appropriate. Student's t test, one-way analysis of variance (ANOVA) and Mann-Whitney U test, as appropriate were used to compare continuous variables between the groups. All tests were two-tailed and P<0.05 was considered as significant. Statistical softwares PASW Statistics 18, Release 18.0.0, (IBM SPSS Statistics, Somers NY, USA); Systat 12, Version 12.00.08 (Systat Software, Inc, Chicago IL, USA); were used for statistical analysis.
Results
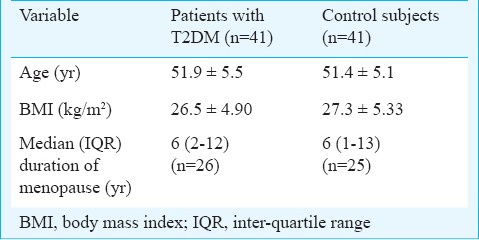
During the study period, 204 patients with T2DM were screened for inclusion in the study. Of these, 41 patients satisfying the inclusion criteria were included in the study (Figure). The baseline demographic characters of patients with T2DM, and control subjects are shown in the Table I. Majority of the patients included in the study were women [31/41 (76%) subjects in each group]; 26/31 women (84%) among study group and 25 of the 31 (81%) women among controls had attained menopause; the median (IQR) duration (years) of menopause was comparable between the two groups. There was no significant difference for normal BMI, overweight and obesity between the two groups.
Figure.
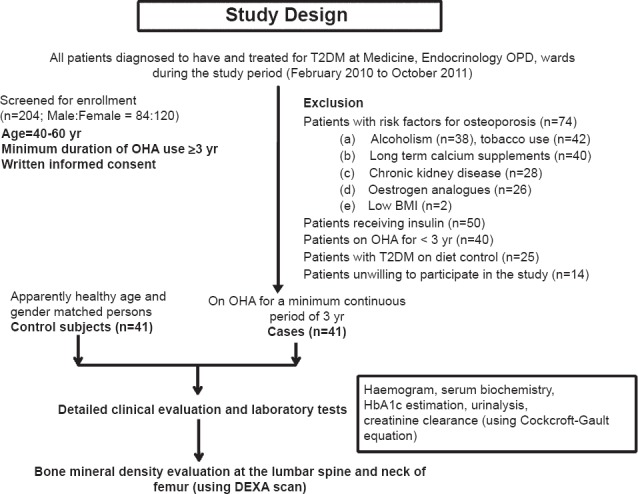
Flow diagram showing the study plan. More than one exclusion criteria were observed in some patients. T2DM, type 2 diabetes mellitus; OPD, out-patient department; OHA, oral hypoglycaemic agents; BMI, body mass index; HbA1c, glycosylated haemoglobin; DEXA, dual energy X-ray absorptiometry.
Table I.
Baseline demographic characteristics in study and control groups
The mean duration of T2DM in study group was 6.44±3.1 yr; mean HbA1c (%) was 8.6±2. Fourteen patients were using either TZD alone or in combination with other OHA; the remaining 27 patients were using other OHA alone or in combination. At the time of evaluation, 21 of 41 (51%) patients in the study group were receiving treatment with atorvastatin alone (n=20), atorvastatin in combination with ezetimibe (n=1) for dyslipidemia; and 19 (46.3%) of the 41 patients were receiving treatment for hypertension. Overall, BMD in postmenopausal women was significantly less at the NOF (0.738±0.098 vs 0.810±0.119; P=0.039) and LSAP (0.810±0.110 vs 0.93±0.193; P=0.008) compared to premenopausal women. There was no significant difference in the BMD between T2DM patients with good and poor glycaemic control at NOF and LSAP.
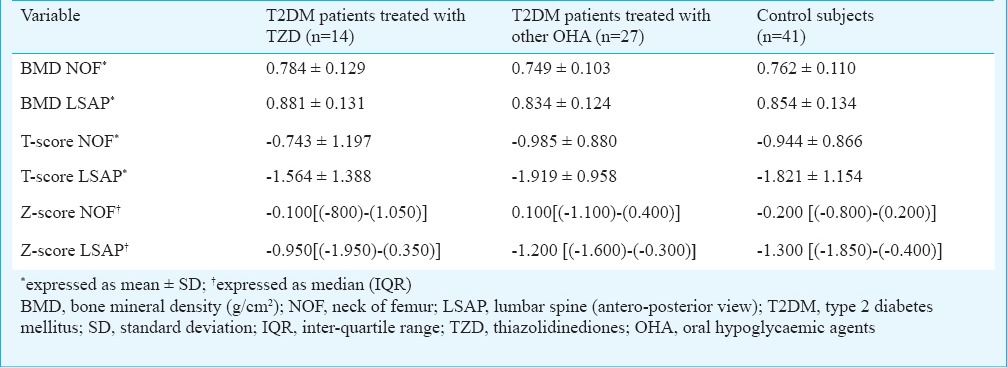
The BMD (g/cm2) and Z-scores at NOF and LSAP were comparable between the study group and controls (Table II). There was no significant difference in the presence of normal BMD (g/cm2) (9/41 vs. 8/41), osteopenia (16/41 vs. 18/41) and osteoporosis (16/41 vs. 15/41) between the study and control groups. On comparing T2DM patients treated with TZD (n=14); those treated with other OHA (n=27) and normal controls (n=41), one-way ANOVA did not show any significant difference in the BMD NOF and LSAP; T-scores at the level of NOF; LSAP view and Z-scores at the level of NOF and LSAP view (Table III).
Table II.
Comparison of BMD between patients with T2DM and healthy control
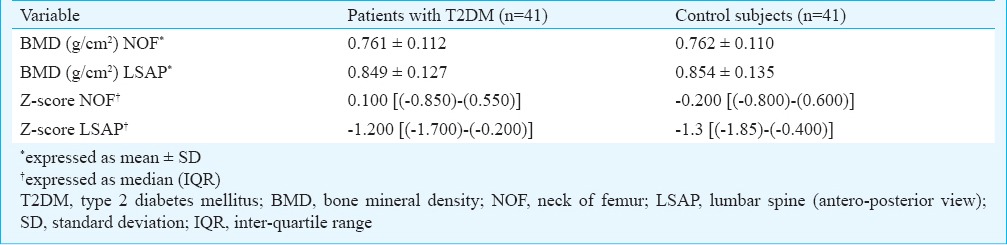
Table III.
Comparison of BMD between patients with type 2 diabetes mellitus treated with thiazolidinediones; those treated with other oral hypoglycaemic agents and control subjects
Among postmenopausal women, osteoporosis was evident in 13 of 26 (50%) T2DM patients and 12 of 25 (48%) control subjects and the difference was not significant. Patients with T2DM as well as controls who had a BMI (kg/m2) less than 25 had a marginally lower BMD at the level of NOF but, not at the level of LSAP when compared to those with BMI more than 25. A curious phenomenon was observed in the behaviour of BMD across the age groups. In the study group, the BMD (both at NOF and LSAP) declined from 40-45 yr through 46-50 yr up to 51-55 yr and again increased in 56-60 yr. In healthy controls the BMD declined from 40-45 yr through 46-50 yr, increased in the 51-55 yr and again declined in 56-60 yr age group. This observation was consistent when both the genders were considered together; and also when only women were considered as a sub-group in both the study and control groups.
Discussion
In the present study, patients receiving OHA for a period of at least three years or more were included. This facilitated inclusion of patients in whom sufficient time had elapsed for detecting the effect of the OHA on BMD. Stringent exclusion criteria were employed in the present study to exclude patients who were at risk of developing osteoporosis due to various other diseases and conditions. Further, patients with type 1 diabetes mellitus (T1DM) were also excluded. T1DM is known to be associated with low BMI, which in turn is a risk factor for osteoporosis13 and T1DM manifests at a much young age than T2DM and can affect the peak bone mass (which in turn may affect BMD)14 at the age group considered for inclusion into the study.
Osteoporosis was observed in 39 per cent of T2DM patients and 36.5 per cent of the normal population. Other studies from India15, and other parts of the world16,17,18,19 also documented a high prevalence of osteoporosis in postmenopausal women with T2DM. These observations suggest that T2DM need not necessarily be a protective factor against development of osteoporosis as was considered previously. In our study, 50 per cent postmenopausal women in the study group and 48 per cent among controls had osteoporosis. In a study20 from Vellore (Tamil Nadu State) which is near Tirupati in south India, high prevalence of osteoporosis was observed among healthy postmenopausal women. This could have been due to the low dietary vitamin D and calcium intake, high prevalence of vitamin D deficiency and higher dietary intake of phytates in this geographic region of the country as documented in an earlier study published from our Institute21. In another study22 from south India, the risk of bone resorption was observed to be greater in early years than late years of menopause. This was attributed to decreased bone resorption risk in late postmenopausal women which might be due to increased follicular stimulating hormone levels.
In a study from Brazil17, the mean age of the patients was a decade older than in the present study and patients with history of fractures and patients with CKD were also included; no control subjects were studied. In spite of excluding patients with previous history of fracture and CKD, the occurrence of osteoporosis observed in the present study was similar to the observations from Brazil17 suggesting that the actual burden of osteoporosis in T2DM patients using OHA and in normal population in India may be much higher. In a study from Turkey16, patients with T2DM and control subjects did not differ on BMDs and T-scores at the hip, LS, and radius. Patients with radial and/or lumbar and/or hip osteoporosis had a longer duration of DM, were older and had a lower BMI16. In our study also, a higher BMI was associated with higher BMD but, no association was observed between the extent of glycaemic control and BMD.
A higher prevalence of osteoporosis was observed in postmenopausal women with T2DM in Saudi Arabia compared with general population18. Racial and ethnic differences may have influenced this phenomenon. A significantly lower BMD, T- and Z-scores at various skeletal regions were reported in non-obese, postmenopausal women with T2DM from China19. In this study19, patients on TZD and premenopausal women were excluded whereas patients receiving OHA, insulin treatment were included. Obese women and control subjects were found to have similar BMDs and T- and Z-scores at various skeletal regions. This study18 also showed a positive correlation between fasting serum insulin levels with BMD at the LS, NOF and Ward's triangle. Age at onset and duration of menopause did not have any significant effect on BMD19. In our study, postmenopausal women with T2DM with BMI less than 25 kg/m2 had marginally lower BMD at NOF with a trend towards significance (P=0.05) but, not at the level of LSAP when compared to patients with T2DM with BMI more than 25 kg/m2. A similar relation was also evident in postmenopausal women in control population (P =0.049).
In other studies16,17,18,19 patients who were using TZD group of agents were excluded. We observed that there was no significant difference in the mean BMD, the median Z-scores and the mean T-scores at various skeletal regions between patients receiving TZD, other OHA and control subjects suggesting that use of oral hypoglycaemic agents for a minimum period of three years has not significantly affected the BMD in patients with T2DM. The pattern of the BMD observed across the age groups in both the study and control groups is contrary to the usual expected pattern of BMD which decreases with increasing age. Whether this behaviour reflects a hitherto undescribed observation or is the consequence of a small sample size needs further clarification.
In the present study, 51 per cent patients with T2DM with dyslipidemia were receiving therapy with statins. Data from some in vitro studies23,24 suggest that statins have a positive pleotropic effect on bone mineral density. But such an efficacy in humans is yet to be clearly demonstrated. The quantum of gain in BMD in humans due to statin therapy in the age group under discussion is not known. Use of statins for simultaneous treatment of dyslipidemia and osteoporosis is not an approved indication at present. As data for statin use among control subjects were not available, adjustment for this confounding factor during analysis could not be done.
Non-availability of group of patients using insulin alone for T2DM for comparison was a limitation for this study. The study included patients attending a tertiary health care facility and the observations from the present study may not reflect the real scenario in the community. The non-availability of the normative data regarding BMD in Indian population is a significant limiting factor. Further studies with larger sample size are required to develop BMD data for Indian population. Given the fact that the higher fracture risk in patients with T2DM is independent of BMD, screening, recognition, and institution of preventive measures for osteoporosis in T2DM patients is warranted so as to preserve a good quality of life in these patients.
References
- 1.Kaveeshwar SA, Cornwall J. the current state of diabetes mellitus in India. Australas Med J. 2014;7:45–8. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Preventive Services Task Force. Screening for Osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356–64. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 3.Meeta, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on post menopausal osteoporosis: An executive summary and recommendations. J Midlife Health. 2013;4:107–26. doi: 10.4103/0976-7800.115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–54. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 5.Lecka-Czernik B. Bone as a target of type 2 diabetes treatment. Curr Opin Investig Drugs. 2009;10:1085–90. [PubMed] [Google Scholar]
- 6.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione (TZD) use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–54. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27:319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulameer SA, Sulaiman SA, Hassali MA, Subramaniam K, Sahib MN. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do? Patient Prefer Adherence. 2012;6:435–48. doi: 10.2147/PPA.S32745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Bone health in healthy Indian population aged 50 years and above. Osteoporos Int. 2011;22:2829–36. doi: 10.1007/s00198-010-1507-8. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 11.Report of a WHO Study Group, WHO Tech Rep Ser 843. Geneva: WHO; 1994. World Health Organization (WHO). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis; pp. 1–129. [PubMed] [Google Scholar]
- 12.Rockville MD, USA: U.S. Department of Health and Human Services, office of the Surgeon General; 2004. Bone health and osteoporosis: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 13.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–6. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leslie WD, Adler RA, El-Hajj Fuleihan G, Hodsman AB, Kendler DL, McClung M, et al. International Society for Clinical Densitometry. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:22–30. doi: 10.1016/j.jocd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarty N, Sarkar P, Pal SK, Banerjee R, Sarkar RN, Debnath NB. A study of bone mineral density in diabetes mellitus in eastern India. J Indian Med Assoc. 2004;102:418–20. [PubMed] [Google Scholar]
- 16.Anaforoglu I, Nar-Demirer A, Bascil-Tutuncu N, Ertorer ME. Prevalence of osteoporosis and factors affecting bone mineral density among postmenopausal Turkish women with type 2 diabetes. J Diabetes Complications. 2009;23:12–7. doi: 10.1016/j.jdiacomp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Viégas M, Costa C, Lopes A, Griz L, Medeiro MA, Bandeira F. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complications. 2011;25:216–21. doi: 10.1016/j.jdiacomp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Al-Maatouq MA, El-Desouki MI, Othman SA, Mattar EH, Babay ZA, Addar M. Prevalence of osteoporosis among postmenopausal females with diabetes mellitus. Saudi Med J. 2004;25:1423–7. [PubMed] [Google Scholar]
- 19.Zhou Y, Li Y, Zhang D, Wang J, Yang H. Prevalence and predictors of osteopenia and osteoporosis in postmenopausal Chinese women with type 2 diabetes. Diabetes Res Clin Pract. 2010;90:261–9. doi: 10.1016/j.diabres.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Paul TV, Thomas N, Seshadri MS, Oommen R, Jose A, Mahendri NV. Prevalence of osteoporosis in ambulatory postmenopausal women from a semiurban region in Southern India: relationship to calcium nutrition and vitamin D status. Endocr Pract. 2008;14:665–71. doi: 10.4158/EP.14.6.665. [DOI] [PubMed] [Google Scholar]
- 21.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PV, Sarma KV, et al. High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am J Clin Nutr. 2007;85:1062–7. doi: 10.1093/ajcn/85.4.1062. [DOI] [PubMed] [Google Scholar]
- 22.Suresh M, Naidu DM. Influence of years since menopause on bone mineral metabolism in South Indian women. Indian J Med Sci. 2006;60:190–8. [PubMed] [Google Scholar]
- 23.Luisetto G, Camozzi V. Statins, fracture risk, and bone remodeling. J Endocrinol Invest. 2009;32:32–7. [PubMed] [Google Scholar]
- 24.Tang QO, Tran GT, Gamie Z, Graham S, Tsialogiannis E, Tsiridis E, et al. Statins: under investigation for increasing bone mineral density and augmenting fracture healing. Expert Opin Investig Drugs. 2008;17:1435–63. doi: 10.1517/13543784.17.10.1435. [DOI] [PubMed] [Google Scholar]


