Abstract
Background & objectives:
Tuberculosis (TB) is a common infection in patients on haemodialysis. There is a definite role of treatment of latent TB (LTB) in these patients. However, diagnosis of LTB in these patients by tuberculin skin test (TST) is unreliable. There is suggestion that interferon gamma release assay (IGRA) will be more reliable test for diagnosis of LTB in this setting. Thus, we evaluated value of IGRA and TST for the diagnosis of LTB in patients on dialysis in an Indian setting.
Methods:
Patients with end stage kidney disease on dialysis were included. Patients with active TB were excluded. Each patient was subjected to TST (induration of ≥10 mm was taken as positive) and QuantiFERON TB Gold In-Tube test (QFT-GIT) for diagnosis of LTB.
Results:
A total of 185 patients were included; 129 (69.7%) were males and mean age was 36.7 ± 12.3 yr. Past history of TB was present in 18 (9.7%) patients. One hundred and thirty four (72.4%) patients had scar of BCG vaccination. QFT-GIT test was positive in 66 (36%), TST in 32 (17%) and both in 13 (7%) patients. Of the 66 patients positive with QFT-GIT, only 13 (19.6%) were positive for TST. Of the 32 patients positive with TST, only 13 (40.6%) were positive with QFT-GIT; 100 (54%) patients were negative for both the tests. Overall, 85 (45.9%) patients were positive for either of the two tests. Poor agreement was shown between the two methods. On logistic regression analysis, odds of QFT-GIT to be positive in patients with BCG vaccination was 1.23 and with history of TB 0.99, both being insignificant. odds of tuberculin skin test to be positive in patients with BCG vaccination was 1.04 and with history of TB 0.99, both again being insignificant.
Interpretation & conclusions:
Our findings showed that more number of patients (36%) on haemodialysis were positive for QuantiFERON Gold In-Tube test as compared to TST (17%). There was poor agreement between the two tests. No significant effect of BCG vaccination and history of TB in past was observed on both tests.
Keywords: Haemodialysis, India, IGRA, latent tuberculosis, QuantiFERON, TB gold test, tuberculin test
Chronic kidney disease (CKD) patients on maintenance haemodialysis (MHD) are a high risk group for tuberculosis (TB)1,2 partly due to progression from latent TB (LTB) to active disease, secondary to impaired immunity associated with CKD3. Screening patients at high risk for development of TB and use of therapy for LTB infection to prevent the development of active tuberculosis is well-accepted strategy for tuberculosis control. We have earlier demonstrated that use of isoniazid (INH) chemoprophylaxis in patients on renal replacement therapy (RRT) significantly reduces the risk of development of TB at a later stage4,5. However, the key question about the best test for the diagnosis of LTB in this clinical situation remains unanswered.
Routine tuberculin skin testing (TST) also called Mantoux test (MxT) in these patients is unreliable, partly because of high rates of anergy among MHD patients6. Further, in conditions, which alter the cutaneous response to TST, like CKD, significance of TST for the diagnosis of LTB is controversial. We had shown that TST was positive only in 10 per cent patients in haemodialysis setting and concluded it to be insensitive test for risk prediction of development of TB at later stage7. TST test has other limitations also including false negative results in patients with malnutrition, concurrent viral illness, inter-individual variability of placement and reading of TST and the requirement of the patient to revisit hospital after 48-72 h for test reading8.
Discovery of the role of T lymphocytes and interferon-γ assay in the immune process has led to the development of an in vitro assay for cell-mediated immune reactions to Mycobacterium tuberculosis. In 2001, the QuantiFERON®-TB test (QFT), interferon γ release assay (IGRA) was approved by the Food and Drug Administration (FDA) as an aid for detecting latent M. tuberculosis infection9. Three IGRAs are currently approved by the US Food and Drug Administration: QuantiFERON-TB Gold (QFT), QuantiFERON-TB Gold In-Tube (QFT-GIT), and the T-SPOT. TB. All IGRA tests measure the cell-mediated immune response to a mixture of synthetic peptides that are derived from M. tuberculosis: early secretory antigenic target-6 and culture filtrate protein-10; the QFT-GIT version also includes the peptideTB7.710. IGRA is said to have a lower rate of false - positive results and avoid cross-reactivity with most non-TB mycobacteria, including subjects with prior Bacille Calmette-Guerin (BCG) vaccination. False negative results are reported to occur less frequently in patients who have defects in cell-mediated immunity as compared to TST11. The CDC guidelines state that ‘an IGRA may be used in place of TST in all situations in which CDC recommends TST as an aid in diagnosing M. tuberculosis infection’12. Considering all these issues, IGRA is expected to perform better as compared with TST in patients on maintenance dialysis. This becomes much more important in a country like India, where BCG vaccination is routinely given in the national immunization programme.
There is paucity of data on utility of IGRA in haemodialysis setting. In a study, using TST, T-SPOT, and QFT-GIT in 167 haemodialysis patients, positivity rate was shown to be 23.5, 60.4, and 45.9 per cent, respectively. TST positivity was poor in all patients, while QFT-GIT was only positive in patients at high risk of TB exposure, whereas positive T-SPOT tests were found in both high- and low-risk groups13. Another study from West while comparing TST and T-SPOT in haemodialysis patients has shown positive results in 12.8 and 35.5 per cent, respectively14. This study was conducted to compare the performance of TST and IGRA for the diagnosis of LTB in haemodialysis patients in Indian setting.
Material & Methods
This cross-sectional study was performed in the department of Nephrology, All India Institute of Medical Sciences (AIIMS), New Delhi, India from May 2007 to August 2010. All patients of end stage kidney disease (ESKD) taken for haemodialysis followed by renal transplant (RT) at our hospital who gave informed written consent were included in the study. Patients who had active TB at the time of inclusion in the study, or received immunosuppressive medication in the last six months, had acute kidney injury or with end stage kidney disease but not for renal transplant with us were excluded. The study protocol was approved by the Institute ethics committee.
The sample size for the study was calculated to compute estimated disagreement between the two methods. To estimate extent of disagreement between the two methods up to 25 per cent with an absolute precision of 10 % and 95 % confidence level, required number of subjects was 750 which was not feasible to enroll during the planned study period. Therefore, it was decided to enroll all the eligible patients into the study during the first two and half year of the three year study period.
Patients of ESKD accepted for haemodialysis and scheduled for renal transplant at our hospital formed the subjects of the study. Patients were dialyzed two or three times a week depending upon their clinical need. Before inclusion, all patients were evaluated by detailed clinical history and thorough physical examination and plain X-ray chest for any evidence of active tuberculosis. Patients who had a BCG vaccination scar confirmed by physicians were regarded as patients with a previous BCG vaccination.
All patients were subjected to TST following standard method15 on non-dialysis day. In short, the test was carried out by intracutaneous inoculation of 0.1 ml tuberculin solution (Span Diagnostic Ltd., India) containing five tuberculin unit (TU) into the volar surface of forearm. Tuberculin was diluted with a special buffer containing Tween-80 as a stabilizer and ready to use solution. Source material had been calibrated against batch RT-23 manufactured by Statens Serum Institute, Denmark. The resulting induration was measured 48 to 72 h after the test. An induration of 10 mm or more was considered positive for the diagnosis of LTB. All patients were also subjected to QFT-GIT test before doing TST as per the manufacturer's instructions (Cellestis Ltd., Carnegie, Victoria, Australia)16. Blood (5 ml) for QFT-GIT test was drawn on non-dialysis days.
Data were recorded on a pre-designed proforma. Categorical variables were summarized by frequency and their percentage. Quantitative variables were assessed for their approximate normal distribution. The variables following approximate normal distribution were compared using Student's t test. For the non-normal variables, Wilcoxon's rank sum test was used to compare median values in different groups. To evaluate the effect of variables, Cox regression analysis was done. STATA 10 (Intercooled version) (Statacorp LP, Texas, USA) was used for statistical analysis. Agreement analysis between the two tests was done using McNemar chi square and kappa tests.
Results
A total of 185 patients fulfilling inclusion criteria were included in the study. Pattern of primary disease in these patients was presumed chronic glomerulonephritis (CGN) in 67 (36.2%) patients, presumed chronic tubulointerstitial disease (TID) in 40 (21.8%), polycystic kidney disease (PKD) in 11 (5.7%), diabetic nephropathy (DN) in seven (4%) and others in 60 (32.3%) cases. Basic demographic details of all the subjects are shown in Table I. Of the 185 patients, QFT-GIT test was positive in 66 (36%) patients and TST in 32 (17%) patients (Table II). Further analysis showed that both results were positive in 13 and both were negative in 100, the overall agreement being 113 patients, namely, 61 per cent. In the remaining 72 patients with disagreement of results, 53 were QFT positive but TST negative as compared with 19 who were TST positive but QFT negative, (P<0.001). (Table II). When both tests were combined for making diagnosis of latent tuberculosis, 85 (46%) patients were positive for either of the two tests. The McNemar chi square test between the two methods was significant (P<0.001), and also, agreement between two tests with kappa was 0.0421 (standard error = 0.0656), both indicating poor agreement between the two methods.
Table I.
Basic demographic details of patients with positive results
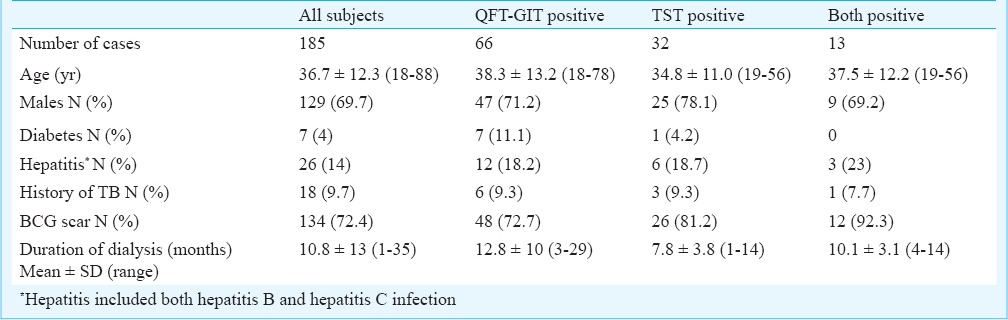
Table II.
Assessment of QFT and TST in study subjects

Logistic regression analysis was done to assess the impact of BCG vaccination and history of tuberculosis on QFT-GIT test and TST. Odds of QFT-GIT test to be positive in patients with BCG vaccination was 1.23 and with history of tuberculosis 0.99, both being insignificant. Odds of tuberculin skin test to be positive in patients with BCG vaccination was 1.04 and with history of tuberculosis 0.99, both again being insignificant.
Discussion
In India tuberculosis is an endemic infection. Considering the number of patients on haemodialysis waiting for renal transplant and with the risk of activation of tuberculosis, burden of tuberculosis is high in these patients. Further, in view of studies showing benefit of chemoprophylaxis4,5, there is a need for accurate diagnosis of latent tuberculosis infection in these patients. We compared QFT-GIT test with tuberculin skin testing for the diagnosis of LTB in patients on maintenance haemodialysis. A higher percentage of patients was found to be positive with QFT-GIT test as compared to TST. There are limited published studies13,14,17,18,19,20,21,22,23,24 in haemodialysis patients comparing IGRA and TST for the diagnosis of latent TB infection and main features of these studies are shown in Table III. Some of these were from low burden countries and a few from intermediate burden countries. There is no published study from high burden country. In low TB burden countries, TST positivity has been shown in the range of 12-26 per cent, while in intermediate TB burden countries, this range is 10-62 per cent. In our study from a high burden country, TST was positive in 17 per cent patients. It is possible that different degree of immunosuppressive state may be one of the factors responsible for different positivity rate in addition to overall burden of tuberculosis in that country. However, this issue has not been addressed in any of the studies.
Table III.
Comparison of IGRA and TST in haemodialysis patients with salient features
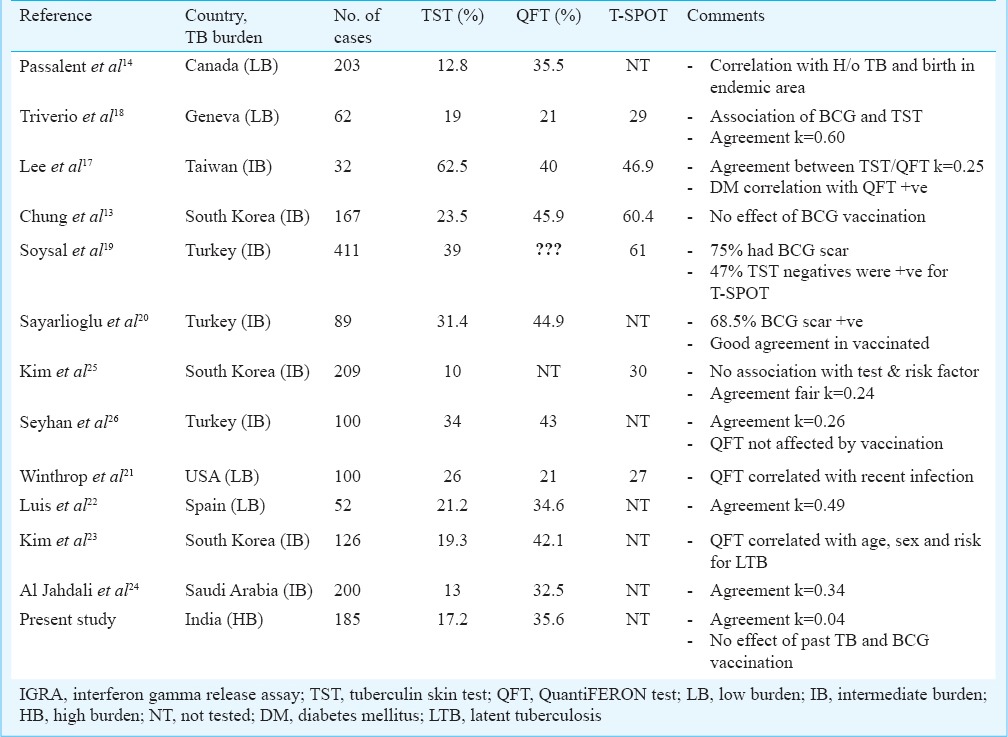
Other than absolute TST positivity, in almost all the studies, including present study, positivity in IGRA has been significantly higher as compared to TST. Only in one study by Lee et al17, TST positivity (62.5%) was higher than both QFT-GIT (40%) and T-spot test (46.9%). In a study by Triverio et al18, agreement between TST and IGRA was fair (k=0.60), while in other three studies agreement was poor (k=0.24, 0.25 and 0.26, respectively)17,20,21. In our study also, there was poor agreement (kappa=0.04) between the two tests.
Different studies have done analysis of factors affecting positivity of these tests. Major factors being analysed had been geographic region, associated diabetes mellitus, past history of tuberculosis and previous BCG vaccination. Lee et al17 showed a positive correlation between diabetes and QFT-GIT. Passalent et al14 showed correlation of tests with birth of the subject in endemic area of tuberculosis and past history of tuberculosis. The major area of concern has been effect of prior BCG vaccination on TST and QuantiFERON test. In a systematic review by Rogerson et al27 the key findings were that compared with the TST, ELISA IGRA positivity was associated more strongly with clinical risk factors for latent TB, while associated less strongly with prior BCG vaccination. This suggests that ELISA IGRA is more sensitive and specific than the TST in the context of end-stage kidney disease. However, in the present study no impact of BCG vaccination was observed on the results of both the tests.
There is only a limited number of studies available on the issue of diagnosis of latent tuberculosis in advanced stage of CKD including dialysis. There are no specific guidelines for the end-stage kidney disease population. The recommendations from the United Kingdom indicate the use of IGRAs with or without a TST in people with chronic kidney disease28. Canadian guidelines for immunosuppressed persons recommend using the TST with or without a supplementary IGRA29 whereas the guidelines from United Kingdom and Switzerland recommend replacing TST with an IGRA28,30,31.
The major limitation of the present study was that only 185 patients could be enrolled against the desirable sample size. There is a need for a large multicentric study to assess the different IGRA and tuberculin skin test in similar high burden population of tuberculosis.
Another area of concern for the diagnosis of latent tuberculosis is that there is no gold standard test for its diagnosis. The best gold standard test will be actual development of active tuberculosis in these patients during follow up. However, other strategies which have been used to address this issue are evaluation of the proportion of positive tests among individuals with active tuberculosis (as a proxy for sensitivity), proportion of negative tests among individuals at low risk for tuberculosis infection (as a proxy for specificity), assessment of the association of test results with risk factors for tuberculosis infection and statistical approach called “latent class analysis”, a method which has been proposed for the assessment of diagnostic tests in the absence of a gold standard32. It follows as a corollary hence that there is little to confirm which of the two tests is actually a true positive as far as the diagnosis of latent TB is concerned. Both the tests seem to use different arms of the immune system to arrive at one conclusion.
In conclusion, our study showed that more number of patients on maintenance haemodialysis in India were positive for interferon γ release assay (QuantiFERRON Gold- In Tube) test as compared to tuberculin skin test. There was poor agreement between the two tests for the diagnosis of latent tuberculosis in this patient population. Prior BCG vaccination and past history of tuberculosis had no effect on these tests. In the absence of a gold standard test for diagnosing latent TB, the question of ‘true positives’ looms large.
Acknowledgment
The authors acknowledge the Indian Council of Medical Research, New Delhi, for financial support.
References
- 1.Garcia-Leoni ME, Martin-Scapa C, Rodeno P, Valderrabano F, Moreno S, Bouza E. High incidence of tuberculosis in renal patients. Eur J Clin Microbiol Infect Dis. 1990;9:283–5. doi: 10.1007/BF01968062. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK. Tuberculosis in chronic renal failure. In: Sharma SK, Mohan A, editors. Tuberculosis. New Delhi: Jaypee Brothers; 2001. pp. 338–47. [Google Scholar]
- 3.Touraine JL, Touraine F, Revillard JP, Brochier J, Traeger J. T-lymphocytes and serum inhibitors of cell mediated immunity in renal insufficiency. Nephron. 1975;14:195–208. doi: 10.1159/000180448. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Gupta S, Dash SC, Bhowmik D, Tiwari SC. Prospective randomised trial of isoniazid prophylaxis in renal transplant recipient. Int Urol Nephrol. 2004;36:425–31. doi: 10.1007/s11255-004-6251-6. [DOI] [PubMed] [Google Scholar]
- 5.Vikrant S, Agarwal SK, Gupta S, Bhowmik D, Tiwari SC, Dash SC, et al. Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transplant Inf Dis. 2005;7:99–108. doi: 10.1111/j.1399-3062.2005.00103.x. [DOI] [PubMed] [Google Scholar]
- 6.Rutsky EA, Rostand SG. Mycobacteriosis in patients with chronic renal failure. Arch Intern Med. 1980;140:57–61. [PubMed] [Google Scholar]
- 7.Agarwal SK, Gupta S, Tiwari SC. Tuberculin skin test for the diagnosis of latent tuberculosis infection during renal replacement therapy in an endemic area: A single center study. Indian J Nephrol. 2010;20:132–6. doi: 10.4103/0971-4065.70842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sester M, Sester U, Clauer P, Heine G, Mack U, Moll T, et al. Tuberculin skin testing underestimates a high prevalence of latent tuberculosis infection in hemodialysis patients. Kidney Int. 2004;65:1826–34. doi: 10.1111/j.1523-1755.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- 9.Rockville, MD: Food and Drug Administration; 2002. [accessed on April 24, 2015]. Food and Drug Administration, Center for Devices and Radiological Health. QuantiFERON®-TB - P010033. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cftopic/pma/pma.cfm?num=p010033 . [Google Scholar]
- 10.Sester M, Sotgiu G, Lange C, Giehl C, Giradi E, Miqliori GB, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta analysis. Eur Respir J. 2011;37:100–1. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 11.Piana F, Codecasa LR, Cavallerio P, Ferrarese M, Miqliori GB, Barbarano L, et al. Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients. Eur Respir J. 2006;28:31–4. doi: 10.1183/09031936.06.00110205. [DOI] [PubMed] [Google Scholar]
- 12.Mazurek M, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 13.Chung WK, Zheng ZL, Sung JY, Kim S, Lee HH, Choi SJ, et al. Validity of interferon-gamma release assays for the diagnosis of latent tuberculosis in haemodialysis patients. Clin Microbiol Infect. 2010;16:960–5. doi: 10.1111/j.1469-0691.2009.02949.x. [DOI] [PubMed] [Google Scholar]
- 14.Passalent L, Khan K, Richardson R, Wang J, Dedier H, Gardam M. Detecting latent tuberculosis infection in hemodialysis patients: A head-to-head comparison of the T-SPOT. TB test, tuberculin skin test, and an expert physician panel. Clin J Am Soc Nephrol. 2007;2:68–73. doi: 10.2215/CJN.01280406. [DOI] [PubMed] [Google Scholar]
- 15.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 16.Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleq AY, et al. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant. 2007;7:2797–801. doi: 10.1111/j.1600-6143.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee SS, Chou KJ, Su IJ, Chen YS, Fang HC, Huang TS, et al. High prevalence of latent tuberculosis infection in patients in end-stage renal disease on hemodialysis: Comparison of QuantiFERON-TB GOLD, ELISPOT, and tuberculin skin test. Infection. 2009;37:96–102. doi: 10.1007/s15010-008-8082-3. [DOI] [PubMed] [Google Scholar]
- 18.Triverio PA, Bridevaux PO, Roux-Lombard P, Niksic l, Rochat T, Martin PY, et al. Interferon-gamma release assays versus tuberculin skin testing for detection of latent tuberculosis in chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:1952–6. doi: 10.1093/ndt/gfn748. [DOI] [PubMed] [Google Scholar]
- 19.Soysal A, Toprak D, Koc M, Arikan H, Akoglu E, Bakir M. Diagnosing latent tuberculosis infection in haemodialysis patients: T-cell based assay (T-SPOT.TB) or tuberculin skin test? Nephrol Dial Transplant. 2012;27:1645–50. doi: 10.1093/ndt/gfr516. [DOI] [PubMed] [Google Scholar]
- 20.Sayarlıoğlu H, Gul M, Eren Dağlı C, Dogan E, Sahin M, Ucar MA, et al. QuantiFERON-TB Gold test for screening latent tuberculosis infection in hemodialysis patients. Tuberk Toraks. 2011;59:105–10. [PubMed] [Google Scholar]
- 21.Winthrop KL, Nyendak M, Calvet H, Oh P, Lo M, Swarbrick G, et al. Interferon- gamma release assays for diagnosing Mycobacterium tuberculosis infection in renal dialysis patients. Clin J Am Soc Nephrol. 2008;3:1357–63. doi: 10.2215/CJN.01010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luis A, Matilde T, Diana F, Mónica R, Luisa P, Alberto P, et al. Value of the tuberculin skin testing and of an interferon-gamma release assay in haemodialysis patients after exposure to M. tuberculosis. BMC Infect Dis. 2012;12:195. doi: 10.1186/1471-2334-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, Jung GS, Kim SK, Chang J, Kim MS, Kim YS, et al. Comparison of the tuberculin skin test and interferon-γ release assay for the diagnosis of latent tuberculosis infection before kidney transplantation. Infection. 2013;41:103–10. doi: 10.1007/s15010-012-0291-0. [DOI] [PubMed] [Google Scholar]
- 24.Al Jahdali H, Ahmed AE, Balkhy HH, Baharoon S, Al Hejaili FF, Hajeer A, et al. Comparison of the tuberculin skin test and Quanti-FERON-TB Gold In-Tube (QFT-G) test for the diagnosis of latent tuberculosis infection in dialysis patients. J Infect Public Health. 2013;6:166–72. doi: 10.1016/j.jiph.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Lee SO, Park IA, Park SJ, Choi SH, Kim YS, et al. Diagnostic usefulness of a T cell-based assay for latent tuberculosis infection in kidney transplant candidates before transplantation. Transplant Infect Dis. 2010;12:113–9. doi: 10.1111/j.1399-3062.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 26.Seyhan EC, Sokucu S, Altin S, Gunluoqlu G, Trablus S, Yilmaz D, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transplant Infect Dis. 2010;12:98–105. doi: 10.1111/j.1399-3062.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Rogerson TE, Chen S, Kok J, Hayen A, Craig JC, Sud K, et al. Tests for latent tuberculosis in people with ESRD: A systematic review. Am J Kidney Dis. 2013;61:33–43. doi: 10.1053/j.ajkd.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 28.British Thoracic Society Standards of Care Committee. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;65:557–70. doi: 10.1136/thx.2009.133173. [DOI] [PubMed] [Google Scholar]
- 29.Canadian Tuberculosis Committee. Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS) Can Commun Dis Rep. 2008;34:1–13. [PubMed] [Google Scholar]
- 30.London: HPA; 2007. Health Protection Agency (HPA). Health Protection Agency Position Statement on the use of interferon gamma release assay (IGRA) tests for tuberculosis (TB) [Google Scholar]
- 31.Berne: SLA; 2011. Swiss Lung Association (SLA). Tuberculosis in Switzerland. [Google Scholar]
- 32.Girardi E, Angeletti C, Puro V, Sorrentino R, Magnavita N, Vincenti D, et al. Estimating diagnostic accuracy of tests for latent tuberculosis infection without a gold standard among healthcare workers. Euro Surveill. 2009;14 pii: 19373. [PubMed] [Google Scholar]


