Abstract
The sickle gene is widespread among many tribal population groups in India with prevalence of heterozygotes varying from 1-40 per cent. Co-inheritance of the sickle gene with β-thalassaemia, HbD Punjab and glucose-6-phosphate dehydrogenase (G6PD) deficiency has also been reported. Most of the screening programmes in India now use high performance liquid chromatography (HPLC) analysis although the solubility test is also sensitive and cheap. Sickle cell disease (SCD) among tribal populations is generally milder than among non-tribal groups with fewer episodes of painful crises, infections, acute chest syndrome and need for hospitalization. This has partly been attributed to the very high prevalence of α-thalassaemia among these tribes as well as higher foetal haemoglobin levels. However, the clinical presentation is variable with many cases having a severe presentation. There is not much information available on maternal and perinatal outcome in tribal women with sickle cell disease. Newborn screening programmes for SCD have recently been initiated in Maharashtra, Gujarat, Odisha and Chattisgarh and monitoring these birth cohorts will help to understand the natural history of SCD in India. Prenatal diagnosis is acceptable by tribal families in India. The Indian Council of Medical Research and the National Rural Health Mission in different States are undertaking outreach programmes for better management and control of the disease.
Keywords: Sickle cell disease, sickle cell gene, tribes
Introduction
Sickle cell disease(SCD) is one of the most common monogenic disorders globally with an autosomal recessive inheritance1. James Herrick, a physician first described the characteristic sickle shaped red cells in a medical student from Grenada in 1910. Linus Pauling and his colleagues showed that sickle haemoglobin (HbS) had an altered electrophoretic mobility and they were the first to define it as a molecular disease in 1949. A few years later in 1957, Vernon Ingram discovered that sickle haemoglobin resulted from a single amino acid substitution in the haemoglobin molecule2,3. The disease results from a single base A>T mutation in the triplet encoding the sixth residue of the β-globin chain, leading to a substitution of valine for glutamic acid and the abnormal haemoglobin S (HbS).
The primary pathophysiology is based on the polymerization of deoxyHbS with formation of long fibers within the RBCs causing a distorted sickle shape which eventually leads to increased haemolysis and vaso-occlusion of sickle red cells. However, the clinical presentation of SCD patients is extremely variable and there are several events that may trigger vaso-occlusion. Recent work has shown the importance of red cell dehydration, abnormal adhesion of RBCs to the vascular endothelium, imflammatory events, and activation of all the cells in the vessel and abnormalities of nitric oxide metabolism in the pathophysiology of this multi-organ disease4.
The sickle gene is an example of balanced polymorphism. Heterozygotes have a selective advantage and are protected against Plasmodium falciparum malaria while there is an increased premature death rate of homozygotes2.
The tribal populations of India
India has the largest concentration of tribal populations globally. They are believed to be the early settlers in the country and are considered to be the original inhabitants. According to the Census of India 20115, the tribal population of India is 8.6 per cent of the total population which is about 67.8 million people. The States of Madhya Pradesh, Maharashtra, Odisha, Gujarat, Rajasthan, Jharkhand, Chhattisgarh, Andhra Pradesh, West Bengal and Karnataka account for around 83 per cent of the total scheduled tribe population in the country and majority of these tribal groups live in rural areas. In all, 461 scheduled tribes have been listed6 and they have their own characteristic cultural patterns, languages and social systems, by and large keeping to themselves. However, Reich et al7 concluded that “several thousand years ago, the entire subcontinent underwent a period of massive intermarriage, shuffling its population's genetic deck so thoroughly that it left clear traces even in the genomes of today's most isolated tribes”.
Prevalence of sickle gene in tribal communities in India
The first description of sickle haemoglobin in India was by Lehman and Cutbush in 1952 in the tribal populations in the Nilgiri hills in south India8. In the same year, Dunlop and Mazumder also reported the presence of sickle haemoglobin in the tea garden workers of Upper Assam who were migrant labourers from tribal groups in Bihar and Odisha9. Since then, many population groups have been screened and the sickle cell gene has been shown to be prevalent among three socio-economically disadvantaged ethnic groups, the scheduled tribes, scheduled castes and other backward classes in India10,11,12,13,14,15,16,17.
The prevalence of sickle cell carriers among different tribal groups varies from 1 to 40 per cent10. Madhya Pradesh has the highest load with an estimated number of 9, 61,492 sickle heterozygotes and 67,861 sickle homozygotes11. Further, 27 of the 45 districts in Madhya Pradesh fall under the sickle cell belt and the prevalence of HbS varies from 10 to 33 per cent11. It has also been estimated that 13,432 pregnancies would be at risk of having a child with sickle cell disease in this State and the expected annual births of sickle homozygotes would be 335818. Gonds and Bhils constitute the largest tribal groups in central India.
In Maharashtra, the sickle gene is widespread in all the eastern districts, also known as the Vidarbha region, in the Satpura ranges in the north and in some parts of Marathawada. The prevalence of sickle cell carriers in different tribes varies from 0 to 35 per cent. The tribal groups with a high prevalence of HbS (20-35 %) include the Bhils, Madias, Pawaras, Pardhans and Otkars. It has also been estimated that Gadchiroli, Chandrapur, Nagpur, Bhandara, Yoetmal and Nandurbar districts would have more than 5000 cases of sickle cell anaemia13.
The entire tribal population of 1,25,000 individuals in the Wayanad district of Kerala was screened, followed by genetic counselling where carriers of HbS were advised not to marry carriers19. A very high prevalence of HbS is seen in these tribes (18.2 to 34.1 %)20.
In Gujarat, the Dhodia, Dubla, Gamit, and Naika tribes have a high prevalence of HbS (13-31 %)10. More recently very extensive population surveys have been done by the Indian Red Cross Society, Gujarat State Branch where 1,68,498 tribals from 22 districts were screened and the overall prevalence of sickle cell carriers was 11.37 per cent21. Some tribal groups in south Gujarat like Chaudry, Gamit, Rohit, Vasava and Kukana have shown both a high prevalence of HbS (6.3 to 22.7%) as well as β-thalassaemia trait (6.3 to 13.6 %)22. These tribal groups would have the likelihood of co-inheriting both these genes.
In a large multicentre study23 where 15200 individuals from 14 primitive tribes from Maharashtra, Gujarat, Tamil Nadu and Odisha were screened, the HbS allele frequency varied from 0.011 to 0.120 and β- thalassaemia allele frequency varied from 0.005 to 0.024. Associated iron deficiency was seen in 26.2 per cent of sickle heterozygotes as well as in 67.7 per cent of sickle homozygotes in this study24.
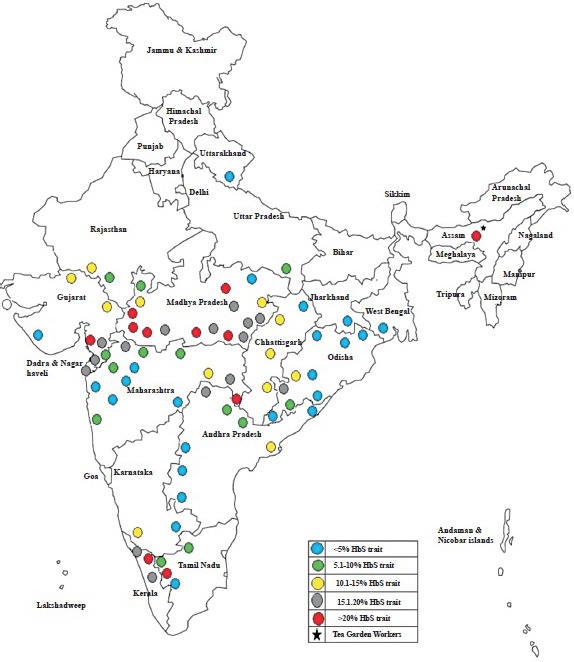
Kaur et al16 have summarized the distribution of HbS in different tribal groups from individual States. Although a large number of tribal groups have been screened for HbS, there are still many gaps in our knowledge about the distribution of the HbS gene in tribal communities in India. The figure shows the district-wise distribution of sickle gene among tribal populations in different States.
Co-inheritance of sickle gene with other Hb defects
Silvestroni and Bianco25 were the first to describe the compound heterozygosity (βs/βthal) for the sickle gene and a β-thalassaemia gene in 1944. Since then, HbS-β thalassaemia cases have been reported from different ethnic groups1.
Apart from HbS, β-thalassaemia is also prevalent in some tribal populations with the frequencies being as high as 6 to 14 per cent in some tribes from Gujarat and Odisha22,26. However, only a few HbS-β-thalassaemia cases have been reported among tribal populations. Seven cases (age 7 to 21 yr) were reported by Mukherjee et al in 201027 and they were either asymptomatic or were mild with only occasional episodes of vaso-occlusive crises. All of them had the β0 mutation, codon 15(G>A) and the presence of associated α-thalassaemia. Earlier studies from Odisha showed the presence of HbS-β+-thalassaemia with the IVS 1-5 (G→C) mutation. These cases had low HbA levels(3-5%)28. With migration for work and some intermixing over the years, co-inheritance of HbS with HbD Punjab, HbE and HbC has also been occasionally reported29,30,31.
Appropriate technologies for screening
Most of the early studies on epidemiology of sickle haemoglobin in different parts of the country used the sickling or the solubility test and in many reports this was followed by Hb electrophoresis to determine the phenotypes. However, in recent years, high performance liquid chromatography (HPLC) analysis has been used in many large programmes to identify carriers of both sickle haemoglobin as well as β-thalassaemia. Capillary electrophoresis has also now been introduced at some centres. Nonetheless, even the simple and cost-effective solubility test has been shown to have a sensitivity and specificity of 97.4 and 100 per cent, respectively in comparison to HPLC and could still serve as a good first line screen for sickle haemoglobin in remote areas where other facilities are not available32.
Newborn screening for sickle cell disorders
Newborn screening for sickle cell disorders was first offered in the New York Programme in 197533, shortly after Robert Guthrie introduced dried blood specimen collection on filter paper34. Susequently, in USA all the States initiated newborn screening and this has also been undertaken in many other countries where the sickle gene is common. Either universal or targeted screening approaches have been used and the technology of choice has been isoelectric focusing (IEF) or HPLC35. Some programmes have now also introduced screening of β-thalassaemias and other haemoglobinopathies. Follow up and care of birth cohorts over several years have helped to understand the natural history of sickle cell disease and reduce early morbidity and mortality in these countries.
In India, newborn screening programmes for sickle cell disorders among tribal and non-tribal populations have only recently been initiated during the last three to five years in south Gujarat, Maharashtra, Chhattisgarh, Odisha and Madhya Pradesh36,37,38,39. In the Kalahandi district in Odisha, 1668 newborns were screened and 19.03 per cent of tribals were sickle heterozygous and 36 babies with sickle cell anaemia were identified37. In Raipur in Chhattisgarh, a pilot study was undertaken where 1158 neonates were screened and 5.2 per cent were sickle heterozygous, five babies had sickle cell anaemia and one had sickle-β-thalassaemia38. In Nagpur in Maharashtra, a targeted screening approach was used where 1162 babies of sickle heterozygous mothers were screened and 536 babies were sickle cell carriers, 88 babies were sickle homozygous, four had sickle -β-thalassaemia and two had HbSD disease39. In Valsad district in south Gujarat, 5467 babies from tribal communities were screened and 12.5 per cent were sickle cell carriers, 33 babies had sickle cell anaemia and 13 had sickle-β-thalassaemia39. In Jabalpur in Madhya Pradesh, newborn screening has just been initiated. In all these studies, heel prick/cord blood samples were collected on Guthrie cards or in EDTA vials and analysed by HPLC. Some of these birth cohorts are being followed up with comprehensive care in the respective sickle cell clinics and will eventually help to understand the natural history of sickle cell disease in India.
Co-inheritance of the sickle gene with G6PD deficiency
Usually G6PD deficiency has no significant effect in patients with sickle cell anaemia. However, a recent study has shown that concomitant G6PD deficiency worsens anaemia and increases blood transfusion requirements in infancy and early childhood upto two years of age but subsequently has little effect40. G6PD deficiency is prevalent in many tribal groups in India10. A cross-section of 15 major tribal communities from different parts of Odisha was randomly screened for haemoglobin variants and G6PD deficiency and high frequencies of sickle cell haemoglobinopathy (0-22.4%) and G6PD deficiency (4.3 to 17.4%) were found with 12 individuals inheriting both these abnormalities41. Among the 14 primitive tribal populations from four different States showing a high frequency of sickle gene, the prevalence of G6PD deficiency varied from 0.7 to 15.6 per cent42. Thus, it may be important to screen for G6PD deficiency in newborn screening programmes for sickle cell disorders.
Clinical presentation and care of sickle cell disease cases in tribal regions
By and large, sickle cell disease in India where the βS gene is linked to the Arab-Indian haplotype which is associated with a mutation in the Gγ-gene leading to increased HbF levels is not as severe as that in African populations. Tribal populations also have a high prevalence of α-thalassaemia. The role of these genetic modifiers in reducing the severity of the disease in tribal groups was first shown by studies done in Odisha43. Subsequently, studies have shown that tribal groups in Gujarat and Maharashtra have a milder presentation than non-tribal populations with the rates of painful crises, infections, acute chest syndrome and hospitalizations being fewer in them. This has partly been attributed to the very high prevalence of α- thalassaemia (90 to 97 %) in some tribes and/or much higher foetal haemoglobin (HbF) levels44,45. However, a few sickle cell disease patients among tribal populations have a more severe disease and have benefitted from hydroxyurea therapy47. The efficacy of hydroxyurea therapy in sickle cell disease patients in different regions in India having the Arab-Indian haplotype have been summarized recently17.
Delivering health care to tribal populations is a challange and a village based model has been described in Bardoli in Gujarat where an outreach programme is being undertaken with the help of a mobile clinical unit and a local villager has also been given basic health care training to regularly visit and monitor sickle cell disease patients and send those with significant complications to the hospital coordinating the programme47. The possibility of comprehensive integrated care of patients with sickle cell disease has also been demonstrated in a remote tribal population in Gudalur in south India where 71 per cent of the 111 patients had at least one annual comprehensive clinic visit and 0.7 painful episodes per year were reported. This study also reported premature deaths in 19 patients at a median age of 23 yr due to acute chest syndrome, sepsis, severe anaemia, stroke, mesenteric infarction or sudden unexplained death48.
There are little data on the maternal and perinatal outcomes of women with sickle cell disease in India. A prospective study from Odisha showed that neonatal outcomes such as low birth weight, perinatal mortality rate, admissions to the neonatal care unit, intrauterine growth retardation and preterm births were significantly higher in sickle cell anemia mothers with successful pregnancies being achieved in 84.44 per cent of cases49. Maternal and perinatal outcomes were also evaluated retrospectively from patients’ case files in women with sickle cell disease in a tribal population in Madhya Pradesh. There were 25 deliveries to women with sickle cell disease and preeclampsia and disseminated intravascular coagulation were common problems. There was no maternal mortality; however, there were five intrauterine foetal deaths and one early neonatal death50.
Prenatal diagnosis
Although there are significant advances in the management of sickle cell disease, yet increased morbidity and early death are not infrequent. Thus, prenatal diagnosis remains an important option for couples at risk of having a child with homozygous sickle cell anaemia, sickle-β-thalassaemia or HbSD disease despite the fact that it is impossible to predict the severity of the disease and many individuals may have a milder clinical presentation. With increasing awareness in the community more couples are opting for prenatal diagnosis51,52. In our experience of around 400 prenatal diagnosis for sickle cell disorders about 20 per cent were tribal couples mainly from South Gujarat, Maharashtra, Madhya Pradesh and Odisha. Chorionic villus sampling at 10 to 12 wk of gestation and DNA analysis by reverse dot blot hybridization or allele specific priming were the methods of choice for prenatal diagnosis. Unlike in the caste populations where a large spectrum of β-thalassaemia mutations are seen, among tribal groups only two β-thalassaemia mutations [(IVS1-5(G>C) and Codon 15(G>A)] are common accounting for around 90 per cent of the β-thalassaemia alleles53. This makes prenatal diagnosis simpler and more cost-effective in tribal populations. For couples who came late, cordocentesis and foetal blood analysis by HPLC were done. These technologies have now been established at regional centres.
Integration of medical genetic services in primary health care
Most of the tribal populations where sickle cell disease is common rely on the primary health care facilities in rural and often remote areas. Thus, the goals of medical genetic services should be to help these people with a genetic disadvantage and their families to have access to quality care as well as social and genetic counselling support to make informed choices for reproduction to have healthy children with the availability of prevention programmes when needed. The Indian Council of Medical Research (ICMR) under its Tribal Health Research Forum (THRF) activities as well as other programmes under the National Rural Health Mission (NRHM) in different States have initiated programmes to enable advances in genetics to reach these communities.
Acknowledgment
Authors thank Shrimati Preeti Pradeep for her assistance.
References
- 1.Serjeant GR, Serjeant BE, editors. Sickle cell disease. 3rd ed. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 2.Stuart MJ, Nagel RL. Sickle cell disease. Lancet. 2004;364:1343–60. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 3.Rees DC, Williams TM, Gladwin MT. Sickle cell disease. Lancet. 2010;376:2018–31. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 4.Odièvre MH, Verger E, Silva-Pinto AC, Elion J. Pathophysiological insights in sickle cell disease. Indian J Med Res. 2011;134:532–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Census of India 2011. Office of the Registrar General and Census Commissioner. Ministry of Home Affairs, Govt of India. [accessed on March 27,2015]. Available from: http://www.censusindia.gov.in .
- 6.Singh KS. Calcutta, India: Anthropological Survey of India; 1992. People of India: An introduction. [Google Scholar]
- 7.Reich D, Thangaraj K, Patterson N, Prince AL, Singh L. Restructuring Indian population history. Nature. 2009;461:489–94. doi: 10.1038/nature08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman H, Cutbush M. Sickle cell trait in southern India. Brit Med J. 1952;1:404–5. doi: 10.1136/bmj.1.4755.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop KJ, Mazumber UK. The occurrence of sickle cell anemia among a group of tea garden labourers in Upper Assam. Indian Med Gaz. 1952;87:387–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia HM, Rao VR. Bombay: Institute of Immunohaematology (ICMR); 1987. Genetic atlas of Indian Tribes. [Google Scholar]
- 11.Rao VR. Genetics and epidemiology of sickle cell anemia in India. Indian J Med Sci. 1988;42:218–22. [PubMed] [Google Scholar]
- 12.Kaur M, Das GP, Verma IC. Sickle cell trait and disease among tribal communities in Orissa, Madhya Pradesh and Kerala. Indian J Med Res. 1997;55:104–9. [PubMed] [Google Scholar]
- 13.Kate SL, Lingojwar DP. Epidemiology of sickle cell disorder in the state of Maharashtra. Indian J Hum Genet. 2002;3:161–7. [Google Scholar]
- 14.Patra PK, Chauhan VS, Khodiar PK, Dalla AR, Serjeant GR. Screening for the sickle cell gene in Chhattisgarh state, India: an approach to a major public health problem. J Community Genet. 2011;2:147–51. doi: 10.1007/s12687-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urade BP. Incidence of sickle cell anemia and thalassemia in Central India. Open J Blood Dis. 2012;2:71–80. [Google Scholar]
- 16.Kaur M, Dangi CBS, Singh M, Singh H, Kapoor H. Burden of sickle cell disease among tribes of India: A burning problem. Int Res J Pharm App Sci. 2013;3:60–80. [Google Scholar]
- 17.Colah R, Mukherjee M, Ghosh K. Sickle cell disease in India. Curr Opin Hematol. 2014;21:215–23. doi: 10.1097/MOH.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RB. Sickle cell disease load in Madhya Pradesh. RMRCT Update. Newslett Regional Med Res Centre Tribals Jabalpur. 2006;3:1–6. [Google Scholar]
- 19.Verma IC. Kochi: Proc. Indo-French Symposium on Recent Trends in Clinical, Diagnostic and Reserch Aspects of hemoglobinopathies; 2004. Nov 21-24, Hemoglobinopathies in India - An overview; pp. 2–4. [Google Scholar]
- 20.Feroze M, Aravindan K. Sickle cell disease in Wayanad, Kerala: Gene frequencies and disease characteristics. Natl Med J India. 2001;14:267–70. [PubMed] [Google Scholar]
- 21.Patel AP, Naik MR, Shah NM, Sharma N, Parmar P. Prevalence of common hemoglobinopathies in Gujarat: An analysis of a large population screening programme. Natl J Community Med. 2012;3:112–6. [Google Scholar]
- 22.Patel AG, Shah AP, Sorathiya SM, Gupte SC. Hemoglobinopathies in South Gujarat population and incidence of anemia in them. Indian J Hum Genet. 2012;18:294–8. doi: 10.4103/0971-6866.107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanty D, Mukherjee MB, Colah RB, Wadia M, Ghosh K, Chottray GP, et al. Spectrum of hemoglobinopathies among the primitive tribes: A multicentric study in India. Asia Pac J Public Health. 2015;27:NP 562–71. doi: 10.1177/1010539513480231. [DOI] [PubMed] [Google Scholar]
- 24.Mohanty D, Mukherjee MB, Colah RB, Wadia M, Ghosh K, Chottray GP, et al. Iron deficiency anaemia in sickle cell disorders in India. Indian J Med Res. 2008;127:366–9. [PubMed] [Google Scholar]
- 25.Silvestroni E, Bianco I. Microdrepanocito-anemia in unsoggetto di razza Bianca. Boll A Acad Med Roma. 1944;70:347. [Google Scholar]
- 26.Balgir RS. The spectrum of haemoglobin variants in two scheduled tribes of Sundaergarh district in north western Orissa, India. Ann Hum Biol. 2005;32:560–73. doi: 10.1080/03014460500228741. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee MB, Nadkarni AH, Gorakshakar AC, Ghosh K, Mohanty D, Colah RB. Clinical, hematologic and molecular variability of sickle cell β- thalassemia in western India. Indian J Hum Genet. 2010;16:154–8. doi: 10.4103/0971-6866.73410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulozik AE, Bail S, Kar BC, Serjeant BE, Serjeant GR. Sickle cell-β+ thalassemia in Orissa state, India. Br J Haematol. 1991;77:215–20. doi: 10.1111/j.1365-2141.1991.tb07980.x. [DOI] [PubMed] [Google Scholar]
- 29.Patel DK, Patel S, Mashon RS, Dash PM, Mukherjee MB. Diverse phenotypic expression of sickle cell hemoglobin C disease in an Indian family. Ann Hematol. 2011;90:357–8. doi: 10.1007/s00277-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 30.Edison ES, Shaji RV, Chandy M, Srivastava A. Interaction of hemoglobin E with other abnormal hemoglobins. Acta Haematol. 2011;126:246–8. doi: 10.1159/000329904. [DOI] [PubMed] [Google Scholar]
- 31.Italia K, Upadhye D, Dabke P, Kangane H, Colaco S, Sawant P, et al. Clinical and hematological presentation among Indian patients with common hemoglobin variants. Clin Chim Acta. 2014;431:46–51. doi: 10.1016/j.cca.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Surve RR, Mukherjee MB, Kate SL, Nagtilak SB, Wadia M, Tamankar AA, et al. Detection of the beta S gene: an evaluation of the solubility test against automated chromatography and haemoglobin electrophoresis. Br J Biomed Sci. 2000;57:292–4. [PubMed] [Google Scholar]
- 33.Dussault JH, Coulombe P, Laberge C, Letarte J, Guyda H, Khoury K. Preliminary report on a mass screening programme for neonatal hypothyroidism. J Pediatr. 1975;86:670–4. doi: 10.1016/s0022-3476(75)80349-0. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie R, Susi A. A simple phenylalanine method for detecting phenyl ketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43. [PubMed] [Google Scholar]
- 35.Benson JM, Therrell BL., Jr History and current status of newborn screening for hemoglobinopathies. Semin. Perinatal. 2010;34:134–44. doi: 10.1053/j.semperi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Mohanty D, Das K, Mishra K. Newborn screening for sickle cell disease and congenital hypothyroidism in western Orissa. Proc 4th Int Conf Sickle Cell Dis Raipur. 2010:29–30. [Google Scholar]
- 37.Panigrahi S, Patra PK, Khodiar PK. Neonatal screening of sickle cell anemia: a preliminary report. Indian J Pediatr. 2012;79:747–50. doi: 10.1007/s12098-011-0682-8. [DOI] [PubMed] [Google Scholar]
- 38.Jain DL, Sarathi V, Upadhye D, Nadkarni AH, Ghosh K, Colah RB. Newborn Screening shows a high incidence of sickle cell anemia in central India. Hemoglobin. 2012;36:316–22. doi: 10.3109/03630269.2012.691434. [DOI] [PubMed] [Google Scholar]
- 39.Italia Y, Krishnamurti L, Mehta V, Raicha B, Italia K, Mehta P, et al. Feasibility of a newborn screening and follow-up programme for sickle cell disease among south Gujarat (India) tribal populations. J Med Screen. 2015;22:1–7. doi: 10.1177/0969141314557372. [DOI] [PubMed] [Google Scholar]
- 40.Benkerrou M, Alberti C, Couque N, Haouari Z, Ba A, Missud F, et al. Impact of glucose-6-phosphate dehydrogenase deficiency on sickle cell anaemia expression in infancy and early childhood: a prospective study. Br J Haematol. 2013;163:646–54. doi: 10.1111/bjh.12590. [DOI] [PubMed] [Google Scholar]
- 41.Balgir RS. Do tribal communities show an inverse relationship between sickle cell disorders and glucose-6-phosphatedehydrogenase deficiency in malaria endemic areas of Central-Eastern India? Homo. 2006;57:163–76. doi: 10.1016/j.jchb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Mohanty D, Colah R, Mukherjee M, editors. New Delhi: Indian Council of Medical Research; 2010. Intervention Programme for Nutritional anemia and hemoglobinopathies amongst some primitive tribal population of India. A National Multicentric study of ICMR (2000- 2005) [Google Scholar]
- 43.Kulozik AE, Kar BC, Serjeant GR, Serjeant BE, Weatherall DJ. The molecular basis of alpha thalassemia in India. Its interaction with the sickle cell gene. Blood. 1988;71:467–72. [PubMed] [Google Scholar]
- 44.Mukherjee MB, Lu CY, Ducrocq R, Gangakhedkar RR, Colah RB, Kadam MD, et al. The effect of alpha thalassemia on sickle cell anemia linked to the Arab-Indian haplotype among a tribal and non-tribal population in India. Am J Hematol. 1997;55:104–9. doi: 10.1002/(sici)1096-8652(199706)55:2<104::aid-ajh9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee MB, Surve RR, Ghosh K, Colah RB, Mohanty D. Clinical diversity of sickle cell disease in western india - influence of genetic factors. Acta Haematol. 2000;103:122–3. doi: 10.1159/000041032. [DOI] [PubMed] [Google Scholar]
- 46.Italia K, Jain D, Gattani S, Jijina F, Nadkarni A, Sawant P, et al. Hydroxyurea in sickle cell disease - a study of clinico - pharamacological efficacy in the Indian haplotype. Blood Cells Mol Dis. 2009;42:25–31. doi: 10.1016/j.bcmd.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Patel J, Patel B, Gamit N, Serjeant GR. Screening for the sickle cell gene in Gujarat, India:a village- based model. J Community Genet. 2013;4:43–7. doi: 10.1007/s12687-012-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nimgaonkar V, Krishnamurti L, Prabhakar H, Menon N. Comprehensive integrated care for patients with sickle cell disease in a remote aboriginal tribal population in southern India. Pediatr Blood Cancer. 2014;61:702–5. doi: 10.1002/pbc.24723. [DOI] [PubMed] [Google Scholar]
- 49.Daigavane MM, Jena RK, Kar TJ. Perinatal outcome in sickle cell anemia: A prospective study from India. Hemoglobin. 2013;37:507–15. doi: 10.3109/03630269.2013.828301. [DOI] [PubMed] [Google Scholar]
- 50.Natu N, Khandelwal S, Kumar R, Dave A. Maternal and perinatal outcome of women with sickle cell disease of a tribal population in central India. Hemoglobin. 2014;38:91–4. doi: 10.3109/03630269.2013.869501. [DOI] [PubMed] [Google Scholar]
- 51.Colah R, Surve R, Nadkarni A, Gorakshakar A, Phanasgaonkar S, Satoskar P, et al. Prenatal diagnosis of sickle syndromes in India: Dilemmas in counseling. Prenat Diagn. 2005;25:345–9. doi: 10.1002/pd.1131. [DOI] [PubMed] [Google Scholar]
- 52.Colah RB, Gorakshakar AC, Nadkarni AH. Invasive and non-invasive approaches for prenatal diagnosis of hemoglobinopathies: Experiences from India. Indian J Med Res. 2011;134:552–60. [PMC free article] [PubMed] [Google Scholar]
- 53.Colah R, Gorakshakar A, Nadkarni A, Phanasgaonkar S, Surve R, Sawant P, et al. Regional heterogeneity of beta thalassemia mutations in the multi ethnic Indian population. Blood Cells Mol Dis. 2009;42:241–6. doi: 10.1016/j.bcmd.2008.12.006. [DOI] [PubMed] [Google Scholar]


