Abstract
Malaria epidemiology is complex due to multiplicity of disease vectors, sibling species complex and variations in bionomical characteristics, vast varied terrain, various ecological determinants. There are six major mosquito vector taxa in India, viz. Anopheles culicifacies, An. fluviatilis, An. stephensi, An. minimus, An. dirus and An. sundaicus. Among these, An. culicifacies is widely distributed and considered the most important vector throughout the plains and forests of India for generating bulk of malaria cases (>60% annually). Major malaria epidemics are caused by An. culicifaices. It is also the vector of tribal malaria except parts of Odisha and Northeastern States of India. An. culicifacies has been the cause of perennial malaria transmission in forests, and over the years penetrated the deforested areas of Northeast. An. culicifacies participates in malaria transmission either alone or along with An. stephensi or An. fluviatilis. The National Vector Borne Disease Control Programme (NVBDCP) spends about 80 per cent malaria control budget annually in the control of An. culicifacies, yet it remains one of the most formidable challenges in India. With recent advances in molecular biology there has been a significant added knowledge in understanding the biology, ecology, genetics and response to interventions, requiring stratification for cost-effective and sustainable malaria control. Research leading to newer interventions that are evidence-based, community oriented and sustainable would be useful in tackling the emerging challenges in malaria control. Current priority areas of research should include in-depth vector biology and control in problem pockets, preparation of malaria-risk maps for focused and selective interventions, monitoring insecticide resistance, cross-border initiative and data sharing, and coordinated control efforts for achieving transmission reduction, and control of drug-resistant malaria. The present review on An. culicifacies provides updated information on vector biology and control outlining thrust areas of research.
Keywords: Anopheles culicifacies, bionomics distribution, India, insecticide resistance, malaria, sibling species, vector genetics
Introduction
Malaria is a major public health problem in India. The epidemiology of malaria is complex on account of multiple vectors, vast and varied terrain, and a number of contextual determinants1,2,3. In Southeast Asia Region of the World Health Organization, India alone contributes nearly 80 per cent malaria cases, and about 95 per cent of the population is estimated to be living at risk of malaria with large concentration of cases in forests and in the hilly and inaccessible terrains4. In 2013, the National Vector Borne Disease Control Programme (NVBDCP) reported 0.88 million microscopically confirmed malaria cases and 440 deaths, and each year these figures are on the decline, although scientific institutions have repeatedly highlighted gross under-reporting of malaria morbidity and mortality4,5,6,7. Plasmodium vivax and P. falciparum are the two main malaria parasites that produce nearly equal proportion of cases (50:50), with a few cases of P. malariae from certain parts of Odisha (formerly Orissa), and sporadic reports of P. ovale. There are six major mosquito vectors in India (Anopheles culicifacies, An. fluviatilis, An. stephensi, An. minimus, An. dirus, and An. sundaicus), and all taxa except An. stephensi are species complex8. Among these, An. culicifacies sensu lato is the most abundant vector. It is widely distributed in rural and peri-urban India. An. culicifacies has played a major role in perennial malaria transmission in forested areas (e.g. Madhya Pradesh), and this vector species has penetrated deforested areas of Northeast9,10,11,12. An. culicifacies is responsible for epidemic malaria in its range of distribution and may cause intense malaria transmission in an estimated 75 million tribal population. An. culicifacies is multiple resistant to insecticides, and its control alone costs about 80 per cent of budget year marked for malaria control of the NVBDCP of the Government of India4. Furthermore, An. culicifacies comprises five sibling species with varying responses to insecticides and transmission potential13. Resurgence of malaria in late 1960s in India and adjoining countries of South Asia was largely the result of failure in the control of An. culicifacies14,15. The present review provides comprehensive information on An. culicifacies, the most important malaria vector in India and the neighbouring countries.
Taxonomy & distribution
An. culicifacies Giles 1901 belongs to subgenus Cellia and series Myzomyia. Three synonyms of An. culicifacies are reported from India. These are the indica Theobald, 1901 of Chennai (formerly Madras); listonii Giles, 1901 from Ellichpur, Maharashtra, and punjabensis James, 1911 from Punjab16. It is in the last three decades that cytogenetic research has revealed the presence of five sibling species of An. culicifacies provisionally designated as A, B, C, D and E17. These sibling species differ in their biology, vectorial capacity and response to malaria control interventions. An. culicifacies s.l. is widely distributed in arid and semi-arid zones ranging from Afghanistan, Bangladesh, Cambodia, Southern China, Eretria, India, Iran, Laos, Myanmar, Nepal, Pakistan, Sri Lanka, Thailand, Vietnam and Yemen (Fig. 1).
Fig. 1.
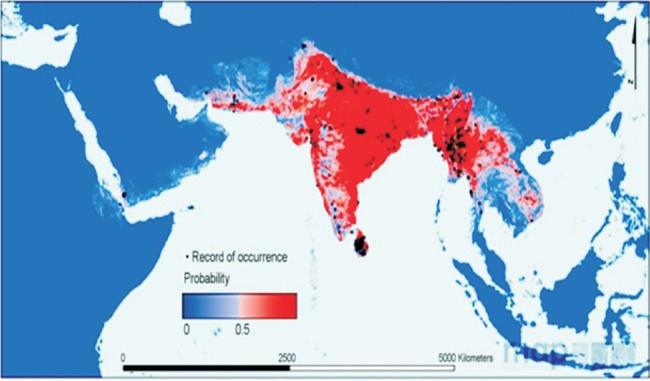
Distribution map of An. (Cellia) culicifacies species complex in the world. Red and blue color depicts the high and low probability of occurrence of this species. Black dots display the sites of data collected (Source: http://www.map.ox.ac.uk/client_media/medialibrary/2011/10/vector_Asia-Pacific_paper.pdf).
Seasonal prevalence
An. culicifacies s.l. is among the most widely distributed mosquito species in India and occurs in all mainland zones including Kashmir and high elevation in the Himalayas excluding islands of Andaman & Nicobar and Lakshadweep (Fig. 2). It is predominantly a vector of unstable malaria in the entire semi-arid and arid zones. Populations of An. culicifacies are low to scanty at higher altitudes and may transmit malaria under favourable conditions, e.g. global warming may bring some ecotones under transmission. It has been reported from 1000 to 2000 meters above mean sea level in Nilgiris hills and Kashmir, respectively18,19,20,21. This mosquito thrives well in plains receiving fair amount of rainfall; heavy rains in various parts of the country (e.g. Maharashtra, Uttrakhand), droughts and floods (e.g. Uttar Pradesh, Bihar), and excessive cold and hot weather in the plains are changing the ecology leading to re-distribution of vector densities capable of malaria transmission. Generally, An. culicifacies populations peak in monsoon and post-monsoon months, and from negligible numbers attain enormously high densities within 4-6 wk resulting in focal to regional epidemics9,10,11,22. It is an important vector of epidemic malaria throughout its range of distribution, although it is a rare species in the Western Ghats.
Fig. 2.
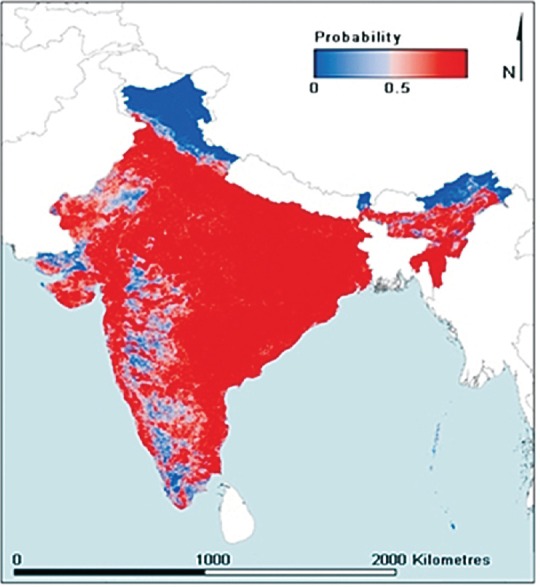
Predictive map of the occurrence of An. (Cellia) culicifacies in India. Red and blue color depicts the high and low probability of its occurrence. (Source: http://www.maps.ox.ac.uk/browse-resources/vector-occurrence/culicifacies/IND/).
Larval ecology
An. culicifacies s.l. is a prolific breeder and its preferred habitats are numerous and varied16,18. Irrigation channels, seepage, unused wells, field channels, waste irrigation water, hoof marks and cart tracks, etc. are some the preferred breeding places. An. culicifacies larvae have also been recorded in varying densities in pools, fallow fields, mining pits, river-bed pools, seaside marsh, turf pools, river edges, perennial streams, tanks, and artificial containers. The most suitable places for the breeding of An. culicifacies are small rocky pools and pits of perennial stream with clear water or with perceptible flow without shade and growth of any vegetation or macroscopic algae; and the least suitable places have highly turbid, stagnant and/or brackish water with good growth of vegetation including floating, sub-merged or vertical vegetation, growth of blue green algae and rich plankton; and the intermediate level of preference is found in fields, and channels with flowing water. An. culicifacies preferentially breeds in sunlit water collections. It breeds profusely in the freshly dug out pits, ornamental waters, unused swimming pools, and rain filled borrow pits along side of railway tracks. Although An. culicifacies is believed to breed in fresh water bodies, but it has adapted to lay eggs and undergo pre-imaginal development in saline/brackish water23.
Sibling species complex
An. culicifacies s.l. was first colonized in the laboratory by the National Institute of Malaria Research (formerly Malaria Research Centre) in 197724. Initially considered to comprise biological races it has now been characterized as species complex with five informally designated species A, B, C, D and E13,17. Techniques are now available to identify these sibling species within the taxon Anopheles culicifacies s.l. (Table I). Among these, the use of diagnostic fixed readable inversions in the ovarian nurse cell's polytene chromosomes unequivocally helps to identify different sibling species. Initially, two sibling species A and B were identified near Delhi; one with standard arrangement in polytene X-chromosome (X +a+b) designated as ‘A’ and the other with two paracentric inversions ‘a’ and ‘b’ with chromosome arrangement (Xab) designated as ‘B’, without any evidence of heterozygotes in natural populations25. Subsequently, examination of polytene chromosomes of populations from other regions of India revealed presence of two fixed inversions, i.e. g1 and h1 in chromosome arm 2 in species B, and two distinct populations with chromosome arrangement Xab; 2g1+h1 and that with Xab; 2+g1h1 were identified which were considered reproductively isolated. The new population with Xab; 2+g1h1 chromosome arrangement was designated as species ‘C’26. Additionally, examination of a few more populations from north India revealed polymorphism for yet another inversion ‘i’ in chromosome 2 of species A which was observed to be fixed in populations from south India. The evidence of deficiency of heterozygotes in northern and central Indian populations for inversion ‘i’ and total absence of heterozygotes in populations of species A with X+a+b; 2+g1+h1 and with X+a+b; 2i1+h1 chromosome arrangement in southern populations provided cytogenetic evidence for species ‘D’ (X+a+b; 2i1+h1)27,28,29.
Table I.
Techniques applied in the identification of An. culicifacies sibling species
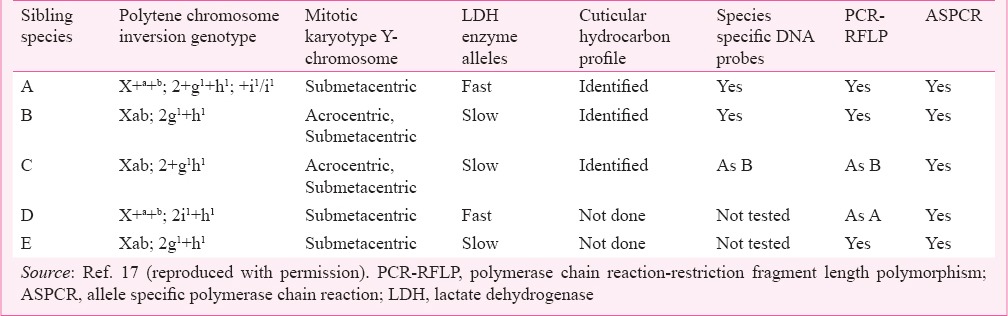
In addition to sibling species A, B, C and D characterized by analyses of polytene chromosome arrangements, sibling species E was identified by Y-chromosome polymorphism in sibling species B populations from Rameshwaram island of Tamil Nadu, south India30. Population with acrocentric Y- chromosome was retained as B while that of submetacentric Y-chromosome karyotype and high sporozoite infectivity was designated as new species E. Both sibling species B and E are homosequential for polytene chromosomes and occur in sympatry but apparently reproductively isolated by pre-mating isolating mechanisms.
Populations of An. culicifacies in range of its distribution other than India have also been examined for sibling species characterization, e.g. in Yeman and Iran, species A was identified31,32, and both A and B are reported occurring sympatrically in Pakistan, of which species A was incriminated33. In Sri Lanka, based on polytene chromosome analysis and DNA probes only species B was identified34, which later confirmed to comprise another sibling species E, the one that was responsible for malaria transmission with high sporozoite infectivity36. In Thailand, both A and B were identified37.
The pre-mating reproductive isolation between sibling species was further substantiated by post-mating isolation mechanisms marked by unidirectional fertility/hybrid male sterility/reduced fertility/atrophied reproductive organs in reciprocal crosses between species A/B, and A/C. Reciprocal crosses between species B and C, however, produced fertile F1 hybrid males and females33,38,39. In addition to these methods, these siblings could also be identified with reasonable accuracy by mitotic karyotype Y- chromosome polymorphism40,41,42,43, electrophoretic variation in lactate dehydrogenase enzyme for fast and slow allele (LdhF & LdhS)44, cuticular hydrocarbon profiles45, and highly repetitive DNA sequences46. PCR-based diagnostic assays have been developed for sequencing 28S-D3 domain47, ITS2-PCR-RFLP (restriction fragment length polymorphism)48, rDNA-ITS2-PCR49, which grouped An. culicifacies sibling species into two distinct groups namely group I (species A/D) and group II (species B/C/E). In another two step PCR assay based on sequence difference within the COII region, A/D specific primers distinguished species A and D, and B/C/E specific primers distinguished B, C and E50, however, this assay could not be used to distinguish species B and E in Sri Lanka51. An efficient and less expensive multiplex PCR–based diagnostic assay using D2 domain of 28S rDNA has been reported which can consistently and accurately discriminate members of the species complex forming two unambiguous monophyly clades of species A/D (group I) and species B/C and E (group 2) were supported by strong bootstrap values52.
Sibling species distribution and sympatricity
Populations of An. culicifacies of diverse origin have been studied for distribution and abundance of its sibling species, A, B, C, D and E in India17. Among these, species B is the most predominant spread throughout the country and occurs sympatrically with A or C or D (Fig. 3). Species A and B are sympatric in north and south India with predominance of species A in the north and species B in the south. In eastern Uttar Pradesh, north Bihar and northeastern States, species B is either predominant or the only prevalent species. Species B and C are predominant in the western and eastern regions. Species D is sympatric with species A and B in northwestern region, and with species A, B and C in central India and a few areas in southern India. Species E is sympatric with species B in southern Tamil Nadu including Rameshwaram islands and Sri Lanka, and there are reports of expansion of distribution range of species A, D and E in Odisha and that of species E in Madhya Pradesh but these investigations lack the component of chromosome analyses for diagnostic confirmation53,54,55. The proportions of sibling species, however, varied in different geographical zones and seasons, e.g. in Delhi where species A and B are sympatric, A was predominant throughout the year but proportions of B increased in post-monsoon months56. In Alwar (Rajasthan), amongst sibling species A, B, C and D, species B increased in post-monsoon months, proportions of D remained the same throughout the year and densities of species C remained very low57.
Fig. 3.
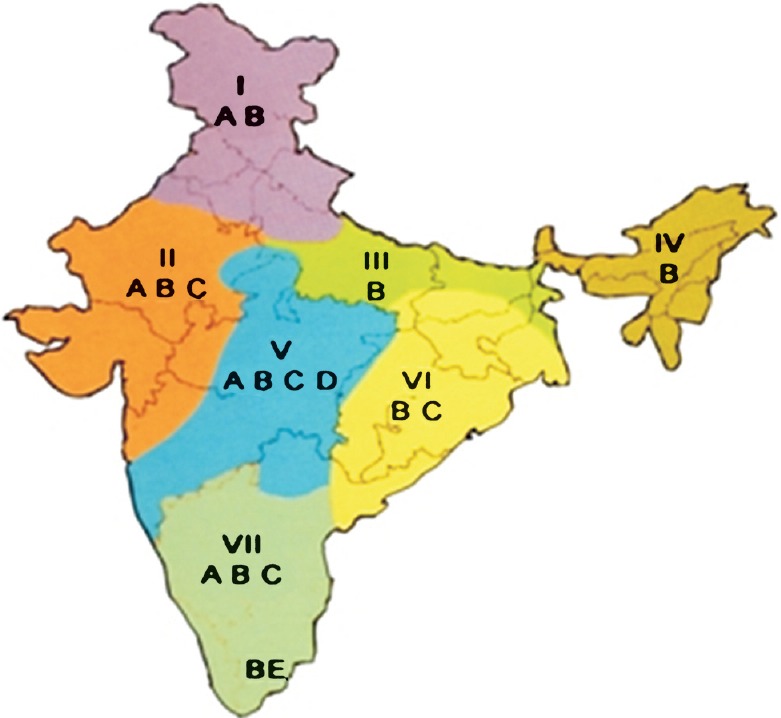
Map of India showing geographical distribution of An. culicifacies complex (sibling species A, B, C, D, and E), and stratification (Divisions I-VII) for vector control options. (Source: Ref. 57).
Bionomical characteristic
Five sibling species spread across India have distinct biological characteristics and role in malaria transmission (Table II). All members of An. culicifacies complex except E were predominantly zoophilic13. Species A had relatively high anthropophilc index (0-4%) compared to species B and D (0-1%), species C had intermediate level of anthropophilic index (0-3%), and species E, had the highest anthropophilic index (80%). All member species largely rest indoors human dwellings preferentially on roof ceilings after feeding on cattle, but also rests outdoors18. All are night biting species with different peak biting activity. The biting activity of A, B and C was observed all through the night except for D for which there was no biting after midnight. The peak biting activity of species A and B occurred between 2200 till 2300 h whereas for species C, it was seasonal; in April it occurred between 1800 till 2100 h and shifted to second quarter of the night in December17.
Table II.
Bionomical characteristics of An. culicifacies sibling species and role in malaria transmission in India
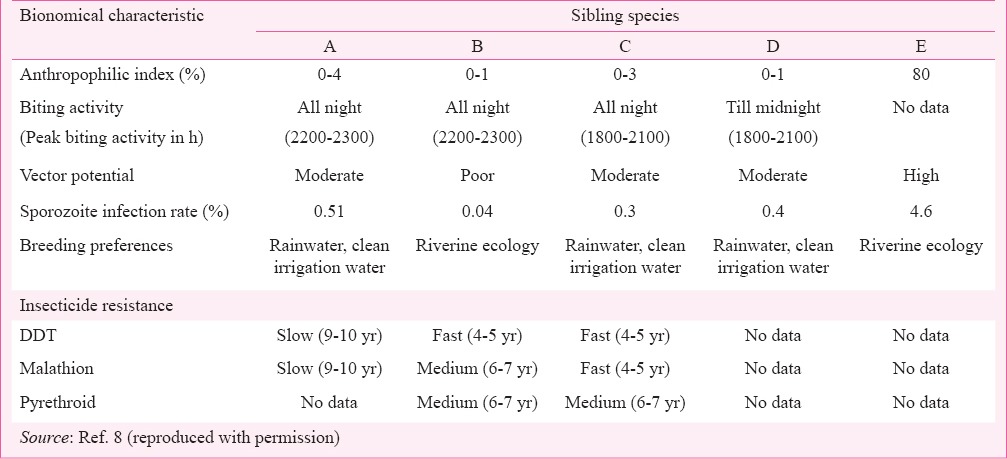
An. culicifacies s.l. has been incriminated by detection of gut and salivary gland infections by numerous independent investigators across its range of distribution throughout India16,18. However, the seasonal infection rate varied from moderate to high in northwestern States, low to moderate in Deccan plateau, low rates in Gangetic plains and east central region, and very low rates in northeastern States18. The sporozoite infectivity rates also varied among sibling species. Immunoradiometric based investigations revealed that sibling species A, C and D are vectors of P. vivax and P. falciparum (including drug resistant strains), and cumulative sporozoite infection rates were recorded 0.51, 0.3 and 0.4 per cent, respectively58,59. Species B is a non-vector or poor vector evidenced by the low prevalence of malaria where species B is predominantly prevalent such as in eastern districts of Uttar Pradesh and southern Indian States60. Species E is the most efficient vector and maintains endemic to epidemic malaria. These observations were further supported by comparative reproductive fitness for which sibling species B was observed to be less fit than species A and C of the complex as well as susceptibility to malaria parasite development61. Species A is susceptible to sporogony marked by higher oocyst and sporozoite rate than C and B; in species B parasite development is inhibited by oocyst encapsulation mechanism62,63. Variations to development of insecticide resistance have also been reported among sibling species but with slower development of resistance to DDT in species A than species B and C, and faster development of resistance to malathion in C than B and at slower rate in A than B (Table II)64,65.
Vector control
Indoor residual spraying (IRS) is the main stay for vector control in the country. For control of An. culicifacies, currently three rounds of spraying of malathion (25%WP, 2 g/m2) are undertaken in DDT resistant areas. In areas with double resistance, i.e. DDT and malathion, two rounds of synthetic pyrethroid insecticides, i.e. deltamethrin (2.5%, 20 mg/m2), or cyfluthrin (10% WP) or lambdacyhalothrin (10% WP) or alphacypermethrin (5% WP) or bifenthrin (10% WP) each are sprayed as 25 mg/m2. Malathion and/or synthetic pyrethroids are also sprayed to control epidemic malaria in complex emergency situations. In urban and industrial areas 5 per cent malathion thermal fogging is undertaken to contain build up of vector population.
The understanding of genetics of An. culicifacies species complex for its sibling species composition, distribution and behavioural characteristics has helped develop stratification for optimizing control efforts and saving costs (Fig. 3). Taking cognizance of the biological differences of sibling species, the rural India is stratified into seven divisions (excluding urban metropolitan cities) for benefit of prioritizing vector control options (Table III)53,54,55,56,57.
Table III.
Distribution of An. culicifacies sibling species and stratification for vector control options in India

No specific strategy has been proposed for species D for its limited distribution and occurrence in low numbers often in sympatricity with other sibling species. In areas with predominance of species E like Rameshwaram Island and Odisha existing interventions should continue. In areas where A, C, and D are sympatric, the choice of insecticide should be guided by the susceptibility status of the predominant sibling species. In addition to stratification, it is strongly advised to develop stratification at the district/block level to economize field operations. For example, in Shankargarh block of Allahabad district, Uttar Pradesh, the prevalence of malaria had direct correlation with cumulative abundance of sibling species A (64%) plus C (11%)66. In general, it is proposed to integrate bioenvironmental methods; larvivorous fish (Guppy and Gambusia) in particular which have been applied successfully in different ecological terrains for control of vector breeding in malaria endemic States Gujarat, Maharashtra and Karnataka67,68,69,70.
Challenges in malaria control
India has reported appreciable decline in malaria cases from two million in 2001 to less than one million in 2013 and notable decline in malaria deaths4. However, there is a steady rise in P. falciparum proportions with large concentration of cases in tribal populations/forest belts, hilly and difficult/inaccessible areas. Malaria transmission is perennial affecting all age groups and with serious consequences to high risk groups, particularly with heavy death toll in pregnant mothers and infants4,7,71. An. culicifacies transmitted malaria is highly uneven, maintains unstable to intermediate stability and brings periodical epidemics. This vector is responsible for malaria resurgence in India, Pakistan and Sri Lanka, although malaria is at the verge of elimination in Sri Lanka14,15,72,73,74.
An. culicifacies maintains endemic malaria throughout rural India and its control has become a formidable task consuming huge resources year after year. Malaria control is particularly problematic in forested and degraded forests as in some areas spraying is difficult and remains unsupervised. Malaria transmission in forests is usually prolonged; for example, An. culicifacies was incriminated for over 10 months in a year in Balaghat area in Madhya Pradesh75,76. An. culicifacies s.l. has grown multi-resistant to DDT, HCH (hexachlorocyclohexane) and malathion, in most parts of the country and in some States increased resistance to pyrethroids77,78,79,80. Molecular characterization revealed a low frequency of the kdr allele (mostly in heterozygous condition) in field populations that were resistant to DDT and pyrethroids77,78,79,80. This species is invasive and expanding its territory by penetrating degraded forests of northeastern States of the country, formerly domain of An. minimus and An. dirus11,12. In addition, global warming, climate change, vector ecology, population migration, deforestation, developmental projects, and poor infrastructure have led to the opportunities for vector proliferation and increased malaria receptivity in the country83,84.
The main strategy for malaria control in rural India continues to be indoor residual spraying (IRS) of insecticide based on the vector susceptibility status. It is the need of time to develop approaches for management of insecticide resistance for increased duration of its efficacy against target vector species by insecticide rotation, mosaic application, and integrating bio-environmental approaches85,86,87,88. IRS has become less effective and operationally difficult on account of poor acceptance by communities89. Instead of IRS, implementation of insecticide-treated netting materials / long-lasting insecticidal nets (LLINs) and biological control may produce substantial transmission reduction in malaria transmitted by An. culicifacies90,91. Most LLINs, however, employ pyrethroid insecticide against which already incipient resistance has been reported. There is an urgent need to develop innovative strategies against resistant An. culicifacies populations through research and development in key areas identified from field operations92,93,94,95.
Priority areas of research
An. culicifacies is the best studied mosquito species for its sibling species identification, range of distribution and relationship with malaria transmission in India. Yet, study of chromosomes (ovarian polytene chromosomes for A, B, C, D, and mitotic chromosomes for B and E) is still the only technique that can distinguish sibling species A, B, C, D and E. No other probe distinguished each species unequivocally. PCR-based diagnostic assays distinguished only two distinct groups namely group I (species A/D) and group II (species B/C/E). Efforts to distinguish species of each group A/D and that of B/C/E based on PCR assays are still far from perfect and could not be used reliably51.
The present distribution of sibling species requires further research for understating population genetics structure in high risk areas of malaria. Recent findings revealed invasion by An. culicifacies sibling species A, D and E in addition to prevalent B and C in Odisha54, and species ‘E’ in Madhya Pradesh55. Similarly, in Assam (northeast India), species A is also observed to occur besides species B (Nanda N, personal communication). Additional investigations on distribution and bionomical characteristics of species E including insecticide susceptibility status, biting time to delimit its role in malaria transmission would be desirable in problem areas.
The crossing experiments between species A and B revealed post-mating barriers marked by unidirectional hybrid male sterility/reduced fertility/atrophied reproductive organs. Similar observations are deemed necessary to establish the extent of post-zygotic isolation by crossing experiments between other member sibling species. The inter-cross fertility data and the existence of possible morphological differences between sibling species A, B, C, D and E should be accorded priority to designate binomial nomenclature similar to other anopheline species vector taxa96,97,98.
An. culicifacies is highly adaptive, holds enormous behavioural plasticity and a fast invading species in areas hitherto found with low vector density, e.g. in deforested pockets in eastern and northeast India and breeding in altered ecological habitats23,99,100,101, and believed to be undergoing significant genetic differentiation with obvious implications in its control102. There is a need to understand species-specific bionomics and insecticide susceptibility status before undertaking IRS for the control of An. culicifacies transmitted malaria.
The primary emphasis in the control of An. culicifacies should be on the innovative integrated methods that are cost-effective, safe and sustainable. It should, therefore, be realized that insecticides should be used sparingly and IRS when it becomes absolutely essential, e.g. epidemic situations. The routine vector control should rely on the bioenvironmental/integrated vector management methods. Field research should be undertaken on malaria control in various ecotypes such as the rural malaria, forest malaria, irrigation malaria, peri-urban malaria, industrial malaria, border malaria, coastal malaria by implementation of integrated vector management (IVM) methods involving the communities. The minimum area for the demonstration of new technologies should be a Primary Health Centre/Community Health Centre and extended to cover a district. This would require mapping of malaria risk areas for An. culicifacies sibling species distribution, potential mosquito breeding habitats, site of contracting infection and control of outdoor malaria transmission. Insecticide incorporated plastic sheeting and insecticide treated hammock should be field tested in areas not responding to treated bed nets/LLINs103,104.
Along with bionomics of An. culicifacies, socio-economic research should continue on human settlements, housing structure, vocations, sleeping habits, migration pattern, and community participation in vector control. Intensive information, education and communication (IEC) for various target groups, health system strengthening and capacity building including technology transfer to the control programme should constitute a continuing activity of the NVBDCP.
Conclusions
In the past decades, a wealth of data on the biology and control of An. culicifacies has been generated. An. culicifacies populations build up during monsoon and post monsoon season, and bring periodical focal outbreaks and epidemics throughout its range of distribution. Recent studies utilizing the molecular techniques for identification of sibling species complex have helped in understanding bionomics and role of each sibling species in malaria transmission. An. culicifacies is an invasive species and its further spread is supported by the changing environmental determinants. Therefore, entomological surveillance should be integral to malaria surveillance. An. culicifacies rapidly develops multiple insecticide resistance, and inter alia it is playing an increasing role in the transmission of P. vivax and P. falciparum, and spread of multi-drug resistant malaria. Research leading to newer evidence-based interventions, community based integrated vector management strategies should be strengthened in tackling the emerging challenges in the control of An. culicifacies transmitted malaria. The priority research areas in vector control may include developing malaria-risk maps, evidence based focused and selective vector control, tackling problems in vector control that arise in the field, protection from contracting malaria in the settlements in forests including inaccessible areas, personal protection measures for individuals and communities, reduction in malaria receptivity legislative measures and biological control, vector surveillance, monitoring insecticide resistance and developing countermeasures, protecting target areas from vector invasion, cross-border synchronized vector control, and establishing priority in vector control to eliminate drug-resistant malaria foci.
Acknowledgment
The authors thank Drs Neeru Singh, T. Adak, K. Raghavendra, O.P. Singh, N. Nanda, A. Das, A. Kumar and S.K. Ghosh for providing valued literature and consultations. Thanks are also due to Prof. S. Manguin (Montpellier, France) for ctitical review of the manuscript and suggestions. This manuscript has been approved by the Institute Publication Screening Committee and bears the publication number 21/2014.
References
- 1.Singh V, Mishra N, Awasthi G, Dash AP, Das A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009;25:452–7. doi: 10.1016/j.pt.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Das A, Anvikar AR, Cator LJ, Dhiman RC, Eapen A, Mishra N, et al. Malaria in India: the centre for the study of complex malaria in India. Acta Trop. 2012;121:267–73. doi: 10.1016/j.actatropica.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma VP. Determinants of malaria in South Asia. In: Casman EA, Dowlatabadi H, editors. The contextual determinants of malaria. Pittsburgh PA: Carnegie Mellon University, USA; 2002. pp. 110–32. [Google Scholar]
- 4.Malaria: magnitude of the problem. National Vector Borne Disease Control Programme. Ministry of Health & Family Welfare, Government of India. [accessed on April 6, 2015]. Available from: http://www.nvbdcp.gov.in .
- 5.Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, et al. Adult and child malaria mortality in India: a nationally representative mortality study. Lancet. 2010;376:1768–74. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377:252–69. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- 7.Sharma VP. Hidden burden of malaria in Indian women. Malar J. 2009;8:281. doi: 10.1186/1475-2875-8-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dev V, Sharma VP. The dominant mosquito vectors of human malaria in India. In: Manguin S, editor. Anopheles mosquitoes - new insights into malaria vectors. Croatia: In Tech Publications; 2013. pp. 239–71. [Google Scholar]
- 9.Singh N, Mishra AK, Chand SK, Sharma VP. Population dynamics of Anopheles culicifacies and malaria in the tribal area of central India. J Am Mosq Control Assoc. 1999;15:283–90. [PubMed] [Google Scholar]
- 10.Mathur KK, Harpalani G, Kalra NL, Murthy GG, Narasimham MV. Epidemic of malaria in Barmer district (Thar desert) of Rajasthan during 1990. Indian J Malariol. 1992;29:1–10. [PubMed] [Google Scholar]
- 11.Bhuyan M, Das NG, Chakraborty BC, Talukdar PK, Sarkar PK, Das SC, et al. Role of Anopheles culicifacies during an outbreak of malaria in Garubandha P.H.C., Assam. Assam J Commun Dis. 1997;29:243–6. [PubMed] [Google Scholar]
- 12.Das NG, Gopalakrishnan R, Talukdar PK, Baruah I. Diversity and seasonal densities of vector anophelines in the relation to forest fringe malaria in district Sonitpur, Assam (India) J Parasit Dis. 2011;35:123–8. doi: 10.1007/s12639-011-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barik TK, Sahu B, Swain V. A review on Anopheles culicifacies: from bionomics to control with special reference to Indian subcontinent. Acta Trop. 2009;109:87–97. doi: 10.1016/j.actatropica.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- 15.Akhtar R, Dutt AK, Wadhwa V, editors. Advances in Asian human-environment research. New York: Springer; 2010. Malaria in South Asia: eradication and resurgence during the second half of the twentieth century; p. 241. [Google Scholar]
- 16.Nagpal BN, Sharma VP. New Delhi: Oxford & IBH Publishing; 1995. Indian anophelines; p. 416. [Google Scholar]
- 17.Geneva: WHO; 2007. World Health Organization (WHO). Anopheles species complexes in South and South East-Asia. SEARO Technical Publication No. 57; p. 102. [Google Scholar]
- 18.Rao TR. New Delhi: Malaria Research Centre, Indian Council of Medical Research; 1984. The anophelines of India; p. 596. [Google Scholar]
- 19.Russell PF, Jacob VP. On the epidemiology of malaria in the Nilgiris district, Madras Presidency. J Malar Inst India. 1942;4:349–92. [Google Scholar]
- 20.Jacob VP. Some aspects of malaria in Jammu and Kashmir state. Indian J Malariol. 1950;4:251–60. [PubMed] [Google Scholar]
- 21.Rao TR, Dhanda V, Bhat HR, Kulkarni SM. A survey of haematophagous arthropods in Western Himalayas, Sikkim and hill districts of West Bengal. A general account. Indian J Med Res. 1973;61:1421–61. [PubMed] [Google Scholar]
- 22.Covell G, Bailey JD. The factors influencing the normal autumnal incidence of malaria in Lakana Taluka, Larkana district. Rec Mal Surv India. 1930;1:549–65. [Google Scholar]
- 23.Ramasamy R, Surendran SN. Global environment changes and salinity adaptation in mosquito vectors. [accessed on January 20, 2014]. Available from: http://www.amazon.com/Environment-Changes-Salinity-Adaptation-Mosquito/dp/3848422905 .
- 24.Ansari MA, Mani TR, Sharma VP. A preliminary note on the colonization of Anopheles culicifacies Giles. J Commun Dis. 1977;9:206–7. [Google Scholar]
- 25.Green CA, Miles SJ. Chromosomal evidence for sibling species of the malaria vector Anopheles (Cellia) culicifacies Giles. J Trop Med Hyg. 1980;83:75–8. [PubMed] [Google Scholar]
- 26.Subbarao SK, Vasantha K, Adak T, Sharma VP. Anopheles culicifacies complex: evidence for a new sibling species C. Ann Entomol Soc Am. 1983;76:985–8. [Google Scholar]
- 27.Subbarao SK, Vasantha K, Sharma VP. Cytotaxonomy of certain malaria vectors of India. In: Service MW, editor. Biosystematics of haematophagous insects. Oxford: Clarendon Press; 1988. pp. 25–37. [Google Scholar]
- 28.Suguna SG, Tewari SC, Mani TR, Hiriyan J, Reuben R. A cytogenetic description of a new species of the Anopheles culicifacies complex. Genetica. 1988;1989; 78:225–30. doi: 10.1007/BF00055642. [DOI] [PubMed] [Google Scholar]
- 29.Vasantha K, Subbarao SK, Sharma VP. Anopheles culicifacies complex: population cytogenetic evidence for species D (Diptera: Culicidae) Ann Entomol Soc Am. 1991;84:531–6. [Google Scholar]
- 30.Kar I, Subbarao SK, Eapen A, Ravindran J, Satyanarayana TS, Raghavendra K, et al. Evidence for a new malaria vector species, species E, within the Anopheles culicifacies complex (Diptera: Culicidae) J Med Entomol. 1999;36:595–600. doi: 10.1093/jmedent/36.5.595. [DOI] [PubMed] [Google Scholar]
- 31.Akoh JI, Beidas MF, White GB. Cytotaxonomic evidence for the malaria vector species A of the Anopheles culicifacies complex being endemic in Arabia. Trans R Soc Trop Med Hyg. 1984;78:698–700. [Google Scholar]
- 32.Zaim M, Subbarao SK, Manouchehri AV, Cochrane AH. Role of Anopheles culicifacies s. l. and An. pulcherrimus in malaria transmission in Ghassreghand (Baluchistan), Iran. J Am Mosq Control Assoc. 1993;9:23–6. [PubMed] [Google Scholar]
- 33.Mahmood F, Sakai RK, Akhtar K. Vector incrimination studies and observations on species A and B of the taxon Anopheles culicifacies in Pakistan. Trans R Soc Trop Med Hyg. 1984;78:607–16. doi: 10.1016/0035-9203(84)90219-0. [DOI] [PubMed] [Google Scholar]
- 34.Abhayawardana TA, Dilrukshi RK, Wijesuriya SR. Cytotaxonomical examination of sibling species in the taxon Anopheles culicifacies Giles in Sri lanka. Indian J Malariol. 1996;33:74–80. [PubMed] [Google Scholar]
- 35.de Silva BG, Gunasekera MB, Abeyewickreme W, Karunanayake EH. Field analysis of Anopheles culicifacies Giles complex for the presence of sibling species A. Proc Sri Lankan Assoc Adv Sci. 1993;49:24. [Google Scholar]
- 36.de Silva BG. Anopheles culicifacies complex: geographical distribution of sibling species and existing methods for their identification. Vidyodaya J Sci. 2009;14:1–28. [Google Scholar]
- 37.Baimai V, Kijchalao U, Rattanarithikul R. Metaphase karyotypes of Anopheles of Thailand and Southeast Asia: V. The Myzomyia Series, subgenus Cellia (Diptera: Culicidae) J Am Mosq Control Assoc. 1996;12:97–105. [PubMed] [Google Scholar]
- 38.Miles SJ. Unidirectional hybrid male sterility from crosses between species A and species B of the taxon Anopheles (Cellia) culicifacies Giles. J Trop Med Hyg. 1981;84:13–6. [PubMed] [Google Scholar]
- 39.Subbarao SK, Vasantha K, Sharma VP. Studies on the crosses between the sibling species of the Anopheles culicifacies complex. J Hered. 1988;79:300–3. [Google Scholar]
- 40.Vasantha K, Subbarao SK, Adak T, Sharma VP. Karyotypic variations in Anopheles culicifacies complex. Indian J Malariol. 1982;19:27–32. [Google Scholar]
- 41.Vasantha K, Subbarao SK, Adak T, Sharma VP. Anopheles culicifacies: mitotic karyotype of species C. Indian J Malariol. 1983;20:161–2. [Google Scholar]
- 42.Suguna SG, Tewari SC, Mani TR, Hiriyan J, Reuben R. Anopheles culicifacies species complex in Thenpennaiyar riverine tract, Tamil Nadu. Indian J Med Res. 1983;77:455–9. [PubMed] [Google Scholar]
- 43.Subbarao SK, Nanda N, Chandrahas RK, Sharma VP. Anopheles culicifacies complex: cytogenetic characterization of Rameshwaram islands populations. J Am Mosq Control Assoc. 1993;9:27–31. [PubMed] [Google Scholar]
- 44.Adak T, Subbarao SK, Sharma VP, Rao SR. Lactate dehydrogenase allozyme differentiation of species in the Anopheles culicifacies complex. Med Vet Entomol. 1994;8:137–40. doi: 10.1111/j.1365-2915.1994.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 45.Milligan PJ, Phillips A, Molyneux DH, Subbarao SK, White GB. Differentiation of Anopheles culicifacies Giles (Diptera: Culicidae) sibling species by analysis of cuticular components. Bull Entomol Res. 1986;76:529–37. [Google Scholar]
- 46.Gunasekera MB, de Silva BG, Abeyewickreme W, Subbarao SK, Nandadasa HG, Karunanayake EH. Development of DNA probes for the identification of sibling species A of the Anopheles culicifacies (Diptera: Culicidae) complex. Bull Entomol Res. 1995;85:345–53. [Google Scholar]
- 47.Singh OP, Goswami G, Nanda N, Raghavendra K, Chandra D, Subbarao SK. An allele-specific polymerase chain reaction assay for the differentiation of members of the Anopheles culicifacies complex. J Biosci. 2004;29:275–80. doi: 10.1007/BF02702609. [DOI] [PubMed] [Google Scholar]
- 48.Goswami G, Raghavendra K, Nanda N, Gakhar SK, Subbarao SK. PCR-RFLP of mitochondrial cytochrome oxidase subunit II and ITS2 of ribosomal DNA: markers for the identification of members of the Anopheles culicifacies complex (Diptera: Culicidae) Acta Trop. 2005;95:92–9. doi: 10.1016/j.actatropica.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Manonmani AM, Sadanandane C, Sahu SS, Mathivanan A, Jambulingam P. rDNA-ITS2-PCR assay for grouping the cryptic species of Anopheles culicifacies complex (Diptera: Culicidae) Acta Trop. 2007;104:72–7. doi: 10.1016/j.actatropica.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Goswami G, Singh OP, Nanda N, Raghavendra K, Gakhar SK, Subbarao SK. Identification of all members of the Anopheles culicifacies complex using allele-specific polymerase chain reaction assays. Am J Trop Med Hyg. 2006;75:454–60. [PubMed] [Google Scholar]
- 51.Surendran SN, Hawkes NJ, Steven A, Hemingway J, Ramasamy R. Molecular studies of Anopheles culicifacies (Diptera: Culicidae) in Sri Lanka: sibling species B and E show sequence identity at multiple loci. Eur J Entomol. 2006;103:233–7. [Google Scholar]
- 52.Raghavendra K, Cornel AJ, Reddy BP, Colins FH, Nanda N, Chandra D, et al. Multiplex PCR assay and phylogenetic analysis of sequences derived from D2 domain of 28S rDNA distinguished members of the Anopheles culicifacies complex into two groups, A/D and B/C/E. Infect Genet Evol. 2009;9:271–7. doi: 10.1016/j.meegid.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Tripathy A, Samanta L, Das S, Paride SK, Marai N, Hazra RK, et al. Distribution of sibling species of Anopheles culicifacies s.l. and Anopheles fluviatilis s.l. and vectorial capacity in eight different malaria endemic districts of Orissa, India. India Mem Inst Oswaldo Cruz. 2010;105:981–7. doi: 10.1590/s0074-02762010000800006. [DOI] [PubMed] [Google Scholar]
- 54.Das M, Das B, Patra AP, Tripathy HK, Mohapatra N, Kar SK, et al. Anopheles culicifacies sibling species in Odisha, Eastern India: First appearance of Anopheles culicifacies E and its vectorial role in malaria transmission. Trop Med Int Health. 2013;18:810–21. doi: 10.1111/tmi.12112. [DOI] [PubMed] [Google Scholar]
- 55.Sharma AK, Tyagi V, Singh S, Veer V, Agrawal OP, Sukumaran D. Distribution of Anopheles culicifacies and detection of its sibling species E from Madhya Pradesh: Central India. J Arthropod-Borne Dis. 2014;8:182–96. [PMC free article] [PubMed] [Google Scholar]
- 56.Subbarao SK, Vasantha K, Adak T, Sharma VP. Seasonal prevalence of sibling species A and B of the taxon Anopheles culicifacies in villages around Delhi. Indian J Malariol. 1987;24:9–15. [PubMed] [Google Scholar]
- 57. New Delhi, India: NIMR; 2002. National Institute of Malaria Research (NIMR). Anopheles culicifacies and An. fluviatilis complexes and their control Technical Report series No. NIMR/TRS/2009-January/2002. [Google Scholar]
- 58.Subbarao SK, Adak T, Vasantha H, Joshi H, Raghvendra K, Cochrane AH, et al. Susceptibility of Anopheles culicifacies species A and B to Plasmodium vivax and Plasmodium falciparum as determined by immunoradiomertric assay. Trans R Soc Trop Med Hyg. 1988;82:394–7. doi: 10.1016/0035-9203(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 59.Subbarao SK, Sharma VP. Anopheline species complexes & malaria control. Indian J Med Res. 1997;106:164–73. [PubMed] [Google Scholar]
- 60.Subbarao SK, Nanda N, Raghavendra K. Malariogenic stratification of India using Anopheles culicifacies sibling species prevalence. ICMR Bull. 1999;29:75–80. [Google Scholar]
- 61.Sharma A, Parasher H, Singh OP, Adak T. Species B of Anopheles culicifacies (Diptera: Culicidae) is reproductively less fit than species A and C of the complex. Acta Trop. 2009;112:316–9. doi: 10.1016/j.actatropica.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Adak T, Kaur S, Singh OP. Comparative susceptibility of different members of the Anopheles culicifacies complex to Plasmodium vivax. Trans R Soc Trop Med Hyg. 1999;93:573–7. doi: 10.1016/s0035-9203(99)90052-4. [DOI] [PubMed] [Google Scholar]
- 63.Kaur S, Singh OP, Adak T. Susceptibility of species A, B, and C of Anopheles culicifacies complex to Plasmodium yoelii yoelii and Plasmodium vinckei petteri infections. J Parasitol. 2000;86:1345–8. doi: 10.1645/0022-3395(2000)086[1345:SOSABA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 64.Subbarao SK, Vasantha K, Sharma VP. Responses of Anopheles culicifacies sibling species A and B to DDT and HCH in India: implications in malaria control. Med Vet Entomol. 1988;2:219–23. doi: 10.1111/j.1365-2915.1988.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 65.Raghavendra K, Subbarao SK, Vasantha K, Pillai MK, Sharma VP. Differential selection of malathion resistance in Anopheles culicifacies A and B (Diptera: Culicidae) in Haryana state, India. J Med Entomol. 1992;29:183–7. doi: 10.1093/jmedent/29.2.183. [DOI] [PubMed] [Google Scholar]
- 66.Tiwari SN, Prakash A, Subbarao SK, Roy A, Joshi H, Sharma VP. Correlation of malaria endemicity with An. culicifacies sibling species composition and malaria antibody profile in distort Allahabad (U.P) Indian J Malariol. 1994;31:48–56. [PubMed] [Google Scholar]
- 67.Sharma VP, Sharma RC, Gautam AS. Bio-environmental control of malaria in Nadiad, Kheda district, Gujarat. Indian J Malariol. 1986;23:95–117. [PubMed] [Google Scholar]
- 68.Sharma VP. Community-based malaria control in India. Parasitol Today. 1987;3:222–6. doi: 10.1016/0169-4758(87)90066-4. [DOI] [PubMed] [Google Scholar]
- 69.Sharma VP, Ghosh A, editors. Proceedings of the MRC-CICFRI Workshop. Delhi: Malaria Research Centre (ICMR); 1994. Larvivorous fishes of inland ecosystems; p. 224. [Google Scholar]
- 70.Ghosh SK, Tewari SN, Sathyanarayan TS, Sampath TR, Sharma VP, Nanda N, et al. Larvivorous fish in wells target the malaria vector sibling species of the Anopheles culicifacies complex in vaillages in Karnataka, India. Trans R Soc Trop Med Hyg. 2004;99:101–5. doi: 10.1016/j.trstmh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Singh N, Mehra RK, Srivastava N. Malaria during pregnancy and infancy, in an area of intense malaria transmission in central India. Ann Trop Med Parasitol. 2001;95:19–29. doi: 10.1080/00034980020035889. [DOI] [PubMed] [Google Scholar]
- 72.Nanda N, Yadav RS, Subbarao SK, Joshi H, Sharma VP. Studies on Anopheles fluviatilis and Anopheles culicifacies sibling species in relation to malaria in forested hilly and deforested riverine ecosystems in northern Orissa, India. J Am Mosq Control Assoc. 2000;16:199–205. [PubMed] [Google Scholar]
- 73.Dutt AK, Dutta HM, Parera C. New York: Springer; 2010. Malaria in South Asia: eradication and resurgence during the second half of the twentieth century; pp. 29–41. [Google Scholar]
- 74.Zaidi IH, Kazmi JH. New York: Springer; 2010. Malaria in South Asia: eradication and resurgence during the second half of the twentieth century; pp. 123–39. [Google Scholar]
- 75.Singh N, Singh OP, Sharma VP. Dynamics of malaria transmission in forested and deforested regions of Mandla district, Central India (Madhya Pradesh) J Am Mosq Control Assoc. 1996;12:225–34. [PubMed] [Google Scholar]
- 76.Singh N, Chand SK, Bharti PK, Singh MP, Chand G, Mishra AK, et al. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 2013;8:e73730. doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raghavendra K, Vasantha K, Subbarao SK, Pillai MK, Sharma VP. Resistance in Anopheles culicifacies sibling species B and C to malathion in Andhra Pradesh and Gujarat States, India. J Am Mosq Control Assoc. 1991;7:255–9. [PubMed] [Google Scholar]
- 78.Singh OP, Raghavendra K, Nanda N, Mittal PK, Subbarao SK. Pyrethroid resistance in Anopheles culicifacies in Surat district, Gujarat, India. Curr Sci. 2002;82:547–50. [Google Scholar]
- 79.Bhatt RM, Sharma SN, Barik TK, Raghavendra K. Status of insecticide resistance in malaria vector, Anopheles culicifacies in Chhattisgarh state, India. J Vector Borne Dis. 2012;49:36–8. [PubMed] [Google Scholar]
- 80.Mishra AK, Chand SK, Barik TK, Dua VK, Raghavendra K. Insecticide resistance status in Anopheles culicifacies in Madhya Pradesh, Central India. J Vector Borne Dis. 2012;49:39–41. [PubMed] [Google Scholar]
- 81.Singh OP, Bali P, Hemingway J, Subbarao SK, Dash AP, Adak T. PCR-based methods for the detection of L1014 kdr mutation in Anopheles culicifacies sensu lato. Malar J. 2009;8:154. doi: 10.1186/1475-2875-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoti SL, Vasuki V, Jambulingam P, Sahu SS. kdr allele-based PCR assay for detection of resistance to DDT in Anopheles culicifacies sensu lato Giles population from Malkangiri District, Orissa, India. Curr Sci. 2006;91:658–61. [Google Scholar]
- 83.Sharma VP, Prasittisuk C, Kondrashin AV. 1991. Forest malaria in South East Asia; p. 18. [Google Scholar]
- 84.Kalra NL. Forest malaria vectors in India: ecological characteristics and epidemiological implications. In: Sharma FP, Kondrashin AV, editors. Forest malaria in South East Asia. Proceedings of an informal consultative meeting of WHO/MRC 1991. New Delhi: Malaria Research Centre; 1991. Feb 18-22, pp. 93–114. [Google Scholar]
- 85.World Health Organization. Global plan for insecticide resistance management in malaria vectors (GPIRM) [accessed on July 2, 2012]. Available from: http://www.who.int/malaria/vector_control/ivm/gpirm .
- 86.Sharma SK, Upadhyay AK, Haque MA, Tyagi PK, Kindo BK. Impact of changing over of insecticide from synthetic pyrethroids to DDT for indoor residual spray in a malaria endemic area of Orissa, India. Indian J Med Res. 2012;135:382–8. [PMC free article] [PubMed] [Google Scholar]
- 87.Raghavendra K, Barik TK, Reddy BP, Sharma P, Dash AP. Malaria vector control: from past to future. Parasitol Res. 2011;108:757–79. doi: 10.1007/s00436-010-2232-0. [DOI] [PubMed] [Google Scholar]
- 88.Sharma RC, Gautam AS, Bhatt RM, Gupta DK, Sharma VP. The Kheda malaria project: the case for environmental control. Health Policy Plann. 1991;6:262–70. [Google Scholar]
- 89.Dev V, Sharma VP, Hojai D. Malaria transmission and disease burden in Assam: challenges and opportunities. J Parasit Dis. 2009;33:13–22. doi: 10.1007/s12639-009-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sreehari U, Mittal PK, Razdan RK, Ansari MA, Rizvi MM, Dash AP. Efficacy of PermaNet 2.0 against Anopheles culicifacies and Anopheles stephensi malaria vectors in India. J Am Mosq Control Assoc. 2007;23:220–3. doi: 10.2987/8756-971X(2007)23[220:EOPAAC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 91.Sharma SK, Upadhyay AK, Haque MA, Tyagi PK, Raghavendra K, Dash AP. Wash-resistance and field evaluation of alphacypermethrin treated long-lasting insecticidal net (Interceptor) against malaria vectors, Anopheles culicifacies and Anopheles fluviatilis in a tribal area of Orissa, India. Acta Trop. 2010;116:24–30. doi: 10.1016/j.actatropica.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Sharma VP. Battling malaria iceberg incorporating strategic reforms in achieving Millennium Development Goals & malaria elimination in India. Indian J Med Res. 2012;136:907–25. [PMC free article] [PubMed] [Google Scholar]
- 93.Bhatia R, Rastogi RM, Ortega L. Malaria successes and challenges in Asia. J Vector Borne Dis. 2013;50:239–47. [PubMed] [Google Scholar]
- 94.Pennetier C, Bouraima A, Chandre F, Piameu M, Etang J, Rossignol M, et al. Efficacy of Olyset Plus, a new long-lasting insecticidal net incorporating permethrin and piperoniyl butoxide against multi-resistant malaria vectors. PLoS One. 2013;8:e75134. doi: 10.1371/journal.pone.0075134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corbel V, Chabi J, Dabire RK, Etang J, Nwane P, Pigeon O, et al. Field efficacy of a new mosaic long lasting mosquito net (PermaNet 30) against pyrethroid-resistant malaria vectors: a multi centre study in Western and Central Africa. Malar J. 2010;9:113. doi: 10.1186/1475-2875-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sallum MA, Peyton EL, Wilkerson RC. Six new species of the Anopheles leucosphyrus group, reinterpretation of An.elegans and vector implications. Med Vet Entomol. 2005;19:158–99. doi: 10.1111/j.0269-283X.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 97.Manguin S, Garros C, Dusfour I, Harbach RE, Coosemans M. Bionomics, taxonomy, and distribution of the major malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: an updated review. Infect Genet Evol. 2008;8:489–503. doi: 10.1016/j.meegid.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gunathilaka N, Fernando T, Hapugoda M, Wickremasinghe R, Wijeyerathne P, Abeyewickreme W. Anopheles culicifacies breeding in polluted water bodies in Trincomalee district of Sri Lanka. Malar J. 2013;12:285. doi: 10.1186/1475-2875-12-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma SK, Tyagi PK, Padhan K, Upadhyay AK, Haque MA, Nanda N, et al. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh district, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100:917–25. doi: 10.1016/j.trstmh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 101.Tyagi V, Sharma AK, Yadav R, Agrawal OP, Devanathan S, Veer V. Characteristics of the larval breeding sites of Anopheles culicifacies sibling species in Madhya Pradesh, India. Int J Malar Res Rev. 2013;1:47–53. [Google Scholar]
- 102.Sunil S, Singh OP, Nanda N, Raghavendra K, Reddy BP, Subbarao SK. Analysis of population genetic structure of Indian Anopheles culicifacies species A using microsatellite markers. Parasit Vectors. 2013;6:166. doi: 10.1186/1756-3305-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chandre F, Dabire RK, Hougard JM, Djogbenou LS, Irish SR, Rowland M, et al. Field efficacy of pyrethroid treated plastic sheeting (durable lining) in combination with long lasting insecticidal nets against malaria vectors. Parasit Vectors. 2010;3:65. doi: 10.1186/1756-3305-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grietens KP, Xuan XN, Ribera J, Duc TN, Bortel Wv, Ba NT, et al. Social determinants of long lasting insecticidal hammock use among the Ra-glai ethnic minority in Vietnam: implications for forest malaria control. PLoS One. 2012;7:e29991. doi: 10.1371/journal.pone.0029991. [DOI] [PMC free article] [PubMed] [Google Scholar]


