Abstract
Background & objectives:
In India, malaria is a major public health problem in States having predominantly tribal population. The objective of this analysis was to find out the incidence of malaria in various States/districts having varied proportions of tribal population using National Vector Borne Disease Control Programme (NVBDCP) data.
Methods:
States and districts were classified into three categories based on proportions of Scheduled Tribes (ST) population as <10, 10-29.9 and 30 per cent + ST population. Five year average (2008-2012) of all important malaria indicators collected by NVBDCP was taken to normalize the effect of annual fluctuations in malaria incidence.
Results:
State level analysis revealed that ten States/UTs with 30 per cent or more tribal population comprising only three per cent of total population, contributed 14 per cent of total malaria, 21 per cent Plasmodium falciparum and 29 per cent of deaths due to malaria. Similarly, district level analysis showed that districts with 30 per cent or more tribal population comprising about eight per cent country's population contributed to 46 per cent of total malaria cases, 70 per cent P. falciparum and 47 per cent malarial deaths in the country.
Interpretation & conclusions:
Our analysis showed that the neglect of the ethnic communities in tribal areas would be detrimental to the overall reduction of morbidity and mortality due to malaria. The fight against the increasing burden of malaria in tribal belt requires adoption of multiple approaches and socio-economic development of the tribal communities.
Keywords: Malaria control, Plasmodium falciparum, P. vivax, Scheduled Tribes, tribal malaria
Malaria is a major public health problem in India though it is both a preventable and treatable disease. As per the WHO estimates 207 million cases of malaria occurred globally in 2012 (uncertain range 135-287 million) and 6,27,000 deaths (uncertain range 4,73,000- 7,89,000); about 80 per cent of these cases were found in African countries and 13 per cent in South East Asia Region (SEAR) countries1. India contributes to 61 per cent of malaria cases and 41 per cent of malaria deaths in SEAR countries2. In India, approximately 539 million people reside in high transmission areas, i.e. defined as more than one case per 1000 population1. Malaria is a major health problem in rural/tribal areas of the central, eastern and north-eastern states, particularly in 16 states including seven north eastern states and nine states of central India [National Vector Borne Disease Control Programme (NVBDCP) report, 2010-2014]3. These are all tribal dominated states having large population of ethnic groups4.
Tribal population in India is mostly residing in areas which are remote and difficult to reach due to typical geographical situations usually due to forest, hills, valleys and perennial streams5,6. The presence of various malaria parasites and vector species, climatic diversity favouring growth and proliferation of the parasite and vector as well as a highly susceptible human population have resulted in high malaria transmission in tribal areas5,7. The utilization of health services is poor among them and they have their orthodox health beliefs. The proportions of tribal population vary considerably among Indian states/Union Territories (UTs). Records revealed that no Scheduled Tribe (ST) population was enumerated in Census 2011 in the five states/UTs viz. Punjab, Chandigarh, Haryana, Delhi and Puducherry, whereas more than 50 per cent of total population was classified as ST population in six states/UTs, viz, Dadra Nagar Haveli, Arunachal Pradesh, Meghalaya, Nagaland, Mizoram and Lakshadweep8. Thus, it will be important to determine whether proportion of tribal population has any positive correlation with malaria prevalence. The objective of this analysis was to find out the incidence of malaria in various states/districts having varied proportions of tribal population by using the retrospective NVBDCP data (2008-2012) of 35 states and UTs segmented in 620 districts.
Material & Methods
The NVBDCP collects and compiles the data of malaria both monthly and annually from all the states for monitoring the trends and taking public health actions and planning. Five year average (2008-2012) of all important malaria indicators was taken to normalize the effect of annual fluctuations at district level. These indicators were total blood slide examined, total malaria positive cases, Plasmodium falciparum and P. vivax cases and total malarial deaths.
According to Census of India, 20118, the country is administratively divided into 28 states and 7 UTs (Fig. 1). The states/UTs are further divided into 640 districts for administrative purposes. Malaria information is available for 620 census districts, but these districts are clubbed into 609 districts in NVBDCP data set. The analyses were carried out at both state and district level. However, for comparison purpose, states and districts were classified into three categories, based on proportions of ST population, viz. category-I (<10% ST population), category-II (10-29.9% ST population) and category-III (30%+ ST population).
Fig. 1.
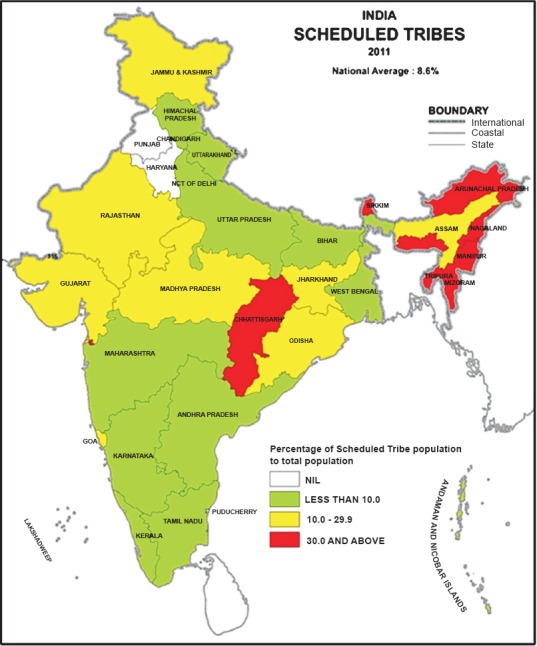
Map showing states in three categories of ST population.
Data were analyzed with the help of MS-excel 2008 (Microsoft, Redmond, WA, USA) and SPSS 20 (IBM Corp., Armonk, NY, USA). Odds ratios (OR) were computed to assess the association of tribal population in the districts with malaria. Category-I (districts with less than 10% ST population) was treated as a reference category.
Results
State level analyses: The distribution of tribal population in India showed that more than 80 per cent of country's tribal population resided in 10 states viz. Madhya Pradesh, Maharashtra, Odisha, Rajasthan, Gujarat, Jharkhand, Chhattisgarh, Andhra Pradesh, West Bengal and Karnataka. Of these states, Madhya Pradesh and Maharashtra contributed to one-fourth of total tribal population. Similarly, more than 80 per cent of country's total malaria cases were reported from 10 states, viz. Odisha, Jharkhand, Chhattisgarh, Madhya Pradesh, West Bengal, Maharashtra, Gujarat, Assam, Uttar Pradesh and Rajasthan. Eight states were common in both 10 tribal concentrated and malarious states/UTs in the country.
Of the 35 Indian states/UTs, 17 states/UTs had tribal population less than 10 per cent, whereas proportion of tribal population varied between 10-29.9 per cent in eight states/UTs and 30 per cent or more in 10 states/UTs. Table I shows that 17 states/UTs with less than 10 per cent tribal population comprised about 70 per cent of total population and 29 per cent of tribal population. Eight states with 10-29.9 per cent tribal population comprised about 27 per cent of India's total population and 55 per cent of total ST population and 10 states/UTs with 30 per cent or more tribal population contributed only three per cent of total population, but about 16 per cent of country's tribal population. Category-II, states/UTs contributed 59, 68 and 49 per cent of total malaria, P. falciparum and P. vivax cases, respectively (Table I). Similarly, category-III states/UTs also contributed considerably to total malaria (14%), P. falciparum (21%) and P. vivax (7%) cases. While 27 per cent of the population in category II States/UTs contributed to 45 per cent of malaria related deaths, only three per cent of population in category III states/UTs contributed to 29 per cent of deaths.
Table I.
Distribution of blood slide examined, Plasmodium vivax, P. falciparum and total malaria cases in States/UTs by categories of per cent ST population of India (average of 2008-2012)
As per NVBDCP guideline, about 10 per cent population should be screened for malaria annually, i.e. annual blood slide examination rate (ABER) should be 10 per cent3. Table I showed that annually 8.8 per cent of population was tested for malaria. However, the proportion of ABER increased consistently by proportion of ST population. Similarly, the slide vivax rate (SVR), slide falciparum rate (SFR) and slide positivity rate (SPR) also increased with proportion of tribal population. The SPR was 0.6 for category-I states/UTs and it was 2 and 4.4 per cent for category-II and III States, respectively. Values of annual parasite incidence (API) for the three categories were 0.44, 2.56 and 5.02, respectively.
District level analyses: Similar to the state level analyses, district level analyses were done to assess the relationship between proportion of ST population and malaria incidence. District level analyses provided micro level insights. For comparison purpose, districts were also divided into three categories, viz. category-I (districts with <10% ST population), category-II (districts with 10-29.9% ST population) and category-III (districts with 30+% ST population). Of the 609 programme districts, 381 (62.6%) had <10 per cent ST population, 104 (17.1%) and 124 (20.3%) districts had 10-29.9 and 30 per cent + ST population, respectively. Category-I districts comprised about 76 per cent total and 19 per cent ST population. Conversely, the proportions of country's total and ST population were 17, 33 and 8, 49 per cent in category-II and category-III districts, respectively. About three-fourth population of India residing in category-I districts contributed to one-third of malaria cases and 11 per cent Pf and 57 per cent Pv cases, whereas, about eight per cent of population residing in category-III districts contributed to 46 per cent of total malaria cases, 70 per cent of Pf and 22 per cent of Pv cases. about 47 per cent malaria deaths in the country also occurred in this eight per cent of population, conversely, 31 and 23 per cent malaria deaths, respectively were reported from category-I and category-II districts (Table II).
Table II.
Distribution of districts, population, ST population, blood slide examined, malaria cases and malaria deaths by categories of districts (average of 2008-2012)
The ABER was 7.1 for category-I districts, which increased to 13.3 and 15.3 in category-II and III districts, respectively. SFR was 0.1 per cent in category-I and it increased to 0.5 and 3.4 per cent in category-II and III districts. Similarly, SPR increased from 0.7 to 1.1 and 4.4 per cent in category-I to category-II and III districts, respectively. API also increased considerably from 0.51 for category-I to 1.44 and 6.77 in category-II and III, respectively. The analysis of SPR and API during the five years period (2008-2012) also revealed that malaria incidence was considerably higher throughout the period in category-III districts as compared to districts from other categories (Fig. 2A, B).
Fig. 2a.
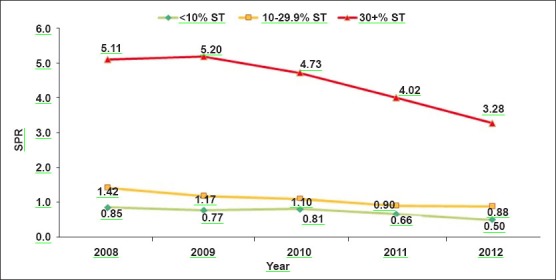
Trends of slide positivity rate (SPR) over 2008-2012 among different categories of districts.
Fig. 2b.
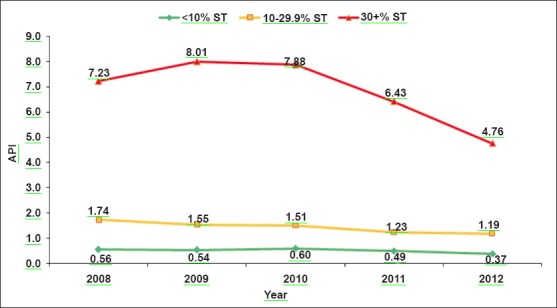
Trends of annual parasite index (API) over 2008-2012 among different categories of districts.
To study the significance of these differences between the three categories of districts, odd-ratios were computed treating category-I (<10% ST population) as a reference category. The analysis demonstrated that category-III (districts with 30+ ST population) districts had significantly higher SVR (OR=1.7; 95% CI 1.4-2.1), SFR (OR=30.0; 95% CI 23.5-38.2), SPR (OR=6.5; 95% CI 5.7-7.3) and API (OR=13.4; 95% CI 11.9-15.1) as compared to category-I (Fig. 3). Category-II districts also presented higher values for all malaria indicators except SVR as compared to category-I.
Fig. 3.
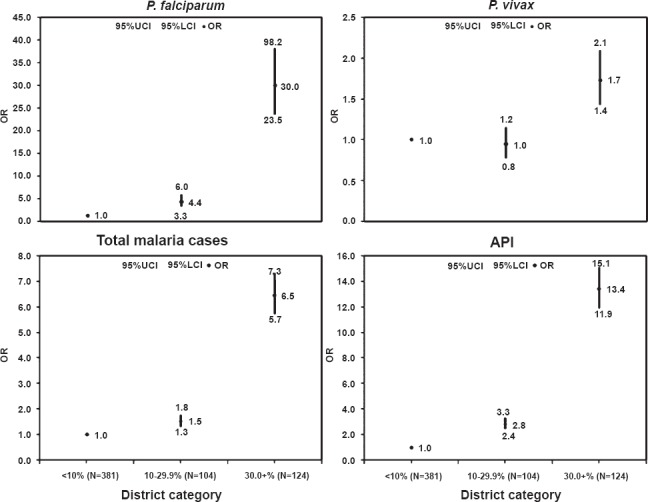
Odds ratio for Pf, Pv total malaria cases and API among different categories of districts. Figures present odds ratios (OR) with respect to category –I (districts with <10% ST population) on Y axis and different districts categories (i.e. districts with <10. 10-29.9 and >30% ST population) on X axis. UCI, upper confidence limit; LCI, lower confidence limit.
Discussion
Understanding the multifaceted determinants of tribal malaria is important as many factors play a role in malaria transmission directly or indirectly. The tribal villages are intersected by numerous hill streams and their tributaries which support mosquito breeding throughout the year5,7,9. The rainfall, water logging and rise in the pooling are major problem of the tribal areas. Moreover, ethnic communities prefer to go to spiritual healer for treatment6,9,10,11 or to untrained and unlicensed practitioners (quacks). Many efficient primary and secondary vectors (Anopheles culicifacies, An. baimaii, An. minimus, An. fluviatilis, An. nivips and An. sundaicus) are present in tribal areas11,12,13,14,15, along with rising insecticide resistance16,17,18. An inadequate health care infrastructure with inadequate spray coverage are the factors that maintain malaria as one of the most important causes of illness and death in most of tribal dominated parts of India19,20.
Rapid diagnostic tests (RDTs) allow the rigorous monitoring of malaria burden and reduce dependence on traditional microscopy21,22. However, RDT supply is limited for prompt diagnosis and treatment. Further, most RDTs have a technical concern. The detection of low density parasitaemia is unreliable and leaving a reservoir of disease only detectable by microscopy/PCR.
However, wide scale testing is currently only possible practically using RDTs. Hence, only good quality RDTs are to be used for optimal results23.
Artemisinin based combination therapy (ACT) is highly effective, works rapidly and has very few adverse effects24. Although the States have adopted ACTs in tribal districts, yet only a small fraction of the population actually receives them6,20. Further, the constant movement of people in and out of the forest makes it at times difficult to maintain contact with people for diagnosis and treatment25,26. The primary health centres (PHCs) responsible for providing health services to the villages are often understaffed and not able to cope with the high prevalence of malaria26,27.
Our study had some limitations. First, the data were aggregated at the district level, so local effect could not be detected. Secondly, ST vs non-ST wise information was not available in the Programme data, hence any kind of comparison between incidence of malaria between tribal and non-tribal population within a category was not possible. Thirdly, in case of SPR, it could be possible that a proportion of the clinical malaria cases never made it to the surveillance system, thus the actual number of malaria cases particularly in the category III States/districts might be much higher. Another major limitation was that information on other risk factors such as behaviour/traditional belief, cultural habits and environmental factors in relation to malaria was not available. Thus, in-depth studies are required for each of the three categories to develop evidence based appropriate malaria control strategies.
The most effective way to control malaria is robust and sensitive surveillance so that both symptomatic or asymptomatic cases may be treated promptly to prevent the spread of disease28,29. The implementation of RDTs, ACTs, long lasting insecticidal nets (LLINs) along with a mix of preventive strategies by timely procuring efficacious insecticide in adequate quantities for complete coverage and integrated vector management are required30.
The neglect of the ethnic communities and tribal areas would be detrimental to the overall reduction of morbidity and mortality due to malaria. The fight against the increasing burden of malaria in tribal belt will require adoption of multiple approaches and socio-economic development of the tribal population.
The present analysis indicates malaria as a major problem in tribal areas, hence these tribal dominated States/UTs and districts need special attention. Improving socio-economic status of the community in the long run will have a great impact on the incidence of malaria.
References
- 1.World malaria report 2013. Geneva: World Health Organization; 2013. [accessed on March 11, 2014]. WHO. Available from: www.who.int/iris/bitstream/10665/97008/1/9789241564694_eng.pdf . [Google Scholar]
- 2.World malaria report 2011. Geneva: World Health Organization; [accessed on March 11, 2014]. WHO. Available from: http://www.who.int/malaria/world_malaria _report_2011/9789241564403_eng.pdf . [Google Scholar]
- 3.Malaria situation in India. Geneva: World Health Organization; [accessed on March 11, 2014]. National Vector Borne Disease Control Programme, (2010-2014) Available from: http://www.nvbdcp.gov.in/Doc/malaria-situation-March14.pdf . [Google Scholar]
- 4.Singh N, Singh MP, Wylie BJ, Hussain W, Kojo YA, Shekhar C, et al. Malaria prevalence among pregnant women in two districts with differing endemicity in Chhattisgarh, India. Malar J. 2012;11:274–87. doi: 10.1186/1475-2875-11-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh N, Singh OP, Sharma VP. Dynamics of malaria transmission in forested and deforested region of Mandla district, Central India, Madhya Pradesh. J Am Mosq Cont Assoc. 1996;12:225–34. [PubMed] [Google Scholar]
- 6.Sundararajan R, Kalkonde Y, Gokhale C, Greenough PG, Bang A. Barriers to malaria control among marginalized tribal communities: a qualitative study. PLoS One. 2013;8:e81966. doi: 10.1371/journal.pone.0081966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh N, Chand SK, Bharti PK, Singh MP, Chand G, Mishra AK, et al. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 2013;8:e73730. doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Census 2011. Primary census abstract for scheduled tribes. [accessed on June 2, 2014]. Available from: http://www.censusindia.gov.in/2011census/population_enumeration.aspx .
- 9.Singh N, Mishra AK, Chand SK, Sharma VP. Population dynamics of Anopheles culicifacies and malaria in tribal area of central India. Am J Mosquito Control Assoc. 1999;15:283–90. [PubMed] [Google Scholar]
- 10.Singh N, Singh MP, Saxena A, Sharma VP, Kalra NL. Knowledge, attitude, beliefs and practices (KABP) study related to malaria and intervention strategies in ethnic tribals of Mandla (Madhya Pradesh) Curr Sci. 1998;75:1386–90. [Google Scholar]
- 11.Nanda N, Bhatt RM, Sharma SN, Rana PK, Kar NP, Sharma A, et al. Prevalence and incrimination of Anopheles fluviatilis species S (Diptera: Culicidae) in a malaria endemic forest area of Chhattisgarh state, central India. Parasit Vectors. 2012;5:215–20. doi: 10.1186/1756-3305-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Potential of Anopheles philippinensis-nivipes complex mosquitoes as malaria vector in north-east India. J Environ Biol. 2005;26:719–23. [PubMed] [Google Scholar]
- 13.Dev V. Anopheles minimus: its bionomics and role in the transmission of malaria in Assam, India. Bull World Health Organ. 1996;74:61–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Jambulingam P, Sahu SS, Manonmani A. Reappearance of Anopheles minimus in Singhbum hills of East-Central India. Acta Trop. 2005;96:31–5. doi: 10.1016/j.actatropica.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Das M, Das B, Patra AP, Tripathy HK, Mohapatra N, Kar SK, et al. Anopheles culicifacies sibling species in Odisha, eastern India: First appearance of Anopheles culicifacies E and its vectorial role in malaria transmission. Trop Med Int Health. 2013;18:810–21. doi: 10.1111/tmi.12112. [DOI] [PubMed] [Google Scholar]
- 16.Mishra AK, Chand SK, Barik TK, Dua VK, Raghavendra K. Insecticide resistance status in Anopheles culicifacies in Madhya Pradesh, central India. J Vector Borne Dis. 2012;49:39–41. [PubMed] [Google Scholar]
- 17.Sahu SS, Patra KP. A study on insecticide resistance in Anopheles fluviatilis and Anopheles culicifacies to HCH and DDT in the Malkangiri district of Orissa. Indian J Malariol. 1995;32:112–8. [PubMed] [Google Scholar]
- 18.Sahu SS, Gunasekaran K, Raju HK, Vanamail P, Pradhan MM, Jambulingam P. Response of malaria vectors to conventional insecticides in the southern districts of Odisha State, India. Indian J Med Res. 2014;139:294–300. [PMC free article] [PubMed] [Google Scholar]
- 19.Singh N, Dash AP, Krongthong T. Fighting malaria in Madhya Pradesh (Central India): Are we loosing the battle? Malar J. 2009;8:93. doi: 10.1186/1475-2875-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N, Shukla MM, Chand G, Bharti PK, Singh MP, Shukla MK, et al. Epidemic of Plasmodium falciparum malaria in Central India, an area where chloroquine has been replaced by artemisinin-based combination therapy. Trans R Soc Trop Med Hyg. 2011;105:133–9. doi: 10.1016/j.trstmh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Bharti PK, Silawat N, Singh PP, Singh MP, Shukla MM, Chand G, et al. The usefulness of a new rapid diagnostic test, First Response® Combo Malaria Ag (pLDH/HRP2) card test for malaria diagnosis in forested belt of Central India. Malar J. 2008;7:126. doi: 10.1186/1475-2875-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh N, Bharti PK, Singh MP, Mishra S, Shukla MM, Sharma RK, et al. Comparative evaluation of bivalent malaria rapid diagnostic tests versus traditional methods in field with special reference to heat stability testing in Central India. PLoS One. 2013;8:e58080. doi: 10.1371/journal.pone.0058080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geneva: World Health Organization; 2012. [accessed on March 26, 2014]. WHO. Malaria rapid diagnostic test performance. Results of WHO product testing of malaria RDTs: round 4. Available from: http://www.finddiagnostics.org/export/sites/default/resource-centre/reports_brochures/docs/RDTMalariaRd4_Web3.pdf . [Google Scholar]
- 24.White NJ. Malaria-time to act. N Engl J Med. 2006;355:1956–7. doi: 10.1056/NEJMp068214. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, Chand SK, Mishra AK, Nagpal AC. Migration malaria associated with forest economy in central India. Curr Sci. 2004;87:1696–9. [Google Scholar]
- 26.Singh N, Chand SK, Mishra AK, Bharti PK, Singh MP, Ahluwalia TP, et al. Epidemiology of malaria transmission in an area of low transmission in Central India. Am J Trop Med Hyg. 2006;75:812–6. [PubMed] [Google Scholar]
- 27.Singh N, Shukla MM, Mishra AK, Singh MP, Paliwal JC, Dash AP. Malaria control using indoor residual spraying and larvivorous fish: a case study: in Betul, Central India. Trop Med Int Health. 2006;11:1512–20. doi: 10.1111/j.1365-3156.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- 28.Sturrock HJ, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med. 2013;10:e1001467. doi: 10.1371/journal.pmed.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R, Godson II, Singh S, Singh RB, Isyaku NT, Ebere UV. High prevalence of asymptomatic malaria in apparently healthy schoolchildren in Aliero, Kebbi state, Nigeria. J Vector Borne Dis. 2014;51:128–32. [PubMed] [Google Scholar]
- 30.Muturi EJ, Burgess P, Novak RJ. Malaria vector management: where have we come from and where are we headed? Am J Trop Med Hyg. 2008;78:536–7. [PubMed] [Google Scholar]


