Abstract
Background & objectives:
The northeastern States of India are co-endemic for Plasmodium falciparum and P. vivax malaria. The transmission intensity is low-to-moderate resulting in intermediate to stable malaria. Malaria control prioritized P. falciparum being the predominant and life threatening infection (>70%). P. vivax malaria remained somewhat neglected. The present study provides a status report of P. vivax malaria in the northeastern States of India.
Methods:
Data on spatial distribution of P. vivax from seven northeastern States (Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland and Tripura) were analysed retrospectively from 2008–2013. In addition, cross-sectional malarial surveys were conducted during 1991-2012 in malaria endemic pockets across the States of Assam, Meghalaya, Mizoram and Tripura to ascertain the prevalence of P. vivax in different age groups.
Results:
Vivax malaria was encountered in all northeastern States but there existed a clear division of two malaria ecotypes supporting ≤30 and >30 per cent of total malaria cases. High proportions of P. vivax cases (60–80%) were seen in Arunachal Pradesh and Nagaland in the north with alpine environment, 42-67 per cent in Manipur, whereas in Assam it varied from 23-31 per cent with subtropical and tropical climate. Meghalaya, Tripura and Mizoram had the lowest proportion of P. vivax cases. Malaria cases were recorded in all age groups but a higher proportion of P. vivax consistently occurred among <5 yr age group compared to P. falciparum (P<0.05). P. vivax cases were recorded throughout the year with peak coinciding with rainy season although transmission intensity and duration varied.
Interpretation & conclusions:
In northeast India, P. vivax is a neglected infection. Estimating the relapsing pattern and transmission dynamics of P. vivax in various ecological settings is an important pre-requisite for planning malaria elimination in the northeastern States.
Keywords: Malaria burden, malaria control, Plasmodium vivax, spatial distribution, transmission dynamics
Northeastern States of India are co-endemic for both Plasmodium falciparum and P. vivax malaria, and in the past contributed 10 per cent of cases and 20 per cent malaria-attributable deaths in India1. Malaria epidemiology is complex due to high aboriginal population, varied terrain, rich forest cover and favourable climatic conditions for transmission. Anopheles minimus and An. baimaii are the two most efficient malaria vectors with strong predilection for human host2. Both vectors were repeatedly incriminated throughout its range of distribution. In addition, An. nivipes, An. maculatus and An. culicifacies are also suspected to contribute some cases3. In 2013, the National Vector Borne Disease Control Programme (NVBDCP) reported 0.88 million malaria cases in the country with nearly equal number of P. falciparum and P. vivax cases4. The proportion of P. falciparum and P. vivax, however, varied greatly inter alia from one ecotype to another due to climate variability and malaria control interventions5.
During widespread malaria outbreaks in early 1970s epidemiological studies revealed that P. falciparum was mainly confined to the northeastern States and its liquidation was considered important to protect rest of the country from P. falciparum invasion. With this objective, an additional component of Plasmodium falciparum Control Programme (PfCP) was launched in the northeastern region beginning 1976 for intensification of control interventions6. Despite this programme in the decade that followed P. falciparum spread and invaded States in the mainland affecting >120 million people. Therefore, PfCP was terminated after 11 years of operation. During this period, P. vivax remained a neglected parasite. Malaria control relied on indoor residual spraying (IRS), case detection and treatment of P. vivax and P. falciparum. In addition, all fever cases were given chloroquine presumptive treatment. P. vivax responded well to chloroquine therapy and malaria-attributable mortality was solely ascribed to P. falciparum infection confirmed by blood-smear examination report6. This scenario changed gradually with appearance of reports of resistance in P. vivax to chloroquine and cases of severe P. vivax malaria from India and many other malaria endemic countries7,8,9. In India, information on P. vivax strains and their relapsing pattern remained scanty except one study from Delhi10. We undertook this study to provide a status report of vivax malaria in the northeastern States of India.
Material & Methods
Study area: The northeast region of India comprises eight States: Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland and Tripura, and State of Sikkim. All States are malaria endemic except Sikkim, therefore, this study was restricted only to seven States. The forest cover varies from 40 per cent in plain valleys to nearly 80 per cent in hill States of Nagaland and Arunachal Pradesh. Assam is the major constituent State of the northeast with more than 70 per cent population (~32 million) contributing >50 per cent malaria cases1. Northeast India shares vast international border with China (South Tibet) in the north, Myanmar in the East, Bangladesh in the southwest and Bhutan to the northwest (Fig. 1). The entire population is largely classified as tribal with at least 220 ethnic groups, rich in cultural heritage, fauna and flora and major river systems. The population density varies from 13 per sq. kilometer in Arunachal Pradesh to 340 per sq. kilometer in Assam living predominantly in the countryside (84%), and literacy rate is 68.5 per cent11.
Fig. 1.
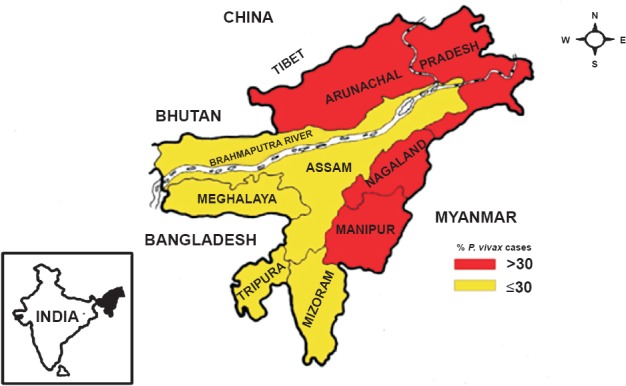
Distribution of P. vivax malaria in northeastern States of India based on pooled data from 2008–2013. International borders are demarcated by bold line and State boundaries are colour coded showing relative abundance of P. vivax (% of total malaria reported cases). Inset is the map of India showing geographical location of northeast region (Source: Ref. 4).
Climate data: Climate in the region varies from temperate to tropical in plains and alpine in the high mountain reaches. In the plain valleys, it is predominantly sub-tropical with hot and humid summers, heavy monsoons, and mild winters. Most parts receive on an average 2-3 m of rainfall during April–September associated with pre-monsoon activity and southwest monsoon.
Malaria control: Transmission is perennial in most parts with seasonal peak during April-September. Malaria is unevenly distributed with varying transmission intensities. Presently malaria control in northeastern States is based on (i) DDT indoor residual spraying two rounds at a concentration of 1g/m2 done on selective basis in areas reporting high incidence of malaria and deaths, and (ii) early case detection by microscopic examination of blood smear or the rapid diagnostic test and prompt treatment with chloroquine (CQ) and 14 day primaquine (instead of 5-day PQ therapy in P. vivax) as per recommendations of WHO since 2007, and impregnation of community-owned mosquito nets with synthetic pyrethroid and/or supply of long-lasting insecticidal net (LLIN) distributed gratis among high-risk groups12,13.
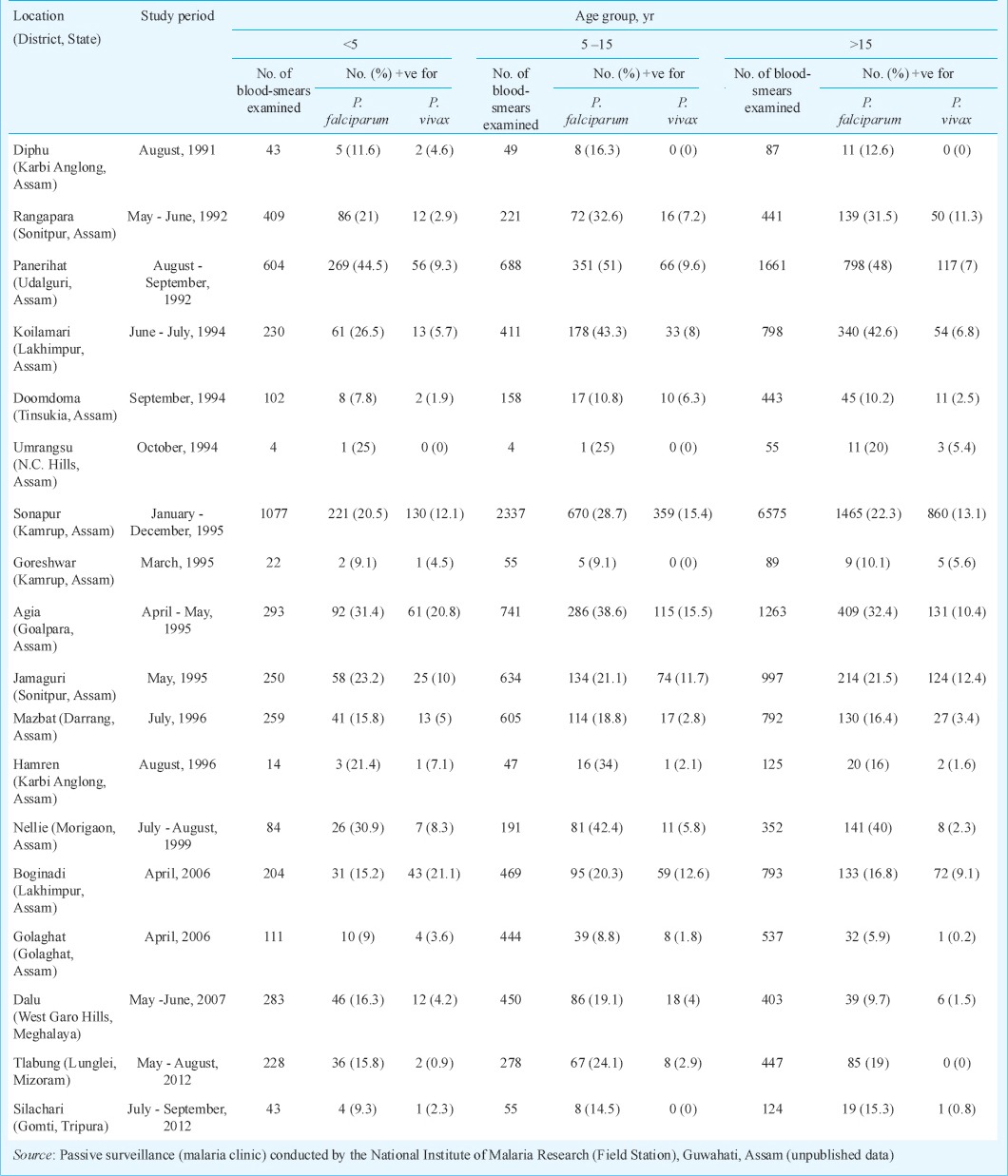
Data collection and analyses: This study was a retrospective data analyses of malaria based on State disease surveillance with particular reference to distribution of P. vivax in northeastern States of India. Data for respective State for the period from 2008–2013 were accessed online from the Directorate of National Vector Borne Disease Control Programme of Government of India4. For district-wise stratification of P. vivax malaria, retrospective data for the period from 2000-2013 were analyzed for the State of Assam (State Health Directorate of Assam, personal communication). In addition, cross-sectional malaria prevalence surveys were conducted by the National Institute of Malaria Research, Guwahati (Field Station) during 1991-2012 mostly during high transmission season in ethnic communities in States of Assam, Meghalaya, Mizoram and Tripura to ascertain malaria prevalence by parasite species in different age groups (unpublished data). Furthermore, in 1992 mass and contact surveys were undertaken on the monthly basis in the Sonapur Primary Health Centre (PHC), a typical foothill PHC of Kamrup district of Assam, to ascertain seasonal malaria positivity in febrile and afebrile cases. Malaria data were thus analyzed to study, (i) vivax malaria distribution in the northeastern States, (ii) seasonal transmission of vivax malaria, (iii) results of cross-sectional surveys for distribution of malaria parasite species in three age groups i.e. <5, 5-15 and >15 yr, and (iv) meteorological data and seasonal prevalence of P. falciparum and P. vivax malaria in afebrile and febrile cases in Assam State only. All microscopically confirmed malaria positive cases were administered anti-malarial drugs as per prevailing national drug policy13.
Data on relative prevalence of malaria parasite species in different age groups were analysed by chi-square test using Stata v10 (http://www.stata.com/).
Results
Data on malaria cases and per cent contribution of P. vivax malaria for the period 2008-2013 in the northeastern States are given in Table I. Vivax malaria is encountered in all States but the number of cases and proportion of parasite species varied from State to State depending on environmental determinants. However, there was a clear division of two malaria ecotypes in the northeast region in respect of P. vivax cases, i.e. with ≤30 and >30 per cent of total malaria cases (Fig. 1). High proportions of P. vivax (60-80%) were seen in Arunachal Pradesh and Nagaland in the north with alpine environment. In Manipur, P. vivax cases varied from 42-67 per cent, whereas in Assam cases varied from 23-31 per cent with subtropical to tropical climate. Meghalaya, Tripura and Mizoram have the lowest population of P. vivax cases (Fig. 2). These proportions were rather consistent over several years.
Table I.
Number of malaria cases with percentages of P. vivax in northeastern States of India
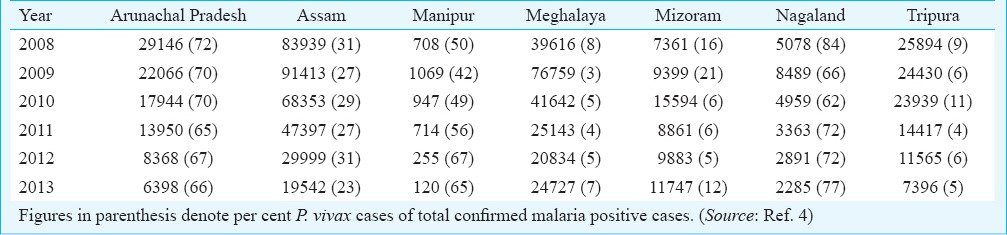
Fig. 2.
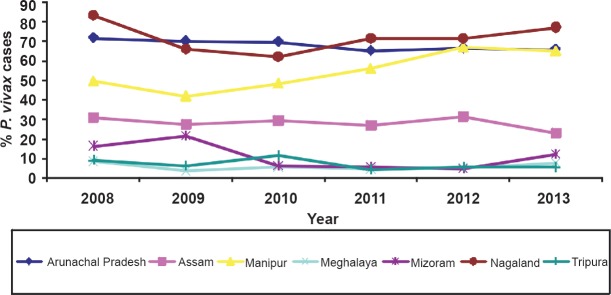
Relative abundance of P. vivax malaria in northeastern States of India during 2008-2013. Arunachal Pradesh and Nagaland showed the highest percentage (60-80%) of vivax malaria, and progressive increase in vivax cases was noted in Manipur beginning 2011 reaching at par with other States. In Assam, this percentage is less than half (≤30%). In Meghalaya and Tripura the percentage of vivax malaria is the lowest (~10%). These percentage figures in these States were almost stable from 2008-2013 except that for Manipur. (Source: Ref. 4)
Data on monthly distribution in 2012 of P. vivax cases in northeastern States are presented in Fig. 3. P. vivax cases were recorded throughout the year with distinct peak coinciding with months of rainfall but transmission intensity and duration varied between States. In Arunachal Pradesh and Nagaland, it was from June-September; in Assam it was from May–September, and in Meghalaya it was form August-November. For all other States, cases were very few with very little variation. Results of cross-sectional malaria prevalence surveys conducted in Assam, Meghalaya, Mizoram and Tripura are presented in Table II. It was observed that P. vivax malaria was prevalent in all age groups but cases were significantly higher in children <5 and relatively less in >15 yr age group (P<0.05).
Fig. 3.
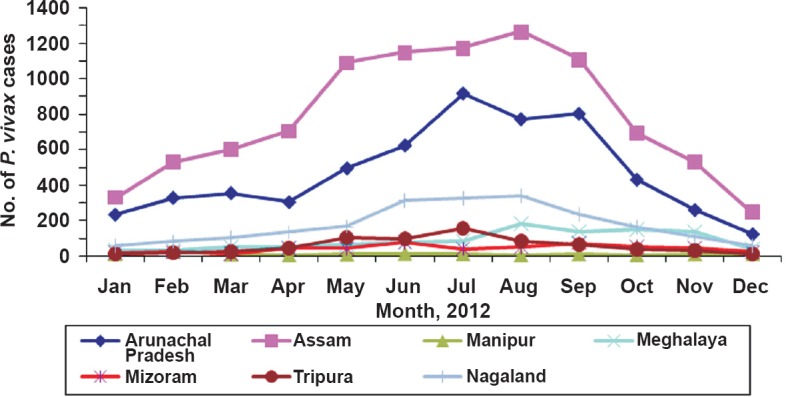
Monthly distribution of P. vivax malaria in northeastern States of India based on 2012 data (Source: State Health Directorates of all seven States, personal communication).
Table II.
Results of cross-sectional malaria prevalence surveys in ethnic communities of northeast India
malaria data for each district of Assam for the period from 2000-2013 were analyzed to study distribution of vivax malaria within the State. The State was divisible in two contiguous sections, i.e. with ≤30 per cent of vivax malaria in districts located south of Brahmaputra river and >30 per cent in districts located on north bank of total reported cases by the State disease surveillance (Fig. 4).
Fig. 4.
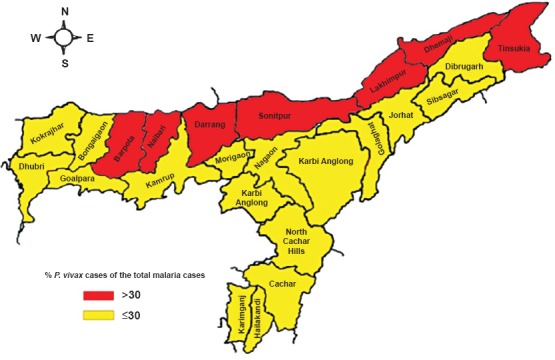
Distribution of P. vivax malaria in Assam for data pooled from 2000–2013. District boundaries are colour coded showing lower proportions of vivax malaria in southern districts and higher in the north (Source: State Health Directorate of Assam, personal communication).
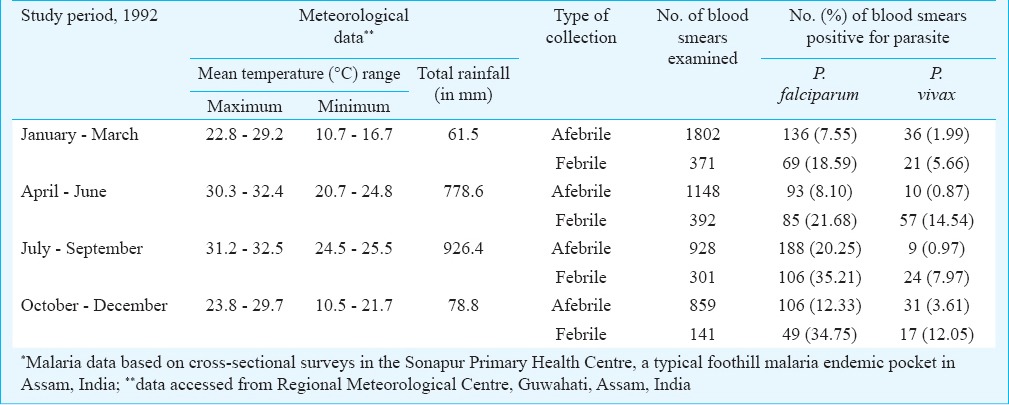
The relative smear positivity of P. vivax and P. falciparum in afebrile and febrile malaria cases in four quarters of the year (January-March, April-June, July-September and October-December) was studied in a typical foothill malaria endemic pocket in the Sonapur Primary Health Centre of Kamrup district of Assam (Table III). Data analyses revealed P. vivax malaria in both afebrile and febrile subjects but was more pronounced in febrile group in all four quarters. There was, however, comparatively higher abundance of P. vivax malaria in afebrile subjects during dry months of October–March (parasite rate 1.99%–3.61%) compared with wet season (April–September) in which parasite rate varied from 0.87–0.97 per cent.
Table III.
Meteorological data and relative smear positivity of malaria parasite species in afebrile and febrile cases*
Discussion
The northeast region of India is of strategic importance and categorized high-risk for sharing vast international borders with neighbouring countries. Chloroquine resistance in P. falciparum was first detected in 1973 in the Karbi Anglong district, Assam14. Studies on drug resistance have revealed that northeast region is an established route for migration and spread of drug-resistant P. falciparum malaria to rest of the country15.
The present analysis revealed that P. vivax malaria in northeast India was substantial and likely to perpetuate. With worldwide reported transmission reduction and many countries heading for malaria elimination, control of P. vivax malaria is gaining eminence due to its inherent biological characteristics/parasite resilience16. As and when the national control programme embarks upon malaria elimination the presented data on spatial distribution and seasonal abundance of malaria parasite species would be vital in planning malaria control interventions as the strategy for elimination of P. falciparum and P. vivax would be different. For example, P. falciparum elimination would depend heavily on artemisinin based combination therapy (ACT); whereas P. vivax elimination would require robust surveillance for case detection and radical treatment to prevent relapses17.
There are many research gaps which need to be addressed for control of P. vivax malaria in continuing efforts for achieving substantial transmission reduction. There are virtually no reports related to relapsing pattern, severe malaria, drug resistance, deaths due to P. vivax malaria specific to northeast region18,19. malaria was eliminated from Greece about 60 years ago but in 2011 there was a case of severe vivax malaria20. Such a situation can arise in the northeastern States as well. In the background of prevailing vivax malaria situation and the malaria elimination strategy that may be implemented in the years to come, priority areas of field-based research have been identified for the speedy elimination of vivax malaria from the northeastern region21,22,23. These include micro-stratification of vivax malaria24; vivax refractory strains for population replacement25; P. vivax strains and their relapsing pattern26,27,28; clinical trials with 8-aminoquinolines for radical cure of vivax malaria29; mixed infections30; mass primaquine (PQ) administration to liquidate vivax malaria from the community31,32; glucose-6-phosphate dehydrogenase (G6PD) deficiency and prevalence of duffy antigen in various ethnic groups33; severe vivax malaria34; chloroquine resistance in P. vivax malaria35; reliable and highly sensitive methodology for detection of P. vivax and mixed infections36; haemoglobinopathies in various ethnic groups and its relationship with antimalarial drugs37; newer treatments for preventing relapses38; post-genomic era research and malaria vaccine trials, etc.39,40,41.
In conclusion, the burden of P. vivax malaria is enormous in northeast India. For control of P. vivax malaria, it is of utmost importance to strengthen health systems for robust surveillance to ensure case detection and treatment, cross-border initiative for coordinated control interventions along inter-State and international borders, and targeting high-risk foci with enhanced vector control interventions in time and place to interrupt transmission. Given the heterogeneity in transmission intensities of the causative parasites, there is scope for additional research specific to northeast India related to parasite biology, and detection and treatment of hypnozoites to ensure radical cure, system biology approaches facilitating field evaluation of effective vaccine against this relapsing malaria to reduce parasite load that is likely to persist resulting in continued transmission.
Acknowledgment
The authors thank Drs Neena Valecha (National Institute of Malaria Research, New Delhi), Naman Shah (Department of Epidemiology, University of North Carolina, USA) and Simon Hay (Department of Zoology, University of Oxford) for critical review and valued inputs, and State Programme Officers of Arunachal Pradesh, Assam, Manipur, Meghalaya and Mizoram, Nagaland and Tripura for data access and discussions on the subject. Technical assistance of the Integrated Disease Vector Control project staffs for field based data collection is duly acknowledged.
References
- 1.Dev V, Bhattacharyya PC, Talukdar R. Transmission of malaria and its control in the Northeastern Region of India. J Assoc Physicians India. 2003;51:1073–6. [PubMed] [Google Scholar]
- 2.Dev V, Sharma VP. The dominant mosquito vectors of human malaria in India. In: Manguin S, editor. Anopheles mosquitoes - New insights into malaria vectors. Croatia: INTECH Publications; 2013. pp. 239–71. [Google Scholar]
- 3.Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Role of the prevalent Anopheles species in the transmission of Plasmodium falciparum and P. vivax in Assam state, north-eastern India. Ann Trop Med Parasitol. 2004;98:559–68. doi: 10.1179/000349804225021361. [DOI] [PubMed] [Google Scholar]
- 4.National Vector Borne Disease Control Programme, Malaria situation in India. Ministry of Health & Family Welfare. [accessed on August 20, 2014]. Available from: http://www.nvbdcp.gov.in/Doc/mal_situation_July2014.pdf .
- 5.Joshi H, Prajapati SK, Verma A, Kang S, Carlton JM. Plasmodium vivax in India. Trends Parasitol. 2008;24:228–35. doi: 10.1016/j.pt.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Ray AP, Narasimham MVVL, Kondrachin AV, Bill AK. Ten years of operation in India (1978-1988) Delhi: PfCP/Directorate of NMEP/WHO/SIDA; 1988. P. falciparum containment programme (PFCP) p. 290. [Google Scholar]
- 7.Dua VK, Kar PK, Sharma VP. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1:816–9. doi: 10.1111/j.1365-3156.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 8.Gosling RD, Hsiang MS. Malaria and severe anemia: Thinking beyond Plasmodium falciparum. PLoS Med. 2013;10:e1001576. doi: 10.1371/journal.pmed.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price RN, Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–91. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adak T, Sharma VP, Orlov VS. Studies on the Plasmodium vivax relapse pattern in Delhi, India. Am J Trop Med Hyg. 1998;59:175–9. doi: 10.4269/ajtmh.1998.59.175. [DOI] [PubMed] [Google Scholar]
- 11.Shillong: North Eastern Council Secretariat, Ministry of Development of North Eastern Region, Government of India; 2009. India's Northeast paradise unexplored; p. 33. [Google Scholar]
- 12.National Vector Borne Disease Control Programme, Malaria control strategies. Government of India, Ministry of Health & Family Welfare. [accessed on May 20, 2012]. Available from: http://www.nvbdcp.gov.in/malaria11.html .
- 13.National Drug Policy on Malaria 2012. Directorate of National Vector Borne Diseases Control Programme, Directorate General of Health Services, Government of India. [accessed on May 20, 2012]. Available from: http://www.nvbdcp.gov.in .
- 14.Sehgal PN, Sharma MID, Sharma SL, Gogoi S. Resistance to chloroquine in falciparum malaria in Assam State, India. J Commun Dis. 1973;5:175–80. [Google Scholar]
- 15.Shah NK, Dhillon GPS, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: Changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos Mda S, Nicolete VC, et al. Epidemiology of disappearing Plasmodium vivax malaria: A case study in Rural Amazonia. PLoS Negl Trop Dis. 2014;8:e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruno M, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, et al. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valecha N, Joshi H, Eapen A, Ravindran J, Kumar A, Prajapati SK, et al. Therapeutic efficacy of chloroquine in Plasmodium vivax from areas with different epidemiological patterns in India and their Pvdhfr gene mutation pattern. Trans R Soc Trop Med Hyg. 2006;100:831–7. doi: 10.1016/j.trstmh.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Mishra N, Singh JPN, Srivastava B, Arora U, Shah NK, Ghosh SK, et al. Monitoring antimalarial drug resistance in India via sentinel sites: outcomes and risk factors for treatment failure, 2009-2010. Bull World Health Organ. 2012;90:895–904. doi: 10.2471/BLT.12.109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spanakos G, Alifrangis M, Schousboe ML, Patsoula E, Tegos N, Hansson HH, et al. Genotyping Plasmodium vivax isolates from the 2011 outbreak in Greece. Malar J. 2013;12:453. doi: 10.1186/1475-2875-12-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 22.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- 23.Sharma VP. Battling malaria iceberg incorporating strategic reforms in achieving Millennium Development Goals & malaria elimination in India. Indian J Med Res. 2012;136:907–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Gething PW, Elyazar IRF, Moyes CL, Smith DL, Battle KE, Guerra CA, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adak T, Singh OP, Nanda N, Sharma VP, Subbarao SK. Isolation of a Plasmodium vivax refractory Anopheles culicifacies strain from India. Trop Med Int Health. 2006;11:197–203. doi: 10.1111/j.1365-3156.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 26.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battle KE, Karhunen MS, Bhatt S, Gething PW, Howes RE, Golding N, et al. Geographical variation in Plasmodium vivax relapses. Malar J. 2014;13:144. doi: 10.1186/1475-2875-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JR, Nandy A, Maji AK, Addy M, Dondorp AM, Day NP, et al. Genotyping of Plasmodium vivax reveals both short and long latency relapse patterns in Kolkata. PLoS One. 2012;7:e39645. doi: 10.1371/journal.pone.0039645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajgor DD, Gogtay NJ, Kadam VS, Kamtekar KD, Dalvi SS, Chogle AR, et al. Efficacy of 14 day primaquine regimen in preventing relapses in patients with Plasmodium vivax malaria in Mumbai, India. Trans R Soc Trop Med Hyg. 2003;97:438–40. doi: 10.1016/s0035-9203(03)90082-4. [DOI] [PubMed] [Google Scholar]
- 30.Ebrahimzadeh A, Fouladi B, Fazaeli A. High rate of detection of mixed infections of Plasmodium vivax and Plasmodium falciparum in South-East of Iran, using nested PCR. Parasitol Int. 2007;56:61–4. doi: 10.1016/j.parint.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Fernando D, Rodrigo C, Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malar J. 2011;10:351. doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galappaththy GN, Omari AA, Tharyan P. Primaquine for preventing relapses in people with Plasmodium vivax malaria. Cochrane Database Syst Rev. 2007;24:CD004389. doi: 10.1002/14651858.CD004389.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Bienzle U, Lucas AO, Ayeni O, Luzzatto L. Glucose -6-phosphate dehydrogenase and malaria. Lancet. 1972;299:107–10. doi: 10.1016/s0140-6736(72)90676-9. [DOI] [PubMed] [Google Scholar]
- 34.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, et al. Severe Plasmodium vivax malaria: A report on serial cases from Bikaner in Northwestern India. Am J Trop Med Hyg. 2009;80:194–8. [PubMed] [Google Scholar]
- 35.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–83. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baird KJ, Maquire JD, Price RN. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol. 2012;80:203–70. doi: 10.1016/B978-0-12-397900-1.00004-9. [DOI] [PubMed] [Google Scholar]
- 37.Richer J, Chudley AE. The hemoglobinopathies and malaria. Clin Genet. 2005;68:332–6. doi: 10.1111/j.1399-0004.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- 38.Cuentas AL, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta, SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomized, phase 2b dose-selection study. Lancet. 2014;383:1049–58. doi: 10.1016/S0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- 39.Advances in 39 P. vivax Malaria Research. The New york Academy of Sciences. [accessed on September 3, 2013]. Available from: http://www.nyas.org .
- 40.Shanks GD. Control and elimination of Plasmodium vivax. Adv Parasitol. 2012;80:301–41. doi: 10.1016/B978-0-12-397900-1.00006-2. [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Li Y, Shaw KS, Learn GH, Plenderleith LJ, Malenke JA, et al. African origin of the malaria parasite Plasmodium vivax. Nat Commun. 2014;5:3346. doi: 10.1038/ncomms4346. [DOI] [PMC free article] [PubMed] [Google Scholar]


