Abstract
Background and objectives:
Epidemiology and transmission of malaria vary within the tribal areas with the variation in topography, forest cover and type of forest. For the control of disease, understanding of the dynamics of transmission in the varied ecological situation is essential. This study was carried out in the two distinct tribal areas- Baiga Chak (thick forested area) of Dindori district and Bichhia block (forest fringe area) of Mandla district, Madhya Prasdesh, India, to understand the epidemiology and transmission dynamics of malaria.
Methods:
Mosquitoes were collected using hand catch and whole night collections to determine the proportion of vectors, their density and seasonality. Vector incrimination was done by sporozoite ELISA and feeding preferences of vector by gel diffusion method. Active fever surveys were carried out fortnightly to determine the age specific malaria parasite rates among the inhabitants of two areas.
Results:
Density of Anopheles culicifacies was significantly higher in Bichhia while the density of An. fluviatilis was higher in Baiga Chak. An. culicifacies was incriminated from both the areas while An. fluviatilis was incriminated from Baiga Chak only. Malaria slide positivity rate (SPR) was significantly higher (OR=3.7 95%CI, 3.1-4.4) in Baiga Chak (28.2%) than Bichhia (9.6%).
Interpretation & conclusions:
The features of malaria transmission in tribal areas differed from those reported in rural or semirural population. Site-specific and region-specific studies are required to develop appropriate intervention measures to control malaria.
Keywords: Anopheles culicifacies, An. fluviatilis, Baiga Chak, malaria, Mandla, Plasmodium falciparum, tribals
Malaria is a global health problem and India contributes substantially to the global burden of malaria1. About 60 per cent of malaria cases in India are confined to tribal areas2. Anopheles culicifacies and An. fluviatilis are efficient vectors in the tribal dominated areas. An. culicifacies is highly resistant to DDT3. Consequently, DDT was withdrawn from many tribal areas in 2001 and replaced by synthetic pyrethroid as indoor residual spray (IRS) (National Vector Borne Disease Control Programme, personal communication). Studies on impact of topography and ecological variations on the dynamics of malaria transmission are available only from a few tribal areas. In Mandla district of Madhya Pradesh (MP), density of An. fluviatilis was found to be relatively higher in forested villages than in deforested villages, resulting in variation in parasite rate and parasite proportion3. In Rourkela (Odisha), An. fluviatilis was absent in plain ecotype villages as compared to forest villages where species were prevalent with An. culicifacies resulting in significant variation in parasite rates in the two sets of tribal villages4. Similarly, in Koraput district (Odisha) marked variation in parasite rate was found between top hill and foot hill villages, and foot hill and plain villages of the two primary health centres (PHC). Within each PHC, both the hyperendemic and hypoendemic situations of malaria were recorded5. Thus ecological variations are known to have direct impact on vector prevalence and thereby malaria. Even in one topographic area distribution of vectors and malaria infection may not be homogeneous because of many factors6,7.
Mandla and Dindori are highly malarious districts in MP, India, contributing together 12 per cent malaria in the State while their population is only 2.6 per cent of the StateState population. Bajag community health centre (CHC) in Dindori district contributs about 40 per cent malaria in the district though its population is 12 per cent of the district's population [State Vector Borne Disease Control Programme (SVBDCP), 2004, unpublished annual report]. In view of this, a study was undertaken to understand the site specific transmission dynamics of malaria to develop evidence based appropriate strategy for malaria control.
Material & Methods
Study area and population: The study was carried out by the team of National Institute for Research in Tribal Health (NIRTH), Jabalpur, MP, in two areas namely Baiga Chak in Dindori district and Bichhia CHC in Mandla district of Madhya Pradesh during June 2004-December 2007. Both these districts are tribal designated. In Baiga Chak, a particularly vulnerable tribal group (PVTG) Baiga is predominating while in Bichhia, Gonds are dominant besides other tribes. Baiga Chak is known for its rugged terrain and vast tracts of forest interspersed with streams and foothills. Mean sea level height of the area is 980 m. Average rainfall is 1500 mm per annum. Baiga Chak is spread over in three CHCs in dense evergreen forest of saal (Shorea robusta) consisting of 52 villages. Villages are located 30-35 km away from the main road. Houses in villages are scattered and solitarily located in undulating terrain.
The area was under the DDT spray [Indoor residual spray (IRS)] till 2001. One round of synthetic pyrethroids (K-otherine & alphacypermethrin) was sprayed in 2002 and 2003. No IRS was carried out in 2004. Regular (two rounds) spray of alphacypermethrin was undertaken from 2005 onwards. One additional round of alphacypermethrin was sprayed in March 2006 (District Malaria Officer Dindori, personal communication).
In Mandla, the terrain is mainly plain and forest is of mixed vegetation mainly bamboo, sagwan (Tectona grandis), mahua (Madhuca indica) and saal (Shorea robusta) and other deciduous trees. River Banjar and its tributaries which reduced to several small ponds and pools in summer months are available around study villages. Mean sea level height of the area is 495 m and average rainfall for the last five years is 1529 mm. Houses are built in plain terrain and are compact unlike Baiga Chak.
The area was under the DDT spray till 2001. No spray was undertaken in 2002-2004. For the first time in 2005, two rounds of alphacypermethrine were sprayed. In 2006 one round of alphacypermethrine was sprayed and no spray was carried out in 2007 (District Malaria Officer Mandla, personal communication).
Almost all the houses in both the areas were kuccha. Geographical distance between these two sites is about 150 km. Within each site, village to village geographical distance is 3-12 km. Agriculture is rain fed and over 90 per cent rainfall occurs from mid June to September in both the areas. On the basis of rainfall pattern four seasons of the year have been classified as - summer (March - June), monsoon (July-September), post monsoon (October-November) and winter (December- February).
Vector composition and vector density: Mosquito collections were carried out in four villages at each site between 0600-0800 h. Indoor man hour density (MHD) was determined by hand catch method once in a month following standard protocol8.
To determine the composition of possibly exophilic vectors, whole night mosquito catches using light trap, animal bait and human landing collections (HLC) on team members engaged in the collection were carried out between 1800-0600 h. In each area collections were made in two villages once in a month, one village was selected for indoor and another for outdoor trap catches. Efforts were made to install a light trap at a fixed place to rule out any place bias except during heavy rain. In a village only one light trap was used each night. Hourly collected mosquitoes were kept separately and identified using the key of Christopher9.
Malaria sporozoite detection: Specimens of An. culicifacies and An. fluviatilis were analyzed for the presence of sporozoite by ELISA technique using monoclonal antibodies against Plasmodium falciparum (Pf) and P. vivax (Pv) circumsporozoite protein as well as positive control10.
Insecticide susceptibility status: Insecticide susceptibility tests were carried out following WHO standard protocol8.
Feeding preference of vector species: Blood elutes from fully fed and semi gravid specimens were taken on Whatman filter paper and analysed using gel diffusion technique11 for determining human blood index(HBI).
Active fever survey and parasitological parameters: Door to door active fever surveys were carried out fortnightly. Blood smears of all the individuals having fever at the time of surveillance or history of fever between two visits were prepared using finger prick blood. Both thick and thin smears were prepared. Radical treatment was given to all malaria positive cases as per the National Vector Borne Disease Control Programme (NVBDCP) criteria12,13. Blood slides were stained with Jaswant Singh Bhattacharjee (JSB) field stain14. At least 100 microscopic fields of thick smears were examined for the presence of malaria parasite. All the malaria positive and 10 per cent of negative slides were cross checked for quality control.
Sample size & sampling: Earlier studies carried out by the NIRTH, Jabalpur, in tribal areas of Madhya Pradesh showed that SPR varied from 10-40 per cent in different areas (unpublished data of NIRTH). Assuming SPR as 20 per cent, and at the 95 % level of confidence and 5% absolute error, a minimum of 250 blood slides of fever/malaria suspected cases were required for examination. At any point of time about 10 per cent of the population may have fever related symptoms, thus about 2,500 individuals were covered to have a sample of 250 fever/febrile cases per season. Further, assuming average population of tribal village as about 500, five villages per study site were covered to meet the desired sample size. Thus, five villages which were approachable throughout the year and having a population near to desired sample size were selected conveniently from each study site. Five villages having a population of 2900 living in 576 houses [average household (HH) size 5.03] were selected from Bajag CHC (which is highly malarious)15. Five villages having a population of 2100 living in 422 houses (average HH size 4.97) from CHC Bichhia were selected. The entomological data were also collected from these villages.
The study protocol was approved by the institutional ethical committee.
Data analysis: Slide positivity rate was calculated as percentage of slide positive for any stage of the malaria parasite among slide examined. Data were analysed using statistical software SPSS (version17) (SPSS Inc., USA). For non-categorical data, student t test and ANOVA were applied and for categorical data odd ratio and chi2 test were used. The significance level was considered at P<0.05 and 95 % confidence intervals were calculated.
Results
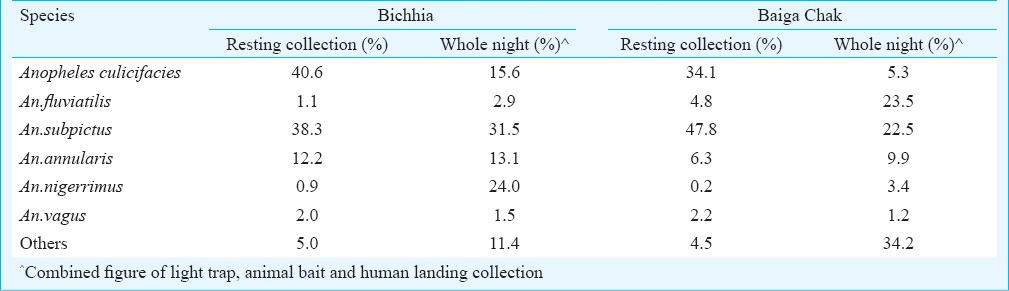
Vector and anopheline species: In all, 4504 and 8273 specimens of anophelines were caught from indoor resting collections from Baiga Chak and Bichhia spending 296 and 282 h, respectively. Collected mosquitoes belonged to 15 and 16 anopheline species respectively from Baiga Chak and Bichhia. In Baiga Chak, An. subpictus (47.8%) was the predominant species followed by An. culicifacies (34.1%), while in Bichhia villages An. culicifacies (40.6%) was the predominant species followed by An. subpictus (38.3%). Proportion of An. fluviatilis was 4.8 and 1.1 per cent of all anophelines in Baiga Chak and Bichhia, respectively (Table I).
Table 1.
Vector and anopheline prevalence in study areas
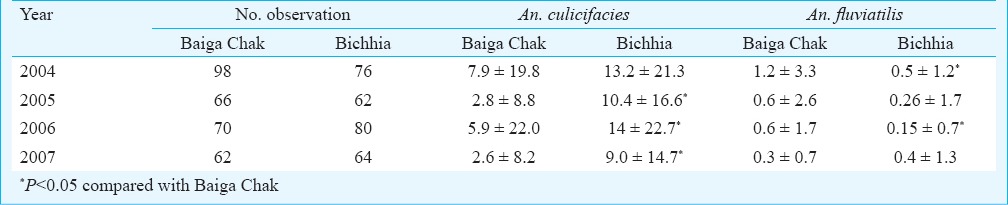
Man hour density (MHD): MHD of anophelines and An. culicifacies were significantly more in Bichhia (29.4 and 11.9) than in Baiga Chak (15.2 and 5.2) (P<0.01), while the density of An. fluviatilis was significantly more in Baiga Chak (0.7) as compared to 0.3 in Bichhia (P<0.01). MHDs of vector species varied from year to year (Table II).
Table II.
Year-wise man hour density (MHD) of Anopheles culicifacies and An. fluviatilis
Mean month-wise MHD of vector species is shown in Fig. 1A, B. Density of An. culicifacies showed two peaks in Bichhia (March–April and August - September) while in Baiga Chak only one sharp peak was recorded between July-September. Overall, highest densities of anophelines and An. culicifacies were recorded in monsoon season followed by post monsoon, summer and winter seasons which were 53.5, 12.9, 7.2 and 6.6 for anophelines and 18.0, 4.7, 4.3 and 4.1 for An. culicifacies respectively. Seasonal variation in density was significant (P<0.01) both anophelines and An. culicifacies. On the contrary, the MHD of An. fluviatilis was relatively more in post monsoon months (1.7) followed by winter (0.55) and it was lowest in summer season. Further analysis revealed that 86.5 and 92.6 per cent of anopheline and An. culicifacies collected during the year were found in monsoon months in Baiga Chak, while in Bichhia corresponding numbers were 65.5 and 50.6 per cent, respectively. Around 90 per cent of An. fluviatilis was recorded from post monsoon and winter months in both the areas.
Fig. 1A.
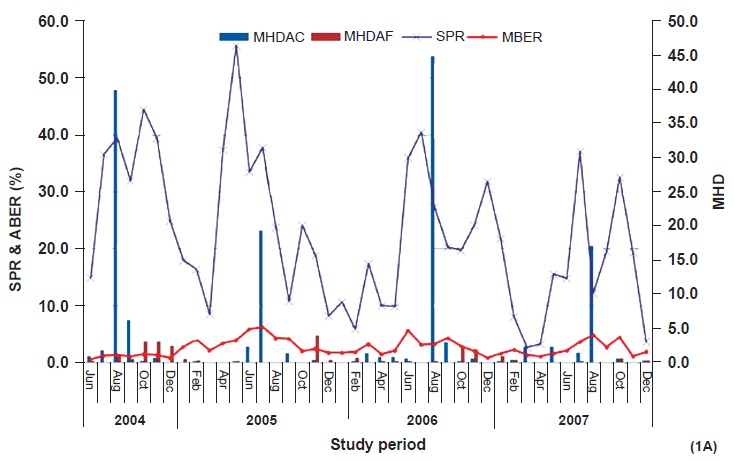
Month-wise blood examination rate (MBER), slide positivity rate (SPR) and man hour density (MHD) of An. culicifacies (MHDAC) and An. fluviatilis (MHDAF) in Baiga Chak (June 2004 - December 2007).
Fig. 1B.
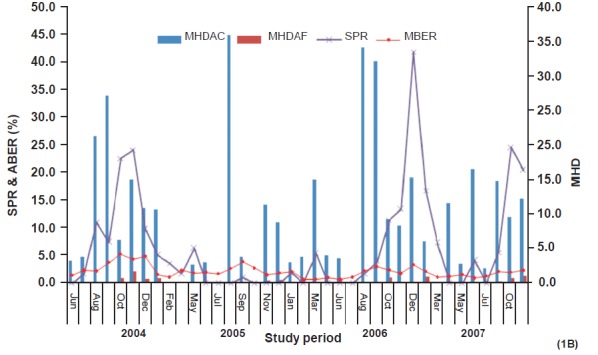
Month-wise blood examination rate (MBER), slide positivity rate (SPR) and man hour density of An. culicifacies (MHDAC) and An. fluviatilis (MHDAF) in Bicchia (June 2004 - December 2007).
Whole night collections:
Light trap collections - In light trap catches 690 and 2644 anopheline specimens were collected from Baiga Chak (72 nights) and Bichhia (77 nights), respectively. An. fluviatilis (26.8%) was the predominant species followed by An. subpictus (15.7%), An. splendidus (14.5%) and An. annularis (10.3%) in Baiga Chak, while An. niggerimus (31.2%) was the predominant species followed by An. subpictus (30.1%) and An. annularis (13.2%) in Bichhia. Proportion of An. culicifacies was only 2.3 and 10.6 per cent, respectively in these two areas. Per trap /night catches of anophelines were significantly more in Bichhia (34.3) than Baiga Chak 9.6 (P<0.01). A similar trend was recorded for An. culicifacies as 3.6 and 0.2 (P<0.01). However, per night per trap catches of An. fluviatilis were significantly more (P<0.01) in Baiga Chak (2.4) than 1.1 in Bichhia. Season-wise per night / trap catches of An culicifacies were significantly more during monsoon season in both Baiga Chak 2.6 (P<0.01) and Bichhia 17.0 (P<0.01). But catches of An. fluviatilis were relatively more in post monsoon season as 12.6 in Baiga Chak (P<0.01) and 1.8 in Bichhia (P<0.01) compared with other seasons.
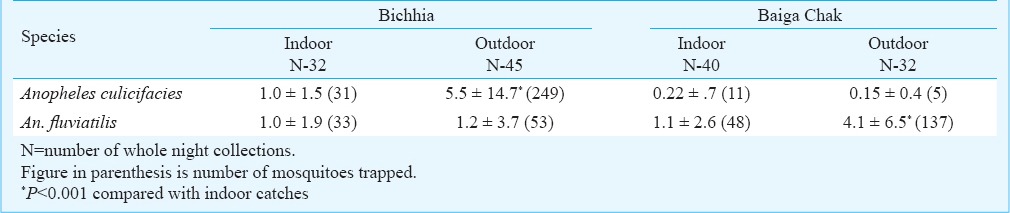
Of the 149 light trap catches, 72 were located indoors (40 in Baiga chak and 32 in Bichhia) and 77 were outdoors (32 in Baiga Chak and 45 in Bichhia). In Baiga Chak per night per trap catches of An. culicifacies were more in indoor while in Bichhia significantly (P<0.001) more catches were from outdoor trap. Regarding An. fluviatilis there was no significant variation found in Bichhia while in Baiga Chak significantly (P<0.001) more catches were recorded in outdoor trap (Table III).
Table III.
Average light trap catches of vectors in study areas (indoor and outdoor)
Animal bait collection - Overall 1278 and 2880 anophelines were collected on animal bait in Baiga Chak (73 nights) and Bichhia (81 nights), respectively. An. subpictus remained the predominant species at both place followed by An. culicifacies in Bichhia (20%) and An. fluviatilis in Baiga Chak (22.0%). Per night per bait collection of An. culicifacies was significantly more (P<0.001) in Bichhia (7.1) than Baiga Chak (1.1), whereas catches of An. fluviatilis were significantly more in Baiga Chak (3.8) than Bichhia (0.9). Both the vectors remained active throughout the night but peak activity was recorded between 2000-2300 i.e. 3rd to 5th h (Fig. 2A, B).
Fig. 2A.
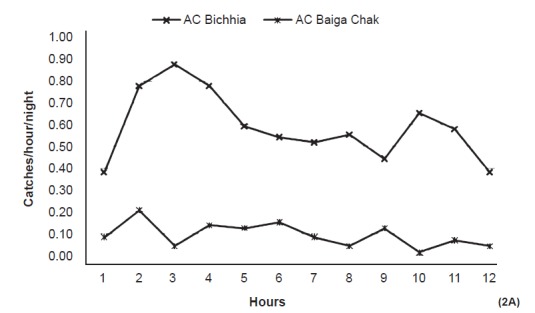
Catches of An. culicifacies (AC) on animal bait (per night/hour) in the study area (12 hours from 1800 to 0600).
Fig. 2B.
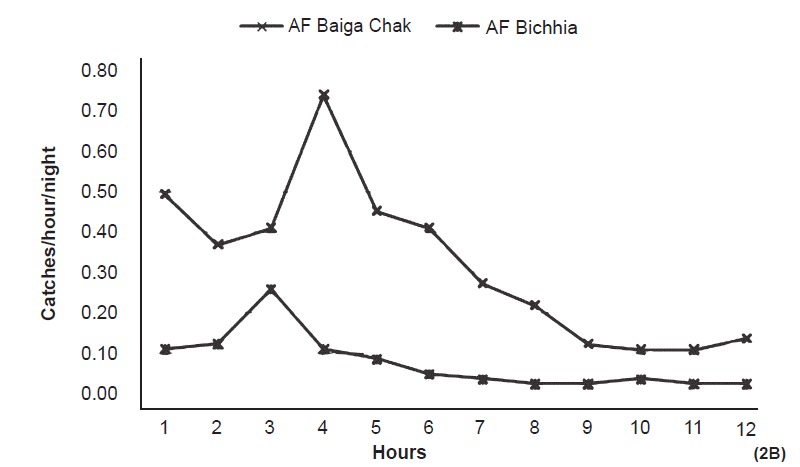
Catches of An. fluviatilis (AF) on animal bait (per night/hour) in the study area (12 hours from 1800-0600 h).
In HLC, 47 and 107 anophelines were caught from Baiga Chak and Bichhia, respectively. An. subpictus was the predominant species in both the areas. Proportions of An. culicifacies and An. fluviatilis were 21.3 and 15 per cent in Baiga Chak while in Bichhia proportions of these species were 19.6 and 2.8 per cent, respectively.
Vector incrimination: A total of 269 and 261 specimens of An. culicifacies from Baiga Chak and Bichhia were tested for sporozoite antigens by ELISA. Only one specimen was found positive for P. falciparum infection from Baiga Chak during the July month (sporozoite rate=0.4%). Two specimens from Bichhia were found positive for Pf infection (sporozoite rate=0.8%). Both these specimens were caught in December from the cattle shed and animal bait. Only one specimen of An. fluviatilis among 140 analyzed was positive for malaria infection from Baiga Chak (sporozoite rate=0.8) and none among 24 from Bichhia.
Insecticide susceptibility test - Forty and 70 specimens of An. culicifacies (fully fed) caught from Bichhia and Baiga Chak were exposed to deltamethrin 0.05 per cent. Cent per cent mortality was recorded. In case of DDT 4 per cent, 60 and 125 specimens were exposed from Bichhia and Baiga Chak, respectively. Corrected mortality of 32 and 15 per cent, respectively were recorded from these two areas.
Human blood index (Feeding preferences) - Overall, 301 specimens of An. culicifacies and 38 of An. fluviatilis collected from Bichhia were tested for the human blood index. None was found positive for human serum. Similarly, from Baiga Chak 140 specimens of An. culicifacies and 245 of An. fluviatilis were tested. One and three specimens, respectively were found positive for human serum from the Baiga Chak area (HBI = 0.7% and 1.2%, respectively).
Parasite rates - During the study period (June 2004 - December 2007) 4681 and 1902 blood slides were collected from the villages of Baiga Chak and Bichhia, respectively. Among these, 1322 (28.2%) and 183 (9.6%) were found positive for malaria parasite. Proportions of P. falciparum infection were 90 and 88.5 per cent, respectively for Baiga Chak and Bichhia villages. Slide positivity rate (SPR) was significantly higher in Baiga Chak than in Bichhia (OR=3.7, 95% CI, 3.1 - 4.4: χ2 =265.8, P<0.01).
Annual blood examination rate (ABER) - ABER were more in Baiga Chak than Bichhia (59, 42.5, 31.7 and 28.2 and 26.4, 27, 21.0 and 16.1 for the years 2004, 2005, 2006 and 2007, respectively). Monthly blood examination rate (MBER) is shown in Fig. 1A,B.
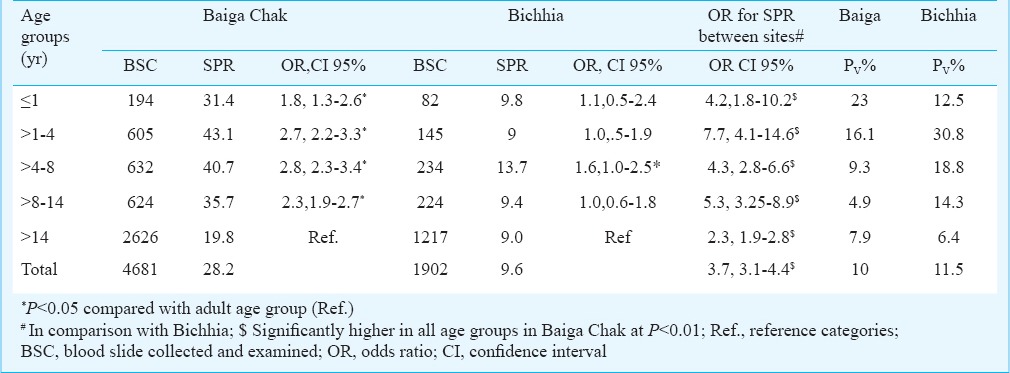
Slide positivity rate: Malaria was prevalent in all the study villages in both the areas. In Baiga Chak villages, SPR varied from 25.6 to 34.7 per cent while in Bichhia villages SPR varied from 5.1 to 12.3 per cent. Age group wise analysis for the Baiga Chak area revealed that children in the age group of 1-4 yr were the worst affected with highest SPR (43%) followed by >4-8 yr group (41%), while in Bichhia no clear cut pattern was seen and all age groups were found equally affected with the slightly higher SPR in >4-8 yr of age (Table IV). The SPR was significantly low in adults in comparison to other age groups in Baiga Chak (P<0.01). Comparison between children (<14 yr) and adults (>14 yr) showed that in Baiga Chak, the risk of malaria infection was twice in children (39.0%) than in adults (19.8%) (OR= 2.6, 95% Cl, 2.27-2.96, P <0.01). Such variation was insignificant in Bichhia where the corresponding values were 10.8 and 9 per cent, respectively (OR= 1.23, Cl 95%, 1.1-1.3). Overall, the age group wise SPR was significantly more in all age groups in Baiga Chak than in Bichhia. P. vivax infection was recorded more frequently among infants and younger children and its presence gradually decreased with the increasing age in both the areas. Gender-wise SPR was more in males (24.4%) than in females (21.3%). Gender-wise variation in SPR was significant in Baiga Chak only, (P<0.05).
Table IV.
Age group wise slide positivity rate (SPR) and P. vivax proportion (Pv%)
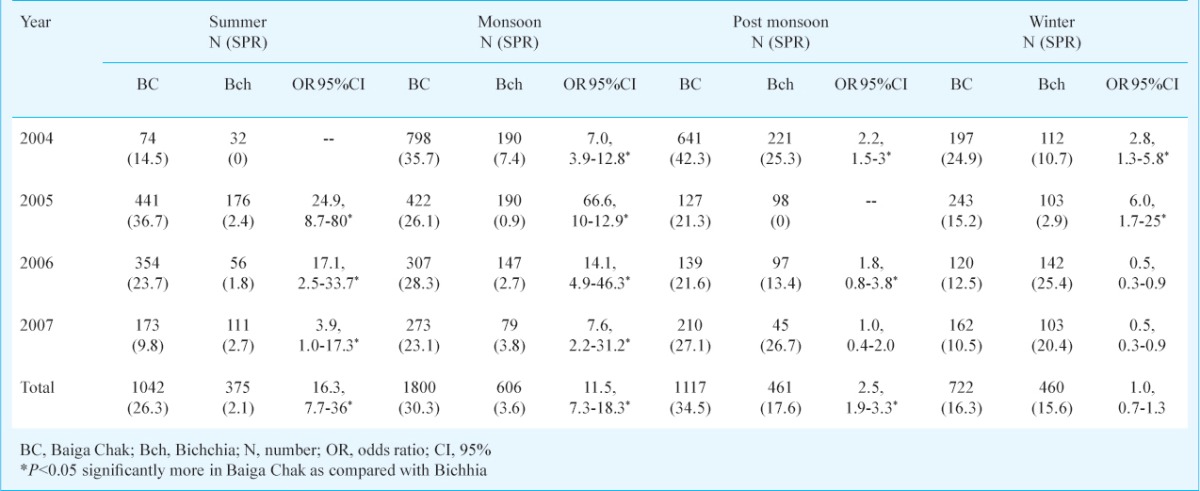
Season and month wise parasite rate: The highest SPR was found in the post monsoon season followed by monsoon in Baiga Chak, while highest SPR was recorded in the post monsoon followed by winter in Bichhia. Except winter season, SPR was significantly higher in all the seasons in Baiga Chak than Bichhia (Table V). Month wise SPR is shown in Fig. 1A,B. In Baiga Chak, SPR was recorded in all the months with a dip in February to April, while in the Bichhia sharp peak of SPR was recorded during November and December months.
Table V.
Year-wise slide positivity rate in different seasons in study areas
Discussion
The epidemiology of malaria is a complex interaction among host, vector and parasite factors that are specific to each location in which malaria occurs16. The present study was carried out in two tribal areas which were about 150 km away from each other and their inhabitants were also different ethnic tribes to understand the variation in epidemiology and dynamics of malaria transmission. Baiga Chak is located at higher altitude as compared to Bichhia and altitude of place plays an important role in malaria transmission17. An association of the prevalence of malaria with higher altitude in the tribal areas has been reported earlier3,5. Age dependent variation in parasite rate between the two areas was the result of the differential malariogenic potential of areas. Review of age specific pattern of malaria revealed that as the transmission increased there was a shift of peak malaria prevalence towards younger age groups regardless of seasonality18. Further, because of repeated infections, immunity is acquired with increasing age which provides protections against the infection in higher ages19,20,21,22. A high level of chloroquine (CQ) resistance in P. falciparum was reported earlier from Baiga Chak15, which could be one of the compounding factors along with other differential abiotic factors for maintaining high prevalence. In Baiga Chak though CQ was replaced with artimisnin based combination therapy (ACT) for treatment of falciparum malaria in 200723, ACT remained in short supply till the end of the study. Consequently, CQ remained the only alternative. Higher parasite rates in males were probably due to their outdoor activities in the field and forest and higher exposure to mosquitoes.
Although in resting collections An. culicifacies was the predominant vector species at both the sites, but its large proportion coinciding with monsoon season suggested that rains might have made the environment conducive for maintaining and proliferation of vector population. However, because of undulating and hilly terrain in Baiga Chak, rain water drains quickly and paddy fields which are potential sites to support An. culicifacies24 are also rare, hence its population declines sharply. On the contrary in Bichhia, the terrain is plain and water bodies made during monsoon persist for long durations and various pools formed in river bed support breeding of An. culicifacies in other seasons.
Higher proportion of An. fluviatilis in Baiga Chak is related to several small streams covered under the shade of dense canopy of saal forest, making ideal breeding surfaces for the species. Such a condition is very supprotive for the breeding and survival of An. fluviatilis25. Higher proportion of An. fluviatilis in whole night collections than indoor resting collections in Baiga Chak suggests that the species is exophilic in nature.
In both the areas, An. culicifacies was found positive for malaria sporozoite though in small number suggested that the species was actively transmitting malaria. An. culicifacies in Baiga Chak was found during monsoon season. An. culicifacies is known to maintain malaria transmission from July to October26 and accordingly, two rounds of spray was recommended under NVBDCP for interruption of malaria transmission (NVBDCP). Late winter transmission of malaria in February and early summer transmission in March by An. culicifacies has already been recorded in Central India26,27. This species was also found responsible for malaria transmission throughout the year in forested areas28.
Though only one sporozoite positive specimen of An. fluviatilis was found in Baiga Chak during the winter season, higher proportion and density of species in post monsoon and winter season which coincided with higher parasite rate in the area, suggested its role in malaria transmission. In human landing collections also, proportion of An. fluviatilis was relatively more in Baiga Chak, which was further confirmed by higher human blood index in An. fluviatilis from Baiga Chak. In the tribal areas higher incidence of malaria was reported with high prevalence of An. fluviatilis3,4.
Although there are limitations in the interpretation of our results because of the small number of infective vectors, yet these results provide information that may be relevant to future studies. The strength of the present study is its prospective community based design and scientific approach in entomological and epidemiological surveillance. Another lacuna was that entomological inoculation rate (EIR) could not be determined to corroborate with the variation in the level of transmission as very few infective mosquitoes were found. However, high parasite rate in younger age groups throughout the study period indicated the intense transmission during this period. Transmission of malaria parasite from human to mosquitoes depends on the availability of mature infectious gametocytes in the peripheral blood. The other limitation was because of the programmatic nature of the intervention, the spray campaigns were not implemented rigorously with high levels of household coverage. Site-specific and region-specific studies are required about vector behaviour and EIR to develop appropriate intervention measures to control malaria. In conclusion, to maximize the effectiveness of limited resources, regions should be prioritized for spraying and supplying long lasting insecticide treated nets according to the current information on vector and disease.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for financial assistance. Authors thank Dr V.M. Katoch, Secretary DHR and DG, ICMR, for his constant support and encouragements and Entomology team of NIRTH for collecting the data.
References
- 1.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, et al. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- 3.Singh N, Singh OP, Sharma VP. Dynamics of malaria transmission in forested and deforested regions of Mandla district, Central India (Madhya Pradesh) J Am Mosq Control Assoc. 1996;12:225–34. [PubMed] [Google Scholar]
- 4.Sharma SK, Tyagi PK, Pradhan K, Upadhyay AK, Haque MA, Nanda N, et al. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundergarh district, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100:917–25. doi: 10.1016/j.trstmh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopalan PK, Das PK, Pani SP, Jambulingam P, Mohapatra SS, Gunasekaran K, et al. Parasitological aspects of malaria persistence in Koraput district Orissa, India. Indian J Med Res. 1990;91:44–51. [PubMed] [Google Scholar]
- 6.Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78:1401–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–66. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 8.Geneva: WHO; 1975. [accessed on August 7, 2013]. World Health Organization (WHO). Manual on practical entomology in malaria, Part-II: Methods and techniques. Available from: http//whqlibdoc.who.int/offset/WHO_OFFSET_13_%28PART2%29pdf . [Google Scholar]
- 9.Christophers SR. Vol. 4. Taylor and Francis; 1993. The fauna of British India including Ceylon and Burma, Diptera, London family Culicidae tribe Anophelinae. [Google Scholar]
- 10.Writz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera. Culicidae) from Papua New Guinae. J Med Entomol. 1987;24:433–7. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 11.Collins RT, Dash BK, Agarwala RS, Dhal KB. An adaptation of gel diffusion technique for identifying the source of mosquito blood meals. Indian J Malariol. 1986;23:81–9. [PubMed] [Google Scholar]
- 12.Delhi: National Anti Malaria programme, Directorate of Health Services, Government of India; 2001. National Anti Malaria Programme. National drug policy on malaria. [Google Scholar]
- 13.Delhi: National Anti Malaria programme, Directorate of Health Services, Government of India; 2005. National Anti Malaria Programme. National drug policy on malaria. [Google Scholar]
- 14.Singh J, Bhattacharji LM. Rapid staining of malarial parasites by a water soluble stain. Indian Med Gaz. 1944;79:102–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Bharti PK, Alam MT, Boxer R, Shukla MM, Gautam SP, Sharma YD, et al. Therapeutic efficacy of chloroquine and sequence variation in pfcrt gene among patients with falciparum malaria in central India. Trop Med Int Health. 2010;15:33–40. doi: 10.1111/j.1365-3156.2009.02425.x. [DOI] [PubMed] [Google Scholar]
- 16.Bruce-chwatt LJ. Malaria and its control: present situation and future prospects. Annu Rev Public Health. 1987;8:75–110. doi: 10.1146/annurev.pu.08.050187.000451. [DOI] [PubMed] [Google Scholar]
- 17.Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461–70. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 18.Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, et al. Age patterns of malaria vary with severity, transmission intensity and seasonality in sub -Saharan Africa: a systematic review and pooled analysis. PLoS One. 2010;5:e8988. doi: 10.1371/journal.pone.0008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith T, Beck HP, Kitua A, Mwankusye S, Felger I, Fraser-Hurt N, et al. Age dependence of the multiplicity of Plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans R Soc Trop Med Hyg. 1999;93(Suppl 1):15–20. doi: 10.1016/s0035-9203(99)90322-x. [DOI] [PubMed] [Google Scholar]
- 20.Baird JK. Host age as a determinant of natural acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–11. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 21.Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–37. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 22.Jones TR, Baird JK, Bangs MJ, Annis BA, Purnomo, Basri H, et al. Malaria vaccine study site in Irian Jaya, Indonesia: Plasmodium falciparum incidence measurement and epidemiologic considerations in sample size estimation. Am J Trop Med Hyg. 1994;50:210–8. doi: 10.4269/ajtmh.1994.50.210. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Shukla MM, Chand G, Bharti PK, Singh MP, Shukla MK, et al. Epidemic of Plasmodium falciparum malaria in central India, an area where chloroquine has been replaced by artemisinin-based combination therapy. Trans R Soc Trop Med Hyg. 2011;105:133–9. doi: 10.1016/j.trstmh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Singh N, Singh OP, Soan V. Mosquito breeding in rice fields and its role in malaria transmission in Mandla district M.P. Indian J Malariol. 1989;26:191–8. [PubMed] [Google Scholar]
- 25.Sharma VP, Prasittisuk C, Kondrashin AV. Magnitude of forest related malaria in the WHO Southeast Asia region. Forest malaria in southeast Asia. In: Sharma VP, Kondrashin AV, editors. proceedings of an informal consultative meeting. Malaria Research Centre: Delhi; 1991. pp. 29–53. [Google Scholar]
- 26.Vaid BK, Nagendra S, Paithane PK. Spring transmission of malaria due to Anopheles culicifacies in North Western Madhya Pradesh. J Commun Dis. 1974;6:270–1. [Google Scholar]
- 27.Kulkarni SM. Feeding behavior of anopheline mosquitoes in an area endemic for malaria in Bastar district, Madhya Pradesh. Indian J Malariol. 1987;24:163–71. [PubMed] [Google Scholar]
- 28.Singh N, Mishra AK, Chand SK, Sharma VP. Population dynamics of Anopheles culicifacies and malaria in the tribal area of central India. J Am Mosq Control Assoc. 1999;15:283–90. [PubMed] [Google Scholar]


