Abstract
Background & objectives:
Dengue (DEN) is a rapidly spreading arboviral disease transmitted by Aedes mosquitoes. Although it is endemic in India, dengue virus (DENV) infection has not been reported from tribal areas of Madhya Pradesh. Investigations were conducted to establish the aetiology of sudden upsurge of cases with febrile illness in June 2013 from tribal villages of Mandla district of Madhya Pradesh, India.
Methods:
The rapid response team of the National Institute for Research in Tribal Health, Jabalpur, conducted clinical investigations and field surveys to collect the samples from suspected cases. Samples were tested using molecular and serological tools. Collected mosquitoes were identified and tested for the presence of virus using semi nested reverse transcriptase-polymerase chain reaction (nRT-PCR). The sequences were analysed to identify serotype and genotype of the virus.
Results:
Of the 648 samples collected from 18 villages of Mandla, 321 (49.53%) were found to be positive for dengue. The nRT-PCR and sequencing confirmed the aetiology as dengue virus type 2. Eighteen per cent of patients needed hospitalization and five deaths were attributed to dengue. The virus was also detected from Aedes aegypti mosquito, which was incriminated as a vector. Phylogenetic analysis revealed that the dengue virus 2 detected belonged to cosmopolitan genotype of the virus.
Interpretation & conclusions:
Dengue virus serotype 2 was detected as the aetiological agent in the outbreak in tribal villages of Mandla district of Madhya Pradesh. Conducive man-made environment favouring mosquitogenic conditions and seeding of virus could be the probable reasons for this outbreak. Urgent attention is needed to control this new threat to tribal population, which is already overburdened with other vector borne diseases.
Keywords: Dengue, DENV, ELISA, IgM, Madhya Pradesh, nRT-PCR, serotype-2, tribal population
Dengue (DEN) incidence has increased by 30-fold in the last 50 years; with more than 100 countries reporting the presence of the disease1 and with the estimates of approximately 100 million annual cases, DEN is an important re-emerging arboviral disease1. Over a billion people residing in South East Asia are at risk of DEN infection2. India is known to be endemic for DEN; in the past, outbreaks mostly occurred in urban and semi-urban areas, but recently the outbreaks are also reported from the rural areas from different parts of the world3,4,5,6,7,8.
Dengue viruses (DENV) are single stranded RNA viruses belonging to genus Flavivirus of family Flaviviridae and have four distinct serotypes (DENV 1-4), that are further classified into genotypes and clades phylogenetically9. The infecting serotypes/genotypes are known to affect severity and complications of DEN10. Moreover, minor genetic changes in arboviruses RNA may result in increase in transmission and severity resulting in epidemic situations11 thus, it is important to characterize the DENV at molecular level.
Mosquitoes of Aedes species are vector of DENV. Aedes aegypti regarded as principal vector, is a day biting, anthropophilic mosquito that prefers clean water for egg laying8. The spread and establishment of the vector to the newer areas because of changing environmental conditions is considered as a reason for endemicity and re-emergence2,12,13. With all four DENV serotypes circulating all round the year causing morbidity and mortality, it has emerged as a threat for public health in India14.
About 21 per cent of the population of Madhya Pradesh is tribal that comprises 14.7 per cent of the total tribal population of the country15. Malaria, haemoglobinopathies, tuberculosis, fluorosis and hepatitis are the major health problems among tribals16. But DENV outbreak has not been reported from the tribes of the region earlier. We investigated the sudden upsurge of cases with febrile illness reported from tribal villages in Mandla district, Madhya Pradesh, India, in June 2013 with the objective to identify aetiology and also looked into the possible reasons.
Material & Methods
Study area and population: Mandla is a tribal dominated (57.2 % of the population) district situated in the east-central of Madhya Pradesh, India17. The sudden upsurge of cases with high grade fever and vomiting were reported in the second week of June 2013, from village Bakori which is about 10 km from Mandla. The village has a population of about 1500 residing in Kacha and Pakka houses, mainly comprising Gonds and Baigas tribal population. The village is well connected by road. June to September are the months when the area receives maximum rain17, and the temperature ranges between 32-38°C with relative humidity of 75 per cent18.
Field investigations and sample collection: The State Health Authorities noticed the upsurge of fever cases and suspecting involvement of waterborne diseases requested our institute (National Institute for Research in Tribal Helath, NIRTH, Jabalpur) to investigate. The rapid response team (RRT) of the institute visited the village and conducted clinical investigations by organizing health camp. House-to-house fever and mosquito surveys were conducted and mosquito larvae were also collected following National Vector Borne Disease Control Programme (NVBDCP) guidelines8. Water samples were collected from a potable water source from the village. The samples collected by RRT were transported maintaining the cold chain (4°C) to the virology laboratory of the institute at Jabalpur. Subsequently, suspected cases were also reported from 17 other tribal villages of the district till September 2013.
Field visits were made every fortnight to the village Bakori and other villages of Mandla district. Blood samples from patients with fever were also sent from primary health care centers, district hospital of Mandla and Netaji Subhash Chandra Bose Medical College and Hospital, Jabalpur (NSCBMC&H) to this laboratory. The demographic and clinical information was collected in predefined formats19. The admitted patients were treated at district hospital Mandla and NSCBMC&H and records were maintained by the hospitals.
Seventy four blood samples were collected during the first visit and in subsequent visits 134 samples were collected by the RRT. Four hundred and forty blood/serum specimens were referred to the laboratory of NIRTH, Jabalpur, for diagnosis from the affected area, from June to September 2013. The study was approved by the institute's ethics committee.
Collected larvae were reared in the laboratory to adults. The adults were identified using standard key20 and stored at -70°C in pools (10/pool) till tested.
Sample processing: Serum was separated from the blood by centrifugation at 4°C and tested immediately or stored at -70°C for later use. Samples collected at the first visit (n=74) were screened for DEN, chikungunya (CHIK) and hepatitis A (HAV) and hepatitis E virus (HEV). DEN IgM in the samples collected after 5th day of illness were diagnosed using NVBDCP recommended DEN IgM ELISA kit (NIV, India). CHIK IgM was detected using CHIKV IgM ELISA kit (NIV, India). Commercially available kits for IgM of HAV (GB, Germany) and IgM of HEV (MP biomedical, USA) were used for diagnosis.
The initial field and laboratory investigations helped in devising the “case definition” of probable cases and the samples received thereafter were tested for DEN. The samples collected from patients during the acute phase of illness were screened either for DENV NS1 protein using commercially available diagnostic kit (J. Mitra & Co., India) as per manufacturer's instruction and/or by semi-nested reverse transcriptase-polymerase chain reaction (nRT-PCR) for DENV RNA using the protocol described by Lanciotti et al21. The samples for nRT-PCR were selected at regular intervals for more than three months to monitor circulating serotype(s). The mosquitoes for viral RNA detection were processed as described earlier3.
Three hundred and eleven samples were tested by IgM ELISA, 405 samples were tested for NS1 antigen and 45 were tested by nRT-PCR, only a few samples were tested by more than one test. For establishing genotype, RT-PCRs were done using primers for envelop-non-structural gene junction region22. The PCR products were sequenced as described earlier23. The sequences were curated manually and submitted to the GenBank.
The water samples (n=7) collected were tested for the presence of coliform bacteria following H2S strip method24.
Data analysis: The reported symptoms, clinical findings, demographic data along with laboratory results were recorded for initial analysis, and for statistical analysis, SPSS v20 (IBM, USA) was used. For phylogenetic analysis, sequences obtained were curated and were assembled with 24 nos. NCBI database downloaded sequences using BioEdit v7.2.5 (Tom Hall Ibis Biosciences, USA). The multiple sequence alignment and phylogenetic analysis were performed using CLUSTAL W & MEGA5 software25 and sequence of DENV-3 used as out group. The Maximum likelihood phylogenetic tree, with Kimura 2-parameter corrections using E/NS1 gene junction sequences was generated employing MEGA version 5 tool, with 1000 bootstrap replicates
Results
Field investigations: The house-to-house surveys and clinical investigations revealed that most of the cases had more than one symptom of DEN like illness with a few cases indicating signs of hepatitis. The larval breeding was detected in cement tanks, mud-pots and large plastic containers in addition to rain water accumulated in discarded tyres, etc. Adult Ae. aegypti (n=7, 2 fed females and 5 males) and larvae (n=330) were collected from the Bakori village. Container index (CI) of 42.1 and Breteau index (BI) of 80 were recorded in first visit at Bakori.
Laboratory investigations: Based on the clinical history samples collected on day one were tested for DEN, CHIK (n=74), HEV and HAV (n=22), of which 34/74 (45.94%) were positive for DEN, three (13.63%) were found reactive for HEV IgM and none for CHIK and HAV IgM by ELISA. The water samples collected from potable water sources were found negative for coliforms.
A total 648 serum samples, including samples from the first visit, were tested and 321 (49.53%) found positive for DEN infection. One hundred and thirty seven samples were DEN IgM positive; 175 were NS1 antigen positive Thirty nine samples were positive by nRT-PCR and all were DENV 2.
In all, samples from the 18 villages of district Mandla were tested. The age of the suspected cases varied from 1 to 94 yr with a median age of 35 yr. Eighty eight per cent of suspected and 90 per cent of confirmed DEN cases were adults. Overall, more males (n=182, 56.69%) were affected as compared to females (n=139, 43.30%), however, this difference was not significant [OR =1.20 (CI 95% = 0.88-1.67) P=0.23]. The gender adjusted age-wise analysis revealed that the age group of 15-24 yr was most affected [OR =1.90 (CI 95% = 1.06-3.40) P=0.03] (Fig. 1).
Fig. 1.
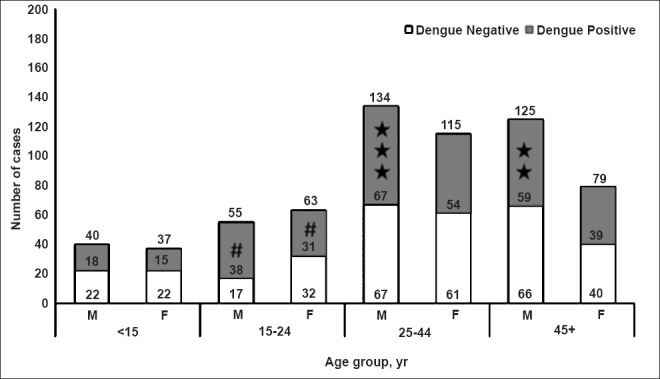
Age, sex distribution of the suspected and confirmed dengue cases from Mandla district (X axis, age groups in years, M= males; F, females. The number in and on the top of bars indicate total cases, Y axis, number of cases; #, the most affected group [OR= 1.90 (95%CI = 1.06-3.4) P=0.03];★, death cases.
The DENV was detected from the fed female mosquitoes collected from the field, but the collected male mosquitoes and the adults that immerged from collected larvae were negative for DENV RNA.
The sequences were submitted to GenBank (E/NS1 junction sequences Acc. no: KF850531, KF887485- KF887489 and C-preM region sequences, GenBank accession no KP420176- KP420180). The sequence analysis of E/NS1 junction revealed that all six sequences, including sequence from mosquito from this study were 100 per cent homologous and were 98 per cent similar at the nucleotide level and 100 per cent similar at the amino acid level with the closest DENV2 isolated from Jammu (GenBank Acc. no AY593239). Further, when sequences of C-preM region were compared, this virus showed highest homology to DENV2 detected at Pondicherry (Puducherry) (India) (Acc. No. JN935383.1) and Gwalior (India) (Acc. No DQ448237.1). The phylogenetic tree constructed using the E/NS1 gene junction sequences revealed that virus belonged to cosmopolitan genotype of DENV2 (Fig. 2).
Fig. 2.
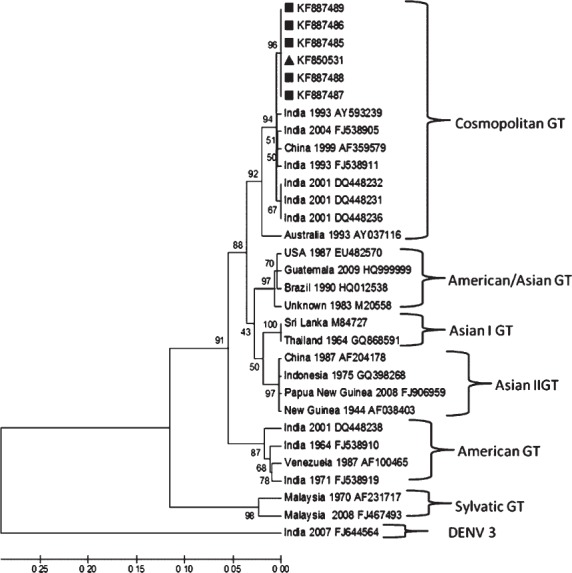
Maximum likelihood phylogenetic tree, generated with Kimura 2-parameter using CLUSTAL W & MEGA 5 software with 1000 bootstrap replicates. Twenty four reported sequences of DENV2 were downloaded from NCBI and were assembled with the six curated (marked with ■= human, ▲ = mosquito) sequences of E/NS1 gene junction of DENV2 from this study. DENV3 used as out group. The analysis reveals that virus belongs to cosmopolitan genotype of DENV2.
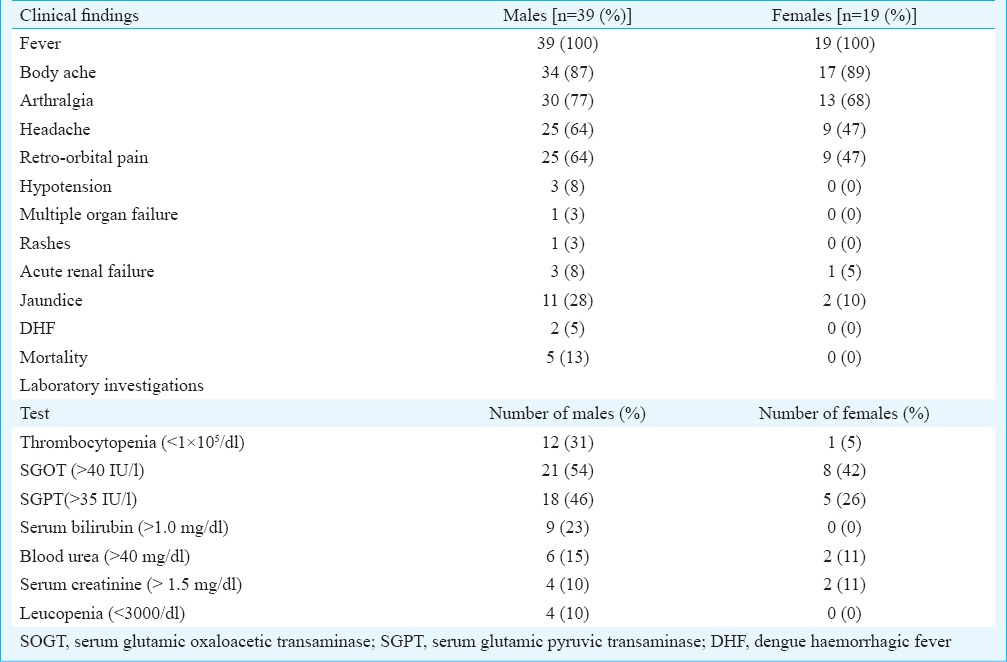
Clinical features: The analysis of clinical symptoms revealed that all suspected cases had at least two of the typical symptoms of DEN. Among confirmed DEN cases 296/321 (92.2%) had fever, 169 (52.6%) had headache/retro-orbital pain, 167 (52.0%) had body ache/joint pain, nine (2.8%) had rash, and another nine (2.8%) had petechiae. The analysis of 58 hospitalized DEN patients done by abstracting records of hospitals revealed that the majority were adults (97.6%) and males (68%). The details of clinical findings and laboratory investigations are given in the Table. Adult males had more complications and severe symptoms. DENV infection was confirmed by laboratory tests in five fatal cases. All fatal cases were adult males.
Table.
Clinical and laboratory findings of hospitalized patients with dengue fever (n=58)
Epidemiological features: The first confirmed DEN case [30 yr old female (index case)] occurred in the last week of May 2013, i.e. about three weeks prior to the peak of the outbreak. The outbreak continued for about eight weeks in and around Bakori village (Mandla town, Phoolsagar). No case was noted in week 10 and 11, but the cases were again detected in the weeks 12-14. The suspected cases were from 17 villages and samples from 11 villages were found positive. These villages were about 50 km from Bakori. No cases were detected after 14th week from this area.
Discussion
The DEN is the most common and rapidly spreading mosquito borne viral infection world over. In the past 20 years only a few reports of DEN have been documented from the central India3,23,26,27,28, probably due to lack of diagnostic facilities.
Vector borne and waterborne diseases are common in tribal areas of central India29,30. In majority of DEN outbreak studies in the past males outnumbered the females in DEN positivity14, however, in a recent outbreak investigation in Narsinghpur3 it was noted that both sexes were equally affected. In this outbreak also similar findings were observed. Occurrence of DEN in adults than in children is known14 and in this study also similar observations were noted.
In the majority of infected people, DEN is an auto-limited disease that resolves in 5-7 days. However, globally about 500,000 people develop a severe form, leading to approximately 20,000 deaths annually1. Serotype and genotype related severity of the disease is a known phenomenon10. The DENV2 is presumed to be, causing severe and complicated disease10,31. In this study, all cases were DENV2 infected and presented a wide array of symptoms and varied haematological patterns. As observed earlier 3,14 adults, especially males had more complications. All five fatal cases were adult males.
The index case had history of visiting the area from where DEN was reported in the past. It is possible that the index case has facilitated the transport of the virus in the new area. Prompt and exact diagnosis of index case/s would have helped to avoid upsurge of cases in the following weeks.
The cases initially were only reported from village Bakori and owing to the extensive mosquito control the cases in this village dropped eight week onwards. Because of the movement of the patients from the outbreak area to nearby villages, clustered cases of the same virus were recorded from other villages.
The entomological studies conducted in the area revealed high BI and CI and favourable mosquitogenic conditions. Studies have demonstrated that elevated BI and CI contribute to upsurge of cases3,8. Molecular detection of virus denoted Ae. aegypti as vector, but transovarial transmission was not detected during this outbreak.
Globally, DENV2 is divided into six genotypes and is regarded as most genetically diverse serotype among DENVs32,33. Earlier phylogenetic study from India showed that American genotype that was circulating prior to 1970s was replaced by Cosmopolitan genotype of DENV234. We also detected Cosmopolitan genotype in this outbreak.
In the absence of efficient vaccine, vigilant vector control programme coupled with timely and accurate laboratory diagnosis, even in far to reach areas is the only way to control such outbreaks. Health authorities need to be alert to check invasion of re-emerging infections like DEN into newer areas especially in tribal populations, who have poor accessibility to the health system.
Acknowledgment
Authors thank the Secretary, Department of Health Research (DHR), Ministry of Health & Family Welfare and the Director-General, ICMR for financial support under Viral Diagnostic Network project (no. VIR/43/2011-ECD-1) and Directorate of National Vector Borne Disease Control Programme for providing dengue IgM kits. Help by Dr R.K. Sharma and Shri M.P. Singh in statistical analysis of data is acknowledged. The technical help by Shriyut M.J. Ukey and L. Sahare and staff of virology laboratory is also acknowledged.
References
- 1.World Health Organization. Dengue and severe dengue. Fact sheet No. 117. Updated September 2013. [accessed on April 12, 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.Dash AP, Bhatia R, Sunyoto T, Mourya DT. Emerging and re-emerging arboviral diseases in Southeast Asia. J Vector Borne Dis. 2013;50:77–84. [PubMed] [Google Scholar]
- 3.Barde PV, Kori BK, Shukla MK, Bharti PK, Chand G, Kumar G, et al. Maiden outbreaks of dengue virus 1 genotype III in rural central India. Epidemiol Infect. 2015;143:412–8. doi: 10.1017/S0950268814000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World health Organization. Global strategy for dengue prevention and control, 2012-2020: WHO report. [accessed on April 12, 2014]. Available from: http://apps.who.int/iris/bitstream/10665/75303/1/9789241504034_eng.pdf?ua=1 .
- 5.Mehendale SM, Risbud AR, Rao JA, Banerjee K. Outbreak of dengue fever in rural areas of Parbhani district of Maharashtra (India) Indian J Med Res. 1991;93:6–11. [PubMed] [Google Scholar]
- 6.Kumar A, Sharma SK, Padbidri VS, Thakare JP, Jain DC, Datta KK. An outbreak of dengue fever in rural areas of northern India. J Commun Dis. 2001;33:274–81. [PubMed] [Google Scholar]
- 7.Dubot-Pérès A, Vongphrachanh P, Denny J, Phetsouvanh R, Linthavong S, Sengkeopraseuth B, et al. An epidemic of dengue-1 in a remote village in rural Laos. PLoS Negl Trop Dis. 2013;7:e2360. doi: 10.1371/journal.pntd.0002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Vector Borne Disease Control Programme, Ministry of Health & Family Welfare, New Delhi, India. [accessed on April 7, 2014]. Available from: http://www.nvbdcp.gov.in .
- 9.Zhang C, Mammen MP Jr, Chinnawirotpisan P, Klungthong C, Rodpradit P, Monkongdee P, et al. Clade replacements in dengue virus serotypes 1 and 3 are associated with changing serotype prevalence. J Virol. 2005;79:15123–30. doi: 10.1128/JVI.79.24.15123-15130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, et al. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4:e617. doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubler DJ. Dengue. In: Monath TPM, editor. Epidemiology of arthropod-borne viral disease. Boca Raton (FL): CRC Press; 1988. pp. 223–60. [Google Scholar]
- 12.Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: WHO; 1997. World Health Organization (WHO) [Google Scholar]
- 13.Gubler DJ. Dengue urbanization and Globalization: The unholy trinity of the 21st century. Trop Med Health. 2011;39(Suppl 4):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarti A, Arora R, Luxemburger C. Fifty years of dengue in India. Trans R Soc Trop Med Hyg. 2012;106:273–82. doi: 10.1016/j.trstmh.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Bisai S, Saha KB, Sharma RK, Muniyandi M, Singh N. An overview of tribal population in India. Tribal Health Bull. 2014;20:1–126. [Google Scholar]
- 16.Jabalpur, India: Tribal Health Research Forum; [accessed on April 11, 2014]. Regional Medical Research centre for Tribals (RMRCT) Available from: http://www.rmrct.org/ICMR_forum%20Tr...Health/priority%20area%20of%20research.htm . [Google Scholar]
- 17.Mandla District. Madhya Pradesh, India. [accessed on April 11, 2014]. Available from: http://mandla.nic.in.
- 18.India Meteorological Department, Mandla. [accessed on April 11, 2014]. Available from: http://www.imd.gov.in/section/hydro/distrainfall/webrain/mp/mandla.txt .
- 19.Regional Medical Research centre for Tribals (RMRCT) [accessed on April 11, 2014]. Available from: http://www.rmrct.org/
- 20.Barraud PJ. London: Taylor and Francis; The fauna of British India including Ceylon and Burma. Diptera. vol. V. Family Culicidae. Tribes Megarhinini and Culicini; pp. 1–452. [Google Scholar]
- 21.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanakaratne N, Wahala WM, Messer WB, Tissera HA, Shahani A, Abeysinghe N, et al. Severe dengue epidemics in Sri Lanka, 2003-2006. Emerg Infect Dis. 2009;15:192–9. doi: 10.3201/eid1502.080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barde PV, Godbole S, Bharti PK, Chand G, Agarwal M, Singh N. Detection of dengue virus 4 from Central India. Indian J Med Res. 2012;136:491–4. [PMC free article] [PubMed] [Google Scholar]
- 24.Manja KS, Maurya MS, Rao KM. A simple field test for the detection of faecal pollution in drinking water. Bull World Health Organ. 1982;60:797–801. [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahadev PV, Prasad SR, Ilkal MA, Mavale MS, Bedekar SS, Banerjee K. Activity of dengue-2 virus and prevalence of Aedes aegypti in the Chirimiri colliery area, Madhya Pradesh, India. Southeast Asian J Trop Med Public Health. 1997;28:126–37. [PubMed] [Google Scholar]
- 27.Baruah K, Singh PK, Mohalia MM, Dhariwal AC. A study on dengue outbreak during 2009 in Bhopal and Indore districts of Madhya Pradesh, India. J Commun Dis. 2010;42:273–9. [PubMed] [Google Scholar]
- 28.Ukey P, Bondade S, Paunipagar P, Powar R, Akulwar S. Study of seroprevalence of dengue fever in Central India. Indian J Community Med. 2010;35:517–9. doi: 10.4103/0970-0218.74366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh N, Dash AP, Thimasarn K. Fighting malaria in Madhya Pradesh (Central India): are we losing the battle? Malar J. 2009;8:93. doi: 10.1186/1475-2875-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anvikar AR, Dolla C, Dutta S, Rao VG, Gadge VS, Shukla GP, et al. Role of Escherichia coli in acute diarrhoea in tribal preschool children of Central India. Paediatr Perinat Epidemiol. 2008;22:40–6. doi: 10.1111/j.1365-3016.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 31.Neeraja M, Lakshmi V, Teja VD, Lavanya V, Priyanka EN, Subhada K, et al. Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South India. Arch Virol. 2014;159:1567–73. doi: 10.1007/s00705-014-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rico-Hesse R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology. 1990;174:479–93. doi: 10.1016/0042-6822(90)90102-w. [DOI] [PubMed] [Google Scholar]
- 33.Rico-Hesse R. Dengue virus evolution and virulence models. Clin Infect Dis. 2007;44:1462–6. doi: 10.1086/517587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar SR, Patil JA, Cecilia D, Cherian SS, Barde PV, Walimbe AM, et al. Evolution, dispersal and replacement of American genotype dengue type 2 viruses in India (1956-2005): selection pressure and molecular clock analyses. J Gen Virol. 2010;91:707–20. doi: 10.1099/vir.0.017954-0. [DOI] [PubMed] [Google Scholar]


