Abstract
Context:
Alpha-2 (α2) adrenergic receptor agonists, clonidine and dexmedetomidine, are widely used as adjuvants during anesthesia for analgesic, sedative, sympatholytic, and cardiovascular stabilizing effects.
Aims:
We compared effects of clonidine and dexmedetomidine (as propofol adjuvants) on intra-operative hemodynamics, recovery time, and postoperative cognitive function impairment.
Subjects and Methods:
Forty-five American Society of Anesthesiologists I and II patients, scheduled for laparoscopic cholecystectomy were divided into three groups (n = 15). Group C patients received bolus of clonidine 3 μg/kg followed by a continuous infusion; Group D patients received dexemedetomidine 1 μg/kg and a continuous infusion; and Group P patients received a bolus of normal saline followed by an infusion. Intra-operative mean arterial pressure (MAP) and pulse rate (PR) were measured throughout the surgery. Bispectral index was maintained at 55 ± 5 by titrating propofol infusion rate. The time between the interruption of anesthesia and eye opening (recovery time) was measured. Cognitive function was assessed using short mental status questionnaire at 15, 30, 45, and 60 min postoperatively.
Results:
The sympathetic response to laryngoscopy and extubation on MAP and PR were significantly reduced with the use of clonidine and dexmedetomidine (P < 0.05). The recovery was delayed (P < 0.05) with both the drug combinations and it was more pronounced with dexmedetomidine (P < 0.05). Dexmedetomidine group showed cognitive impairment in a postoperative period lasting up to an hour.
Conclusions:
When co-administered with propofol, both clonidine, and dexmedetomidine attenuate sympathetic response to laryngoscopy and extubation but cause delay in the recovery from anesthesia. Dexmedetomidine causes impairment of postoperative cognitive functions.
Keywords: Clonidine, cognitive dysfunction, dexmedetomidine, hemodynamics, recovery time
INTRODUCTION
The use of α2 adrenoceptor agonists, clonidine and dexmedetomidine, as an anesthetic adjuvant is well documented.[1,2,3] These drugs are known to have amnesic and analgesic properties, therefore, reduce anesthetic and opioid requirement during surgery. They also decrease sympathetic tone causing attenuation of neuroendocrine and hemodynamic responses to anesthesia and surgery, providing greater hemodynamic stability intra- and postoperatively.[1,2,3] The α2 adrenoceptors belong to G-protein coupled family of transmembrane receptors and are present at both pre- and post-synaptic autonomic ganglia in the central and peripheral nervous systems. Binding of agonists, endogenous (norepinephrine) or exogenous (clonidine and dexmedetomidine), results in G-protein coupling with the inhibition of both adenylyl cyclase and phospholipase C activity and subsequent effects.[4]
Both, clonidine and dexmedetomidine are imidazoline compounds and act by same mechanism, nevertheless there is a difference in the α2 selectivity, dexmedetomidine being 8 times more α2 selective than clonidine, has an elimination half-life, which is 4 times less and distribution half-life, which is 2 times less than clonidine making dexmedetomidine more desirable for continuous infusion.[4] Although providing a good hemodynamic control both the drugs, however, have been seen to prolong recovery from anesthesia when used along with propofol, and, in addition, dexmedetomidine has been reported to affect the cognitive function after an infusion in healthy volunteers, a property which is not observed with the use of clonidine at low-dose infusions.[5,6,7,8] There are no studies comparing this property of the two drugs. Therefore, the purpose of this study was to compare the effects of clonidine and dexmedetomidine on intra-operative hemodynamics, recovery time, and postoperative cognitive function impairment when administered as propofol adjuvants.
SUBJECTS AND METHODS
Study design
We designed a randomized, double-blinded, control study to evaluate the effects of clonidine and dexmedetomidine (as propofol adjuvants) on intra-operative hemodynamics, recovery time, and postoperative cognitive function impairment.
Patient demographics - inclusion and exclusion criteria
After obtaining an Institutional Ethical Committee approval and individual written informed consent, 45 adult, American Society of Anesthesiologists (ASA) grade I and II patients, of both genders, undergoing laparoscopic cholecystectomy were included in the study. All the patients were assessed for eligibility and were explained about the study protocol and the questions they were going to be asked in the postoperative period. Using sealed opaque envelopes patients were randomly divided into three groups (15 subjects in each group). Group P - patients received propofol and saline infusion; Group C - patients received propofol and clonidine infusion; and Group D - patients received propofol and dexmedetomidine infusion. Both patients and anesthesiologists were blinded to the administered drug. Patients older than 50 years, on β blockers, taking asthma treatment, allergic to any drug, with any degree of heart block or with a history of psychiatric illness, pregnant, and lactating females were excluded from the study.
Patient drug treatment
Patients received oral lorazepam 2 mg, the night and the morning before the surgery along with oral ranitidine 150 mg. On arrival in the operation theater after the establishment of intravenous (IV) and monitoring lines, all patients were given IV injection glycopyrrolate 0.2 mg and injection ondensetron 4 mg. Group C patients received an initial bolus dose of clonidine 3 μg/kg over 15 min followed by a continuous infusion of 1.5 μg/kg/h, Group D patients received dexemedetomidine 1 μg/kg over 15 min followed by a continuous infusion of 0.6 μg/kg/h, and Group P patients received a bolus of normal saline over 15 min at the same rate followed by an infusion, by the researcher. After the bolus dose patients were induced with IV propofol 2 mg/kg given by the anesthesiologist in a separate IV line, to facilitate intubation IV rocuronium 0.6 mg/kg was administered, and for analgesia IV fentanyl 2 μg/kg was given. All the patients were given diclofenac suppository 2 mg/kg per rectally after the induction.
Anesthesia maintenance/management during and postsurgery
Maintenance of anesthesia was done with propofol infusion along with N2O: O2 (60:40). Propofol was started at 3 mg/kg/h and then titrated to maintain bispectral index (BIS) of 55 ± 5. Intermittent doses of rocuronium were given to maintain muscle relaxation after every 20 min or on the return of spontaneous respiration, whichever was earlier. Electrocardiography, pulse rate (PR), oxygen saturation, end-tidal CO2 were continuously measured, and mean arterial pressure (MAP) was measured every 5 min. A decrease in MAP >20% was intervened with injection mephentermine 3–6 mg and IV fluid boluses, bradycardia (PR <40/min) was intervened with injection atropine 0.6 mg.
At the end of surgery, both the infusions and N2O flow were stopped. Before stopping the infusion, it was made sure that at least 30 min have passed since the last dose of muscle relaxant. Patients were called by name and asked to open their eyes. Time from stopping of infusion to making an attempt to open the eyes was noted. After which neuromuscular block was reversed with neostigmine 0.05 mg/kg and glycopyrrolate 0.02 mg/kg and patient was extubated and shifted to the recovery room. Assessment of cognitive function was done using short mental status questionnaire (SMSQ) test,[9] which was modified slightly to suit our requirements, at 15, 30, 45, and 60 min after extubation [Tables 1 and 2]. Propofol required to maintain the BIS of 55 ± 5 was noted as mg/kg/h at every 15 min, and an average was calculated.
Table 1.
Short mental status questionnaire[9]

Table 2.
Short mental status questionnaire (interpretation)[8]

Statistical analysis
Samples were taken on the basis of frequency of patient visit in the hospital. A total of 15 patients in each group provided a confidence level of 95% with a confidence interval ranging 1–15. Paired Student's t-test was performed to assess the difference between arterial pressure and PR variation during surgery within each group, and Unpaired Student's t-test was used to measure difference of arterial pressure and PR variation, recovery time, and propofol requirement between the three groups. Significance was established at P < 0.05. The Chi-square test was performed to assess the difference in cognitive function between different groups, and P < 0.05 was considered significant.
RESULTS
All three groups were comparable regarding patients’ age, weight, sex ratio, ratio of ASA grade I and II, and duration of surgery [Table 3].
Table 3.
Demographics of the patients recruited for the study
Effect of clonidine and dexmedetomidine on intra-operative hemodynamics
When preoperative arterial pressure and PRs were compared with arterial pressure and PR during laryngoscopy, pneumoperitoneum (PP 10 min), and extubation; a statistically significant increase in the MAP was observed for Group P, whereas, the increase was not significant for Group C and D [Table 4 and Figure 1a]. There was a significant increase in the PR in Group P during laryngoscopy, (PP 10 min) and extubation where as a decrease was found in Group C and Group D [Table 4 and Figure 1b].
Table 4.
Comparison of MAP and PR variation during surgery within each group
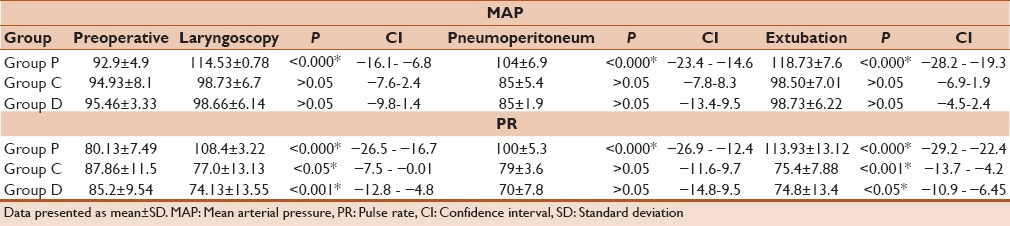
Figure 1.
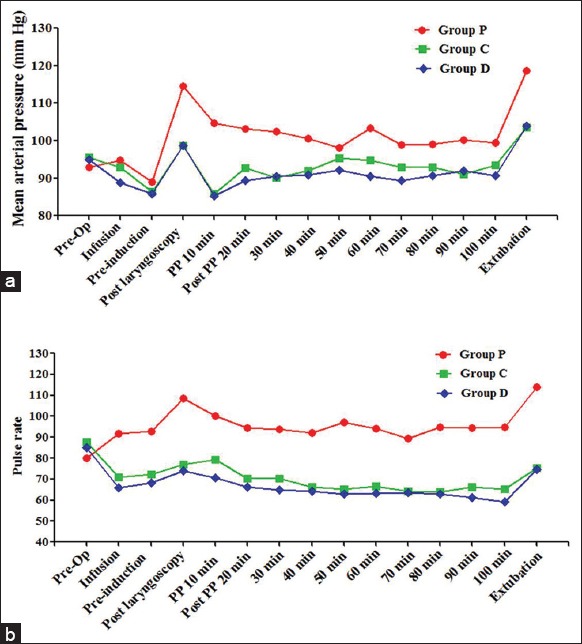
Variation of mean arterial pressure (MAP) and pulse rate (PR) during surgery. (a) Variation of MAP during surgery, hypertensive response during laryngoscopy, and extubation in Group P was significantly higher when compared to preoperative values (b) variation of PR during surgery, PR during laryngoscopy, and extubation was significantly higher in Group P; and significantly lower in Group C and D when compared to preoperative values
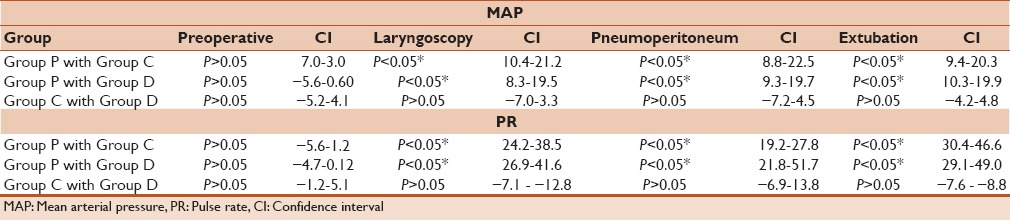
When preoperative arterial pressure and PRs were compared between Group P, Group C, and Group D, no significant difference was observed for both parameters. However, when arterial pressure and PR variation were compared during laryngoscopy, (PP 10 min) and extubation between three groups a statistically significant difference was observed, when Group P was compared to Group C, as well as when Group P was compared with Group D [Table 5]. In both drug combinations, arterial pressure and PR were significantly lower as compared to propofol alone. Although when both these parameters were compared between Group C and Group D there was no significant difference [Table 5].
Table 5.
Comparison of MAP and PR between the three groups at various time points
Effect of clonidine and dexmedetomidine on anesthetic requirement and recovery time
Propofol requirement was reduced by 45% and 40% with simultaneous administration of clonidine and dexmedetomidine, respectively, which was statistically significant compared to propofol [Table 6 and Figure 2a]. The recovery time (time required to respond to verbal commands) in propofol group was 556 ± 117.5 s, which was significantly shorter than the other two groups. In Group C, the recovery time was 662 ± 236.72 s, which was significantly shorter than Group D group where the recovery was most prolonged with 1288 ± 276.46 s [Table 6 and Figure 2b].
Table 6.
Average propofol requirement during surgery in each group and recovery time (time from the stoppage of drug to making an attempt to eye opening)

Figure 2.
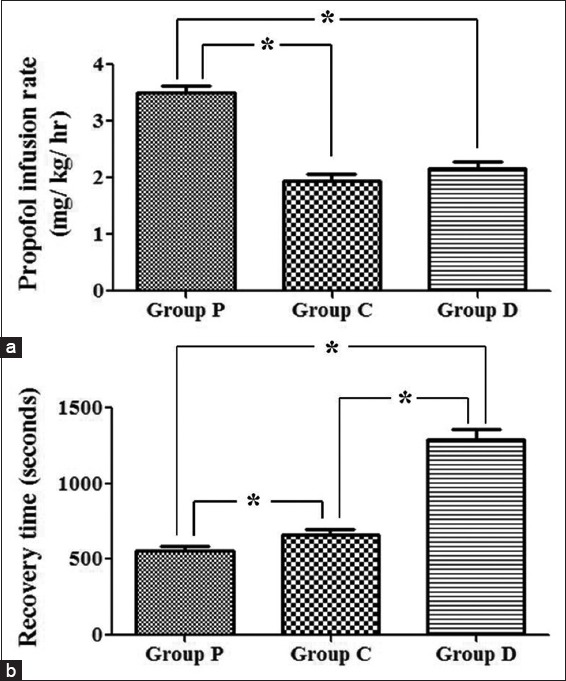
Comparison of anesthetic infusion rate and recovery time between Group P, C, and D. (a) Propofol infusion rate was highest in Group P and it significantly lower in Group C and D compared to Group P (b) patients in group D took the longest time to regain normal cognition, which was significantly higher than Group P and C. Recovery time in Group C was also significantly higher when compared to Group P
Effect of clonidine and dexmedetomidine on postoperative cognitive function impairment
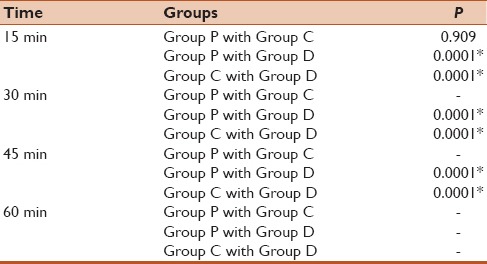
On assessing the cognitive functions, it was observed that 73% patients in Group P and 66% patients in Group C had normal cognitive functions at 15 min while at 30 min 100% of the patients in both the groups had regained normal cognition [Figure 3]. Whereas, in Group D 100% patients till 30 min had a moderate degree of cognition impairment and in around 50% of the patients it persisted for up to 45 min. However, at 60 min almost all the patients in all the three groups had regained normal cognition including 100% in Group P and Group C and 66% in Group D [Figure 3]. There is no statistically significance difference between Group P and Group C at any time point. However, a comparison between either Group P or Group C with Group D revealed a statistically significant difference in normal cognition at each time point till 45 min. However, no statistical test could be applied at 60 min time point as by this time most of the patients in all the three groups have regained normal cognitive functions [Table 7 and Figure 3].
Figure 3.
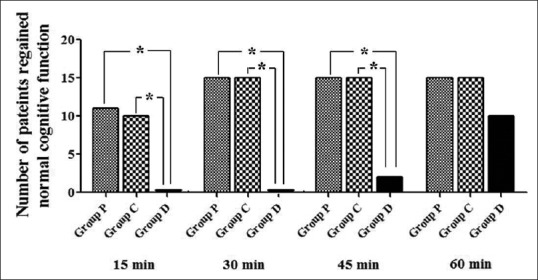
Number of patients with normal cognitive functions in Group P, C, and D at different time points. At 30 min, all the patients in Group P and C had regained normal cognition, and none in Group D were showing normal cognitive functions. Till 60 min, only 10 patients were showing normal cognitive function in Group D, and 5 were still displaying improper cognition
Table 7.
Comparison of number of patients regained normal cognition between the three groups at different time points
DISCUSSION
Dexmedetomidine has been used extensively intravenously with the recommended bolus dose of 1 μg/kg followed by an infusion ranging from 0.2 to 0.7 μg/kg/h.[10,11] There is a paucity of studies, which have compared the dose equivalence of clonidine and dexmedetomidine. Hence, the dose of clonidine was selected on the basis of previous studies, which have sorted out the its most effective dose of for intra-operative use.[12,13,14] The dose of clonidine was considered equipotent to that of dexmedetomidine. Rapid administration of both clonidine and dexmedetomidine can lead to bradycardia and hypotension, so the bolus dose was administered over a period of 15 min followed by the infusion.[10,13]
Both clonidine and dexmedetomidine give intra-operative hemodynamic stability, which has been investigated in many studies before.[10,11,15] They act presynaptically to attenuate norepinephrine release and postsynaptically to inhibit sympathetic activity causing a decrease in PR and blood pressure.[11] This property of clonidine and dexmedetomidine has been evaluated in many studies previously all showing similar and replicable results.[10,15] In the present study, we observed that both the drugs cause a decrease of MAP and PR, which is favorable during laparoscopic cholecystectomy. The decrease in MAP and PR after both the drugs were administered of similar magnitude with no added advantage of more selective agonist dexmedetomidine over clonidine. Our results are in concert with the results of Taittonen et al.,[15] where it is reported that both clonidine and dexmedetomidine provide similar metabolic and hemodynamic effects.
Hypotension and bradycardia are the major side effects of α2 agonists.[16,17] In the present study, one patient in Group C and 2 patients in Group D experienced brief periods of bradycardia during insufflation, which responded promptly to atropine without any need for interruption of either clonidine or dexmedetomidine infusion. The requirement of propofol to maintain the similar level of BIS score was reduced significantly with clonidine (45%) and dexmedetomidine (40%) compared to the control group. The results are consistent with previous studies showing a reduction in the requirement of maintenance dose of propofol with concomitant use of clonidine (20–40%) and dexmedetomidine (30–50%).[3,6,18,19,20] The sedative effect of α2 agonists is mediated through the locus ceruleus in the brain stem, where they decrease sympathetic outflow and increases parasympathetic outflow.[4] As the mechanism for producing the sedative effect is different from propofol, there is a possible synergism upon combined administration of α2 agonists and propofol with respect to their sedative effects. The propofol sparing effect of clonidine and dexmedetomidine may be beneficial for reducing the propofol dosage and hence avoiding adverse effects mainly including myocardial depression, metabolic acidosis, and impaired platelet aggregation.[21]
The time of eye opening (recovery time) was significantly prolonged in patients receiving clonidine compared to propofol alone. The delayed recovery was further significantly higher in patients receiving dexmedetomidine compared to those receiving propofol with clonidine, as well as those receiving propofol alone. The delayed recovery with dexmedetomidine as propofol adjuvant is consistent with previous reports.[5] With clonidine however contrasting results have been documented. Goyagi et al.[6] and Higuchi et al.[19] have reported a delayed emergence, which is supportive of the findings observed in the present study on the other hand Bellaοche et al.[22] have found no delay with the use of clonidine in the propofol based anesthesia. Such discrepancy in results among studies might arise due to different clonidine doses administered and the presence or absence of premedication in the control patients. However, in the previous studies, clonidine was used as premedication oral or IV before induction and not as an infusion during the surgery. A striking finding of the present study, is that, the delay in recovery is much more evident with dexmedetomidine compared to clonidine suggesting an explanation involving α2 receptor selectivity and pharmacodynamics of the drug.
Postoperative impairment of cognition, as judged by SMSQ, was impaired to a moderate degree in the dexmedetomidine group. In clonidine group, only 40% patients had mild impairment of cognition at 15 min and all the patients had normal cognition at 30 min. On the other hand, not only the dexmedetomidine group had a moderate degree of impaired cognition, it took almost an hour for the full recovery of cognition. Hall et al.[7,8] have evaluated the effect of clonidine and dexmedetomidine on cognitive function on healthy subjects in two different studies. In one study, it was reported that clonidine infusion up to 2 μg/kg/h for 60 min did not cause an impairment of cognitive functions in healthy subjects, a significant impairment was observed at 4 μg/kg/h infusion (plasma concentration above 1.5 ng/ml).[8] Small dose dexmedetomidine infusion, on the other hand, caused an impaired performance on cognition testing, which lasted for at least 1 h after termination of infusion.[7]
A similar finding has been reported by Bustillo et al.,[23] where dexmedetomidine infusion was used for sedation during embolization of cerebral arteriovenous malformations and found that although patients were awake and following simple commands 10 min after the discontinuation of the infusion of dexmedetomidine, they were unable to undergo cognitive testing even after 45 min of discontinuation. Although it has been reported that dexmedetomdine affects complex cortical processing, the cause of cognitive impairment is unknown. One possibility suggested by Bustillo et al.[23] is that dexmedetomidine enters cerebrospinal fluid (CSF) reaching 4% ± 1% of the plasma concentration peaked in CSF 2–10 min after the end of infusion and remained elevated up to 45 min. In the present study, therefore, very well demonstrates the difference in the effect of clonidine and dexmedetomidine as propofol adjuvants on the cognitive functions, thereby, providing valuable information regarding their use as anesthetic adjuvants especially in elderly patients where postoperative cognitive dysfunction is very common, and in situations where there is a need to perform cognitive functions intra-operatively such as during certain types of neurosurgical (awake craniotomy) and interventional neuroradiologic procedures.
Although, the present study was very convincingly compares the various effects of using clonidine and dexmedetomidine as propofol adjuvant, however, there are certain limitations. First, owing to its long elimination half-life clonidine is not being used for continuous sedation, and although it has been used as a continuous infusion in different studies, there is no consensus on applied dose regimen for infusion. Second, the plasma concentrations of the drugs were not measured therefore, no comments on the pharmacokinetic interactions could be made. Third, propofol was administered using a manually controlled infusion rather than the recommended target controlled infusion. Furthermore, the recovery assessment was done only by a verbal stimulus, which was not standardized, and the cognitive function assessment test scale that was used was very simple which did not provide a numerical value.
CONCLUSION
Our study, thus, demonstrates that the use of α2 agonists provide hemodynamic stability by reducing the sympathetic response to laryngoscopy and extubation, and have a propofol-sparing effect, however, both drugs delay emergence from propofol based anesthesia suggesting that propofol sparing effect is not associated with faster recovery. Cognitive impairment was also observed, when both drugs were used as adjuvants. Dexmedetomidine infusion at the recommended dose impairs cognitive functions, which do not improve even after 45 min of discontinuation. Clonidine at the dose of 3 mcg/kg bolus followed by an infusion of 1.5 mcg/kg/h provides good intra-operative conditions, without much affecting the cognitive functions, needs further evaluation.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Kulka PJ, Tryba M, Zenz M. Dose-response effects of intravenous clonidine on stress response during induction of anesthesia in coronary artery bypass graft patients. Anesth Analg. 1995;80:263–8. doi: 10.1097/00000539-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 2.De Kock M, Crochet B, Morimont C, Scholtes JL. Intravenous or epidural clonidine for intra- and postoperative analgesia. Anesthesiology. 1993;79:525–31. doi: 10.1097/00000542-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Kang WS, Kim SY, Son JC, Kim JD, Muhammad HB, Kim SH, et al. The effect of dexmedetomidine on the adjuvant propofol requirement and intraoperative hemodynamics during remifentanil-based anesthesia. Korean J Anesthesiol. 2012;62:113–8. doi: 10.4097/kjae.2012.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma D, Rajakumaraswamy N, Maze M. alpha2-adrenoceptor agonists: Shedding light on neuroprotection? Br Med Bull. 2004;71:77–92. doi: 10.1093/bmb/ldh036. [DOI] [PubMed] [Google Scholar]
- 5.Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth Analg. 2008;107:1871–4. doi: 10.1213/ane.0b013e3181887fcc. [DOI] [PubMed] [Google Scholar]
- 6.Goyagi T, Tanaka M, Nishikawa T. Oral clonidine premedication reduces induction dose and prolongs awakening time from propofol-nitrous oxide anesthesia. Can J Anaesth. 1999;46:894–6. doi: 10.1007/BF03012982. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Robertson D, Rockwood K, Stolee P. A short mental status questionnaire. Can J Aging. 1982;1:16–20. [Google Scholar]
- 10.Sağýroğlu AE, Celik M, Orhon Z, Yüzer S, Sen B. Dýfferent doses of dexmedetomidine on controlling haemodynamic responses to tracheal intubation. Int J Anesthesiol. 2010;27:2. [Google Scholar]
- 11.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 12.Jamadarkhana S, Gopal S. Clonidine in adults as a sedative agent in the intensive care unit. J Anaesthesiol Clin Pharmacol. 2010;26:439–45. [PMC free article] [PubMed] [Google Scholar]
- 13.Samantaray A, Rao MH, Chandra A. The effect on post-operative pain of intravenous clonidine given before induction of anaesthesia. Indian J Anaesth. 2012;56:359–64. doi: 10.4103/0019-5049.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marinangeli F, Ciccozzi A, Donatelli F, Di Pietro A, Iovinelli G, Rawal N, et al. Clonidine for treatment of postoperative pain: A dose-finding study. Eur J Pain. 2002;6:35–42. doi: 10.1053/eujp.2001.0270. [DOI] [PubMed] [Google Scholar]
- 15.Taittonen MT, Kirvelä OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. Br J Anaesth. 1997;78:400–6. doi: 10.1093/bja/78.4.400. [DOI] [PubMed] [Google Scholar]
- 16.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu R, Chen Y, Cottingham C, Peng N, Jiao K, Limbird LE, et al. Enhanced hypotensive, bradycardic, and hypnotic responses to alpha2-adrenergic agonists in spinophilin-null mice are accompanied by increased G protein coupling to the alpha2A-adrenergic receptor. Mol Pharmacol. 2010;78:279–86. doi: 10.1124/mol.110.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards MJ, Skues MA, Jarvis AP, Prys-Roberts C. Total i.v. anaesthesia with propofol and alfentanil: Dose requirements for propofol and the effect of premedication with clonidine. Br J Anaesth. 1990;65:157–63. doi: 10.1093/bja/65.2.157. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi H, Adachi Y, Arimura S, Ogata M, Satoh T. Oral clonidine premedication reduces the awakening concentration of propofol. Anesth Analg. 2002;94:609–14. doi: 10.1097/00000539-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Fudickar A, Bein B. Propofol infusion syndrome: Update of clinical manifestation and pathophysiology. Minerva Anestesiol. 2009;75:339–44. [PubMed] [Google Scholar]
- 22.Bellaïche S, Bonnet F, Sperandio M, Lerouge P, Cannet G, Roujas F. Clonidine does not delay recovery from anaesthesia. Br J Anaesth. 1991;66:353–7. doi: 10.1093/bja/66.3.353. [DOI] [PubMed] [Google Scholar]
- 23.Bustillo MA, Lazar RM, Finck AD, Fitzsimmons B, Berman MF, Pile-Spellman J, et al. Dexmedetomidine may impair cognitive testing during endovascular embolization of cerebral arteriovenous malformations: A retrospective case report series. J Neurosurg Anesthesiol. 2002;14:209–12. doi: 10.1097/01.ANA.0000017492.93942.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]


