Abstract
Context:
Ventilator-associated pneumonia (VAP) is a common nosocomial infection occurring in intensive care unit (ICU) settings. VAP occurs due to interplay of three factors - impaired host defense, access of large numbers of pathogenic bacteria to the lower respiratory tract and the virulence of the organism. Knowledge of colonizing microbial flora and their antibiogram in ventilated patients is of great importance in timely institution of empirical therapy, so that mortality and morbidity due to VAP can be reduced.
Subjects and Methods:
A prospective study was performed over a period of 6 months in a multi-specialty hospital to determine the various pathogens in respiratory secretions and to determine the prevalence of multidrug resistance (MDR).
Results:
Pseudomonas aeruginosa (26%), Acinetobacter (26%), Klebsiella pneumoniae (26%), followed by Escherichia coli (15%), Staphylococcus aureus (6%) and Citrobacter spp. (1.5%) were the common pathogens isolated in our study. In all, 72.73% (48/66) bacterial isolates were isolated from medical ICU, while 25.76% (17/66) were isolated from surgical ICU. Only one strain (Acinetobacter) was isolated from pediatric ICU. Fifty-seven (86.36%) of the 66 pathogens in our study were MDR.
Conclusion:
There is increasing colonization of pathogenic bacteria in ventilated patients admitted in ICUs, which are predominantly MDR. These colonizers may cause infection resulting in VAP. Judicious use of antibiotics, guided by local antibiotic resistance profile coupled with strict infection control practices alongside application of VAP bundle are important measures to prevent these pathogens from causing VAP in ICU patients.
Keywords: Multidrug resistance, pathogens, ventilator-associated pneumonia
INTRODUCTION
Ventilator-associated pneumonia (VAP) is defined as pneumonia occurring after more than 48 hours following endotracheal intubation and mechanical ventilation (MV).[1] It is the second most common hospital-acquired infection (HAI), accounting for 15% of HAIs and has the highest morbidity and mortality.[2]
There is a dire need of epidemiological studies on patients on MV, so that there is greater knowledge of local microbial flora and their antibiotic profiles for rational usage of antibiotics. Hence, this study was undertaken to determine the prevalence of pathogenic bacteria in respiratory secretions of ventilated patients and to know their antibiotic susceptibility patterns.
SUBJECTS AND METHODS
This study was a prospective observational study conducted in three intensive care units (ICU) - surgical ICU (SICU), medical ICU (MICU) and pediatric ICU (PICU) of a multi-specialty hospital in central India for duration of six months from January to June 2012. The study was approved by the Ethics committee of our hospital, and informed consent was taken from each patient's attendant. Postoperative patients requiring ventilation were admitted to the SICU. These included patients with head injury, intracranial bleeding and patients requiring neurosurgery. Patients with medical conditions, except pediatric cases, necessitating ventilation were admitted to the MICU. The underlying illnesses of patients in MICU included renal failure, malignancies, diabetic ketoacidosis, respiratory distress, septicemia, stroke, poisoning, etc., Children less than 14 years of age were admitted to the PICU, mainly for birth asphyxia, sepsis, acute respiratory distress syndrome (ARDS) and gastroenteritis.
During the study period, there were a total of 91 patients who were admitted to ICUs and were intubated and mechanically ventilated. Among them, only 53 patients who were ventilated for more than 48 hours were eligible for inclusion in the study (39 patients from MICU, 13 patients from SICU and 1 patient from PICU), and respiratory secretions were collected from them. The first sample of endotracheal aspirate was collected after 48 hours of intubation and subsequently samples were collected every 48 hours to see significant colonization. Samples of broncho-alveolar lavage (BAL) were collected from patients who underwent bronchoscopy for specific indication. A total of 129 samples were collected from these 53 patients, which included 27 BAL and 102 endotracheal aspirates. A total of 66 samples (49 endotracheal aspirates and 17 BAL secretions) showed significant growth when cultured using standard microbiological techniques.[3] Significant growth was considered when >105 colonies were obtained from tracheal secretions and >104 colonies were obtained from BAL.[4] The antibiotic susceptibility of these clinical isolates were determined by the Kirby-Bauer disk diffusion method and analyzed according to Clinical and Laboratory Standards Institute 2011 document.[5] In our study, multidrug resistance (MDR) definition for Gram-negative organisms was taken as non-susceptible to more than one agent in at least 3 antimicrobial categories.[6] Staphylococcus was considered as MDR if (i) it was methicillin-resistant and (ii) non-susceptible to more than one agent in at least 3 antimicrobial categories.[6]
RESULTS
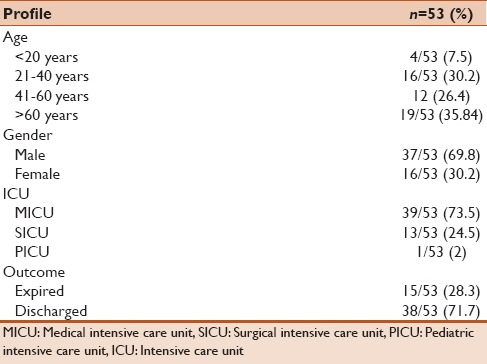
The mean age of the patients from whom pathogenic organisms were isolated was found to be 50.26 years. Majority of the patients (35.8%) were more than 60 years of age, whereas only 7.5% were less than 20 years of age. The rest of the patients were in the age group of 20–60 years. Total 37 males and 16 females were enrolled. The crude mortality rate of the patients was determined to be 28.3%. A more elaborate description has been given in Table 1. Culture positivity seen in these patients was found to be 51.1% (66 isolates out of 129 samples). The most common bacterial agents isolated were Pseudomonas aeruginosa (26%), Acinetobacter (26%), Klebsiella pneumoniae (26%) followed by Escherichia coli (15%), Staphylococcus aureus (6%) and Citrobacter spp. (1.5%).
Table 1.
Demographic profile of 53 patients enrolled in the study
Comparison of bacterial isolates from MICU, SICU and PICU
Out of the total 66 bacterial isolates, 72.73% (48/66) isolates were obtained from MICU, while 25.76% (17/66) were isolated from SICU. Only one organism (Acinetobacter spp.) was isolated from PICU. In MICU, Enterobacteriaceae - E. coli, K. pneumoniae, C. freundii accounted for 41.67% (20/47) isolates, non-fermenters (P. aeruginosa and Acinetobacter spp.) for 48.93% (23/47) and Staphylococcus spp. for 8.5% (4/47) of bacterial isolates. In SICU, non-fermenters were the predominant pathogens, accounting for 58.82% (10/17) and Enterobacteriaceae comprised the rest.
Antimicrobial susceptibility profile of the pathogens
Table 2 shows the antibiotic resistance among the Enterobacteriaceae. Highest resistance was observed against β-lactams (96.4% isolates were resistant to ampicillin and 100% isolates were resistant to amoxicillin-clavulanic acid); 89.3% isolates were resistant to 3rd and 4th generation cephalosporins and 82% to ciprofloxacin. However, they were relatively less resistant to amikacin (17.86%), gentamicin (64.3%) and piperacillin-tazobactam (53.6%). Among carbapenems, 32% isolates were resistant to ertapenem, 14.3% to imipenem and 42.8% to meropenem.
Table 2.
Antibiotic resistance of Gram-negative organisms from 53 patients enrolled in the study
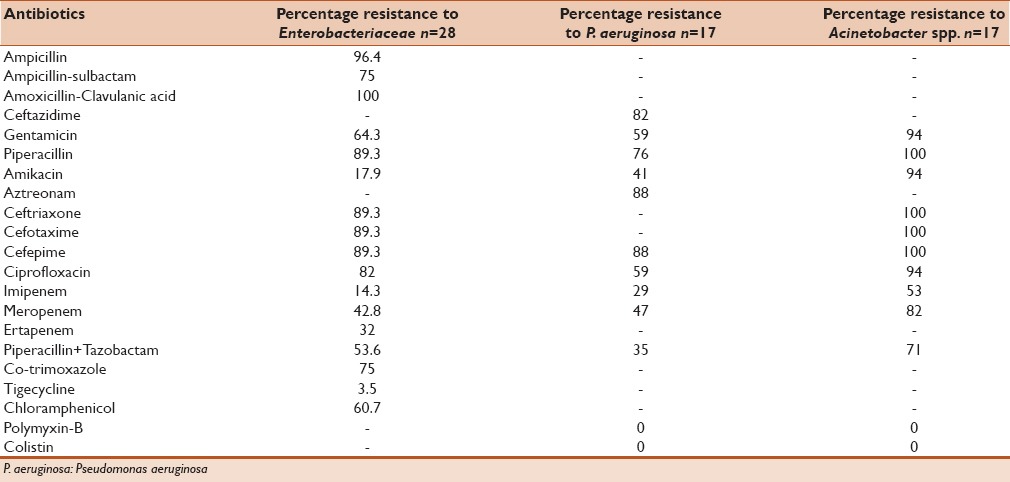
Among the non-fermenting Gram-negative bacilli, [Table 2] 82% P. aeruginosa isolates were resistant to ceftazidime, 88% to cefepime, 76% to piperacillin, 59% to gentamicin, 41% to aztreonam, 59% to ciprofloxacin, 29% to imipenem, 47% to meropenem and 35% to piperacillin + tazobactam. All Acinetobacter isolates were resistant to 3rd and 4th generation cephalosporins and piperacillin. Total 94% isolates were resistant to gentamicin, amikacin and ciprofloxacin. Resistance was high even against carbapenems - 53% and 82% isolates were resistant to imipenem and meropenem, respectively. Total 71% isolates were resistant to piperacillin + tazobactam. All non-fermenters were found to be sensitive to polymyxin and colistin.
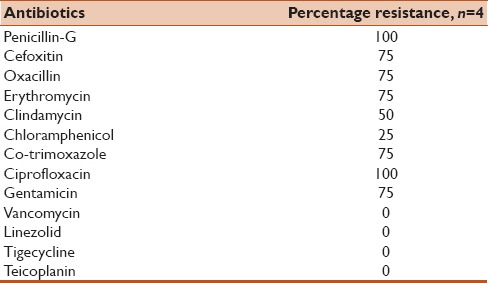
In Staphylococcus spp., 75% isolates were methicillin-resistant. However, no resistance was observed against vancomycin and teicoplanin [Table 3].
Table 3.
Antibiotic resistance of Staphylococcus spp. isolated from 53 patients enrolled in the study
Three of the total number of patients who were mechanically ventilated in the study period developed VAP (11.5 per 1,000 ventilator days). Two patients were from MICU, having diabetic ketoacidosis with ARDS and chronic renal failure with chronic obstructive pulmonary disease as the underlying illnesses; they developed late-onset VAP with MDR P. aeruginosa and Acinetobacter spp., respectively. The third patient also developed late-onset VAP; he was a case of head injury from SICU, and the etiological agent was methicillin-resistant S. aureus. The first two patients recovered and were extubated after 14 and 17 days, respectively and the third patient expired after 21 days of hospital stay (Crude mortality in proven VAP cases = 33.3%).
DISCUSSION
VAP is an important nosocomial infection among ICU patients, causing high morbidity and mortality. According to the National Nosocomial Infection Surveillance Program, the incidence of VAP is 7.6 cases per 1,000 patient ventilator days.[7] It is associated with increased morbidity, prolonged hospitalization and increased health care costs.
VAP occurs due to interplay of three factors - impaired host defense, access of pathogenic bacteria in sufficient numbers to the lower respiratory tract and the virulence of the organism.[8] The organism may gain access into the lungs by one of several routes i. e., micro aspiration of oropharyngeal secretions (most common), aspiration of gastric contents, inhalation, hematogenous spread, direct inoculation and exogenous penetration (e. g., pleural space).
Very little is known about colonizing micro-organisms, which eventually cause VAP, and their antimicrobial resistance in central India. In our study, VAP was diagnosed in only 3 patients as per the CDC definition (11.5 per 1,000 ventilator days).[4] All the three cases were of late-onset VAP and the etiological agents were MDR bacteria. A study conducted in Pondicherry, India, showed a high incidence rate of 22.94 per 1,000 ventilator days.[9] In other Asian countries, the incidence rate is relatively less, ranging from 9 to 12 per 1,000 ventilator days.[10,11]
Many studies have been undertaken in Indian subcontinent regarding the etiological agents of VAP, as well as their increasing antibiotic resistance causing nosocomial infections. Most common VAP pathogens are P. aeruginosa, Acinetobacter spp., E. coli, K. pneumoniae and S. aureus. These organisms are often MDR owing to the production of extended spectrum β-lactamases (ESBL), AmpC β-lactamases (AmpC) or metallo β-lactamases (MBL).[12] Microbiological investigations are of great importance for developing appropriate antimicrobial therapy and for standardizing empirical therapies to be used in the future. In this context, the culture of endotracheal aspirate has similar importance for diagnosis as compared to invasive techniques of BAL and a protected specimen brush, and it is also a simpler and less expensive technique.[13]
In our study, 72.73% (48/66) bacterial strains were isolated from MICU, while 25.76% (17/66) were isolated from SICU. Only one strain (Acinetobacter spp.) was isolated from PICU. Of the patients who developed VAP, two were from MICU, whereas only one was from SICU. This is in contrast to the study by Craven (2000), where SICUs were found to have higher rates of VAP compared to MICUs.[14] However, Torres et al., found that the type of ICU population did not influence the incidence of VAP.[15]
In a study by Joseph et al., it was observed that non-fermenters (77.8%) were the most predominant pathogens causing VAP in the CCU, while in the MICU along with non-fermenters (48.3%), members of Enterobacteriaceae (24.1%) and Gram-positive bacteria (24.1%) were most commonly causing VAP.[16] Although our study was on the colonizing pathogens in significant numbers, we observed that non-fermenters (61.11%) were the predominant isolates from SICU while in the MICU, along with non-fermenters (47.91%), Enterobacteriaceae (41.66%) were the most common organisms isolated. The knowledge of this difference in colonizing pathogens in different ICU settings will help in judicious usage of appropriate empirical antibiotics for treatment of the infection.
Fifty-seven (86.36%) of the total 66 isolates in our study were MDR. These MDR pathogens included all isolates of E. coli and C. freundii and Acinetobacter spp., 82.35% isolates of Klebsiella pneumoniae, 70.58% isolates of P. aeruginosa and 75% isolates of Staphylococcus spp. In another study done by Joseph et al., 37 (78.7%) of the 47 VAP pathogens were MDR. MDR pathogens such as P. aeruginosa, A. baumannii and S. aureus (42.9% of them being MRSA) were the common organisms causing VAP in their study.[17] This highlights the need for treatment of the VAP cases with second-line antibiotics effective against these MDR pathogens. This finding also emphasizes the need for stringent preventive measures for VAP including strict adherence to infection control practices and implementation of VAP bundle, as these MDR colonizers later become the source of established VAP.
In our study, the crude mortality rate of VAP patients was 33.3%. The mortality rate was lower in the Pondicherry study where it was 16.2%.[16] However in a few studies performed in Brazil, mortality rates were higher ranging between 32.1% and 70.9%.[17,18] It is important to notice that co-morbid conditions like cardiac surgery, acute lung injury and immune-compromised status significantly affect prognosis in VAP patients, and consequently, the mortality rates are high.
In general, significant colonization by pathogenic bacteria increased with the number of days of MV. Total 55 out of 66 isolates (83.3%) were from patients who were on MV for more than 4 days. All the three patients who ultimately developed VAP were on ventilator for ≥2 weeks.
The limitation of the study is that it is of short duration and is from a single tertiary care center of a semi-urban population. The microbiological profile thus reflects local environment and cannot be generalized to other health care settings. More such studies of longer durations and involving multiple centers can be helpful in making generalized recommendations.
To conclude, we found that colonizing bacteria in significant numbers were isolated in high frequency from mechanically ventilated patients of our ICUs, and mainly consisted of MDR bacteria. These bacteria can later be the etiological agents of VAP in these patients. The implementation of rational protocols for the use of empirical antibacterial agents, based on the knowledge of local microbiological patterns, rapid delivery of results of culture and susceptibility assays are essential strategies, which coupled with strict infection control practices and regular application of VAP bundle may help in decreasing VAP-related mortality rates and morbidity by MDR bacteria in the ICUs. The bacteriological approach for the management of VAP avoids the problem of overtreatment by separating colonizers from infecting pathogens. The antibiotic susceptibility pattern of these isolates will help the clinicians to choose the appropriate antimicrobial agents.
Source of funding
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Davis KA. Ventilator-associated pneumonia: A review. J Intensive Care Med. 2006;21:211–26. doi: 10.1177/0885066606288837. [DOI] [PubMed] [Google Scholar]
- 2.Feil S. Guidelines and critical pathways for severe hospital-acquired pneumonia. Chest. 2001;119:412S–8. doi: 10.1378/chest.119.2_suppl.412s. [DOI] [PubMed] [Google Scholar]
- 3.Collee JG, Marr W. Culture of bacteria. In: Marmion BP, Fraser AG, Simmons A, editors. Mackie and McCartney's Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 113–30. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Guidelines for preventing health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR. 2004;53 No. RR-3. [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty first Informational supplement. CLSI document. 2011 M100-S2. [Google Scholar]
- 6.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JR, Peterson KD, Andrus ML, Tolson JS, Goulding JS, Dudeck MA, et al. ; NHSN Facilities. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Weber DJ, Rutala W, Mayhall CG. Fishman's text book of pulmonary diseases and disorders. Nosocomial Respiratory tract infection and Gram negative pneumonia. In: Fishman AP, Fishman JA, Kaiser LR, Senior RM, Elias JA, Elias J, et al., editors. 3rd ed. Ch. 143. Vol. 2. New York: McGraw-Hill Publication; 1998. pp. 2213–29. [Google Scholar]
- 9.Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: Incidence and risk factors. J Infect Dev Ctries. 2009;3:771–7. doi: 10.3855/jidc.396. [DOI] [PubMed] [Google Scholar]
- 10.Aly NY, Al-Mousa HH, Al Asar el SM. Nosocomial infections in a medical-surgical intensive care unit. Med Princ Pract. 2008;17:373–7. doi: 10.1159/000141500. [DOI] [PubMed] [Google Scholar]
- 11.Suka M, Yoshida K, Uno H, Takezawa J. Incidence and outcomes of ventilator-associated pneumonia in Japanese intensive care units: The Japanese nosocomial infection surveillance system. Infect Control Hosp Epidemiol. 2007;28:307–13. doi: 10.1086/511997. [DOI] [PubMed] [Google Scholar]
- 12.Noyal MJ, Menezes GA, Harish BN, Sujatha S, Parija SC. Simple screening tests for detection of carbapenemases in clinical isolates of nonfermentative Gram-negative bacteria. Indian J Med Res. 2009;129:707–12. [PubMed] [Google Scholar]
- 13.Cook D, Mandell L. Endotracheal aspiration in the diagnosis of ventilator-associated pneumonia. Chest. 2000;117:195S–7. doi: 10.1378/chest.117.4_suppl_2.195s. [DOI] [PubMed] [Google Scholar]
- 14.Craven DE. Epidemiology of ventilator-associated pneumonia. Chest. 2000;117:186S–7. doi: 10.1378/chest.117.4_suppl_2.186s. [DOI] [PubMed] [Google Scholar]
- 15.Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–8. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 16.Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: Role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4:218–25. doi: 10.3855/jidc.634. [DOI] [PubMed] [Google Scholar]
- 17.Rocha Lde A, Vilela CA, Cezario RC, Almeida AB, Gontijo Filho P. Ventilator associated pneumonia in an adult clinical-surgical intensive care unit of a Brazilian university hospital: Incidence, risk factors, etiology, andantibiotic resistance. Braz J Infect Dis. 2008;12:80–5. doi: 10.1590/s1413-86702008000100017. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues PM, Carmo Neto Ed, Santos LR, Knibel MF. Ventilator-associated pneumonia: Epidemiology and impact on the clinical evolution of ICU patients. J Bras Pneumol. 2009;35:1084–91. doi: 10.1590/s1806-37132009001100005. [DOI] [PubMed] [Google Scholar]


