Abstract
Purpose of review
To summarize the current knowledge regarding mechanisms linking the complement system to transplant injury, highlighting findings reported since 2013.
Recent findings
Building upon the documentation that complement activation is a pathogenic mediator of post-transplant ischemia-reperfusion (IR) injury, emerging evidence indicates blocking either the classical or lectin pathways attenuates IR injury in animal models. Immune cell-derived and locally activated complement, including intracellular C3 positively modulates allo-reactive T cell activation and expansion, while simultaneously inhibiting regulatory T cell induction and function, together promoting transplant rejection. While alloantibody-initiated complement activation directly injures target cells, complement-dependent signals activate endothelial cells to facilitate T cell dependent inflammation. Complement activation within allografts contributes to progressive chronic injury and fibrosis.
Summary
The complement cascade, traditionally considered relevant to transplantation only as an effector mechanism of antibody-initiated allograft injury, is now understood to damage the allograft through multiple mechanisms. Complement activation promotes post-transplant IR injury, formation and function of allo-antibody, differentiation and function of alloreactive T cells, and contributes to chronic progressive allograft failure. The recognition that complement impacts transplant injury at many levels provides a foundation for targeting complement as a therapy to prolong transplant survival and improve patient health.
Keywords: complement, T cells, antibody mediated rejection, ischemia reperfusion
INTRODUCTION
The complement system is traditionally considered a component of the innate immune system. In the context of transplantation, complement activation is a well-recognized effector mechanism underlying alloantibody mediated rejection (1, 2). Evidence published since the late 1990s has expanded our understanding of complement’s role in allograft injury. Complement participates in the pathogenesis of ischemia-reperfusion (IR) injury, modulates alloreactive T cell immunity, and contributes to chronic allograft failure. Herein we will summarize the current state of knowledge regarding complement and transplant injury, highlighting new findings published since 2013.
OVERVIEW OF COMPLEMENT
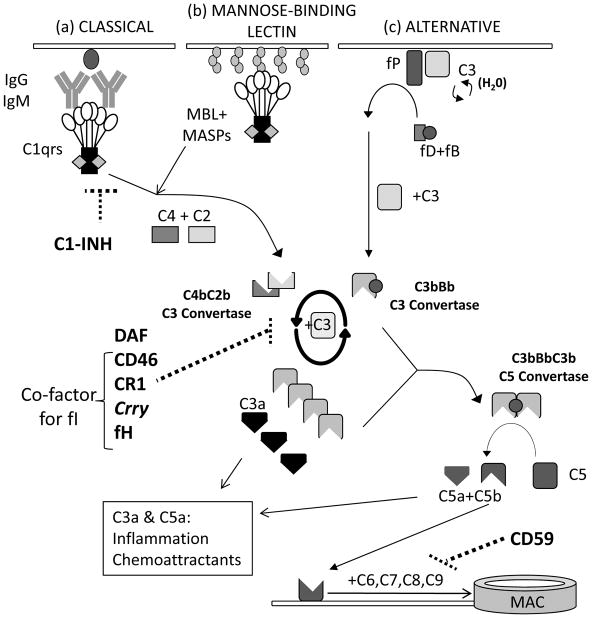
The complement system is comprised of soluble and membrane-bound proteins, including zymogens, receptors and regulators that link innate and adaptive immunity. The complement cascade, outlined in Figure 1, can be activated via the lectin pathway, the classical pathway, and the alternative pathway (3). Convergence at a central amplification step forms multimeric C3 convertases (3) which cleave C3 to C3a and C3b, the latter initiating formation of the C5 convertase and, ultimately, the membrane attack complex (MAC, C5b-9). Soluble and surface bound split products, including C3a, C3b, iC3b, C3dg and C5a mediate inflammation by directly lysing target cells, serving as chemoattractants, functioning as opsonins, and activating innate immune cells, including macrophages and neutrophils (4). Regardless of the initiating pathway, cascade amplification predominantly occurs at the C3 convertase step and is driven by the alternative pathway (Figure 1). Circulating/systemic complement proteins are produced by the liver but complement proteins are also produced by tissue-resident [e.g. tubular cells in the kidney (5)] and migratory/immune cells, including T cells and antigen presenting cells [APCs (6)]. Under physiological conditions, complement activation is highly regulated by several membrane-bound and soluble regulatory proteins to prevent injury to self-cells (4) (see Figure 1). The protective effects of complement regulators can be overcome under pathogenic conditions although precise mechanisms remain to be elucidated.
Figure 1.
Overview of the complement cascade and its regulators. Complement activation can be initiated by the classical pathway that is triggered by cross-linking, cell-bound subclasses of IgG and IgM antibodies (a), the mannose binding lectin (MBL) pathway triggered by carbohydrates present on bacteria surface (b), and the alternative pathway that undergoes spontaneous activation on cell surfaces (c). All three pathways converge into one key amplification step to form multimeric C3 convertases which cleave C3 to C3a and C3b, the latter initiating formation of the C5 convertase. Subsequently C5 cleavage yields C5a and C5b, ultimately forming the membrane attack complex (MAC, C5b-9) on the target cells. Complement activation/amplification is restrained on self-cells by several membrane-bound and soluble regulatory proteins. Surface-expressed regulators include Decay Accelerating Factor (DAF or CD55, accelerates the decay of cell-surface assembled C3 convertases), CD46 [membrane cofactor protein, MCP, co-factor for factor I (soluble) that inactivates C3b to iC3b], Crry, the murine homologue of CD46 that has both decay accelerating and co-factor activities, complement receptor 1, (CR1, CD35, binds to C3b/C4b and has co-factor activity) and CD59 (protectin, inhibits formation of the MAC). Factor H is a soluble complement regulator that exhibits both decay accelerating and co-factor activity. C1 inhibitor (C1-INH) disassociates the C1qrs complex (among other actions) limiting classical pathway activation. C1-INH: C1 inhibitor; CR1: complement receptor 1 (CD35); DAF: decay accelerating factor (CD55), fB: factor B; fD: factor D; fH: factor H; fI: factor I; fP: properdin; MAC: membrane attack complex C5b-9; MBL: mannose-binding lectin; MASP: mannan-binding serine peptidase.
COMPLEMENT AND ISCHEMIA-REPERFUSION INJURY
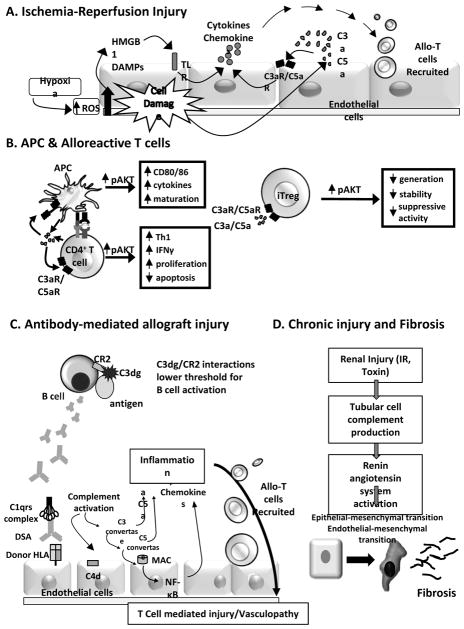
Post-transplant ischemia induces tissue hypoxia, mitochondrial damage and ATP depletion, followed by the generation of free oxygen radicals and endothelial damage upon reperfusion (7). Subsequent inflammation is partially dependent upon complement activation (8–11). Work performed in murine kidney transplant models revealed that donor kidney-derived C3, and not systemic recipient C3, is the predominant complement source driving IR injury (12). Data from animal models and humans suggest that donor brain death upregulates complement activation in the donor kidney prior to organ removal (13). The mechanisms through which complement mediates IR injury include signals transmitted via C3a/C5a interactions with their receptors, C3aR/C5aR (14), including (but not limited to) C3a/C3aR dependent production of chemokines by renal tubular epithelial cells (15). The complement-dependent inflammation associated with IR injury can amplify adaptive alloimmunity (16) and can facilitate T cell infiltration into the allograft (17, 18), together potentially resulting in negative long-term consequences to the transplanted organ (Figure 2A).
Figure 2. Mechanisms through which complement mediates transplant injury.
A) Ischemia-Reperfusion Injury. Following reperfusion the generation of reactive oxygen species (ROS) is associated with graft-derived complement production and local activation as well as directly causing endothelial damage and release of damage-associated molecular patterns (DAMPs, e.g. HMGB1). Subsequent Toll-like receptor (TLR) signaling synergizes with and amplifies complement activation yeilding C3a and C5a. These anaphylatoxins signal through their receptors on endothelial cells (among other targets) inducing chemokine release and facilitating T cell infiltration into the allograft. B) APC & Alloreactive T cells. Cognate interactions between APCs and T cells yield immune cell complement production which activates through the alternative pathway to yield C3a and C5a. The anaphylatoxins bind to their receptors on both partners to induce APC maturation, and effector T cell proliferation/expansion, survival, and differentiation. The same signals inhibit generation, stability, and function of Treg. C) Antibody-mediated allograft injury. Alloantigen-bound C3dg (a C3b cleavage product) binds to B cell-expressed complement receptor 2 (CD21) to facilitate antigen presentation to lowers the threshold for B cell activation. Donor specific antibodies that bind to endothelial cells initiate the classical pathway activation, leading to complement deposition/activation, local C3a/C5a production and MAC formation. The latter results in non-canonical NF-κB signaling that initiates a proinflammatory gene program, enhancing recruitment of allreactive T cells and development of vasculopathy. D) Chronic graft injury and fibrosis. Intra-graft complement production/activation to activation of the renin-angiotensin system and epithelial-to-mesenchymal transition. C3a/C5a produced by endothelial cells induce endothelial-to-mesenchymal transition and renal firbrosis.
Understanding the signals that initiate complement activation following post-transplant IR injury has the potential to guide development of preventative therapies. A 2013 publication showed significantly reduced kidney injury in mannan-binding lectin serine peptidase 2 (MASP2)-deficient mice implicating the lectin pathway (19). Cardiac IR injury also results in mannose lectin pathway-dependent complement activation initiated by binding of natural IgM reactive to tissue-expressed neo-antigens (including non-muscle myosin heavy chain II) that are upregulated/exposed by hypoxia (20, 21). Complement activation via the classical pathway also contributes to murine liver IR injury (22). Blocking complement activation with recombinant C1-INH (inhibits C1qrs, Figure 1) was effective in preventing IR injury in an animal model (23). Therapeutic use of C1-INH improved survival and oxygenation in lung transplant patients with early signs of primary graft dysfunction (24) supporting the need to more broadly test the efficacy of this agent to prevent IR injury in human transplant recipients.
Regardless of the activation pathway, amplification of the complement cascade initiated by IR is alternative pathway-dependent (Figure 1) and results in deposition of C3b on the ischemic graft cells (8–11). To target this mechanism, one research team conjugated a human complement-regulatory protein CD35 (complement receptor 1, CR1, binds to C3b/C4b and blocks complement activation at the C3 convertase step, Figure 1) to a myristoylated peptidyl tail such that when administered by intravenous perfusion of the harvested organ ex vivo it self-inserts into the lipid bilayer of the endothelial cell membranes (25). The approach inhibited local complement activation and limited post-transplant kidney IR injury in rats (26). A human version, mirococept (APT070), is being tested for its efficacy to prevent post-transplant DGF (25). Murine kidney IR injury was analogously prevented through treatment with a protein comprised of the complement regulator Crry (Figure 1) fused to complement receptor 2 (CR2). The CR2 component binds to the activation products iC3b/C3d/C3dg and thereby targets Crry-mediated complement inhibition specifically to sites of complement activation (13). In an effort to target complement activation/amplification downstream of the C3 convertase, the humanized anti-C5 monoclonal antibody (mAb) Eculizumab is being tested for efficacy in preventing post-transplant kidney DGF (NCT01403389; NCT01919346).
COMPLEMENT AND ALLOREACTIVE T CELLS
Building upon the paradigm-shifting observation that WT mice do not reject allografts from C3-deficient donors (27), work from several groups uncovered an unexpected role for immune cell-derived complement as a regulator of T cell immunity (Figure 2B). These studies showed that alternative pathway complement components are produced by T cells and APCs during cognate interactions (including but not limited to allo-reactions) (6, 28, 29). Locally produced C3a and C5a bind to their receptors expressed on the T cell and the APC, resulting in signals that induce T cell proliferation, inhibit T cell apoptosis and drive APC upregulation of costimulatory molecules and cytokines, together amplifying T cell immunity. C5a also has T cell chemoattractant properties via directly binding to C5aR on the T cells (30) and indirectly, through upregulating chemokines (17). Experiments performed in BM chimeric mice indicate that these effects are mediated through immune cell-derived, rather than systemic, complement (6, 31).
The effects of complement on alloreactive T cells impact transplant rejection. WT mice reject heart allografts deficient in the complement regulator DAF with accelerated kinetics through a process that is complement and T cell dependent (31). DAF physiologically restrains complement activation so its deficiency results in increased local production of C3a and C5a which amplify alloreactive T cell responses, explaining the above observations (29, 32). DAF deficiency also accelerates T cell-dependent skin graft rejection (28) and murine graft versus host disease (GVHD) (33). Conversely, blocking C5a/C5aR interactions modulate T cell-dependent rodent kidney transplant rejection (34) and murine GVHD (33). Together with the observations that anti-C5 monoclonal antibody a) synergizes with CTLA4-Ig to prevent T cell priming, b) limits T cell trafficking to an allograft and c) prolongs transplant survival in mice (17), the body of work supports the conclusion that complement is a physiologically important regulator of pathogenic T cell immunity that causes allograft rejection in animal models.
Analogous mechanisms apply to human T cells. C3a and C5a are generated during in vitro cultures of human T cells responding to allogeneic DCs and mediate alloimmune T cell activation and expansion through the similar mechanisms delineated in the murine models (35, 36). Pharmacological C5aR blockade reduced human anti-mouse GVHD scores, and inhibited T cell responses in NOD/SCID/γcnull mouse recipients of human peripheral blood mononuclear cells (35). Consistent with a role for complement as a crucial mediator of human T cells, patients genetically deficient in C3 have impaired Th1 differentiation (37), and Compstatin, a C3 antagonist, was shown to inhibit human CD4 T cell proliferation and polarization (38). One unique difference between mice and humans delineated in 2013 is that human CD4+ T cells store intracellular C3. Upon T cell receptor (TCR) activation cathepsin L-mediated production of C3 cleavage leads to autocrine C3a-C3aR signaling that sustains T cell homeostasis and Th1/17 effector cell differentiation (39). Another publication from the same group showed that C3a/C3aR signaling synergizes with lipopolysaccharide (LPS)-transmitted signals on human monocytes to induce inflammasome activation, secretion of IL-1β and differentiation of IL-17-producing alloreactive T cells (40). Together, the findings substantiate a key role for complement in human effector T cell immunity.
Three 2013 publications showed that immune cell-derived complement inhibits induction, function and stability of regulatory T cells (Treg), including those required for induction/maintenance of allograft tolerance (41–44) (Figure 2B). Genetic or pharmacological blockade of C3aR/C5aR signal transduction in thymic-derived or natural Treg (nTreg) augments their in vitro and in vivo suppressive activity, abrogates autoimmune colitis, and prolongs allogeneic skin graft survival. Mechanisms involve C3a/C3aR and C5a/C5aR-induced phosphorylation of AKT in the Treg and, as a consequence, phosphorylation of the transcription factor Foxo1, which results in lowered nTreg Foxp3 expression. Genetic deficiency or pharmacological blockade of C3aR/C5aR signaling also augments generation of Treg from naive precursors (induced or iTreg), stabilizes Foxp3 expression in iTreg, resists iTreg conversion to IFNγ/TNFα-producing effector T cells (42, 43).
Immune cell-derived complement similarly modulates human Treg generation and function in vitro and in vivo (42). Building upon previously published evidence that coengagement of the TCR and the complement regulator CD46 promote regulatory IL-10 production (45), these new translational results underscore the crucial role of complement in modulating the balance between pathogenic and protective adaptive T cell responses. They provide proof-of-concept that C3a/C3aR and C5a/C5aR ligations are viable targets for inhibiting alloreactive Teff and facilitating iTreg-mediated transplant tolerance. Whether complement antagonists can therapeutically control T cell alloreactivity while simultaneously promoting Treg-induction, function and stability to improve transplant outcomes in humans remains to be determined.
COMPLEMENT AND ANTIBODY-MEDIATED TRANSPLANT INJURY
It has been known for decades that complement regulates IgG production (46). The mechanism involves antigen-bound C3dg (a C3b cleavage product) binding to B cell-expressed CR2 (CD21), which facilitates antigen presentation to B cells, and lowers the threshold for B cell activation (47, 48). C3-deficient mice fail to produce high-affinity IgG responses against major histocompatibility antigens in skin grafts (49), confirming relevance to transplantation. Helper signals provided by T follicular helper (TFH) cells to support the differentiation of antigen-specific B cells into IgG-producing memory and plasma cells (reviewed in (50)). Whether complement specifically impacts this process remains to be determined. Additional evidence for a link between complement and humoral immunity derives from a 2013 publication in which the authors demonstrated that complement-dependent IR injury amplifies development of humoral immune responses through a factor B-dependent mechanism (16).
Complement activation is an established effector mechanism of alloantibody-mediated graft injury in rodent transplant models (2, 51) (Figure 2C). Donor specific antibodies (DSA) initiate complement activation via the classical pathway. Subsequent anaphylatoxin production and MAC formation mediate inflammation and injury (52). Similar mechanisms apply to human transplant recipients with donor-specific anti-HLA antibodies (1, 52–54).
A 2013 publication performed in a humanized mouse model of vascular transplantation provided new mechanistic insight into how complement links alloantibodies to allograft injury (55). The investigators demonstrated that DSA bound to the donor human vascular endothelial cells, caused complement deposition, activation, and MAC formation, and led to non-canonical NF-κB signaling. The complement activation did not result in cell lysis but instead initiated a pro-inflammatory gene program that facilitated recruitment of alloreactive T cells required for the development of the allograft injury (Figure 2C).
Building upon the mechanistic studies, the availability of Eculizumab, a mAb that blocks the C5 convertase, led to clinical trials testing distal complement inhibition as a means to prevent and/or treat antibody-mediated rejection (ABMR) in humans (off label, not FDA approved). Eculizumb plus plasma exchange reduced the incidence of ABMR in 26 sensitized kidney transplant recipients compared to a historical control group treated with a plasma exchange-based protocol alone (56). Eculizumab also successfully reversed established antibody-mediated rejection in a small cohort (57) although not universally (58). Later observations that ABMR can be resistant to Eculizumab (56) suggest that complement-mediated inflammation upstream of C5 or non-complement mediated mechanisms (e.g. Fc receptor dependent) contribute to disease pathogenesis. Other reagents are being developed and tested as well. Among them, C1-INH partially inhibited ABMR in baboons (23) and a novel peptide inhibitor of C1 (PIC1) inhibited antibody-initiated classical pathway and lectin pathway complement activation in rodents (59, 60).
With the recognition that DSA initiates complement activation and injury via the classical pathway, investigators hypothesized that detection of serum anti-HLA antibodies capable of binding C1q would enhance the prognostic utility of serum alloantibody analysis in kidney transplantation (61, 62). The latest iteration of the single antigen bead technology currently used for detecting anti-HLA antibodies (63) additionally identifies those antibodies that bind C1q. A 2013 population-based study of 1016 kidney transplant recipients suggested that, amongst patients with anti-HLA antibodies, those that were C1q+ had the worst graft survival (64). Similar associations were documented in heart (65) and in lung transplants (66) recipients. The clinical importance of C1q binding remains unclear as other reports suggest that C1q binding adds little prognostic information beyond standard DSA testing (67, 68)
COMPLEMENT AND CHRONIC ALLOGRAFT INJURY
Mechanisms of late graft failure are complex and involve immune and non-immune mechanisms (69), but late graft loss is routinely associated with pathological evidence of progressive glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis (70, 71). Evidence suggests that intra-graft complement activation contributes to this pathogenic process (Figure 2D). C3-deficient kidney isografts transplanted into WT recipients were protected from toxin-induced tubular damage, proteinuria and progressive renal failure, despite the presence of abundant circulating C3 (72). Follow-up work showed that C3 is implicated in the activation of the renin-angiotensin system and in the epithelial to mesenchymal transition (73, 74) supporting the concept that synthesis of complement components by renal epithelial cells is one critical mediator of tubular damage in proteinuria-associated renal disease. A 2013 publication suggested that cyclosporine A-induced microparticle release causes activation of alternative pathway complement in endothelial cells and kidney allograft injury (75). In a 2014 paper, the investigators showed that endothelial-to-mesenchymal transition and associated renal fibrosis are linked to C3a/C5a via AKT activation (76). Together, these results raise the possibility that kidney-derived complement participates in the development of kidney post-transplant IF/TA. Other murine studies indicate complement activation contributes to chronic allograft injury in other organs, including obliterative bronchiolitis following lung transplantation (77–79).
Associative evidence linking complement to progressive transplant injury in humans derives from studies of complement gene polymorphisms, serum complement concentrations and transplant outcomes in humans. Specific C5 polymorphisms in both the donor and the recipient have been associated with worse late graft function, but interestingly not with the risk of acute rejection (80). Although controversial, some additional evidence suggests that donor kidney expression of a specific polymorphic variant of C3 is associated with worse post-transplant outcomes (81, 82). Low C4 gene copy numbers (<4) in kidney transplant recipients were also associated with increased allograft survival (83). Additional evidence linking complement to chronic human allograft injury includes a proteomic analysis of kidney allograft tissue which showed strong associations between IF/TA and alternative pathway complement components (84). An ongoing study of chronic Eculizumab therapy in kidney transplant recipients (NCT01327573) could potentially provide further insight into the role of complement as a mediator of progressive graft dysfunction and IF/TA.
CONCLUSIONS
The complement system is now firmly established as a pervasive mediator of transplant injury in animal models, and evidence supports that these newly recognized mechanisms apply to human transplant recipients (Figure 2). This success of translational immunology along with the development of pharmacological agents that block human complement components and receptors (85, 86), will permit testing of the intriguing concept that targeting complement in all solid organ transplant recipients will improve graft survival and patient health.
Key points.
The complement system negatively impacts the allograft survival via multiple mechanisms.
Serum complement participates in antibody initiated allograft injury, locally produced, graft-derived, complement mediates IR injury, and immune cell-derived mediates crucially modulates effector and regulatory T cells
Targeting complement has the potential to attenuate alloimmunity and allograft injury thereby improving outcomes in human transplant recipients
Acknowledgments
The authors thank Douglas Mathern (Icahn School of Medicine at Mount Sinai, NY, NY) for assistance with the figure preparation.
This work was supported by NIH grants AI071185 (awarded to PSH). JHS is a recipient of the American Heart association (AHA) grant number 14PRE20460072
P Heeger is a recipient of research grants from Alexion Pharmaceuticals (Cheshire CT) and Apellis Pharmaceuticals (Crestwood KY), 2 companies involved in complement therapeutics.
References
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280(14):735–9. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin WM, 3rd, Valujskikh A, Fairchild RL. Antibody-mediated rejection: emergence of animal models to answer clinical questions. Am J Transplant. 2010;10(5):1135–42. doi: 10.1111/j.1600-6143.2010.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 4.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peake PW, O’Grady S, Pussell BA, Charlesworth JA. C3a is made by proximal tubular HK-2 cells and activates them via the C3a receptor. Kidney Int. 1999;56(5):1729–36. doi: 10.1046/j.1523-1755.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 6.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112(5):1759–66. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–96. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park P, Haas M, Cunningham PN, Alexander JJ, Bao L, Guthridge JM, et al. Inhibiting the complement system does not reduce injury in renal ischemia reperfusion. J Am Soc Nephrol. 2001;12(7):1383–90. doi: 10.1681/ASN.V1271383. [DOI] [PubMed] [Google Scholar]
- 9.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105(10):1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170(3):1517–23. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 11.De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75(3):375–82. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- 12.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 2006;20(2):217–26. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson C, Floerchinger B, Qiao F, Casey S, Williamson T, Moseley E, et al. Donor brain death exacerbates complement-dependent ischemia/reperfusion injury in transplanted hearts. Circulation. 2013;127(12):1290–9. doi: 10.1161/CIRCULATIONAHA.112.000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23(9):1474–85. doi: 10.1681/ASN.2011111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123(1):7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuquay R, Renner B, Kulik L, McCullough JW, Amura C, Strassheim D, et al. Renal ischemia-reperfusion injury amplifies the humoral immune response. J Am Soc Nephrol. 2013;24(7):1063–72. doi: 10.1681/ASN.2012060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M, et al. Anti-complement component C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice. Am J Transplant. 2011;11(7):1397–406. doi: 10.1111/j.1600-6143.2011.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9(8):1784–95. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 19*.Asgari E, Farrar CA, Lynch N, Ali YM, Roscher S, Stover C, et al. Mannan-binding lectin-associated serine protease 2 is critical for the development of renal ischemia reperfusion injury and mediates tissue injury in the absence of complement C4. FASEB J. 2014 doi: 10.1096/fj.13-246306. The investigators show that MASP2 is required for IR injury in mice mouse while C4 is not, indicating the lectin but not the classical pathway initiates the disease process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Carroll MC. Natural IgM-mediated innate autoimmunity: a new target for early intervention of ischemia-reperfusion injury. Expert Opin Biol Ther. 2007;7(10):1575–82. doi: 10.1517/14712598.7.10.1575. [DOI] [PubMed] [Google Scholar]
- 21.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, et al. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010;87(4):618–27. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Saidi RF, Rajeshkumar B, Shariftabrizi A, Dresser K, Walter O. Human C1 inhibitor attenuates liver ischemia-reperfusion injury and promotes liver regeneration. J Surg Res. 2014;187(2):660–6. doi: 10.1016/j.jss.2013.09.009. In this study the authors showed that blocking classical pathway activation with C1-INH post-transplant liver limited IR injury in a mice. As C1-INH is approved for use in humans the results have important translational potential. [DOI] [PubMed] [Google Scholar]
- 23.Tillou X, Poirier N, Le Bas-Bernardet S, Hervouet J, Minault D, Renaudin K, et al. Recombinant human C1-inhibitor prevents acute antibody-mediated rejection in alloimmunized baboons. Kidney Int. 2010;78(2):152–9. doi: 10.1038/ki.2010.75. [DOI] [PubMed] [Google Scholar]
- 24*.Sommer W, Tudorache I, Kuhn C, Avsar M, Salman J, Ius F, et al. C1-esterase-inhibitor for primary graft dysfunction in lung transplantation. Transplantation. 2014;97(11):1185–91. doi: 10.1097/TP.0000000000000034. In this uncontrolled study the investigators showed that C1-INH treatment of primary graft dysfunction following lung transplantation is safe and may improve outcome, supporting a role of complement activation in the pathogenesis of the disease and supporting the need for additional controlled studies. [DOI] [PubMed] [Google Scholar]
- 25.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12(6):431–42. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 26.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17(4):1102–11. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 27.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582–7. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 28.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nataf S, Davoust N, Ames RS, Barnum SR. Human T cells express the C5a receptor and are chemoattracted to C5a. J Immunol. 1999;162(7):4018–23. [PubMed] [Google Scholar]
- 31.Pavlov V, Raedler H, Yuan S, Leisman S, Kwan WH, Lalli PN, et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008;181(7):4580–9. doi: 10.4049/jimmunol.181.7.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160(5):1558–78. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122(6):2234–8. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gueler F, Rong S, Gwinner W, Mengel M, Brocker V, Schon S, et al. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol. 2008;19(12):2302–12. doi: 10.1681/ASN.2007111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune Cell-Derived C3a and C5a Costimulate Human T Cell Alloimmunity. Am J Transplant. 2013 doi: 10.1111/ajt.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Fazekasova H, Wang N, Sagoo P, Peng Q, Khamri W, et al. Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol. 2011;48(9–10):1121–7. doi: 10.1016/j.molimm.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Ghannam A, Fauquert JL, Thomas C, Kemper C, Drouet C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol Immunol. 2014;58(1):98–107. doi: 10.1016/j.molimm.2013.11.010. Making use of T cells from humans deficient in C3, the authors provide further evidence that C3 regulates human Th1 immunity. [DOI] [PubMed] [Google Scholar]
- 38*.Ma Q, Li D, Carreno R, Patenia R, Tsai KY, Xydes-Smith M, et al. Complement component C3 mediates Th1/Th17 polarization in human T-cell activation and cutaneous GVHD. Bone Marrow Transplant. 2014;49(7):972–6. doi: 10.1038/bmt.2014.75. Using human T cells and specific C3 inhibitors, this study confirms a role for C3 in human alloreactive T cell activation and differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–57. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, et al. C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122(20):3473–81. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 42.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14(2):162–71. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting Edge: Receptors for C3a and C5a Modulate Stability of Alloantigen-Reactive Induced Regulatory T Cells. J Immunol. 2013 doi: 10.4049/jimmunol.1300847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210(2):257–68. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11(9):862–71. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140(1):126–45. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160(11):5273–9. [PubMed] [Google Scholar]
- 48.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 49.Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001;72(7):1310–8. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 50.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13(6):412–26. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179(7):4451–63. doi: 10.4049/jimmunol.179.7.4451. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19(1):33–40. doi: 10.1097/MOT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8(11):670–8. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 54.Akiyoshi T, Hirohashi T, Alessandrini A, Chase CM, Farkash EA, Neal Smith R, et al. Role of complement and NK cells in antibody mediated rejection. Hum Immunol. 2012;73(12):1226–32. doi: 10.1016/j.humimm.2012.07.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, et al. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-kappaB signaling in endothelial cells. Circulation. 2013;128(23):2504–16. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11(11):2405–13. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 57.Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9(1):231–5. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 58*.Burbach M, Suberbielle C, Brocheriou I, Ridel C, Mesnard L, Dahan K, et al. Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation. 2014;98(10):1056–9. doi: 10.1097/TP.0000000000000184. The authors report 2 cases of C4d negative, antibody mediated kidney transplant rejection that did not respond to eculizumab. [DOI] [PubMed] [Google Scholar]
- 59.Mauriello CT, Pallera HK, Sharp JA, Woltmann JL, Jr, Qian S, Hair PS, et al. A novel peptide inhibitor of classical and lectin complement activation including ABO incompatibility. Mol Immunol. 2013;53(1–2):132–9. doi: 10.1016/j.molimm.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Sharp JA, Whitley PH, Cunnion KM, Krishna NK. Peptide inhibitor of complement c1, a novel suppressor of classical pathway activation: mechanistic studies and clinical potential. Front Immunol. 2014;5:406. doi: 10.3389/fimmu.2014.00406. This review summarizes development and testing of a novel pharmacological inhibitor of the classical pathway (PIC1), highlighting its efficacy in blocking classical complement pathways in ABO incompatibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95(9):1113–9. doi: 10.1097/TP.0b013e3182888db6. [DOI] [PubMed] [Google Scholar]
- 62.Crespo M, Torio A, Mas V, Redondo D, Perez-Saez MJ, Mir M, et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: Does C1q-fixation matter? Transpl Immunol. 2013 doi: 10.1016/j.trim.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Picascia A, Infante T, Napoli C. Luminex and antibody detection in kidney transplantation. Clin Exp Nephrol. 2012;16(3):373–81. doi: 10.1007/s10157-012-0635-1. [DOI] [PubMed] [Google Scholar]
- 64.Loupy A, Lefaucheur C, Vernerey D, Prugger C, van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 65.Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, Bennett Bain K, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32(10):1034–40. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crespo M, Torio A, Mas V, Redondo D, Perez-Saez MJ, Mir M, et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: does C1q-fixation matter? Transpl Immunol. 2013;29(1–4):28–33. doi: 10.1016/j.trim.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Peacock S, Kosmoliaptsis V, Bradley AJ, Taylor CJ. Questioning the added value of Luminex single antigen beads to detect C1q binding donor HLA-specific antibodies. Transplantation. 2014;98(4):384–6. doi: 10.1097/TP.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 69.Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378(9800):1428–37. doi: 10.1016/S0140-6736(11)60699-5. [DOI] [PubMed] [Google Scholar]
- 70.Kaneku HK, Terasaki PI. Thirty year trend in kidney transplants: UCLA and UNOS Renal Transplant Registry. Clin Transpl. 2006:1–27. [PubMed] [Google Scholar]
- 71.Cravedi P, Perico N, Remuzzi G. Non-immune interventions to protect kidney allografts in the long term. Kidney Int Suppl. 2010;(119):S71–5. doi: 10.1038/ki.2010.427. [DOI] [PubMed] [Google Scholar]
- 72.Sheerin NS, Risley P, Abe K, Tang Z, Wong W, Lin T, et al. Synthesis of complement protein C3 in the kidney is an important mediator of local tissue injury. FASEB J. 2008;22(4):1065–72. doi: 10.1096/fj.07-8719com. [DOI] [PubMed] [Google Scholar]
- 73.Tang Z, Lu B, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J Am Soc Nephrol. 2009;20(3):593–603. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X, Fukuda N, Matsuda H, Endo M, Wang X, Saito K, et al. Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am J Physiol Renal Physiol. 2013;305(7):F957–F67. doi: 10.1152/ajprenal.00344.2013. [DOI] [PubMed] [Google Scholar]
- 75.Renner B, Klawitter J, Goldberg R, McCullough JW, Ferreira VP, Cooper JE, et al. Cyclosporine induces endothelial cell release of complement-activating microparticles. J Am Soc Nephrol. 2013;24(11):1849–62. doi: 10.1681/ASN.2012111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76**.Curci C, Castellano G, Stasi A, Divella C, Loverre A, Gigante M, et al. Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol Dial Transplant. 2014;29(4):799–808. doi: 10.1093/ndt/gft516. In this study performed in a swine transplant model, the authors show IR injury induced C3a and C5a cause kidney endothelial-to-mesenchymal transition (EMT) and fibrosis, and that C1-INH reduced this process. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, Benson HL, et al. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol. 2013;191(8):4431–9. doi: 10.4049/jimmunol.1202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan MA, Maasch C, Vater A, Klussmann S, Morser J, Leung LL, et al. Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc Natl Acad Sci U S A. 2013;110(15):6061–6. doi: 10.1073/pnas.1217991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan MA, Nicolls MR. Complement-mediated microvascular injury leads to chronic rejection. Adv Exp Med Biol. 2013;735:233–46. doi: 10.1007/978-1-4614-4118-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeong JC, Hwang YH, Kim H, Ro H, Park HC, Kim YJ, et al. Association of complement 5 genetic polymorphism with renal allograft outcomes in Korea. Nephrol Dial Transplant. 2011;26(10):3378–85. doi: 10.1093/ndt/gfr025. [DOI] [PubMed] [Google Scholar]
- 81.Brown KM, Kondeatis E, Vaughan RW, Kon SP, Farmer CK, Taylor JD, et al. Influence of donor C3 allotype on late renal-transplantation outcome. N Engl J Med. 2006;354(19):2014–23. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 82.Varagunam M, Yaqoob MM, Dohler B, Opelz G. C3 polymorphisms and allograft outcome in renal transplantation. N Engl J Med. 2009;360(9):874–80. doi: 10.1056/NEJMoa0801861. [DOI] [PubMed] [Google Scholar]
- 83.Bay JT, Schejbel L, Madsen HO, Sorensen SS, Hansen JM, Garred P. Low C4 gene copy numbers are associated with superior graft survival in patients transplanted with a deceased donor kidney. Kidney Int. 2013;84(3):562–9. doi: 10.1038/ki.2013.195. [DOI] [PubMed] [Google Scholar]
- 84.Nakorchevsky A, Hewel JA, Kurian SM, Mondala TS, Campbell D, Head SR, et al. Molecular mechanisms of chronic kidney transplant rejection via large-scale proteogenomic analysis of tissue biopsies. J Am Soc Nephrol. 2010;21(2):362–73. doi: 10.1681/ASN.2009060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2012;44(3):205–17. doi: 10.3109/07853890.2010.535556. [DOI] [PubMed] [Google Scholar]
- 86.Chen G, Chen S, Chen X. Role of complement and perspectives for intervention in transplantation. Immunobiology. 2013;218(5):817–27. doi: 10.1016/j.imbio.2012.09.002. [DOI] [PubMed] [Google Scholar]