ABSTRACT
The transepithelial voltage (Vte) and the volume of isolated posterior midguts of adult female yellow fever mosquitoes (Aedes aegypti) were monitored. In all experiments, the initial Vte after filling the midgut was lumen negative, but subsequently became lumen positive at a rate of approximately 1 mV min−1. Simultaneously, the midgut volume decreased, indicating spontaneous fluid absorption. When the midguts were filled and bathed with mosquito saline, the average rate of fluid absorption was 36.5±3.0 nl min−1 (N=4, ±s.e.m.). In the presence of theophylline (10 mmol l−1), Vte reached significantly higher lumen-positive values, but the rate of fluid absorption was not affected (N=6). In the presence of NaCN (5 mmol l−1), Vte remained close to 0 mV (N=4) and fluid absorption was reduced (14.4±1.3 nl min−1, N=3, ±s.e.m.). When midguts were filled with buffered NaCl (154 mmol l−1 plus 1 mmol l−1 HEPES) and bathed in mosquito saline with theophylline, fluid absorption was augmented (50.0±5.8 nl min−1, N=12, ±s.e.m.). Concanamycin A (10 µmol l−1), ouabain (1 mmol l−1), and acetazolamide (1 mmol l−1) affected Vte in different ways, but all reduced fluid absorption by 60–70% of the value before addition of the drugs.
KEY WORDS: Acetazolamide, Carbonic anhydrase, Concanamycin, Insect, Ouabain, Sodium-potassium pump, V-type proton pump
Summary: Fluid absorption in the isolated midgut of adult female yellow fever mosquitoes is shown to spontaneously proceed at rates expected from in vivo urine excretion, and Na+/K+ pumps, V-type H+ pumps and carbonic anhydrase are shown to be important for the active component of fluid absorption.
INTRODUCTION
Adult female mosquitoes of many species require a blood meal to finalize egg production (cf. Clements, 1992). In female yellow fever mosquitoes (Aedes aegypti), the blood meal can have a volume between 2 and 7 µl (Klowden and Lea, 1979), significantly affecting the weight and mobility of the animal. The largest part of the blood meal (water and salts) is not of use for the female mosquito and must be quickly excreted. Fluid excretion by Aedes aegypti has been studied on whole animals after blood feeding (Williams et al., 1983). Three phases were distinguished. Immediately after the blood meal, the urine flow rate peaks to maximal rates of over 50 nl min−1. This peak phase takes about 20 min, and the excreted urine contains high sodium concentrations. During the post-peak phase (20–70 min after the blood meal), the rate of urine production declines (11 nl min−1 at 26 min). The urine contains less sodium and its potassium concentration rises. During the so-called late phase, urine production falls to values slightly above control levels (2–3 nl min−1). Urine sodium concentration decreases further and urine potassium concentration increases.
Urine production in Malpighian tubules of female yellow fever mosquitoes has been intensively studied with isolated tubules (for a review, see Beyenbach, 2003). The mechanisms of urine production and the hormones that regulate rates of urine production are well described. However, before the Malpighian tubules can excrete parts of the blood meal, the midgut epithelium must absorb the fluid to be excreted. Transepithelial transport in the midgut of larval mosquitoes has been intensively studied (for reviews, see Okech et al., 2008; Onken and Moffett, 2009). However, the midgut of female adult mosquitoes has been studied less. The first study with significance for female adult midgut absorption was the localization of Na+/K+-ATPase in basal membranes and of V-ATPase in apical membranes of the midgut epithelium (Patrick et al., 2006). More recently, Pacey and O'Donnell (2014) investigated ion fluxes across blood-filled midguts isolated at different times after the blood meal and bathed in mosquito saline.
Here, we introduce a technique that isolates empty midguts from female adult yellow fever mosquitoes, transfers them into a bath, fills them with a desired solution and monitors the transepithelial voltage and midgut volume. The observed rates of spontaneous fluid absorption match the rates of urine excretion during the peak phase right after a blood meal (see above). First results with different transport inhibitors indicate that active and passive transport are both important for fluid absorption in the female adult mosquito midgut, and demonstrate the importance of Na+/K+ pumps, V-type H+ pumps and carbonic anhydrase for the active component of fluid absorption driven by active NaCl absorption.
RESULTS
Experiments with mosquito saline in the midgut lumen
The first series of experiments were performed with mosquito saline on both sides of the tissue in order to avoid the presence of any ionic or osmotic gradients that could influence the transepithelial voltage or the rate of fluid absorption (Fig. 1, supplementary material Movie 1). Under these conditions, the influence of cyanide and theophylline, a reagent that is known to increase intracellular cyclic AMP levels by inhibition of phosphodiesterase (Johnson and Nielsen, 1978), was monitored.
Fig. 1.
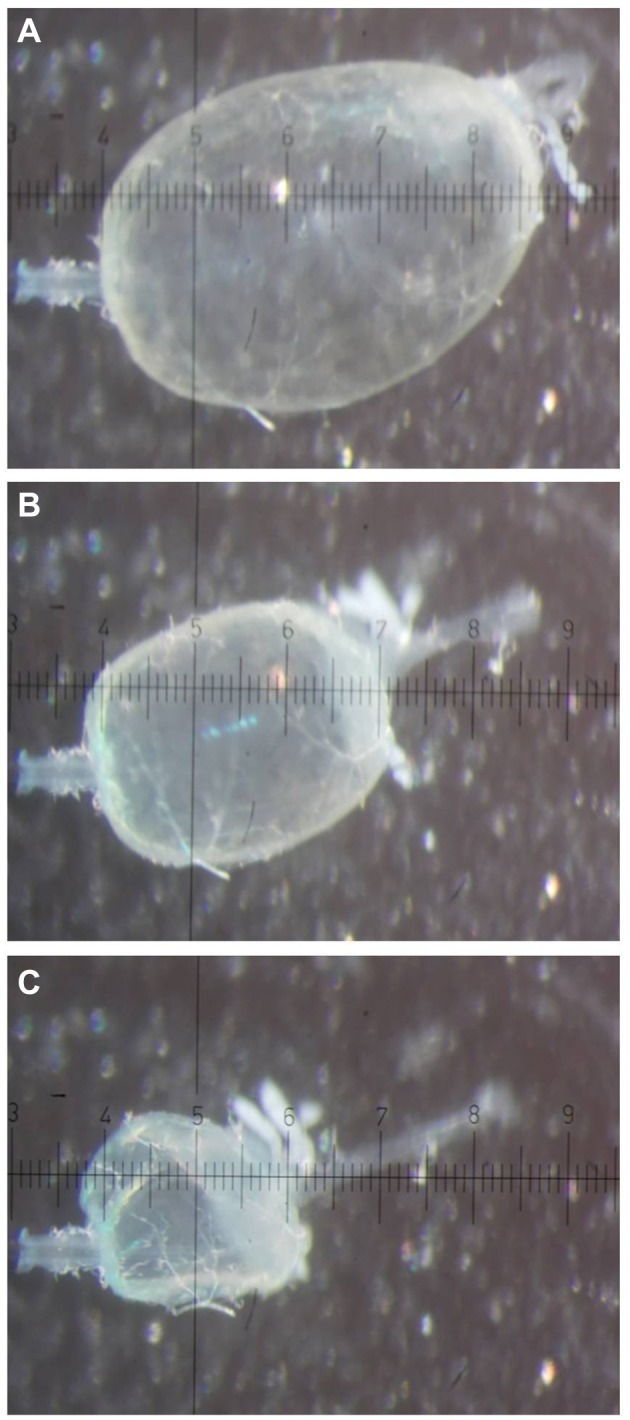
Images of a midgut of a female yellow fever mosquito (Aedes aegypti) after filling with buffered NaCl. Immediately (A), 15 min (B) and 30 min (C) after filling with 154 mmol l−1 NaCl. The midgut was bathed in mosquito saline with theophylline (10 mmol l−1). The pipette (left) was inserted into the anterior midgut. Hindgut and remainders of Malpighian tubules are visible on the right of the posterior midgut. Each scale unit of the ocular micrometer is 40 µm.
After filling the isolated midgut with mosquito saline, a lumen-negative transepithelial voltage (Vte) was recorded (see Fig. 2). The initial Vte was −38±5 mV (N=4, ±s.e.m.). This voltage started to spontaneously depolarize and became lumen positive (+9±4 mV) at a Vte change of 1.00±0.11 mV min−1. Simultaneously, the midgut volume decreased at a rate of 36.5±3.0 nl min−1 (N=4; see also supplementary material Movie 1).
Fig. 2.
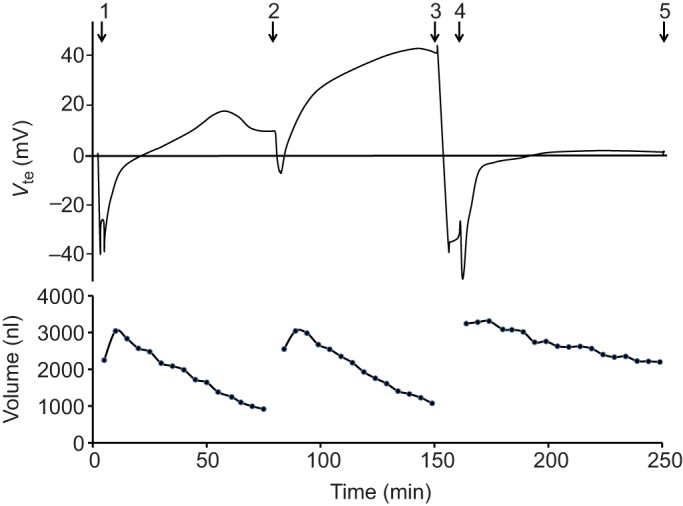
Effects of theophylline and NaCN on transepithelial voltage and volume of the posterior midgut from a female mosquito. Representative time courses of Vte (top) and volume (bottom) of a posterior midgut filled and bathed with mosquito saline. (1) The posterior midgut was filled with mosquito saline. (2) Refilling of the midgut and addition of 10 mmol l−1 theophylline to the bath. (3) Addition of 5 mmol l−1 NaCN to the bath. (4) Refilling of the midgut. (5) Withdrawal of the midgut from the pipette.
In six experiments, the midgut volume and Vte were observed in the presence of theophylline (10 mmol l−1) in the bath (Fig. 2). The initial voltage was less lumen negative (−13±3 mV, N=6), but stabilized at significantly higher (P=0.02), lumen-positive values (+26±5 mV). However, neither the Vte change over time (0.69±0.13 mV min−1) nor the rate of fluid absorption (33.5±3.6 nl min−1) was significantly different from the averages obtained without the drug (see above and Fig. 3).
Fig. 3.
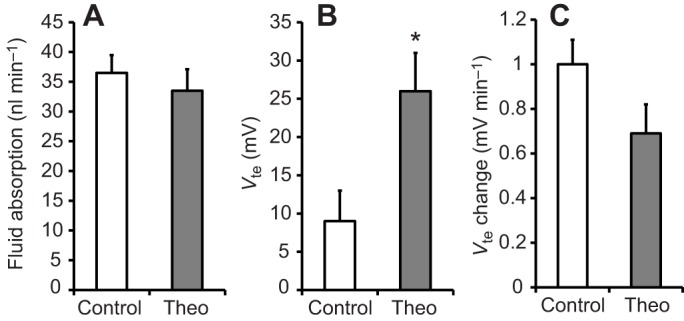
Effect of theophylline on the rate of fluid absorption, transepithelial voltage and change in transepithelial voltage during fluid absorption. Fluid absorption (A), Vte (B) and change in Vte (C) are shown for midguts treated with theophylline (10 mmol l−1) in the mosquito saline bath (Theo) and control samples, which were bathed only in mosquito saline. Differences between groups were tested with Student's t-test. Significant differences were assumed for P<0.05 and are indicated with an asterisk.
In a third series of experiments with mosquito saline in the midgut lumen, NaCN (5 mmol l−1) was added to the bath after observing Vte and fluid absorption in the presence of theophylline (see Fig. 2). Within minutes, Vte dropped significantly (P=0.02) from lumen-positive values (+31±7 mV, N=4, ±s.e.m.) to values near zero mV (+1±1 mV). After re-filling the midgut, fluid absorption (14.4±1.3 nl min−1, N=3, ±s.e.m.) and the Vte change during this period (0.08±0.01 mV min−1) were observed to be significantly reduced (P=0.04 in both cases; see Figs 2 and 4).
Fig. 4.
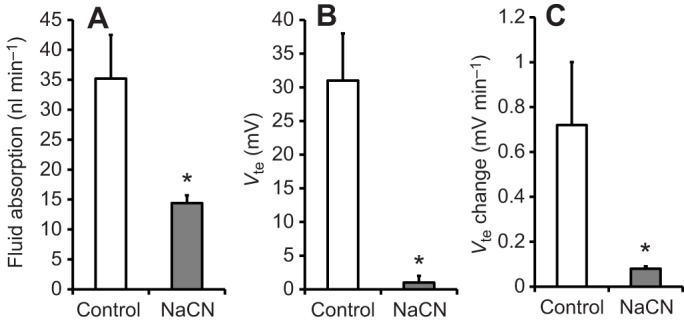
Effect of NaCN on the rate of fluid absorption, transepithelial voltage and change in transepithelial voltage during fluid absorption. Fluid absorption (A), Vte (B) and change in Vte (C) are shown for midguts treated with NaCN (5 mmol l−1) in the bath (mosquito saline with theophylline, 10 mmol l−1, N=3–4). Differences between groups were tested with Student's t-test. Significant differences were assumed for P<0.05 and are indicated with an asterisk.
Experiments with NaCl in the midgut lumen
A second series of experiments were performed with NaCl (‘buffered NaCl’, 154 mmol l−1 plus 1 mmol l−1 HEPES, pH 7.0) in the midgut lumen and with mosquito saline plus theophylline (10 mmol l−1) in the bath. The buffered NaCl mimics the osmolarity of human blood plasma and eliminates nutrients and all other inorganic ions that could modify Vte and/or fluid absorption. Under these conditions, the midgut lumen is hyperosmotic to the bath, and the luminal NaCl concentration is about double that in the bath. Theophylline was used in the bath, because the drug significantly influenced Vte (see above). Under these conditions, we studied the influence of inhibitors of V-ATPase (concanamycin A), Na+/K+-ATPase (ouabain) and carbonic anhydrase (acetazolamide) on Vte and fluid absorption.
After filling the isolated midgut with buffered NaCl, a lumen-negative Vte was recorded in the presence of mosquito saline plus theophylline in the bath (10 mmol l−1, see Fig. 5). On average, the initial Vte was −16±4 mV (N=12, ±s.e.m.). As in the presence of mosquito saline in the midgut lumen, this voltage spontaneously started to decrease and became lumen positive (+28±4 mV) at a Vte change of 1.13±0.25 mV min−1. Simultaneously, the midgut volume decreased at a rate of 50.0±5.8 nl min−1. ANOVA revealed that this average rate of fluid absorption was significantly higher when compared with the fluid absorption in the presence of luminal mosquito saline.
Fig. 5.
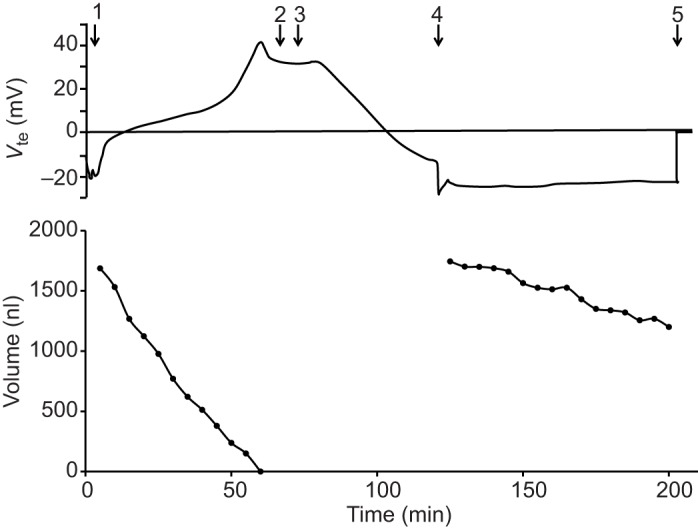
Time course of the effects of DMSO and concanamycin A on transepithelial voltage and volume of the posterior midgut. Representative time courses of Vte (top) and volume (bottom) of a posterior midgut filled with buffered NaCl (154 mmol l−1) and bathed with mosquito saline with theophylline (10 mmol l−1). (1) Filling of the posterior midgut. (2) Addition of DMSO (0.1%) to the bath. (3) Addition of concanamycin A (10 µmol l−1) to the bath. (4) Refilling of the midgut. (5) Withdrawal of the midgut from the pipette.
In three series of experiments, concanamycin A (10 µmol l−1), ouabain (1 mmol l−1) or acetazolamide (1 mmol l−1) were added at the end of a control period in absence of these drugs. After observing the Vte change induced by the drugs, the midgut was re-filled and the subsequent Vte change and fluid absorption were observed in the presence of the drugs.
Concanamycin A significantly reduced (P=0.001) Vte from 36±4 mV to −8±3 mV (N=3, ±s.e.m.) within approximately 45 min (see Fig. 5). After re-filling the midgut with buffered NaCl, Vte measured −22±3 mV. Concanamycin A significantly reduced (P=0.02) the rate of fluid absorption from 58.6±9.0 nl min−1 to 25.2±10.0 nl min−1. The rate of Vte change was also significantly reduced (P=0.03) from 1.22±0.33 mV min−1 to 0.06±0.02 mV min−1. These data are summarized in Fig. 6.
Fig. 6.
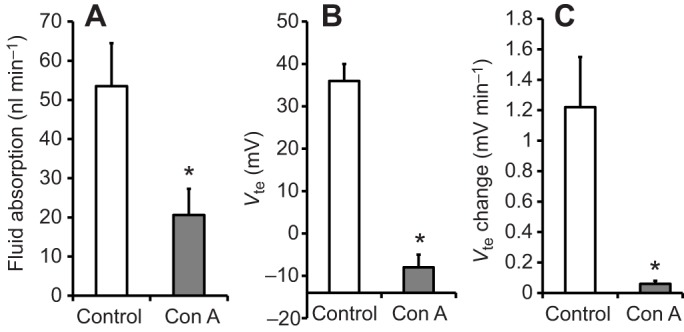
Effect of concanamycin A on the rate of fluid absorption, transepithelial voltage and change in transepithelial voltage during fluid absorption. Fluid absorption (A), Vte (B) and change in Vte (C) are shown for midguts before and after addition of 10 µmol l−1 concanamycin A to the bath (mosquito saline with theophylline, 10 mmol l−1, N=3–4). Posterior midguts were filled with buffered NaCl (154 mmol l−1). Differences between groups were tested with Student's t-test. Significant differences were assumed for P<0.05 and are indicated with an asterisk.
Addition of ouabain to the bathing solution quickly and significantly increased (P=0.047) Vte from +29±8 mV to +57±16 mV (N=4, ±s.e.m.). After re-filling the midgut, a Vte of −3±2 mV was recorded. Ouabain significantly reduced (P=0.0004) the rate of fluid absorption from 53.5±11.0 nl min−1 to 20.6±6.7 nl min−1. The rate of Vte change was also significantly reduced (P=0.02) from 1.14±0.19 mV min−1 to 0.61±0.22 mV min−1. These data are summarized in Fig. 7.
Fig. 7.
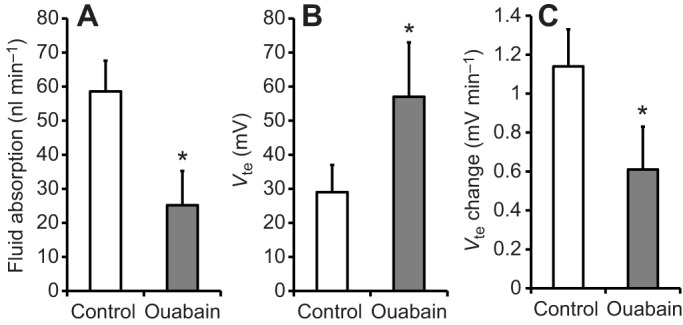
Effect of ouabain on the rate of fluid absorption, transepithelial voltage and change in transepithelial voltage during fluid absorption. Fluid absorption (A), Vte (B) and change in Vte (C) are shown for midguts before and after addition of1 mmol l−1 ouabain to the bath (mosquito saline with theophylline, 10 mmol l−1, N=4). Posterior midguts were filled with buffered NaCl (154 mmol l−1). Differences between groups were tested with Student's t-test. Significant differences were assumed for P<0.05 and are indicated with an asterisk.
Applying acetazolamide to the bath significantly reduced (P=0.01) Vte within 10–15 min from +20±4 mV to +3±1 mV (N=5, ±s.e.m.). After re-filling the midgut with buffered NaCl, a Vte of −7±3 mV was recorded. In four experiments in which fluid absorption and Vte were observed before and after addition of the drug, acetazolamide significantly reduced (P=0.04) the rate of fluid absorption from 42.5±12.7 nl min−1 to 12.9±4.8 nl min−1 (N=4, ±s.e.m.). The rate of Vte change was also significantly reduced (P=0.04) from 0.55±0.20 mV min−1 to 0.09±0.03 mV min−1. These data are summarized in Fig. 8.
Fig. 8.
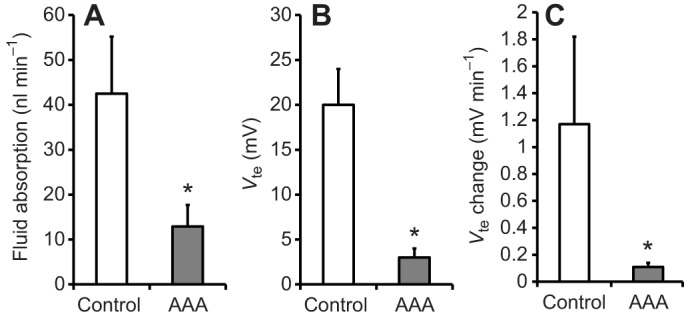
Effect of acetazolamide on the rate of fluid absorption, transepithelial voltage and change in transepithelial voltage during fluid absorption. Fluid absorption (A), Vte (B) and change in Vte (C) are shown for midguts before and after addition of 1 mmol l−1acetazolamide to the bath (mosquito saline with theophylline, 10 mmol l−1, N=4-5). Posterior midguts were filled with buffered NaCl (154 mmol l−1). Differences between groups were tested with Student's t-test. Significant differences were assumed for P<0.05 and are indicated with an asterisk.
DISCUSSION
The central finding of the present studies was that the isolated midgut of adult female yellow fever mosquitoes is able to spontaneously transfer a fluid load of approximately the magnitude of that ingested during a blood meal (cf. Klowden and Lea, 1979), from its lumen to the surrounding bath in a matter of minutes (Fig. 1, supplementary material Movie 1). While the gut was emptying, the transepithelial potential, initially inside-negative, progressively became more inside-positive (Figs 2 and 3).
Methodological aspects
The most important methodological question with regard to the current study is whether the midgut preparations are mounted tightly enough to exclude a leak between the pipette and the tissue. In former studies with larval mosquito midguts, the tissues were tied on the pipettes with human hair (Onken et al., 2004a,b, 2006, 2008). The adult midgut, however, has a thinner wall, and it turned out that human hair cannot improve the seal between midgut and pipette. The pipette was inserted either through the anterior midgut or through the pyloric valve into the posterior midgut. In either approach, it appeared that the muscle tissue contracted onto the pipette shaft, typically forming a pressure-resistant seal and the opposite end of the preparation was sealed by muscle contraction to allow filling of the midgut (see Fig. 1). Although it cannot be completely ruled out that some minor leakage may occur between pipette and tissue, the high transepithelial voltages (in extreme cases below −40 mV or above +80 mV) indicate that the preparations were electrically rather tight. In some experiments, isolated midgut preparations could not be inflated, although a leak was not visible under the microscope. Such preparations were rejected. Also, in preliminary experiments, induction of miniscule leaks with a needle resulted in immediate and rapid deflation and voltage drop. Consequently, those preparations that could be inflated and that deflated at rates similar to the rates of urine production in vivo (Williams et al., 1983), can be considered to have only very minor artificial leaks between the pipette and the wall of the midgut.
Another methodological point that seems to be of importance is our use of a more complete saline compared with other studies on Malpighian tubules and the only other investigation of isolated midguts of adult female yellow fever mosquitoes. In our hands, isolated midguts bathed in a conventional saline did not produce Vte values comparable to those obtained with the more complete saline described here. Interestingly, in a previous study of the larval midgut, removal of amino acids from the saline resulted in a decrease in Vte and in the inability of the tissue to alkalinize the midgut lumen (Izeirovski et al., 2009).
Rates and mechanisms of fluid absorption
The rates of fluid absorption observed in the present study with isolated midguts of female adult Aedes aegypti were in the same order of magnitude as the rates of urine production in adult females during the peak phase after a blood meal (Williams et al., 1983). When the preparations were filled and bathed with mosquito saline, fluid absorption could be driven by two different processes: active solute transport and pressure filtration. Active solute absorption could generate osmotically driven absorption of water between the epithelial cells and/or via aquaporins, which have been shown to be present in the midgut of adult female mosquitoes (Drake et al., 2010).
After filling the midgut preparation, the hydrostatic pressure inside of the midgut must balance the hydrostatic pressure in the bath plus the pressure due to the stretched elastic elements of the midgut wall, which could be partly caused by the network of muscle with which it is invested (Park and Shahabuddin, 2000). The resulting pressure gradient could drive pressure filtration across the midgut wall. The pressure in the midgut could even be considerably increased by peristaltic contractions of the muscle network surrounding the midgut epithelium (see supplementary material Movie 2). Although the pressure in the hemolymph after a blood meal of a female mosquito in vivo must be increased, because of the distension of the body wall, an outward-directed pressure gradient must still compensate for the pressure generated by the elastic midgut wall. Consequently, a pressure gradient that favors fluid absorption should also be present in vivo. It would certainly be interesting to measure these pressures in a future study. Furthermore, the microanatomy of the midgut wall has been shown to have features of a filter, such as different kinds of pores (diameter 7 and 20 nm) in the basement membrane that are considerably enlarged (to 20 and 40 nm, respectively) after a blood meal (Reinhardt and Hecker, 1973).
NaCN and the inhibitors of ion pumps and carbonic anhydrase inhibited fluid absorption by about 60%. This could be because glycolytic energy production is insensitive to cyanide (cf. Irvine and Phillips, 1971) or due to incomplete inhibition by the other drugs. Based on a more detailed analysis of the results with NaCN, an alternative explanation arises. Fig. 9 shows the average volume of three midgut preparations before and after addition of NaCN during the last phase of fluid absorption. Initially, the rate of fluid absorption is almost equal in the presence and absence of NaCN. However, fluid absorption in the presence of cyanide decreases with decreasing volume (and, thus, pressure) of the midguts and completely ceases in a state when the gut wall appears no longer distended. In contrast, in the absence of NaCN, fluid absorption continues at a somewhat lower rate until the midgut appears almost empty. This behavior is consistent with the assumption that cyanide completely inhibits fluid absorption driven by active transport, but that another mechanism, which is not dependent on oxidative metabolism, drives fluid absorption only at higher midgut volumes when the midgut wall is distended. Because addition of NaCN decreased the rates of fluid absorption by approximately 60% (see Figs 2 and 4) and assuming that NaCN does not affect the elasticity of the midgut wall, we conclude that about 60% of fluid absorption might be driven by active solute absorption and about 40% by pressure filtration.
Fig. 9.
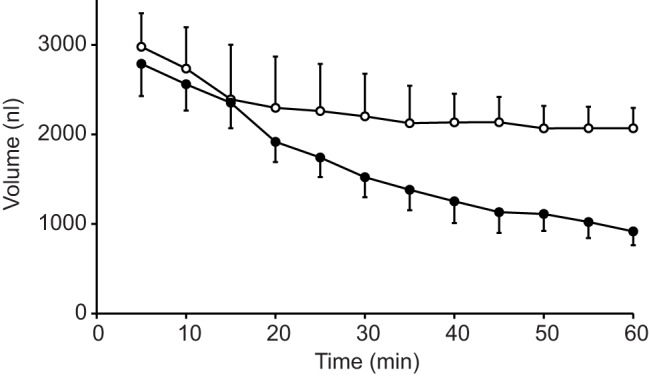
Time course of the effects of NaCN on volume of the posterior midgut. Values are mean (±s.e.m.) volume of three posterior midgut preparations of female mosquitoes filled with mosquito saline and bathed with mosquito saline plus theophylline (10 mmol l−1) in the presence (open circles) and absence (filled circles) of 5 mmol l−1 NaCN. The graph depicts the last 55 min of the three experiments.
When the midguts were filled with buffered NaCl (154 mmol l−1; in the presence of mosquito saline with theophylline in the bath), the rates of fluid absorption were significantly higher than with mosquito saline on both sides of the midgut epithelium. On first sight this may be surprising, because the NaCl solution is considerably hyperosmotic compared with mosquito saline, which should osmotically drive water from the bath into the midgut lumen. However, this situation is more similar to the situation in the female mosquito midgut after a blood meal, because the plasma of a mammal is indeed hyperosmotic to the mosquito hemolymph (cf. Williams et al., 1983). In this situation, a third potential mechanism of fluid absorption must be considered. Because the NaCl concentration in the midgut lumen is almost twice as high as in the bath with mosquito saline under these conditions, passive diffusion of NaCl could drive fluid absorption by solvent drag.
Inhibitors of transporters (concanamycin A, ouabain, acetazolamide; see Figs 5–8) applied under these conditions resulted (as with NaCN, see above) in reductions in the rates of fluid absorption to approximately 60% of the control rates. Because the control rates of fluid absorption were significantly higher with luminal NaCl than with luminal mosquito saline, it appears that fluid absorption driven by both active and passive processes had increased. This could be explained by an increase of passive processes related to passive NaCl diffusion down the concentration gradient and by an increased active solute absorption based on the increased substrate concentration for active transport, but this hypothesis demands further experimental support. Although it is clear that potassium and organic nutrients must be absorbed from blood meals in vivo, the results with luminal NaCl clearly demonstrate that fluid absorption in the adult female midgut does not require these additional solutes. Future studies are needed to analyze the mechanisms of potassium and nutrient absorption in this tissue.
Rates and mechanisms of active NaCl absorption
From the results discussed above, it is evident that active NaCl absorption is a major driving force for fluid absorption in the isolated midgut of adult female mosquitoes. Assuming that the fluid absorbed is iso-osmotic with the midgut contents, the rate of NaCl absorption can be calculated from the rate of fluid absorption. For the control conditions of the experiment shown in Fig. 5 the rate of fluid absorption (38 nl min−1) results in a rate of NaCl absorption of approximately 6000 µmol min−1, which is very close to the magnitude of NaCl excretion in experiments with whole animals (Williams et al., 1983). When we relate this value to the average surface of the midgut during this phase of the experiment (which, like the midgut volume, can be calculated from the dimensions of the midgut), this computes to over 1500 pmol s−1 cm−2. This is about five times of the highest value of Na+ fluxes measured with the scanning ion electrode technique (SIET; Pacey and O'Donnell, 2014) on isolated, blood-filled mosquito midguts. However, it should be considered that the conditions with blood-filled midguts may be considerably different from those just filled with NaCl. Moreover, SIET may not be an ideal method to determine ion fluxes across an epithelium that absorbs solute and water at the same time.
Concanamycin A (Figs 5 and 6), ouabain (Fig. 7) and acetazolamide (Fig. 8) inhibited fluid absorption and influenced the transepithelial voltage in a way that is consistent with the conclusion that apical V-ATPase (Dröse and Altendorf, 1997; Patrick et al., 2006), basal Na+/K+-ATPase (Skou, 1965; Patrick et al., 2006) and carbonic anhydrase (Maren, 1967; del Pilar Corena et al., 2005) are essential for generating active NaCl absorption that drives fluid absorption in the midgut of adult female mosquitoes. This interpretation is also consistent with the finding that ouabain inhibited Na+ absorption in isolated, blood-filled mosquito midguts (Pacey and O'Donnell, 2014). Bafilomycin (another inhibitor of V-ATPases; Dröse and Altendorf, 1997) did not affect Na+ absorption in the latter study, and acetazolamide was shown to affect H+ fluxes, but its potential effect on Na+ absorption was not studied (Pacey and O'Donnell, 2014). Altogether, it is clear that further studies are required to uncover the complete mechanisms of active NaCl absorption in the adult female mosquito midgut. Using the approach of the present investigation, it is necessary to observe fluid absorption and transepithelial voltage after filling the midgut lumen with NaCl and some inhibitors of the transporters that are potentially involved.
The transepithelial voltage has often been used to analyze active transport across epithelia. The results of the current study are complicated by the observation that the transepithelial voltage changes and even reverses polarity during the experiment. The initially lumen-negative voltage and the observation that ouabain hyperpolarizes the lumen-positive voltage at the end of the experiments (see Fig. 7) indicates that Na+/K+-ATPase drives active cation absorption. The final lumen-positive voltage and the observation that concanamycin polarizes the transepithelial voltage to lumen-negative voltages (see Figs 5 and 6) indicates that V-ATPase drives active anion absorption. The effect of acetazolamide on the transepithelial voltage (see Fig. 8) indicates an influence on the absorption of cations and anions. In summary, however, it is evident that further experiments are required to better understand the mechanisms of NaCl absorption and how they are reflected in the transepithelial voltage and its spontaneous changes.
Regulation of NaCl and fluid absorption
It is well known that post prandial urine production by female adult mosquitoes is controlled by hormones that regulate the volume and composition of the urine (cf. Beyenbach, 2003). The results of the present study demonstrate that isolated midguts have the spontaneous capacity to absorb fluid at rates that are similar to urine production in vivo (cf. Williams et al., 1983). The present study used theophylline, a reagent that is commonly known to increase intracellular cyclic AMP in tissues that are equipped with a baseline activity of adenylate cyclase (Johnson and Nielsen, 1978). It became evident that theophylline significantly affected the magnitude of the lumen-positive transepithelial potential difference (see Fig. 3), which may indicate stimulation of V-ATPase. Cyclic-AMP-stimulated V-ATPase has been observed before in other tissues (e.g. Dames et al., 2006). However, the presence of theophylline did not result in an increase of fluid absorption (see Fig. 3).
Summary and future perspectives
In summary, the results of the present investigation have shown that the isolated posterior midgut of female adult yellow fever mosquitoes spontaneously absorbed fluid at a rate that seems to satisfy the physiological requirements in vivo. Fluid absorption is driven by three mechanisms: pressure filtration, active transport and passive diffusion of solute. Active transport relies on active V-ATPase, Na+/K+-ATPase and carbonic anhydrase. Future experiments are required to uncover the complete mechanisms of active NaCl absorption, to understand the background for the dynamic change of the transepithelial voltage during fluid absorption, to analyze absorption of other components of the blood meal (potassium, amino acids, glucose) and to uncover potential mechanisms that may modulate fluid and solute absorption, and peristaltic activity. The results of the present study underline the viability of the isolated tissue. Although it would be desirable to mount the tissue into an Ussing-type chamber for some of the above future tasks, certain questions could actually be better addressed with the preparation used in the present study.
MATERIALS AND METHODS
Animals
The colonies of yellow fever mosquitoes (Aedes aegypti Linnaeus, Vero Beach strain) at Wagner College and Washington State University were maintained in an incubator/climatized room at 27°C, at a humidity of approximately 80% and in a 14 h:10 h light:dark photoperiod. Adult mosquitoes were held in plastic boxes and had access to sucrose (10%). Adult females were blood-fed on rats or through artificial membranes with human or goat blood. Eggs were collected on wet tissue strips 2–4 days after blood feeding. Eggs were hatched and larvae were maintained in a 1:1 mixture of deionized water and tap water. Ground Tetramin flakes (Tetrawerke, Melle, Germany) were used to feed larvae. Water and food was replaced every day. Pupae were collected and transferred to plastic boxes for emergence of adults. Adult female mosquitoes 3–10 days after emergence were used in the present investigation. Prior to the experiments, the animals were fed only with sucrose.
Solutions and chemicals
The mosquito saline used in the present investigation was based on larval Aedes hemolymph composition (Edwards, 1982a,b) and consisted of (mmol l−1): NaCl, 42.5; KCl, 3; MgCl2, 0.6; CaCl2, 5.0; NaHCO3, 5; succinic acid, 5, malic acid, 5; l-proline, 5; l-glutamine, 9.1; l-histidine, 8.7; l-arginine, 3.3; dextrose, 10; HEPES, 25. The pH was adjusted to 7.0 with NaOH. In some preliminary experiments, we used a saline often used with adult mosquitoes (e.g. Beyenbach et al., 2000, consisting of (mmol l−1): NaCl 150, KCl 3.4, CaCl2 1.7, MgCl2 0.6, NaHCO3 1.8, HEPES 25 and glucose 5, pH 7). However, the Vte of the tested midguts was very low. For this reason, we used the mosquito saline with more nutrients and lower NaCl concentration for the experiments in the present study. Mosquito saline was used to bathe the tissue in all experiments. In some experiments, mosquito saline was used to fill the posterior midgut. However, in most experiments, the posterior midgut was filled with 154 mmol l−1 NaCl buffered with 1 mmol l−1 HEPES, pH 7. Acetazolamide, ouabain and theophylline were directly dissolved in mosquito saline. Concanamycin A was predissolved in dimethylsulfoxide (DMSO). The concentration of DMSO (0.1%) used had no significant effect on Vte. All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Preparations and experimental procedures
Adult female Aedes aegypti were cold anesthetized and transferred into a dish, containing a drop of mosquito saline. Wings and legs were rapidly cut off, and the most posterior abdominal segment was nicked with a pair of tweezers and then gently pulled into the drop of saline. In most cases, this procedure isolated the most posterior abdominal segment together with hindgut, midgut, Malpighian tubules and ovaries. Ovaries and Malpighian tubules were trimmed off, and the gut was transferred into mosquito saline in the experimental chamber.
The experimental chamber consisted of a bath with a volume of 100 µl which was gravity perfused with oxygenated mosquito saline at a rate of 2 ml min−1. After transfer to the bath, the tissue was mounted on an L-shaped perfusion pipette with a tip diameter of approximately 50 µm (for the manufacture of these pipettes, see Onken et al., 2004a,b) in one of two ways. Either the anterior midgut was slipped onto the pipette until the pipette tip reached into the posterior midgut, or the pipette tip was gently inserted through the pyloric valve at the posterior end of the posterior midgut.
After mounting, the midgut was filled with mosquito saline or with buffered NaCl through the pipette either by using a syringe pump (model Aladdin, World Precision Instruments, Sarasota, FL) or a Hamilton syringe (25 µl) mounted into a micro syringe burette (model SB2, Micro Metric Instruments Co., Cleveland, OH). Vte was measured with a voltage clamp (model VCC 600, Physiologic Instruments, San Diego, USA) connected via calomel electrodes and agar bridges to the bath and the perfusion pipette, respectively. The voltage was measured with reference to the bath, digitized using a Labtrax 4 AD converter and analyzed with Data-Trax software (World Precision Instruments, Sarasota, FL).
The volume of the posterior midgut was monitored by taking photographs directly after filling and every 5 min afterwards (see Fig. 1). Photographs were taken with a digital camera (model coolpix L27, Nikon, USA) through an eyepiece with ocular micrometer at a magnification where each unit of the ocular micrometer scale was 40 µm. The midgut volume was calculated as the volume of a symmetrical ellipsoid. In the cases when midguts were filled from a Hamilton syringe, it was possible to compare expanded volume from the syringe with inflated volume of the midgut, and good consistency was determined. Videos of midgut fluid absorption (supplementary material Movie 1) and midgut peristalsis (supplementary material Movie 2) were recorded with an eyepiece camera (model DEC-18, World Precision Instruments, FL, USA) and edited with Microsoft MovieMaker.
Initially, a lumen-negative Vte was measured with each midgut preparation (cf. Figs 2 and 5). Simultaneously with the reduction of the midgut volume (fluid absorption), Vte became less lumen negative and ultimately reached lumen-positive values. When the midgut lumen was empty, Vte peaked and stabilized at a somewhat less-positive value (see Figs 2 and 5). In the Results, we report the voltage change during this phase in the presence or absence of drugs in mV min−1. After a control experiment, a drug was added to the bath. We report the effect of the drug on the lumen-positive Vte at the end of the control experiment (see Figs 2 and 5). When the drug had reached a maximal effect on Vte, the midgut was refilled. Refilling the midgut usually resulted in a rapid change in Vte to more lumen-negative values (see Figs 2 and 5).
Statistics
Differences between means were determined with ANOVA or Student's t-test as appropriate. Statistical significance was assumed for P≤0.5. In the Results section, all data are reported as means±s.e.m.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Both authors have equally contributed to conception, design and execution of the experiments, interpretation of the findings, and drafting and revising the article.
Funding
The work of the authors cited here was supported by the National Institutes of Health [RO1 AI 063463] and a Wagner College faculty research grant to H.O. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.119529/-/DC1
References
- Beyenbach K. W. (2003). Transport mechanisms of diuresis in Malpighian tubules of insects. J. Exp. Biol. 206, 3845-3856. 10.1242/jeb.00639 [DOI] [PubMed] [Google Scholar]
- Beyenbach K. W., Pannabecker T. L. and Nagel W. (2000). Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J. Exp. Biol. 203, 1459-1468. [DOI] [PubMed] [Google Scholar]
- Clements A. N. (1992). The Biology of Mosquitoes. Vol. 1: Development, Nutrition and Reproduction. London: Chapman & Hall. [Google Scholar]
- Dames P., Zimmermann B., Schmidt R., Rein J., Voss M., Schewe B., Walz B. and Baumann O. (2006). cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc. Natl. Acad. Sci. USA 103, 3926-3931. 10.1073/pnas.0600011103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pilar Corena P., VanEkeris L., Salazar M. I., Bowers D., Fiedler M. M., Silverman D., Tu C. and Linser P. J. (2005). Carbonic anhydrase in the adult mosquito midgut. J. Exp. Biol. 208, 3263-3273. 10.1242/jeb.01739 [DOI] [PubMed] [Google Scholar]
- Drake L. L., Boudko D. Y., Marinotti O., Carpenter V. K., Dawe A. L. and Hansen I. (2010). The aquaporin gene family of the yellow fever mosquito, Aedes aegypti. PLoS ONE 5, e15578 10.1371/journal.pone.0015578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröse S. and Altendorf K. (1997). Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200, 1-8. [DOI] [PubMed] [Google Scholar]
- Edwards H. A. (1982a). Ion concentration and activity in the haemolymph of Aedes aegypti larvae. J. Exp. Biol. 101, 143-151. [Google Scholar]
- Edwards H. A. (1982b). Free amino acids as regulators of osmotic pressure in aquatic insect larvae. J. Exp. Biol. 101, 153-160. [Google Scholar]
- Irvine H. B. and Phillips J. E. (1971). Effects of respiratory inhibitors and ouabain on water transport by isolated locust rectum. J. Insect Physiol. 17, 381-393. 10.1016/0022-1910(71)90221-6 [DOI] [Google Scholar]
- Izeirovski S., Moffett S. B., Moffett D. F. and Onken H. (2009). The anterior midgut of larval yellow fever mosquitoes (Aedes aegypti): Effects of amino acids, dicarboxylic acids, and glucose on the transepithelial voltage and strong luminal alkalinization. J. Exp. Zool. 311A, 719-726. 10.1002/jez.561 [DOI] [PubMed] [Google Scholar]
- Johnson A. H. and Nielsen R. (1978). Effects of the antidiuretic hormone, arginine vasotocin, theophylline, filipin and A23187 on cyclic AMP in isolated frog skin epithelium (Rana temporaria). Acta Physiol. Scand. 102, 281-289. 10.1111/j.1748-1716.1978.tb06075.x [DOI] [PubMed] [Google Scholar]
- Klowden M. J. and Lea A. O. (1979). Effect of defensive host behavior on the blood meal size and feeding success of natural populations of mosquitoes (Diptera: Culicidae). J. Med. Entomol. 15, 514-517. 10.1093/jmedent/15.5-6.514 [DOI] [PubMed] [Google Scholar]
- Maren T. H. (1967). Carbonic anhydrase: chemistry, physiology and inhibition. Physiol. Rev. 47, 595-781. [DOI] [PubMed] [Google Scholar]
- Okech B. A., Boudko D. Y., Linser P. J. and Harvey W. R. (2008). Cationic pathway of pH regulation in larvae of Anopheles gambiae. J. Exp. Biol. 211, 957-968. 10.1242/jeb.012021 [DOI] [PubMed] [Google Scholar]
- Onken H. and Moffett D. F. (2009). Revisiting the cellular mechanisms of strong luminal alkalinization in the anterior midgut of larval mosquitoes. J. Exp. Biol. 212, 373-377. 10.1242/jeb.023580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken H., Moffett S. B. and Moffett D. F. (2004a). The transepithelial voltage of the isolated anterior stomach of mosquito larvae (Aedes aegypti): pharmacological characterization of the serotonin-stimulated cells. J. Exp. Biol. 207, 1779-1787. 10.1242/jeb.00964 [DOI] [PubMed] [Google Scholar]
- Onken H., Moffett S. B. and Moffett D. F. (2004b). The anterior stomach of larval mosquitoes (Aedes aegypti): effects of neuropeptides on transepithelial ion transport and muscular motility. J. Exp. Biol. 207, 3731-3739. 10.1242/jeb.01208 [DOI] [PubMed] [Google Scholar]
- Onken H., Moffett S. B. and Moffett D. F. (2006). The isolated anterior stomach of larval mosquitoes (Aedes aegypti): voltage-clamp measurements with a tubular epithelium. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 143, 24-34. 10.1016/j.cbpa.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Onken H., Moffett S. B. and Moffett D. F. (2008). Alkalinization in the isolated and perfused anterior midgut of the larval mosquito, Aedes aegypti . J. Insect Sci. 8, 43 10.1673/031.008.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey E. K. and O'Donnell M. J. (2014). Transport of H+, Na+ and K+ across the posterior midgut of blood-fed mosquitoes (Aedes aegypti). J. Insect Physiol. 61, 42-50. 10.1016/j.jinsphys.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Park S. S. and Shahabuddin M. (2000). Structural organization of posterior midgut muscles in mosquitoes, Aedes aegypti and Anopheles gambiae. J. Struct. Biol. 129, 30-37. 10.1006/jsbi.1999.4208 [DOI] [PubMed] [Google Scholar]
- Patrick M. L., Aimanova K., Sanders H. R. and Gill S. S. (2006). P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J. Exp. Biol. 209, 4638-4651. 10.1242/jeb.02551 [DOI] [PubMed] [Google Scholar]
- Reinhardt C. and Hecker H. (1973). Structure and function of the basal lamina and of the cell junctions in the midgut epithelium (stomach) of female Aedes aegypti L. (Insecta, Diptera). Acta Tropica. 30, 213-236. [PubMed] [Google Scholar]
- Skou J. C. (1965). Enzymatic basis for active transport of Na+ and K+ across the cell membrane. Physiol. Rev. 45, 596-617. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Hagedorn H. H. and Beyenbach K. W. (1983). Dynamic changes in flow rate and composition of urine during the post-bloodmeal diuresis in Aedes aegypti (L.). J. Comp. Physiol. B 153, 257-265. 10.1007/BF00689629 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


