ABSTRACT
Sex differences in longevity may reflect sex-specific costs of intra-sexual competition and reproductive effort. As male rhesus macaques experience greater intrasexual competition and die younger, we predicted that males would experience greater oxidative stress than females and that oxidative stress would reflect sex-specific measures of reproductive effort. Males, relative to females, had higher concentrations of 8-OHdG and malondialdehyde, which are markers of DNA oxidative damage and lipid peroxidation, respectively. Older macaques had lower 8-OHdG levels than younger ones, suggesting that oxidative stress decreases in parallel with known age-related declines in reproductive investment. Among males, a recent period of social instability affected oxidative status: males who attacked others at higher rates had higher 8-OHdG levels. Multiparous lactating females with daughters had higher 8-OHdG levels than those with sons. No differences in antioxidant capacity were found. These results lend initial support for the use of oxidative stress markers to assess trade-offs between reproductive effort and somatic maintenance in primates.
KEY WORDS: Oxidative damage, Antioxidant capacity, Maternal investment, Sex bias, TBARS, 8-OHdG
Summary: Measures of oxidative stress in rhesus macaques reveal more DNA oxidative damage in males than females, in mothers raising daughters rather than sons and show enduring costs of aggression for males.
INTRODUCTION
Reactive oxygen species (ROS) are a normal by-product of aerobic cellular metabolism, but oxidative damage to important biomolecules (including DNA) has been linked to pathological processes, reproduction and aging (Costantini, 2014). Oxidative stress is a potential mediator of the relationship between increased reproduction and decreased lifespan (Selman et al., 2012) and inter-individual variation in oxidative stress can provide important insights into the processes that lead to individual and sex differences in reproduction and mortality (Costantini, 2014; Metcalfe and Alonso-Alvarez, 2010).
In this study, we examined whether measures of oxidative stress can reveal sex-specific costs of reproduction in free-ranging male and female rhesus macaques (Macaca mulatta Zimmermann 1780). Male rhesus macaques have a shorter life span than females (Clutton-Brock and Isvaran, 2007), thus males are expected to exhibit higher levels of oxidative damage than females. However, since males experience higher mortality during the mating season while female mortality is higher during the birth season (Hoffman et al., 2008), sex differences in oxidative damage may depend on the time period in which oxidative stress is measured.
In rhesus macaques, reproductive investment peaks in young adults (6–12 years of age; Dubuc et al., 2014; Hoffman et al., 2010) therefore we predicted that younger adults would show greater oxidative damage than older adults. Among lactating females, we hypothesized that levels of oxidative damage would be negatively associated with offspring age, as the energetic costs of lactation in primates tend to be highest at the beginning of infant life (Emery Thompson et al., 2012). We also predicted higher oxidative damage in mothers with daughters relative to mothers with sons, as interbirth intervals are longer (suggestive of greater maternal investment) following the birth of daughters than following the birth of sons (Maestripieri, 2001). For males, we hypothesized that reproductive effort during the preceding mating season (March–June 2013), measured by mean rate of copulations and mean rate of agonistic interactions won would correlate positively with oxidative damage, as higher mating effort leads to loss of energetic condition (Higham et al., 2011). We also examined whether oxidative status related to male agonistic effort during a period of unusually high social instability (22 July–18 August 2013; supplementary material Fig. S1) to test the prediction that investment in high-intensity agonistic competition compromises male oxidative status. As blood collections could only be made several months after the breeding season (December 2013–February 2014), the intervening period of male social instability might be predicted to exert a greater effect on variation in oxidative stress, as aggressive competition during this period was unusually intense, with rates of agonistic behaviors exceeding those observed during the mating season.
In our study, we characterized individual oxidative status in plasma samples via three assays: two assays to measure oxidative damage (DNA oxidative damage and lipid peroxidation) and one assay to assess antioxidant defenses (total antioxidant capacity). We also examined the potential confounding effect of social status in both sexes, as more dominant males were previously found to have lower plasma lipid peroxidation before the start of the mating season (Georgiev et al., 2015).
RESULTS AND DISCUSSION
We collected blood samples (N=31) from our study subjects during the last 2–3 months before the start of the mating season – a period when females were still lactating and males had not yet started to experience increases in androgens, associated with the start of mating (supplementary material Fig. S1). Lactating females had significantly (17.7%) lower plasma concentrations of 8-OHdG, a marker of DNA oxidative damage, than males (F2,28=6.33, adjusted R2=0.26, P=0.005; effect of male sex: β=0.13±0.06, t=2.2, P=0.04; Fig. 1A). Across both sexes (N=31 individuals; 14 males, 17 females), younger adults had higher levels 8-OHdG than older ones (β=−0.02±0.01, t=−2.3, P=0.03; Fig. 1B). The interaction between age and sex was not significant, suggesting that age had a similar effect on oxidative status in both sexes (F3,27=4.22, adj. R2=0.24, P=0.014; interaction age×sex: β=−0.01±0.02, t=−0.55, P=0.59). Sex had a small effect on concentration of malondialdehyde (MDA), which is a marker of lipid peroxidation (F1,30=5.44, adj. R2=0.12, P=0.03), with males having higher levels than females (β=0.04±0.02, t=2.4, P=0.03; supplementary material Fig. S2A). Adding age to the model, however, worsened its fit (F2,30=2.76, adj. R2=0.10, P=0.08) as age was not significantly associated with MDA (β=0.001±0.002, t=0.35, P=0.73; supplementary material Fig. S2B). Neither age nor sex were associated with total antioxidant capacity, measured in Trolox equivalents (F2,31=1.16, adj. R2=0.01, P=0.33; supplementary material Fig. S3).
Fig. 1.
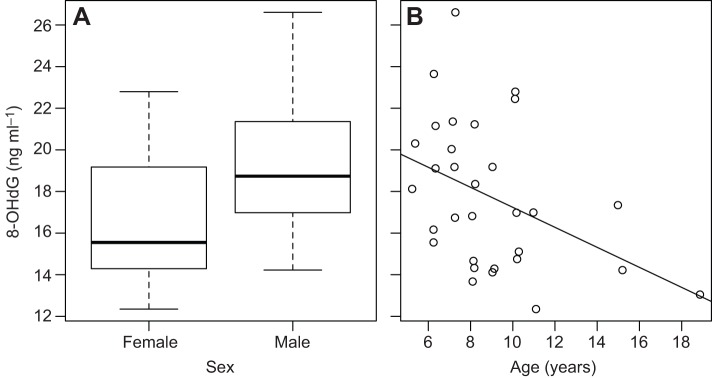
Inter-individual variation in DNA oxidative damage among free-ranging rhesus macaques on Cayo Santiago. Both sex (A) and age (B) independently affected 8-OHdG concentration (an indicator of oxidative damage to DNA). N=31 individuals.
Oxidative status was not related to dominance rank among the females, or among the males (supplementary material Table S1). We thus did not consider the effect of rank further in our analyses. Among lactating females, 8-OHdG levels were not related to offspring age (Kendall rank correlation: τ=0.258, N=17, P=0.16). Mothers with daughters tended to have higher DNA oxidative damage than mothers with sons (W=54, N=17, P=0.08). After excluding the only primiparous mother (who had a son, as well as one of the highest levels of 8-OHdG concentration) from the analysis, the effect of offspring sex became significant: multiparous mothers with daughters (N=9) had significantly higher levels of DNA oxidative damage than multiparous mothers with sons (N=7) (W=53, P=0.02, Fig. 2A). Lipid peroxidation and total antioxidant capacity did not relate to offspring age or to offspring sex (supplementary material Table S1).
Fig. 2.
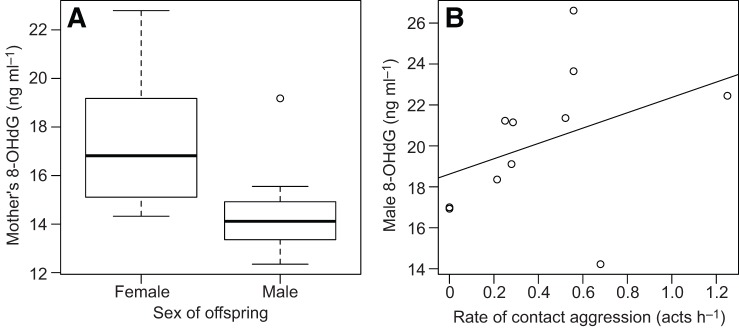
Factors affecting oxidative damage among lactating multi-parous females and adult males. Effect of (A) sex of offspring on oxidative damage among mothers (N=16). (B) Mean rates of contact aggression given during a period of social instability (July–August 2013) on male oxidative damage (N=11 males) measured several months later (December 2013–February 2014). Regression line is for illustrative purposes. Two of the data points in B were offset minimally along the y-axis to avoid over-plotting.
For males, total copulation rates during the 2013 mating season (March–June) did not relate to subsequent levels of 8-OHdG, MDA or total antioxidant capacity (supplementary material Table S1). Male agonistic investment (rate of dominating others and rate of attacking others) during the mating season was not related to any measure of subsequent oxidative status (supplementary material Table S1). Male rates of dominating others during the period of social instability following the overthrow of the alpha male did not correlate with subsequent oxidative status (supplementary material Table S1). Males who physically attacked others at higher rates, however, exhibited higher subsequent 8-OHdG levels (τ=0.5, N=11, P=0.041, Fig. 2B) but not lipid peroxidation or total antioxidant capacity (supplementary material Table S1).
The finding that males had higher levels of 8-OHdG and MDA than lactating mothers supports the hypothesis that sex differences in life span reflect weaker selection on longevity among males in polygynous species (Clutton-Brock and Isvaran, 2007). In rhesus macaques, direct male competition is less pronounced than in many polygynous primates, yet male mortality peaks during the breeding season (Hoffman et al., 2008) and energy availability appears to constrain mating effort (Higham et al., 2011). Genetic data reveal significant, though moderate, reproductive skew, with lifetime reproductive success better predicted by variation in reproductive rate than longevity (Dubuc et al., 2014). Because of constraints imposed by the timing of trapping, both males and females were sampled during the latter part of the birth season, when lactating females were experiencing high reproductive costs (Hoffman et al., 2008). The fact that oxidative damage remained higher in males than in females even during the birth season illustrates that physiological costs of intra-sexual competition for males are significant year round, even in the absence of pronounced direct competition.
In our study, the greater levels of 8-OHdG in younger adults are consistent with the higher reproductive rates experienced in this age class, particularly among males (Dubuc et al., 2014). A similar relationship between age and oxidative damage measured during the non-mating season has been shown among male mandrills, Mandrillus sphinx (Beaulieu et al., 2014). Young adult males may face additional costs as a result of migration out of their natal group. Finally, the youngest animals in our dataset are probably still undergoing somatic growth and this may also contribute to the negative correlation between age and oxidative damage (Nussey et al., 2009). We cannot, however, exclude the possibility of phenotypic correlations, such that the few individuals surviving past the age of 12 may have been those who were best able to avoid or counter oxidative damage.
Multiparous females with daughters had higher levels of 8-OHdG than mothers of sons. This is consistent with maternal investment being higher in daughters than in sons in rhesus macaques (Maestripieri, 2001). The only primiparous mother in our sample had one of the highest levels of 8-OHdG, which is consistent with the observation that first-time mothers experience higher costs of reproduction than multiparous females (Bercovitch et al., 1998; Hinde, 2007).
Among males, rates of copulation and dominance interactions during the preceding mating season did not correlate with oxidative status measured several months later. As oxidative status could only be assessed several months following the mating season, males may have recovered from the somatic damage of the mating season in preparation for the next. Nevertheless, we found that the rate at which males physically attacked others during the month following the overthrow of the alpha male, a period temporarily closer to blood sample collection, was related positively to 8-OHdG levels. Unusually, rates of contact aggression given during this socially unstable month were more than twice as high than during mating season (supplementary material Fig. S4). Considering the males, for which we measured 8-OHdG levels (N=11), total rates of dominating other individuals in decided agonistic interactions were somewhat higher during the period of social instability but not significantly so (Wilcoxon signed rank test: V=11, N=11, P=0.05). Male rates of physically attacking others during the period of social instability, however, were significantly higher than during the mating season (V=10, N=11, P=0.04; supplementary material Fig. S4). Such intense agonistic engagement was likely more salient in affecting the overall condition of males than the competition they experienced during the mating season several months earlier. Indirect measures of social instability have also been linked to increased oxidative damage among high-ranking male mandrills (Beaulieu et al., 2014). Taken together, these findings suggest that intense male–male competition during periods of social instability may result in significant physiological costs, the recovery from which can take more than a few months.
Oxidative stress may result from an increase in production of potentially damaging reactive oxygen species (ROS) and/or a decreased ability to resist damage via antioxidants. In our study, differences in oxidative damage between and within the sexes were most likely related to the former, as levels of antioxidant capacity did not vary with any of the variables we investigated. This is consistent with the high metabolic costs of activities such as lactation and aggression. Additionally, where null effects on oxidative damage were found, as in the case of dominance rank, we can also tentatively conclude that this was not because individuals producing more oxygen radicals were simply more effective at neutralizing them.
Finally, it is worth noting that only one of the results – that males have higher oxidative damage than females – was corroborated by both markers of oxidative damage used in this study: 8-OHdG and MDA. Similar to others (Sharick et al., 2014), we suggest that assessing oxidative damage via multiple bio-markers may provide a more complete, albeit more difficult to interpret, picture of variation in individual oxidative status.
In conclusion, in rhesus macaques, a moderately polygynous species, sex differences in the costs of reproduction are reflected in the pattern of 8-OHdG concentration, a marker of DNA oxidative damage. Within-sex variation in oxidative stress was also associated, to some extent, with variation in maternal investment and in male agonistic effort. This provides promising evidence to compel future work in primates to track changes in oxidative status longitudinally to allow a more detailed, proximate examination of how inter- and intra-individual variation in reproductive effort may relate to oxidative costs.
MATERIALS AND METHODS
Study subjects
We studied the free-ranging, provisioned rhesus macaques on Cayo Santiago, a small island off the coast of Puerto Rico. All experimental procedures were approved by IACUC of the University of Puerto Rico (protocol no. A0100108). Our study group, Group S, numbered 133 individuals including 15 adult males (≥5 years old) and 42 adult females (≥3 years old) during the mating season (28 of which gave birth during the latter part of the study), the remainder being non-reproducing infants, juveniles, and adolescents. We analyzed samples from a total of 34 subjects (17 adult males, 17 lactating females). Sample sizes differ for the various analyses, as not all plasma samples produced reliable readings across the three assays of oxidative status (exclusion criteria for each assay described below). Female subjects had a mean±s.e. age of 9.4±0.9 (range 5.4–18.9) years; and males, 8.6±0.7 (range 5.2–15.2) years. The offspring of female subjects had a mean age of 99.6±10.6 (range 32–174) days.
Behavioral data collection
Methods for behavioral sampling are described in detail elsewhere (Georgiev et al., 2015). Briefly, to calculate male copulation rates and rates of agonistic behaviors, we conducted 5 min focal follows of all adult males in the study group on a random, rotating basis multiple times a day between approximately 07:30 h and 14:00 h. Focal data (430.8 h) for calculating behavioral rates were available for the duration of the mating season (March–June 2013) and for part of the birth season (through to 20 August 2013). Additional observations on male–male agonistic interactions were collected at the group level via all-occurrence sampling (i.e. not via focal animal sampling) in October and November 2013 to monitor any changes in the dominance hierarchy following the overthrow of the alpha male, which occurred around 21 July.
Dominance rank calculations
Male dominance ranks were calculated on the basis of decided dyadic agonistic interactions observed throughout the study. Ranks were calculated via the I&SI method in the DomiCalc package (Schmid and de Vries, 2013) separately for the period before (which included the mating season) and for the period after (during the birth season) the overthrow of the alpha male.
Females were classified as high ranking or low ranking on the basis of existing knowledge of dominance relations between matrilines and individual females. Female rankings in rhesus macaques are maternally inherited (Berman, 1980) and are usually stable over multiple years. All members of a more dominant matriline outrank all members of a less-dominant matriline (reviewed by Berman, 2015). Matrilineal rank is thus a meaningful proxy of individual rank within the group (e.g. Blomquist et al., 2011). In categorizing matrilines as high ranking (the top two matrilineal clusters in the study group) or low ranking (all other females, not directly related to the high-ranking clusters) we relied on outcomes from decided female–female agonistic interactions collected in Group S in 2012 (Mandalaywala et al., 2014). Additionally, we confirmed that inter-matriline dominance relationships established from those data remained unchanged from observations collected by Krishna Balasubramaniam and colleagues in 2013 (Balasubramaniam et al., 2014). Eight of our female study subjects were classified as high-ranking females and 9 as low ranking.
Animal trapping procedure and blood sample collection
The annual trapping season of the Caribbean Primate Research Center (CPRC) on Cayo Santiago ran from December 2013 to February 2014. Subjects of this study were captured between 4 December 2013 and 5 February 2014. Adult males and females with infants were caught in the morning, between 08:00 h and 12:00 h, and sedated with ketamine (∼10 mg kg–1 via intramuscular injection). Blood samples were collected from the femoral vein by veterinary technicians from CPRC. Plasma was separated, flash-frozen and kept on dry ice immediately after centrifugation. Within several hours, samples were transferred to a −80°C freezer. They were later transported on dry ice to The University of New Mexico, where we performed all assays in July 2014.
Oxidative stress assays
Increases in oxidative stress can be harmful to the organism and may result either from increased production of reactive species (which leads to oxidative damage) or from a decrease in antioxidant protection (Costantini, 2014; Finkel and Holbrook, 2000). A variety of measures of oxidative stress have been used in the biomedical field (Halliwell and Whiteman, 2004; Kadiiska et al., 2005) and in ecological research (Costantini, 2008; Hõrak and Cohen, 2010; Monaghan et al., 2009; Speakman and Garratt, 2014). In this study, we quantified both oxidative damage (DNA oxidative damage and lipid peroxidation) and antioxidant capacity in rhesus macaque plasma samples.
To quantify DNA oxidative damage, we measured the concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG). 8-OHdG is the most common product resulting from DNA lesions in the nucleus and the mitochondria induced by free radicals (Valavanidis et al., 2009). This marker is widely used to assess oxidative stress and carcinogenesis, in particular when evaluating the risk of various cancers and degenerative diseases (Evans et al., 2004; Wu et al., 2004). We used the commercially available ‘New 8-OHdG Check’, an enzyme-linked immunosorbent assay (KOG-200S/E, Japan Institute for the Control of Aging/Genox Corp.). Assays were performed following the manufacturer's instruction. We excluded one extreme male outlier and two other male samples which had coefficients of variation (CV) >15% in duplicate determinations. Mean intra-assay CV for the remaining 31 samples (17 females; 14 males) was 4.8%.
As a second measure of oxidative damage, we quantified plasma lipid peroxidation (Del Rio et al., 2005; Niki, 2009) and specifically thiobarbituric acid reactive substances (TBARS), measured in malondialdehyde (MDA) concentration equivalents (nmol ml−1). We used a commercially available calorimetric assay kit (OXItek TBARS assay kit; ZaptoMetrix Corporation 0801192), which has been successfully applied to quantify oxidative status in ecological (Alonso-Alvarez et al., 2010; Bergeron et al., 2011; Torres and Velando, 2007) and clinical (Basu et al., 2014) research. While TBARS assays have been criticized for lack of specificity to MDA (Hõrak and Cohen, 2010; Monaghan et al., 2009) the assay we employed used an acid precipitation step that minimizes interfering substances. One male sample had a CV over 15% and was excluded from the dataset. Mean CV for the remaining samples was 3.3% (N=33 samples; 16 males, 17 females).
Finally, we quantified circulating non-enzymatic antioxidant protection (total antioxidant capacity) with an antioxidant assay kit (Cayman Chemical Company, item no. 709001) in millimolar Trolox equivalents following the manufacturer's instructions. This assay has also been widely used in clinical practice (e.g. Lorente et al., 2015). Mean CV of assay values was 3.3% (N=34 samples; 17 males, 17 females).
Statistics
We log10-transformed values of oxidative status assays before parametric analysis to ensure normality of residual distribution. We fitted three separate multiple regression models with each of the three oxidative stress measures as dependent variables, and individual age and sex as predictors (N=31–34 samples). Initially we also added the interaction between sex and age but as it was not significant, we reported the main effects removing the interaction from the model. Because of the small sample size and associated low statistical power, we conducted bivariate nonparametric analysis for the male (N=11–14) and female (N=16–17) datasets separately. Nonparametric tests were conducted using untransformed assay values. All tests were two-tailed and significance set at 0.05.
Supplementary Material
Acknowledgements
We thank Angelina Ruiz-Lambides and the staff of the CPRC for assistance in data collection and access to long-term demographic data. Field assistance was provided by Samuel Schulte, Diana Christie, Duncan Schulte, Amaury Michel, Stacey McCarthy and Gabriel Stump. Sean Coyne, Michelle Evans, Christine Fleener, Aneila Hogan, Jesus Madrid, Krista Milich, Kevin Rosenfield and Richelle Scales provided additional support. We also thank Krishna Balasubramaniam for sharing female dominance data. Two anonymous reviewers provided helpful comments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.V.G. and D.M. conceived the study. A.V.G. and T.M.M. collected data and samples. A.V.G., M.E.T. and T.M.M. analyzed data/samples. A.V.G. wrote a first draft of the manuscript and all authors contributed to revisions.
Funding
The CPRC is supported by the National Institutes of Health [8 P40 OD012217], the National Center for Research Resources (NCRR) and the Office of Research Infrastructure Programs (ORIP). This study was funded, in part, by NIH [R01-HD067175] to D.M. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of NCRR, ORIP or the NIH. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.121947/-/DC1
References
- Alonso-Alvarez C., Pérez Rodríguez L., García J. T., Viñuela J. and Mateo R. (2010). Age and Breeding Effort as Sources of Individual Variability in Oxidative Stress Markers in a Bird Species. Physiol. Biochem. Zool. 83, 110-118. 10.1086/605395 [DOI] [PubMed] [Google Scholar]
- Balasubramaniam K. N., Dunayer E. S., Gilhooly L. J., Rosenfield K. A. and Berman C. M. (2014). Group size, contest competition, and social structure in Cayo Santiago rhesus macaques. Behaviour 151, 1759-1798. 10.1163/1568539X-00003216 [DOI] [Google Scholar]
- Basu S., De D., Khanna H. D. and Kumar A. (2014). Lipid peroxidation, DNA damage and total antioxidant status in neonatal hyperbilirubinemia. J. Perinatol. 34, 519-523. 10.1038/jp.2014.45 [DOI] [PubMed] [Google Scholar]
- Beaulieu M., Mboumba S., Willaume E., Kappeler P. M. and Charpentier M. J. E. (2014). The oxidative cost of unstable social dominance. J. Exp. Biol. 217, 2629-2632. 10.1242/jeb.104851 [DOI] [PubMed] [Google Scholar]
- Bercovitch F. B., Lebron M. R., Martinez H. S. and Kessler M. J. (1998). Primigravidity, body weight, and costs of rearing first offspring in rhesus macaques. Am. J. Primatol. 46, 135-144. [DOI] [PubMed] [Google Scholar]
- Bergeron P., Careau V., Humphries M. M., Réale D., Speakman J. R. and Garant D. (2011). The energetic and oxidative costs of reproduction in a free-ranging rodent. Funct. Ecol. 25, 1063-1071. 10.1111/j.1365-2435.2011.01868.x [DOI] [Google Scholar]
- Berman C. M. (1980). Early agonistic experience and rank acquisition among free-ranging infant rhesus monkeys. Int. J. Primatol. 1, 153-170. 10.1007/BF02735595 [DOI] [Google Scholar]
- Berman C. M. (2015). Primate kinship: Contributions from Cayo Santiago. Am. J. Primatol. 10.1002/ajp.22383. [DOI] [PubMed] [Google Scholar]
- Blomquist G. E., Sade D. S. and Berard J. D. (2011). Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta). Int. J. Primatol. 32, 193-208. 10.1007/s10764-010-9461-z [DOI] [Google Scholar]
- Clutton-Brock T. H. and Isvaran K. (2007). Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B Biol. Sci. 274, 3097-3104. 10.1098/rspb.2007.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D. (2014). Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology. Berlin; Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Costantini D. (2008). Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238-1251. 10.1111/j.1461-0248.2008.01246.x [DOI] [PubMed] [Google Scholar]
- Del Rio D., Stewart A. J. and Pellegrini N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15, 316-328. 10.1016/j.numecd.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Dubuc C., Ruiz-Lambides A. and Widdig A. (2014). Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behav. Ecol. 25, 878-889. 10.1093/beheco/aru052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M., Muller M. N. and Wrangham R. W. (2012). The energetics of lactation and the return to fecundity in wild chimpanzees. Behav. Ecol. 23, 1234-1241. 10.1093/beheco/ars107 [DOI] [Google Scholar]
- Evans M. D., Dizdaroglu M. and Cooke M. S. (2004). Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res. Rev. Mutat. Res. 567, 1-61. 10.1016/j.mrrev.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Finkel T. and Holbrook N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408, 239-247. 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Georgiev A. V., Muehlenbein M. P., Prall S. P., Emery Thompson M. and Maestriperi D. (2015). Male quality, dominance rank, and mating success in free-ranging rhesus macaques. Behav. Ecol. 26, 763-772. 10.1093/beheco/arv008 [DOI] [Google Scholar]
- Halliwell B. and Whiteman M. (2004). Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 142, 231-255. 10.1038/sj.bjp.0705776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J. P., Heistermann M. and Maestripieri D. (2011). The energetics of male–male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Anim. Behav. 81, 1001-1007. 10.1016/j.anbehav.2011.02.001 [DOI] [Google Scholar]
- Hinde K. (2007). First-time macaque mothers bias milk composition in favor of sons. Curr. Biol. 17, R958-R959. 10.1016/j.cub.2007.09.029 [DOI] [PubMed] [Google Scholar]
- Hoffman C. L., Higham J. P., Mas-Rivera A., Ayala J. E. and Maestripieri D. (2010). Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behav. Ecol. 21, 972-978. 10.1093/beheco/arq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. L., Ruiz-Lambides A. V., Davila E., Maldonado E., Gerald M. S. and Maestripieri D. (2008). Sex differences in survival costs of reproduction in a promiscuous primate. Behav. Ecol. Sociobiol. 62, 1711-1718. 10.1007/s00265-008-0599-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hõrak P. and Cohen A. (2010). How to measure oxidative stress in an ecological context: methodological and statistical issues. Funct. Ecol. 24, 960-970. 10.1111/j.1365-2435.2010.01755.x [DOI] [Google Scholar]
- Kadiiska M. B., Gladen B. C., Baird D. D., Germolec D., Graham L. B., Parker C. E., Nyska A., Wachsman J. T., Ames B. N., Basu S., et al. (2005). Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Rad. Biol. Med. 38, 698-710. 10.1016/j.freeradbiomed.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Lorente L., Martín M. M., Almeida T., Abreu-González P., Ferreres J., Solé-Violán J., Labarta L., Díaz C. and Jiménez A. (2015). Association between serum total antioxidant capacity and mortality in severe septic patients. J. Crit. Care 30, 217.e7-217.e12. 10.1016/j.jcrc.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Maestripieri D. (2001). Female-biased maternal investment in rhesus macaques. Folia Primatol. 72, 44-47. 10.1159/000049920 [DOI] [PubMed] [Google Scholar]
- Mandalaywala T. M., Higham J. P., Heistermann M., Parker K. J. and Maestripieri D. (2014). Physiological and behavioural responses to weaning conflict in free-ranging primate infants. Anim. Behav. 97, 241-247. 10.1016/j.anbehav.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe N. B. and Alonso-Alvarez C. (2010). Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984-996. 10.1111/j.1365-2435.2010.01750.x [DOI] [Google Scholar]
- Monaghan P., Metcalfe N. B. and Torres R. (2009). Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75-92. 10.1111/j.1461-0248.2008.01258.x [DOI] [PubMed] [Google Scholar]
- Niki E. (2009). Lipid peroxidation: Physiological levels and dual biological effects. Free Rad. Biol. Med. 47, 469-484. 10.1016/j.freeradbiomed.2009.05.032 [DOI] [PubMed] [Google Scholar]
- Nussey D. H., Pemberton J. M., Pilkington J. G. and Blount J. D. (2009). Life history correlates of oxidative damage in a free-living mammal population. Funct. Ecol. 23, 809-817. 10.1111/j.1365-2435.2009.01555.x [DOI] [Google Scholar]
- Schmid V. S. and de Vries H. (2013). Finding a dominance order most consistent with a linear hierarchy: an improved algorithm for the I&SI method. Anim. Behav. 86, 1097-1105. 10.1016/j.anbehav.2013.08.019 [DOI] [PubMed] [Google Scholar]
- Selman C., Blount J. D., Nussey D. H. and Speakman J. R. (2012). Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 27, 570-577. 10.1016/j.tree.2012.06.006 [DOI] [PubMed] [Google Scholar]
- Sharick J. T., Vazquez-Medina J. P., Ortiz R. M. and Crocker D. E. (2014). Oxidative stress is a potential cost of breeding in male and female northern elephant seals. Funct. Ecol. 29, 367-376. 10.1111/1365-2435.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J. R. and Garratt M. (2014). Oxidative stress as a cost of reproduction: Beyond the simplistic trade-off model. Bioessays. 36, 93-106. 10.1002/bies.201300108 [DOI] [PubMed] [Google Scholar]
- Torres R. and Velando A. (2007). Male reproductive senescence: the price of immune-induced oxidative damage on sexual attractiveness in the blue-footed booby. J. Animal Ecol. 76, 1161-1168. 10.1111/j.1365-2656.2007.01282.x [DOI] [PubMed] [Google Scholar]
- Valavanidis A., Vlachogianni T. and Fiotakis C. (2009). 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health 27, 120-139. 10.1080/10590500902885684 [DOI] [PubMed] [Google Scholar]
- Wu L. L., Chiou C.-C., Chang P.-Y. and Wu J. T. (2004). Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clinica Chimica Acta 339, 1-9. 10.1016/j.cccn.2003.09.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


