ABSTRACT
F-BAR proteins are known to participate in cytokinesis, but their mechanisms are not well understood. Here we investigated Rga7p, an Schizosaccharomyces pombe F-BAR protein with a RhoGAP domain. Localization of Rga7p to the cytokinetic cleavage furrow depends on its F-BAR domain, actin filaments, the formins Cdc12p and For3p, and the presence of a contractile ring. Rga7p is not required for the constriction of the contractile ring but does participate in the transport of a β-glucan synthetase (Bgs4p) from the late Golgi compartments to plasma membrane that is adjacent to the contractile ring. Cells without Rga7p moved Bgs4p normally from the poles to the Golgi complex near to the cell center, but Bgs4p then moved slowly from the late Golgi compartments to the cleavage site. The late arrival and lower than normal numbers of Bgs4p result in septal defects late in cytokinesis, and in the lysis of separating cells, similar to that in cells with mutations in the cwg1+ gene (which encodes Bgs4p).
KEY WORDS: Bgs4, Cell wall, Cytokinesis, F-BAR proteins, Golgi complex, S. pombe
Summary: F-BAR protein Rga7p is required for the timely delivery of a β-glucan synthetase (Bgs4p) to the cleavage furrow during cytokinesis of fission yeast.
INTRODUCTION
Eukaryotic cells divide by assembling and constricting a contractile ring of actin filaments and myosin motors that is attached to the plasma membrane at the cell center (Glotzer, 2005; Pollard, 2010). Completion of cytokinesis is coupled to the secretory pathway, which provides new cell membrane and proteins of the extracellular matrix (ECM) (Xu and Vogel, 2011). When cells have defects in secretion of ECM, contractile rings can retract before completion, resulting in multinucleate cells (Xu and Vogel, 2011). Yeast cells assemble an extracellular septum that primarily comprises β-D-glucan and α-D-glucan polymers (Cortes et al., 2012; Humbel et al., 2001) that is essential for the contractile ring constriction (Arasada and Pollard, 2014; Liu et al., 1999; Proctor et al., 2012; Sipiczki and Bozsik, 2000). A signaling pathway called the septation initiation network (SIN) couples contractile ring constriction with septum assembly (Simanis, 2015).
The enzyme α-glucan synthetase 1, Ags1p, is the major contributor of α-D-glucans in the primary and the secondary septum in fission yeast (Cortes et al., 2012). Cells with mutations in mok1+/ags1+ lyse during cell separation at the end of cytokinesis.
Schizosaccharomyce pombe has four transmembrane enzymes that synthesize β-D-glucans – Bgs1p, Bgs3p and Bgs4p are required for cell wall integrity during vegetative growth, and Bgs2p is required during spore formation (Cortes et al., 2005, 2002, 2007; Liu et al., 2000, 2002, 1999; Martin et al., 2003, 2000). Bgs1p, Bgs3p and Bgs4p appear to contribute at different stages of septum formation. Bgs1p is the first of these enzymes to be recruited to the cleavage site and synthesizes the primary septum (Cortes et al., 2005, 2007; Martin et al., 2003). Mutations in the cps1+/bgs1+ gene cause cytokinesis defects (Liu et al., 1999; Cortes et al., 2007); ∼40% of contractile rings are poorly anchored so they slide to one end of the cell, and some contractile rings fail to constrict (Arasada and Pollard, 2014). Bgs4p accumulates at the cleavage furrow after the synthesis of the primary septum begins and is essential for closure of the primary septum (Munoz et al., 2013). In a large fraction of cells with mutations in bgs4+/cwg1+, growth of the primary septum is uncoupled from the ingressing plasma membrane and constricting contractile ring. Bgs4p also appears to contribute to the formation of the secondary septum because mutant daughter cells frequently lyse as they separate (Cortes et al., 2005; Munoz et al., 2013).
Cdc15p, a protein with an F-BAR domain, is important for the transport of Bgs1p to the cleavage furrow. Cells depleted of Cdc15p transport Bgs1p to the cleavage site more slowly than normal, resulting in the sliding of assembled contractile rings and a delay in the onset of the contractile ring constriction (Arasada and Pollard, 2014). Three other proteins with F-BAR domains localize to the cleavage furrow of S. pombe, of which two appear to participate in cytokinesis – Imp2p has an SH3 domain, and Rga7p and Rga8p each contain a Rho GTPase-activating protein (RhoGAP) domain (Arasada and Pollard, 2011; Demeter and Sazer, 1998; Martin-Garcia et al., 2014; Roberts-Galbraith et al., 2009; Soto et al., 2010). Mutant Δrga8 cells have no cytokinesis defects (Yang et al., 2003), so Rga8p has no established function during cytokinesis. Deletion mutations show that Imp2p and Rga7p appear to perform different functions during cytokinesis – Δimp2 cells are multi-septated (Demeter and Sazer, 1998), whereas Δrga7 cells appear to lyse near the end of cytokinesis (Martin et al., 2003; Soto et al., 2010). Martin-Garcia and colleagues have recently investigated the role of Rga7p in the stability of the contractile ring, cell separation and septation, but interesting questions remain about its roles in cytokinesis (Martin-Garcia et al. 2014).
Here, we have used quantitative fluorescence microscopy to characterize Δrga7 cells and discovered that the septal defects result from slow transfer of Bgs4p from late Golgi compartments to plasma membrane that is adjacent to the contractile ring. Assembly of Bgs1p in the cleavage furrow appears to be normal in cells lacking Rga7p.
RESULTS
Rga7p is required for septum integrity
Rga7p is a non-essential protein with N-terminal F-BAR and C-terminal RhoGAP domains. To understand its function, we replaced the entire open reading frame of the rga7+ gene with either an ura4+ or a cassette encoding G418 resistance (kanMX6). Both Δrga7 strains were viable at 25°C and 36°C but grew slowly (Fig. 1A) owing to lysis of many cells (Fig. 1B). Many more cells lysed at 36°C than at 25°C (Fig. 1B). Time-lapse imaging at 25°C (Fig. 1C,D) showed that lysis and the release of cytoplasmic contents occurred after septum formation as the daughter cells separated, resulting in the death of either one or both of the daughter cells (Fig. 1C). A few partially lysed Δrga7 cells recovered and continued growing (Fig. 1C,E).
Fig. 1.
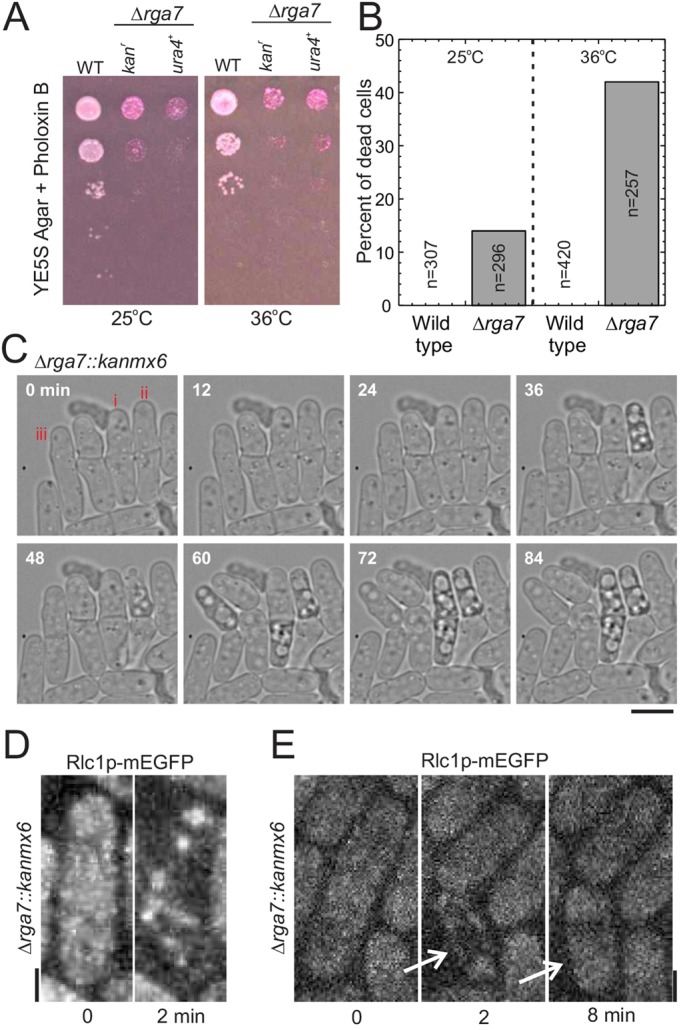
Rga7p is required for successful cell separation. (A) Growth of wild type and two deletion strains with the rga7+ gene replaced with either a kanMX6 or an ura4+ cassette. Cultures of 2×107 cells/ml were serially diluted 10-fold in YE5S medium; 5 µl samples were spotted onto YE5S agar plates supplemented with Phloxin B and grown for 3 days at 25°C or 36°C. (B) Percentage of dead cells in cultures of wild-type cells (open bars) and Δrga7 cells (grey bars) grown in YE5S at 25°C or shifted to 36°C for 3 h. (C) Time-lapse differential interference contrast images of Δrga7 cells dividing on 25%-gelatin pads in EMM5S medium at 25°C. Cells with different fates were labeled – (i) both daughter cells lysed during cell separation, (ii) one of two daughter cells lysed during cell separation, and (iii) one daughter cell lysed during cell separation but recovered and continued growing. (D,E) Maximum intensity projections of fluorescence micrographs showing the fates of Δrga7 cells expressing Rlc1p–mEGFP at the end of cytokinesis. (D) Both daughter cells lyse with the loss of cytoplasmic fluorescence at the end of cell separation. (E) One of the two daughter cells (indicated by arrows) lyses at the end of cell separation (2 min) but then recovers (8 min). Scale bars: 2 µm.
Localization of Rga7p
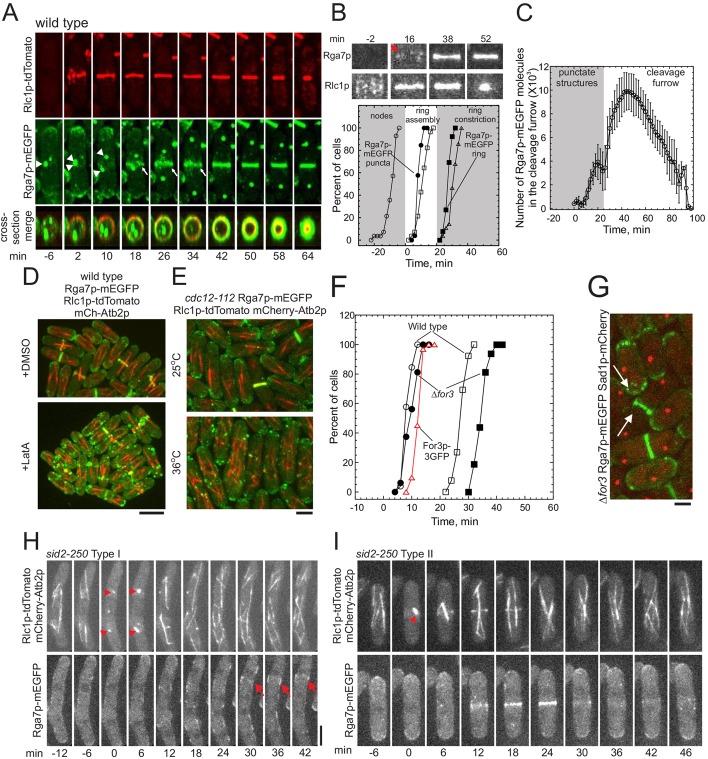
Rga7p tagged with monomeric enhanced green fluorescent protein (Rga7p–mEGFP) concentrates at the poles of interphase cells and at the division site during cytokinesis when expressed from the endogenous promoter at the native locus (Arasada and Pollard, 2011). Closer examination of mitotic cells revealed that Rga7p–mEGFP first localized to punctate, cytoplasmic structures near the cell center before concentrating in the cleavage furrow (Fig. 2A). To determine the timing of these events, we expressed mCherry-tagged α-tubulin and defined the appearance of spindle microtubules between the duplicated spindle pole bodies as cell cycle time 0 min. The appearance of punctate Rga7p–mEGFP structures in the cytoplasm at time 8 min coincided with the formation of a contractile ring by coalescence of nodes marked with Rlc1p–tdTomato, the regulatory light chain 1 of Myo2p (type II myosin) (Fig. 2B). Rga7p–mEGFP began to concentrate in the cleavage furrow as the contractile ring began to constrict at time 27 min (Fig. 2B, lower panel). As the contractile ring constricted, 10,000 Rga7p–mEGFP molecules (Fig. 2C) formed a disc-shaped structure in the cleavage furrow (Fig. 2A).
Fig. 2.
Localization of Rga7p in S. pombe across the cell cycle. All of this data was obtained by using maximum or sum intensity projections of stacks of up to 20 confocal z-sections collected every 2 min for 2 h. Cell cycle time 0 denotes the separation of spindle pole bodies, marked directly with Sad1p–mCherry or indirectly by the appearance of spindle microtubules marked with mCherry–Atb2p. (A) Time series of maximum-intensity-projected fluorescence micrographs at 8-min intervals of a wild-type cell expressing Rlc1p–tdTomato, Sad1p–GFP and Rga7p–mEGFP at 25°C. (Upper panels) Rlc1p–tdTomato, (middle panels) Sad1p–GFP and Rga7p–mEGFP, (lower panel) merged cross-section of the middle of the cell. Arrows mark Rga7p punctate structures, and arrowheads mark the separation of the spindle pole bodies. (B) Top, time series of micrographs of a 35×27-pixel region of interest (ROI) around the centers of wild-type cells expressing either (top) Rga7p–mEGFP or (bottom) Rlc1p–tdTomato during cytokinesis. Times are minutes after spindle pole body separation. Arrowhead marks Rga7p punctate structures. Graph shows time courses of cytokinesis events in wild-type cells determined from the maximum-intensity-projected time-lapse movies. (Open symbols) Cells expressing Rlc1p–mEGFP and Sad1p–mCherry: (Ο, n=18) appearance of Rlc1p–mEGFP in nodes; (☐, n=18) completion of contractile rings; and (▵, n=18) onset of ring constriction. (Filled symbols) Cells expressing Rga7p–mEGFP and Sad1p–mCherry: (●, n=33) appearance of punctate Rga7p structures at the cell center; and (■, n=33) concentration of Rga7p in an equatorial ring. (C) Average numbers (±1 s.d.) of Rga7p–mEGFP molecules at the equators of wild-type cells expressing Rga7p–mEGFP and Sad1p–mCherry. Total numbers of molecules were calculated from measurements of mEGFP fluorescence in sum-projections of 20 confocal z-slices in two ways: (grey region) when Rga7p–mEGFP was concentrated in punctate structures near to the cell center, the ROI was a 22×30-pixel rectangle in the middle of the cell; or (white region) when Rga7p–mEGFP was concentrated in rings and furrows, the ROI was a 7×30-pixel rectangle centered on the equator. (D) Wild-type cells expressing (green) Rga7p–mEGFP, (red) Rlc1p–tdTomato and mCherry–Atb2p (mCh-Atb2p) treated with either (top) 128 µM DMSO or (bottom) 100 µM latrunculin A (LatA) in DMSO for 30 min at 25°C to depolymerize actin. (E) Temperature-sensitive cdc12-112 cells expressing Rga7p–mEGFP, Rlc1p–tdTomato and mCherry–Atb2p at (top) 25°C or (bottom) 3 h at 36°C. (F) Time course of the behavior of For3p–3GFP and Rga7p–mEGFP in wild-type cells (open symbols) and Δfor3 cells (filled symbols) using Sad1p–mCherry in spindle pole bodies to mark time 0. A log rank test was used to determine whether each time course differed significantly from that of wild-type cells. (Δ, red line, n=31) For3p–3GFP at the cell center, (Ο, n=38, ●; n=17, P<0.04) Rga7p–mEGFP in punctate structures and (☐, n=38; ■, n=17, P<0.0001) Rga7p–mEGFP in rings. (G) Micrograph of Δfor3 cells expressing Rga7p–mEGFP (green) and Sad1p–mCherry (red) to mark spindle pole bodies. Arrows highlight Rga7p–mEGFP at a cell tip and cleavage furrow. (H,I) Localization and maintenance of Rga7p–mEGFP in the cleavage furrow depends on the contractile ring. Time series of fluorescence micrographs at 8-min intervals of sid2-250 cells expressing Rlc1p–tdTomato, mCherry–Atb2p and Rga7p–mEGFP after 1 h at 36°C. Upper panels, Rlc1p–tdTomato and mCherry–Atb2p; lower panels, Rga7p–mEGFP. Arrowheads mark the appearance of spindle microtubules. (H) Montage of fluorescent micrographs of a cell that failed to assemble a contractile ring. Arrows mark a Rga7p–mEGFP puncta that did not form a ring. Arrowheads indicate the appearance of spindle microtubules. (I) Montage of a cell that transiently formed a contractile ring associated with ring of Rga7p–mEGFP. Arrowheads indicate the appearance of spindle microtubules. Scale bars: 2 μm (A,G–I); 4 μm (D).
Cells that had been treated with 100 µM latrunculin A (Wu et al., 2006) did not accumulate Rga7p–mEGFP in the cleavage furrow or at the poles (Fig. 2D), so the normal localization of Rga7p–mEGFP depends in some way on actin filaments. Treated cells aggregated Rga7p–mEGFP in large clumps that occasionally colocalized with remnants of contractile rings that were marked with Rlc1p–tdTomato (data not shown).
Because Rga7p–mEGFP is not a component of actin patches that depend on the Arp2/3 complex (Arasada and Pollard, 2011), we tested whether its localization depended on actin filaments that are nucleated by the formin Cdc12p, which is essential for contractile ring formation (Chang et al., 1997), or formin For3p, which is required for actin cable formation (Feierbach and Chang, 2001; Martin and Chang, 2006). At the restrictive temperature of 36°C, most temperature sensitive cdc12-112 cells failed to assemble actin in a cleavage furrow, so cells with two nuclei associated with multiple bundles of microtubules accumulated over time (Fig. 2E). In these cdc12-112 cells, Rga7p–mEGFP remained associated with the poles and did not accumulate in puncta or rings at the cell center. Wild-type cells recruited For3p to the cleavage furrow at time 12 min, 3 min after Rga7p–mEGFP appeared in punctate structures at the cell center (Fig. 2G). In Δfor3 cells at 25°C, Rga7p–mEGFP appeared both at the poles and in the cleavage furrow, but its appearance into an equatorial ring was delayed by 10 min (Fig. 2G). Therefore, both formins contribute to Rga7p localization – Cdc12p-dependent contractile ring assembly is required for Rga7p to move from the cell tips to punctate structures at the cell center, and For3p is required for the timely transfer of Rga7p from these punctate structures into the cleavage furrow.
Because ring constriction is also delayed in Δfor3 cells, we questioned whether formation of a ring of Rga7p–mEGFP depends on cleavage furrow formation. Contractile ring constriction in S. pombe cells depends on the SIN pathway, so we used sid2-250, a temperature-sensitive mutation of this SIN kinase, to prevent ring constriction. After shifting to the restrictive temperature 36°C for 1 h, few mitotic sid2-250 cells assembled complete contractile rings. In cells without contractile rings, Rga7p–mEGFP localized to medial punctate structures but did not form a continuous ring (Fig. 3H). A few sid2-250 cells transiently formed a contractile ring that was accompanied by a ring of Rga7p–mEGFP that persisted for a few minutes after the contractile ring disappeared (Fig. 3I). Thus, localization of Rga7p to the cell center depends on the contractile ring.
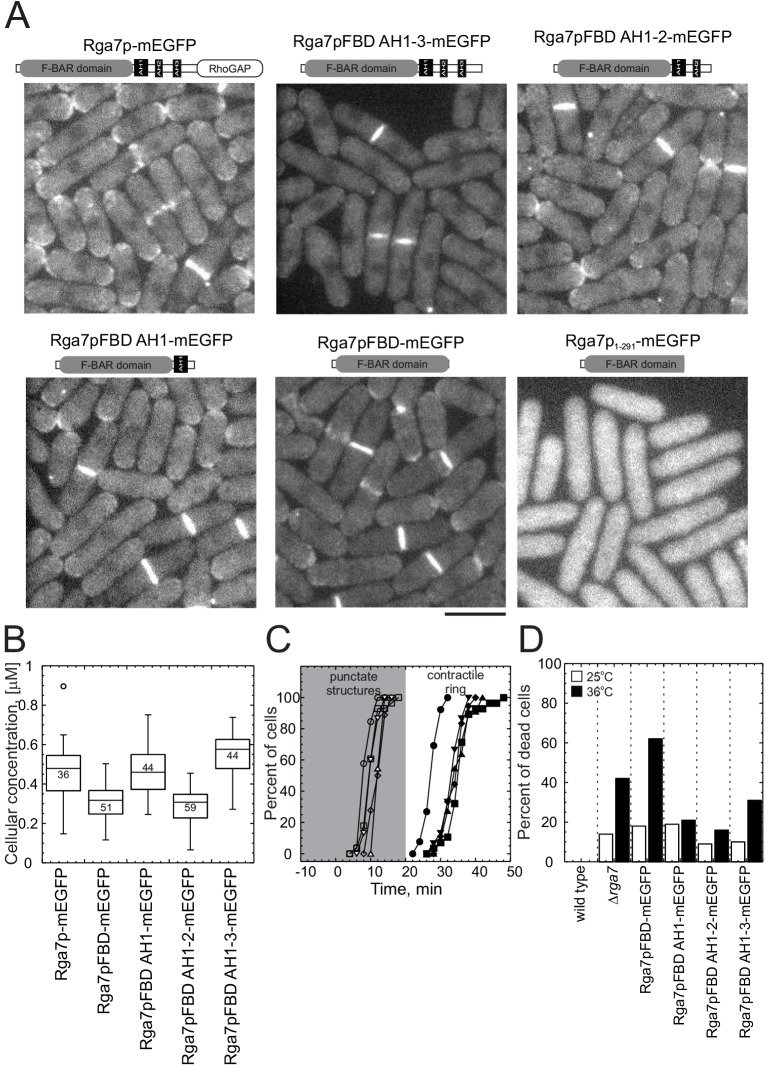
Fig. 3.
Complementation experiments with truncated constructs of Rga7p–mEGFP. Cells that expressed Sad1p–mCherry and full-length Rga7p–mEGFP or one of the following truncated constructs from the native rga7+ locus: Rga7pFBD AH1-3–mEGFP (residues 1–505); Rga7pFBD AH1-2 (residues 1–441); Rga7pFBD AH1 (residues 1–391); Rga7pFBD (residues 1–320); and Rga7p1-291–mEGFP. After growth in mid-log phase (OD595<0.6) in YE5S medium for 36 h, cells were imaged on 25%-gelatin pads in EMM5S with a stack of 20 confocal z-sections every 2 min for 2.5 h at 25°C. (A) Diagrams show the domains of each expressed protein. Sum projections of fluorescence micrographs. (B) Box plots of the total cellular concentrations of each construct measured from the total fluorescence of mEGFP. The line is the median, the whiskers indicate upper and lower bounds and the circles outside the whiskers are outliers. The total number of cells measured is indicated in the box. (C) Time course of the accumulation of cells with full-length or truncated Rga7p–mEGFP at the site of cytokinesis. Sad1p–mCherry was used to determine the cell cycle time. Open symbols, cells with mEGFP fluorescence in punctate structures at the cell center. Filled symbols, cells with mEGFP fluorescence in rings. A log rank test was used to determine if each time course differed significantly from that of cells with full-length Rga7p–mEGFP. (○, n=26; ●, n=26) full-length Rga7p–mEGFP, (◽, n=28, P>0.1; ■, n=28, P<0.0001) Rga7pFBD–mEGFP, (▵, n=11, P<0.0001; ▴, n=11, P<0.0001) Rga7pFBD AH1–mEGFP, (◊, n=18, P<0.0001; ♦, n=18, P<0.0001) Rga7pFBD AH1-2–mEGFP, and (▿, n=15, P<0.0001; ▾, n=15, P<0.0001) Rga7pFBD AH1-3–mEGFP. (D) Percentage of dead cells in cultures of strains, depending on full-length or truncated Rga7p–mEGFP. Cells were grown in YE5S at (black bars) 25°C or (white bars) shifted to 36°C for 3 h. Scale bar: 5 μm (A).
Domain analysis of Rga7p
We identified the N-terminal 320 amino acids of Rga7p as an F-BAR domain, based on sequence comparisons with crystal structures of three F-BAR proteins – cdc42 interacting protein 4, CIP4; formin binding protein 17, FBP17 (Shimada et al., 2007); and Fer/Cip4 homology domain-only protein 2, FCHO2 (Henne et al., 2007). Residues 506–692 at the C-terminus of Rga7p form a Rho2p-specific RhoGAP domain (Soto et al., 2010). Sequence analysis of the residues between these two domains with Heliquest (Gautier et al., 2008) identified no obvious homology with other proteins, but did identify three potential amphipathic helices – amphipathic helix (AH)1 (residues 331–369), AH2 (residues 392–409) and AH3 (residues 442–462).
To determine the parts of Rga7p required for its localization and function, we truncated the C-terminus of Rga7p to create a deletion series lacking the GAP domain and one or more putative amphipathic helices (Fig. 3A). All of the truncated Rga7p constructs were tagged with mEGFP and expressed from the endogenous promoter in the native locus. Based on the total cellular fluorescence, these strains expressed each truncation mutant at approximately the same level (within a factor of 2) as full-length Rga7p (Fig. 3B).
All of the truncated Rga7p proteins that included the full F-BAR domain accumulated in medial punctate structures and in the cleavage furrows (Fig. 3A), although the mutants took up to 10 min longer to form a continuous ring in the cleavage furrow than the full length protein (Fig. 3C). All of the truncation mutants that lacked the RhoGAP domain were susceptible to lysis, like Δrga7 cells, and cells that depended on just the Rga7p F-BAR domain were even more susceptible than Δrga7 cells to lysis during separation (Fig. 3D). Fewer cells lysed if the Rga7p construct included both the F-BAR domain and all or part of the middle domain (Fig. 3D). Thus, only the F-BAR domain is required for localization, but both the middle domain and the RhoGAP domain are required for Rga7p function in cells.
Role of Rga7p in cytokinesis
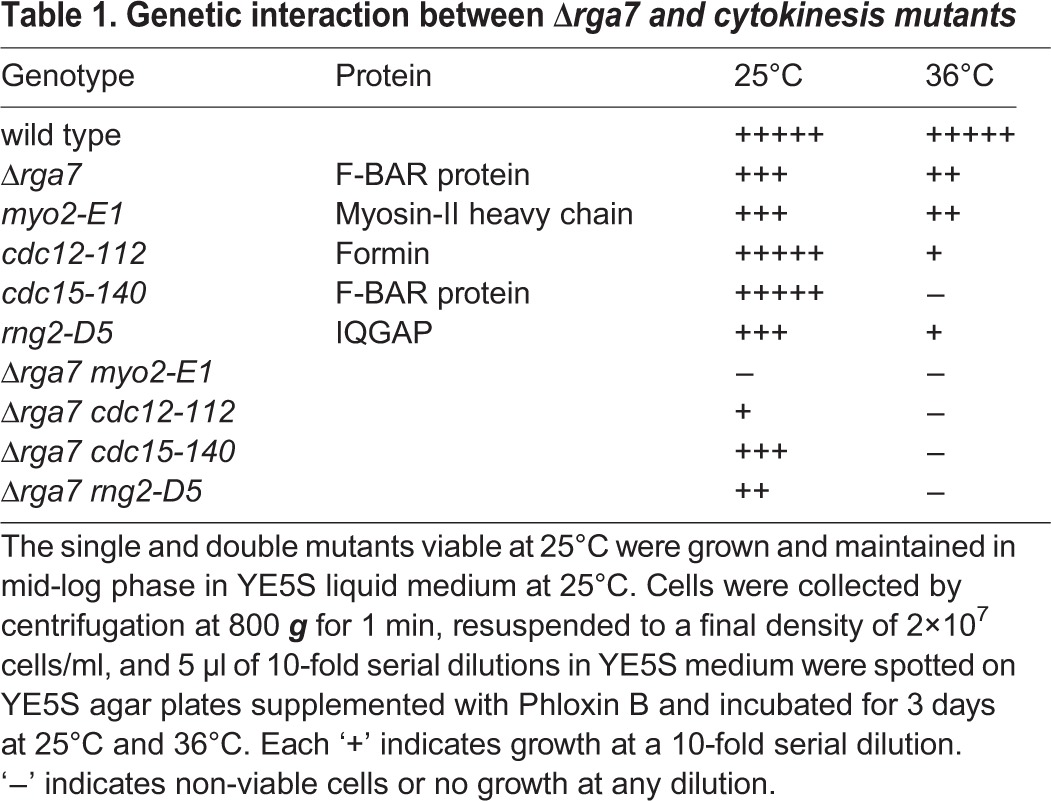
Genetic interactions between Δrga7 and mutations of genes involved in contractile ring assembly and constriction –including rng2-D5, cdc12-112, myo2-E1, cdc15-140 and cdc4-8 – support a role for Rga7p during cytokinesis (Table 1). We observed the strongest negative interaction with myo2-E1, a temperature-sensitive mutation of the myosin-II heavy chain with defective motor activity (Balasubramanian et al., 1998; Lord and Pollard, 2004). No spores carrying mutations in both the genes were viable. Double mutants of Δrga7 with rng2-D5 or with cdc15-140 were viable at 25°C but synthetically lethal at higher temperatures.
Table 1.
Genetic interaction between Δrga7 and cytokinesis mutants
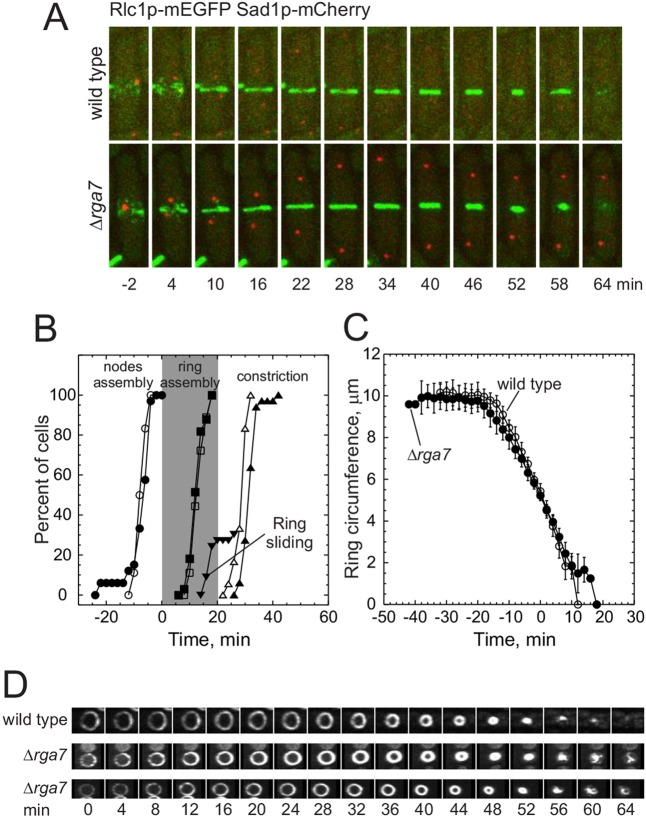
To understand the role of Rga7p in contractile ring assembly and disassembly, we imaged cells and measured the timing of cytokinesis events in wild-type and Δrga7 cells expressing Rlc1p–mEGFP and a spindle pole body protein, Sad1p–mCherry (Fig. 4A). Wild-type and Δrga7 cells accumulated Rlc1p nodes at the cell center and assembled a contractile ring at the normal time, between time 0 and 12 min relative to spindle pole body separation (Fig. 4A,B). This was expected given that Rga7p localizes to the cell center after the contractile ring assembles (Fig. 1B). However, the onset of the contractile ring constriction was delayed by 6 min in Δrga7 cells (Fig. 4A,B). In 30% of Δrga7 cells, the contractile rings slid toward a pole, beginning at time 16 min, until they stopped sliding and began to constrict (Fig. 4A,B). Contractile rings in Δrga7 cells constricted slightly slower (at a rate of 0.55±0.07 μm/min, n=28) than they did in wild-type cells (0.70±0.08 μm/min, n=14, P<0.0001) (Fig. 4C). Contractile rings constricted at similar rates in Δrga7 cells that separated successfully and those that failed in cell separation. Usually, all contractile rings marked with Rlc1p–mEGFP constricted uniformly to a single point and dispersed into the cytoplasm, but contractile rings in Δrga7 cells disassembled and released strands of Rlc1p–mEGFP during the final stages of constriction (Fig. 4D).
Fig. 4.
Contractile ring dynamics in Δrga7 cells. Wild-type and Δrga7 cells expressing Rlc1p–mEGFP and Sad1p–mCherry were grown exponentially in YE5S medium at 25°C and imaged by collecting stacks of 21 z-slices at 2 min intervals at 25°C. Spindle pole bodies marked with Sad1p–mCherry separated at time 0. (A) Time series of maximum intensity projections of fluorescence micrographs of cells expressing (green) Rlc1p–mEGFP and (red) Sad1p–mCherry. Top panel, wild-type cell; and bottom panel, Δrga7 cell. (B) Time courses of the cytokinesis events in (open symbols) wild-type and (filled symbols) Δrga7 cells. A log rank test was used to determine if each time course differed significantly from that of wild-type cells. (○, n=18; ●, n=33, P>0.1) appearance of Rlc1p–mEGFP in cortical nodes; (◽, n=18; ■, n=33, P>0.1) completion of contractile ring assembly; (▵, n=18; ▴, n=33, P<0.0001) onset of ring constriction; and (▾, n=10) onset of ring sliding. (C) Time course of contractile ring constriction measured as the circumference of Rlc1p–mEGFP fluorescence in reconstructions of cross-sections of the middle of cells. The mid points of individual time courses were aligned, and the circumference of the ring at each time point was averaged (±1 s.d.). (○, n=14) wild-type cells; (●, n=28) Δrga7 mutant cells. P<0.0001 compared with wild type, unpaired two-tailed t-test. (D) Time series of fluorescence micrographs (maximum intensity projection) showing reconstructions of the cross-sections of the middle of a wild-type and two Δrga7 cells expressing Rlc1p–tdTomato at 4 min intervals at 25°C. (Upper panel) Wild-type cell and (middle and lower panel) Δrga7 cell. Scale bar: 2 μm (A).
Contributions of Rga7p to the recruitment of β-glucan synthetases
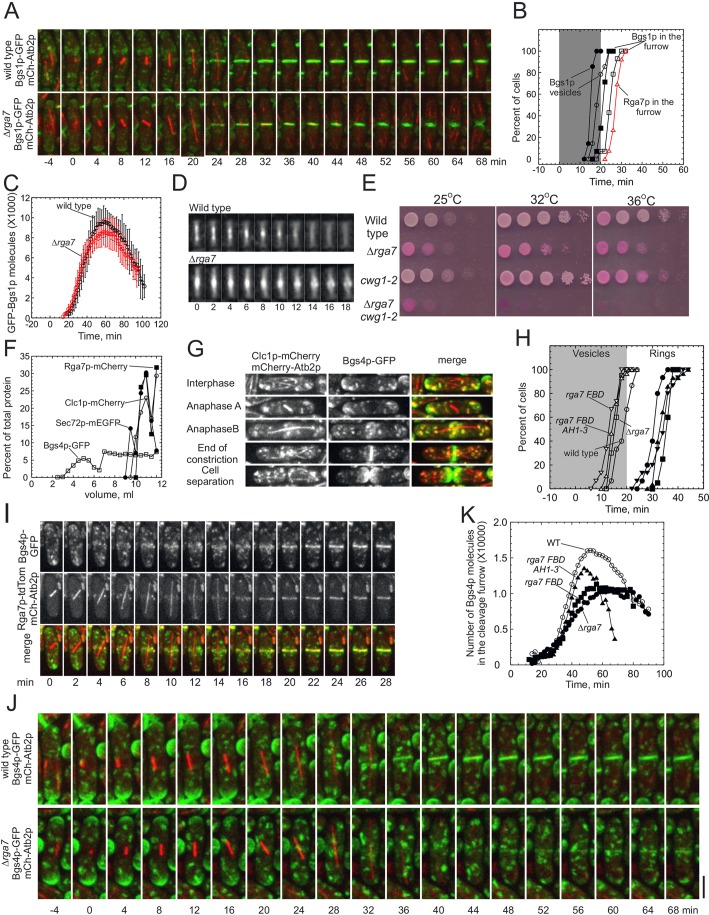
The transmembrane enzyme Bgs1p and its product, the primary septum, are required to anchor the contractile ring and to initiate the contractile ring constriction (Arasada and Pollard, 2014; Cortes et al., 2007; Liu et al., 1999). S. pombe cells recruit Bgs1p to the plasma membrane adjacent to the contractile ring in two steps. First, Bgs1p moves from the cell tips to medial puncta that contain the trans-Golgi membrane markers Sec72p (the homolog of budding yeast SEC7 Arf GEF) and Clc1p (clathrin light chain) (de Leon et al., 2013; Vjestica et al., 2008). Bgs1p is visible in these puncta by time 19 min in wild-type cells and by 16 min in Δrga7 mutant cells. Then, within 6 min, both types of cell start to move Bgs1p from these late Golgi compartments to plasma membrane that is adjacent to the contractile ring, arriving by time 25 min in wild-type cells and by 22 min in Δrga7 cells (Fig. 5B) (Arasada and Pollard, 2014). Rga7p accumulated in punctate structures at the cell center before Bgs1p, but Bgs1p formed a ring in the cleavage furrow before Rga7p, so we measured the timing of the both events in wild type and Δrga7 mutant cells.
Fig. 5.
Targeting β-glucan synthetases GFP–Bgs1p and Bgs4p–GFP to the cleavage furrow in Δrga7 cells. All wild-type and Δrga7 cells expressing fluorescent proteins shown in this figure were grown in mid-log phase (OD595<0.6) for 36 h in YE5S medium at 25°C before imaging on 25%-gelatin pads in EMM5S at 25°C. Stacks of 19–21 z-slices were collected using the green and red channels to image the entire cell. Spindle microtubules marked with mCherry–Atb2p appeared at time 0 unless indicated otherwise. (A) Time series of maximum intensity projections of stacks of confocal z-slices of (upper panel) wild-type and (lower panel) Δrga7 cells expressing (green) GFP–Bgs1p and (red) mCherry–Atb2 (tubulin). (B) Time courses of the accumulation of cells with GFP–Bgs1p or Rga7p–mEGFP in structures in (open symbols) wild-type cells and (filled symbols) Δrga7 cells. A log rank test was used to determine if each time course differed significantly from that of wild-type cells. (Ο, n=15; ●, n=15, P<0.0005) GFP–Bgs1p in vesicles near to the cell center; (☐, n=15; ■, n=15, P=0.0001) GFP–Bgs1p in cleavage furrows; and (Δ, red, n=33) Rga7p–mEGFP in the cleavage furrows. (C) Time courses of the average numbers of GFP–Bgs1p molecules (mean±1 s.d.) in cleavage furrows of (Ο, n=15; peak number 9600±1600) wild-type cells and (●, n=15; peak number 8600±1300, P<0.1 compared with wild type from a two-tailed t-test) Δrga7 cells. The intensity of GFP–Bgs1p fluorescence was measured in 21 optical sections at 2 min intervals for 2 h, normalized and corrected for background. (D) Maximum intensity projections of GFP–Bgs1p fluorescence in a 15×35-pixel ROI around the cell center during the completion of septation and cell separation in (upper panel) one wild-type cell and (lower panel) one Δrga7 cell. Arbitrary time in minutes. (E) Growth assay comparing the viability of wild-type cells, Δrga7 cells lacking rga7+, cwg1-2 cells with a temperature-sensitive mutation in bgs4+ and Δrga7 cwg1-2 cells with mutations in both rga7+ and bgs4+. The same methods as those described in Fig. 1A were used, except growth was for 2 days. (F) Sedimentation-velocity ultracentrifugation of homogenates of wild-type cells expressing clathrin light chain Clc1p–mCherry, Rga7p–mCherry, Bgs4p–GFP or trans-Golgi marker Sec72p–mEGFP. Homogenate samples of 1 ml were centrifuged on 12 ml gradients of 18–60% sucrose for 140 min at 38,000 r.p.m. at 4°C. Fractions of 500 μl were (fraction 1 is the top of the gradient) analyzed by using SDS-PAGE and quantitative immunoblotting: (Ο) Clc1p–mCherry; (☐) Bgs4p–GFP; (■) Rga7p–mCherry; and (●) Sec72p–mCherry. (G) Fluorescence micrographs (maximum intensity projections of 21 confocal z-slices) of five different wild-type cells expressing (first column) Clc1p–mCherry and mCherry–Atb2p, and (second column) GFP–Bgs4p. The third column is a merge of the two fluorescence images. Row 1, interphase; row 2, anaphase A; row 3, anaphase B; row 4, end of contractile ring constriction; row 5, separated daughter cells. (H) Time courses of the accumulation of cells with (open symbols, grey area) GFP–Bgs4p in punctate structures near to the cell center and (closed symbols, white area) GFP–Bgs4p in the cleavage furrows. A log rank test was used to determine whether each time course differed significantly from that of wild-type cells. (Ο, ●, n=15) wild-type cells, (☐, ■, n=22 P<0.001 and P<0.0001) Δrga7 cells, (Δ, ▲, n=28 P<0.0115 and P<0.0003) rga7 FBD AH1-3 -mCherry cells, and (∇, ▾, n=15, P<0.0003 and P<0.001) rga7 FBD -mCherry cells. (I) Time series of maximum intensity projections of stacks of 21 confocal z-slices of a wild-type cell expressing (upper panel) Bgs4p–GFP, (middle panel) Rga7p–tdTomato and mCherry–Atb2p, and (lower panel) merge of both the channels. Time 0 is the first frame in the time series. (J) Time series of fluorescence micrographs (maximum intensity projection of 18 confocal z-slices, 2 min intervals) of (upper panel) a wild-type cell and (lower panel) an Δrga7 cell expressing (green) Bgs4p–GFP and (red) mCherry–Atb2p (tubulin) at 25°C. Spindles formed at time 0 min. Scale bar 2 µm. (K) Time courses of the average numbers of Bgs4p–GFP molecules in cleavage furrows of (open symbols) wild-type cells and (filled symbols) Δrga7 mutant cells. Time 0 is at spindle pole body separation. Bgs4p–GFP fluorescence intensities were measured in 20 optical sections at 2 min intervals for 2 h, normalized and corrected for background. (Ο, n=15) wild-type cells, (●, n=21) Δrga7 cells, (■, n=16) rga7 FBD -mCherry cells and (▲, n=16) rga7 FBD AH1-3 -mCherry cells.
The time course of the accumulation of GFP–Bgs1p in late Golgi compartments at the cell center was similar in wild-type cells and Δrga7 mutant cells, but the peak numbers of Bgs1p molecules were slightly lower in Δrga7 mutant cells (9600±1600 in wild type and 8600±1300 in Δrga7 cells, P<0.1, two-tailed t-test) (Fig. 5A–C). Septa in Δrga7 cells formed perpendicular to the long axis but accumulated defects, such as bends and bulges, late during septum closure formation (Martin-Garcia et al., 2014), indicating a lack of the rigidity needed to maintain a straight septum (Fig. 5A,D) (Munoz et al., 2013).
The rigidity of the primary septum in S. pombe depends on the transmembrane enzyme Bgs4p – the main cell wall β-glucan synthetase (Munoz et al., 2013) and target of the lipopeptide inhibitor aculeacin A (Martins et al., 2011). Irregularities in cells with temperature-sensitive mutations in the bgs4+ gene, cwg1-1 and cwg1-2, or cells with reduced expression of Bgs4p indicate that the primary septum is less rigid than normal, and daughter cells lyse when they separate (Munoz et al., 2013). Given the similar phenotype of Δrga7 mutants, we investigated whether Rga7p has a role in delivering Bgs4p to the cleavage furrow by using four types of experiment.
First, we observed a strong genetic interaction between the deletion of rga7+ and the temperature-sensitive mutation cwg1-2. Cells carrying both mutations grew slowly at 25°C and did not grow at temperatures greater than 25°C (Fig. 5E).
Second, sedimentation-velocity experiments showed that Rga7p–mCherry co-sedimented in a single peak with a high sedimentation coefficient, along with the late Golgi markers Sec72p–mEGFP and Clc1p–mCherry. Bgs4p sedimented in two peaks – 25% of the protein sedimented slowly, and the remaining 75% was distributed in a broad peak from 7 to 12 ml that overlapped the peak of Rga7p–mCherry (Fig. 5F).
Third, fluorescence microscopy showed that Bgs4p trafficked during mitosis from the poles to the middle of cells in punctate structures with clathrin light chain Clc1p (Fig. 5G), a marker of late Golgi compartments (de Leon et al., 2013), and then to the plasma membrane in the cleavage furrow. Bgs1p takes a similar pathway earlier than Bgs4p. In wild-type cells, Bgs4p–GFP accumulated along with Rga7p–tdTomato in medial punctate structures at time 19.2±3 min (relative to separation of the spindle pole bodies) (Fig. 5H). By time 30.1±2.8 min, Bgs4p–GFP started to transfer at 780 molecules/min to the cleavage furrow, along with Rga7p–tdTomato (Fig. 5I).
Bgs4p arrived in late Golgi compartments at the cell center slightly earlier in all the rga7+ mutant cells (Δrga7, rga7-FDB-mCherry and rga7 FBD AH1-3 -mCherry) than in wild-type cells (Fig. 5H), but the subsequent transfer of Bgs4p to a ring adjacent to the plasma membrane varied in the mutants (Fig. 5H,J). The rate of transfer was normal (750 molecules/min) in rga7 FBD AH1-3 -mCherry cells (comprising both the F-BAR and middle domains of Rga7p), but slower in Δrga7 (290 molecules/min) and rga7Δ FDB-mCherry (560 molecules/min) cells. All of the mutant strains initiated this transfer a few minutes later than wild-type cells (Fig. 5H). The peak numbers of Bgs4p molecules that were adjacent to the contractile ring were lower than normal in all of the rga7+ mutant strains (Fig. 5K).
DISCUSSION
We confirmed the following observations of Martin-Garcia et al. (2014). Cells that lack Rga7p have unstable contractile rings, and some lyse when the daughter cells separate. Rga7p cooperates with another F-BAR protein, Cdc15p, to stabilize the contractile ring during constriction. In Δrga7 cells, GFP–Bgs1p concentrates at the center of the cleavage furrow, rather than distributing more uniformly along the plasma membrane in the furrow. The Rga7p F-BAR domain alone is sufficient for concentration of the protein in the cleavage furrow, but the C-terminal middle domains and RhoGAP domains are necessary for full Rga7p function.
This confirmation of previous findings was an excellent starting point to investigate the mechanism of action of Rga7p using quantitative measurements in live cells. We discovered that Rga7p is required to deliver Bgs4p to the cleavage furrow at the normal rate and that trafficking of Bgs4p from the cell tips to the cleavage furrow depends on actin filaments produced by the formins Cdc12p and For3p.
Role of Rga7p in post-Golgi transport of Bgs4p
The fundamental defect in Δrga7 cells is that the β-glucan synthetase Bgs4p transfers more slowly than normal from centrally located late Golgi compartments to the plasma membrane in the cleavage furrow. Normally, Bgs4p moves from the cell tips to centrally located puncta with Rga7p and late Golgi markers Sec72p and Clc1p, before moving to the plasma membrane around the cleavage site. In cells that lack Rga7p, the transfer reaction is slow, so the total number of Bgs4p molecules at the cleavage furrow peaks 10 min late at only 60% of that of wild-type levels. Detecting this subtle defect required quantitative measurements of the time course of Bgs4p accumulation at the cell center. Synthetic lethality between the Δrga7 deletion mutation and the temperature-sensitive mutation cwg1-2 in bgs4+ is consistent with a functional link between Rga7p and Bgs4p.
We propose that the late arrival of Bgs4p at the cleavage site in cells that lack Rga7p compromises septum formation and results in bends in the septa and cytokinesis defects. Consistent with this interpretation, Δrga7 cells and bgs4+ mutants cwg1-1 and cwg1-2 have similar septal defects and a similar tendency to lyse (Cortes et al., 2012; Martin-Garcia et al., 2014; Munoz et al., 2013).
We propose that Rga7p contributes to the budding of vesicles containing Bgs4p from the Golgi complex. The Rga7p F-BAR domain binds to phospholipids (Martin-Garcia et al., 2014) in vitro and localizes Rga7p to late Golgi compartments containing Bgs4p, but the middle and RhoGAP domains also contribute. Like other F-BAR domains (Frost et al., 2008), Rga7p can sense or induce the membrane curvature that is necessary to form vesicles or tubules. However, Rga7p does not act alone because Bgs4p can move slowly to the plasma membrane in its absence. Furthermore, Rga7p moves with Bgs4p to the cleavage furrow, where it may have other functions.
Bgs4p targeting to the cleavage furrow depends on actin filaments and the contractile ring
We discovered that relocalization of Rga7p–mEGFP from the poles to the cell division site depends on the presence of a stable contractile ring. Formin Cdc12p assembles actin filaments for the contractile ring, so cells lacking Cdc12p accumulate multiple nuclei without any septal material (Chang et al., 1997). Rga7p is not recruited to the site of cell division in cdc12-112 mutants that fail to assemble a contractile ring. Furthermore, in SIN mutants that assemble a contractile ring only transiently without constricting, Rga7p accumulates around the equator only while the ring is present. The mechanism is unclear, but the contractile ring might recruit factors that anchor Rga7p at the cleavage furrow.
In cells lacking formin For3p, Rga7p appears at the cleavage furrow 10 min late, so actin filaments assembled by For3p contribute in some way to this process. For3p assembles actin filament cables during interphase (Feierbach and Chang, 2001; Martin and Chang, 2006) but also localizes to the cleavage furrow where it contributes to the timely assembly and constriction of contractile rings (Coffman et al., 2013). For3p binds to activated Rho3p and Cdc42p, both of which are involved in membrane trafficking (Estravis et al., 2011; Martin et al., 2007; Nakano et al., 2002), and Rho3p localizes to the Golgi and endosomes, as well as to the division site (Yu et al., 2013). Furthermore, the synthetic lethal genetic interactions of both Δrga7 and Δfor3 with mutations of the genes for myosin-II essential light chain cdc4+ and IQGAP rng2+ (Table 1) (Coffman et al., 2013) are likely to result from the combined defects in the contractile ring assembly and septum assembly. Indirect effects might delay the delivery of Rga7p to the plasma membrane in Δfor3 cells, but actin filaments polymerized by For3p could help to sort membrane cargo in the Golgi complex or serve as tracks for post-Golgi vesicles.
Parallel pathways for Bgs1p and Bgs4p to the cleavage furrow
The glucan synthetases Bgs1p and Bgs4p rely on different F-BAR proteins – Cdc15p and Rga7p, respectively – for transport to the cleavage furrow. Both of these transmembrane enzymes contribute to septum formation and contractile ring constriction in S. pombe cells (Arasada and Pollard, 2014; Ishiguro et al., 1997; Liu et al., 2002, 1999; Martin-Garcia et al., 2014; Munoz et al., 2013). Both enzymes move at slightly different times from the cell tips to centrally located elements of the Golgi apparatus and then to the cleavage furrow. These movements of Bgs1p depend on Cdc15p, whereas Rga7p contributes to the movements of Bgs4p. Mutation of either F-BAR protein reduces the rate of transfer of its cargo enzyme to the plasma membrane. At the division site, Bgs1p synthesizes the primary septum and helps to anchor the contractile ring, whereas Bgs4p provides material for both the primary and the secondary septum, which stabilizes the cell wall in preparation for separation of the daughter cells. The sequential arrival of the glucan synthetases at the cleavage site seems to be so important that fission yeast have evolved specialized pathways with different timings for Bgs1p and Bgs4p. It will be interesting to learn how other F-BAR proteins participate in secretion of the extracellular matrix during cytokinesis in other eukaryotic organisms.
MATERIALS AND METHODS
Strains, growth conditions and cellular methods
Supplementary material Table S1 lists the strains used in this study. All fluorescent protein tags were integrated at native chromosomal loci, so fusion proteins were expressed under the control of endogenous promoters (Bahler et al., 1998). Cells were grown in YE5S medium and maintained in mid-log phase (OD595<0.6) for 36 h before microscopy analyses. In order to delete the rga7+ gene, wild-type cells were transformed with a kanMX6 or an ura4+ cassette flanked with sequences homologous to the regions on either side of the rga7+ open reading frame. The C-terminal truncations of Rga7p were generated by transforming the wild-type strain with mEGFP-kanMX6 or mCherry-kanMX6 flanked with homologous sequences from the designated position at the rga7+ locus.
Subcellular fractionation
Cell lysates were fractionated by using sedimentation-velocity ultracentrifugation in a sucrose gradient, as described previously for budding yeast (Antebi and Fink, 1992; Arasada and Pollard, 2014; Barrowman et al., 2010). Proteins were quantified by immunoblotting with antibodies against fluorescent protein tags (Arasada and Pollard, 2011).
Microscopy and data analysis
Cells for microscopy analyses were collected from liquid cultures by centrifugation at 800 g and washed twice in EMM5S medium. Cells resuspended in EMM5S medium were spotted on a thin 25%-gelatin pad prepared in EMM5S liquid medium supplemented with 25 nM n-propyl-gallate. Microscopy was performed at room temperature or as noted. Temperature-sensitive mutants were imaged on 35-mm lectin-coated glass-bottomed Petri dishes (WillCo Wells BV) at 36°C. The coverslips in the glass-bottomed Petri dishes were coated with 1 μg/µl of lectin from Glycine Max (Sigma-Aldrich L1395) and air-dried overnight. Cells were collected and washed as described above. Cells were spotted on the coverslip, allowed to settle for 5 min before the Petri dishes were filled with 2 ml of EMM5S medium. Imaging was performed with an Olympus IX-71 microscope with a 100×/NA 1.4 Plan Apo lens (Olympus) and a CSU-X1 (Andor Technology) confocal spinning disk confocal system equipped with an iXON-EMCCD camera (Andor Technology) calibrated to count fluorescent proteins (Wu and Pollard, 2005). Routinely, a stack of up to 21 z-sections spaced at 0.36 μm were imaged every 2 min for 2.5 h. Maximum-intensity, sum projections and other image analyses were performed using ImageJ with custom-made plugins. We used a log rank test [MedCalc software for Windows 7 (10.2), MedCalc Software, Ostend, Belgium] to compare time courses of whole-outcome plots with controls and calculated a P value for each pair of curves. We used a t-test to calculate the statistical significant differences in the rate of contractile ring constriction and in measurements of the average number of molecules.
Counting numbers of molecules in whole cells and at the division site
Stacks of images of wild-type cells and of cells expressing fluorescent fusion proteins were taken under identical conditions at 0.36-μm z-spacing through the entire cell. The total GFP intensity was the sum intensity of all sections with signal (Wu and Pollard, 2005). Images were also corrected for the camera noise and uneven illumination. To correct for the camera noise, cells were imaged without any light. The uneven illumination of the excitation laser was corrected using images of AlexaFluor-488 solution on coverslips. The intensity of wild-type cells was subtracted to remove autofluorescence and background from the measurement. The concentration and the number of molecules were obtained by comparing the intensities of GFP fluorescence with that of cells expressing GFP proteins of known concentrations. We counted Rga7p–mEGFP molecules from the fluorescence in rectangular boxes of 22×30 pixels when in punctate structures and of 7×30 pixels when in rings.
Supplementary Material
Acknowledgements
The authors thank Drs Juan Carlos Ribas, Pilar Perez and Vladimir Sirotkin for the yeast strains, and members of the Pollard lab for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
R.A. and T.D.P. designed the experiments, R.A. performed the experiments, R.A. and T.D.P. analyzed the data, and wrote and paper.
Funding
Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health [grant numbers R01GM026132 and R01GM026338]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.162974/-/DC1
References
- Antebi A. and Fink G. R. (1992). The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3, 633-654. 10.1091/mbc.3.6.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasada R. and Pollard T. D. (2011). Distinct roles for F-BAR proteins Cdc15p and Bzz1p in actin polymerization at sites of endocytosis in fission yeast. Curr. Biol. 21, 1450-1459. 10.1016/j.cub.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasada R. and Pollard T. D. (2014). Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep. 8, 1533-1544. 10.1016/j.celrep.2014.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J., Wu J.-Q., Longtine M. S., Shah N. G., McKenzie A. III, Steever A. B., Wach A., Philippsen P. and Pringle J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S. and Gould K. L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman J., Bhandari D., Reinisch K. and Ferro-Novick S. (2010). TRAPP complexes in membrane traffic: convergence through a common Rab. Nat. Rev. Mol. Cell Biol. 11, 759-763. 10.1038/nrm2999 [DOI] [PubMed] [Google Scholar]
- Chang F., Drubin D. and Nurse P. (1997). cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 137, 169-182. 10.1083/jcb.137.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman V. C., Sees J. A., Kovar D. R. and Wu J.-Q. (2013). The formins Cdc12 and For3 cooperate during contractile ring assembly in cytokinesis. J. Cell Biol. 203, 101-114. 10.1083/jcb.201305022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J. C. G., Ishiguro J., Duran A. and Ribas J. C. (2002). Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115, 4081-4096. 10.1242/jcs.00085 [DOI] [PubMed] [Google Scholar]
- Cortes J. C. G., Carnero E., Ishiguro J., Sanchez Y., Duran A. and Ribas J. C. (2005). The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 118, 157-174. 10.1242/jcs.01585 [DOI] [PubMed] [Google Scholar]
- Cortes J. C. G., Konomi M., Martins I. M., Muñoz J., Moreno M. B., Osumi M., Durán A. and Ribas J. C. (2007). The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol. 65, 201-217. 10.1111/j.1365-2958.2007.05784.x [DOI] [PubMed] [Google Scholar]
- Cortes J. C. G., Sato M., Munoz J., Moreno M. B., Clemente-Ramos J. A., Ramos M., Okada H., Osumi M., Duran A. and Ribas J. C. (2012). Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J. Cell Biol. 198, 637-656. 10.1083/jcb.201202015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon N., Sharifmoghadam M. R., Hoya M., Curto M.-A., Doncel C. and Valdivieso M.-H. (2013). Regulation of cell wall synthesis by the clathrin light chain is essential for viability in Schizosaccharomyces pombe. PLoS ONE 8, e71510 10.1371/journal.pone.0071510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter J. and Sazer S. (1998). imp2, a new component of the actin ring in the fission yeast Schizosaccharomyces pombe. J. Cell. Biol. 143, 415-427. 10.1083/jcb.143.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estravis M., Rincon S. A., Santos B. and Perez P. (2011). Cdc42 regulates multiple membrane traffic events in fission yeast. Traffic 12, 1744-1758. 10.1111/j.1600-0854.2011.01275.x [DOI] [PubMed] [Google Scholar]
- Feierbach B. and Chang F. (2001). Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 11, 1656-1665. 10.1016/S0960-9822(01)00525-5 [DOI] [PubMed] [Google Scholar]
- Frost A., Perera R., Roux A., Spasov K., Destaing O., Egelman E. H., De Camilli P. and Unger V. M. (2008). Structural basis of membrane invagination by F-BAR domains. Cell 132, 807-817. 10.1016/j.cell.2007.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier R., Douguet D., Antonny B. and Drin G. (2008). HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24, 2101-2102. 10.1093/bioinformatics/btn392 [DOI] [PubMed] [Google Scholar]
- Glotzer M. (2005). The molecular requirements for cytokinesis. Science 307, 1735-1739. 10.1126/science.1096896 [DOI] [PubMed] [Google Scholar]
- Henne W. M., Kent H. M., Ford M. G. J., Hegde B. G., Daumke O., Butler P. J. G., Mittal R., Langen R., Evans P. R. and McMahon H. T. (2007). Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure 15, 839-852. 10.1016/j.str.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Humbel B. M., Konomi M., Takagi T., Kamasawa N., Ishijima S. A. and Osumi M. (2001). In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 18, 433-444. 10.1002/yea.694 [DOI] [PubMed] [Google Scholar]
- Ishiguro J., Saitou A., Duran A. and Ribas J. C. (1997). cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J. Bacteriol. 179, 7653-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang H., McCollum D. and Balasubramanian M. K. (1999). Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics 153, 1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Tang X., Wang H. and Balasubramanian M. (2000). Bgs2p, a 1,3-beta-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett. 478, 105-108. 10.1016/S0014-5793(00)01828-7 [DOI] [PubMed] [Google Scholar]
- Liu J., Tang X., Wang H., Oliferenko S. and Balasubramanian M. K. (2002). The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol. Biol. Cell 13, 989-1000. 10.1091/mbc.01-12-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M. and Pollard T. D. (2004). UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol. 167, 315-325. 10.1083/jcb.200404045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. G. and Chang F. (2006). Dynamics of the formin for3p in actin cable assembly. Curr. Biol. 16, 1161-1170. 10.1016/j.cub.2006.04.040 [DOI] [PubMed] [Google Scholar]
- Martin V., Ribas J. C., Carnero E., Duran A. and Sanchez Y. (2000). bgs2+, a sporulation-specific glucan synthase homologue is required for proper ascospore wall maturation in fission yeast. Mol. Microbiol. 38, 308-321. 10.1046/j.1365-2958.2000.02118.x [DOI] [PubMed] [Google Scholar]
- Martin V., Garcia B., Carnero E., Duran A. and Sanchez Y. (2003). Bgs3p, a putative 1,3-beta-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot. Cell 2, 159-169. 10.1128/EC.2.1.159-169.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. G., Rincon S. A., Basu R., Perez P. and Chang F. (2007). Regulation of the formin for3p by cdc42p and bud6p. Mol. Biol. Cell 18, 4155-4167. 10.1091/mbc.E07-02-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia R., Coll P. M. and Perez P. (2014). F-BAR domain protein Rga7 collaborates with Cdc15 and Imp2 to ensure proper cytokinesis in fission yeast. J. Cell Sci. 127, 4146-4158. 10.1242/jcs.146233 [DOI] [PubMed] [Google Scholar]
- Martins I. M., Cortes J. C. G., Munoz J., Moreno M. B., Ramos M., Clemente-Ramos J. A., Duran A. and Ribas J. C. (2011). Differential activities of three families of specific beta(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J. Biol. Chem. 286, 3484-3496. 10.1074/jbc.M110.174300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J., Cortes J. C. G., Sipiczki M., Ramos M., Clemente-Ramos J. A., Moreno M. B., Martins I. M., Perez P. and Ribas J. C. (2013). Extracellular cell wall beta(1,3)glucan is required to couple septation to actomyosin ring contraction. J. Cell Biol. 203, 265-282. 10.1083/jcb.201304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K., Imai J., Arai R., Toh-e A., Matsui Y. and Mabuchi I. (2002). The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J. Cell Sci. 115, 4629-4639. 10.1242/jcs.00150 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. (2010). Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 22, 50-56. 10.1016/j.ceb.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor S. A., Minc N., Boudaoud A. and Chang F. (2012). Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr. Biol. 22, 1601-1608. 10.1016/j.cub.2012.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith R. H., Chen J.-S., Wang J. and Gould K. L. (2009). The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J. Cell Biol. 184, 113-127. 10.1083/jcb.200806044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A., Niwa H., Tsujita K., Suetsugu S., Nitta K., Hanawa-Suetsugu K., Akasaka R., Nishino Y., Toyama M., Chen L. et al. (2007). Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 129, 761-772. 10.1016/j.cell.2007.03.040 [DOI] [PubMed] [Google Scholar]
- Simanis V. (2015). Pombe's thirteen - control of fission yeast cell division by the septation initiation network. J. Cell Sci. 128, 1465-1474. 10.1242/jcs.094821 [DOI] [PubMed] [Google Scholar]
- Sipiczki M. and Bozsik A. (2000). The use of morphomutants to investigate septum formation and cell separation in Schizosaccharomyces pombe. Arch. Microbiol. 174, 386-392. 10.1007/s002030000214 [DOI] [PubMed] [Google Scholar]
- Soto T., Villar-Tajadura M. A., Madrid M., Vicente J., Gacto M., Perez P. and Cansado J. (2010). Rga4 modulates the activity of the fission yeast cell integrity MAPK pathway by acting as a Rho2 GTPase-activating protein. J. Biol. Chem. 285, 11516-11525. 10.1074/jbc.M109.071027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vjestica A., Tang X.-Z. and Oliferenko S. (2008). The actomyosin ring recruits early secretory compartments to the division site in fission yeast. Mol. Biol. Cell 19, 1125-1138. 10.1091/mbc.E07-07-0663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-Q. and Pollard T. D. (2005). Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310-314. 10.1126/science.1113230 [DOI] [PubMed] [Google Scholar]
- Wu J.-Q., Sirotkin V., Kovar D., Lord M., Beltzner C., Kuhn J. R. and Pollard T. D. (2006). Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 174, 391-402. 10.1083/jcb.200602032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. and Vogel B. E. (2011). A secreted protein promotes cleavage furrow maturation during cytokinesis. Curr. Biol. 21, 114-119. 10.1016/j.cub.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Qyang Y., Bartholomeusz G., Zhou X. and Marcus S. (2003). The novel Rho GTPase-activating protein family protein, Rga8, provides a potential link between Cdc42/p21-activated kinase and Rho signaling pathways in the fission yeast, Schizosaccharomyces pombe. J. Biol. Chem. 278, 48821-48830. 10.1074/jbc.M306819200 [DOI] [PubMed] [Google Scholar]
- Yu Y., Li C., Kita A., Katayama Y., Kubouchi K., Udo M., Imanaka Y., Ueda S., Masuko T. and Sugiura R. (2013). Sip1, an AP-1 accessory protein in fission yeast, is required for localization of Rho3 GTPase. PLoS ONE 8, e68488 10.1371/journal.pone.0068488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.