Abstract
The long tendons of the limb extend from muscles that reside in the zeugopod (arm/leg) to their skeletal insertions in the autopod (paw). How these connections are established along the length of the limb remains unknown. Here, we show that mouse limb tendons are formed in modular units that combine to form a functional contiguous structure; in muscle-less limbs, tendons develop in the autopod but do not extend into the zeugopod, and in the absence of limb cartilage the zeugopod segments of tendons develop despite the absence of tendons in the autopod. Analyses of cell lineage and proliferation indicate that distinct mechanisms govern the growth of autopod and zeugopod tendon segments. To elucidate the integration of these autopod and zeugopod developmental programs, we re-examined early tendon development. At E12.5, muscles extend across the full length of a very short zeugopod and connect through short anlagen of tendon progenitors at the presumptive wrist to their respective autopod tendon segment, thereby initiating musculoskeletal integration. Zeugopod tendon segments are subsequently generated by proximal elongation of the wrist tendon anlagen, in parallel with skeletal growth, underscoring the dependence of zeugopod tendon development on muscles for tendon anchoring. Moreover, a subset of extensor tendons initially form as fused structures due to initial attachment of their respective wrist tendon anlage to multiple muscles. Subsequent individuation of these tendons depends on muscle activity. These results establish an integrated model for limb tendon development that provides a framework for future analyses of tendon and musculoskeletal phenotypes.
KEY WORDS: Musculoskeletal development, Tendon, Limb
Highlighted article: The analysis of tendon development in mice that have defective muscle or cartilage developmental programmes reveals a novel integrated model for limb tendon development.
INTRODUCTION
The musculoskeletal system is an efficient assembly of tissues that coordinate to enable movement and provide structural stability (Benjamin and Ralphs, 2000). Functionality of the musculoskeletal system is determined by the precise connection of each muscle to its skeletal insertion, yet little is known about the initial establishment of these connections or how the development and growth of the musculoskeletal tissues are integrated. The exquisite coordination of muscles, tendons and cartilage from early stages of development suggests that these tissues might be coordinated and interdependent during the induction, differentiation and growth of the musculoskeletal system.
In the developing embryo, the induction of axial tendons in the tenogenic compartment, the syndetome, was shown to depend on signals emanating from the adjacent myotome, as disruption of myotome formation in chick and mouse embryos resulted in failure of tendon progenitor induction (Brent et al., 2005; Brent and Tabin, 2004; Chen and Galloway, 2014). By contrast, induction of cranial tendon progenitors was not disrupted in the absence of muscle, but subsequent differentiation of cranial tendons failed in ‘muscle-less’ mutants (Chen and Galloway, 2014; Grenier et al., 2009; Grifone et al., 2008).
Studies of interdependence in the musculoskeletal system of developing limbs uncovered an additional level of complexity during tendon development. While early chick experiments concluded that muscle and tendon form autonomously in the limb (Brand et al., 1985; Chevallier et al., 1977; Shellswell and Wolpert, 1977), a later more comprehensive study identified surprising divergence in the development of distal and proximal limb tendons (Kardon, 1998). Like cranial tendons, induction of limb tendon progenitors was not dependent on muscle. However, although tendon differentiation was not perturbed in the autopod of muscle-less limbs, there was an early loss of zeugopod tendons, and no differentiated tendons were ever observed in the zeugopod of muscle-less limbs (Kardon, 1998). The muscle dependency of limb tendons was also evaluated in the mouse using the Splotch (Sp) mutant, in which disruption of the Pax3 gene results in failure of myoblast migration, thus generating muscle-less limbs (Bober et al., 1994). Similar to chick embryos, in muscle-less Sp embryos limb tendon progenitor induction was not affected but tendon progenitors subsequently failed to differentiate (Bonnin et al., 2005; Schweitzer et al., 2001). It was therefore suggested that mouse tendon development can be divided into two stages: a progenitor, muscle-independent stage followed by a differentiated, muscle-dependent stage (Bonnin et al., 2005; Tozer and Duprez, 2005). However, the early lethality of Sp embryos restricted the scope of this analysis, underscoring a need for analysis of tendon development in other mutants in which limb muscle development is perturbed.
To date, much of the work on interdependency during musculoskeletal development has focused on the effects of muscle loss, while cartilage dependency in tendon development has received considerably less attention. A few studies have suggested that the tendon and cartilage cell fates represent alternative lineages derived from a common tendo-chondro progenitor population and that inhibition of chondrogenesis may drive these progenitors toward a tendon cell fate (Brent et al., 2003, 2005; Lorda-Diez et al., 2009). This assertion was also supported by recent data showing that the tendon and cartilage cells comprising the entheses, which are the skeletal insertions of tendons, are derived from a pool of Sox9 and Scx co-expressing progenitors that then differentiate toward their respective lineages (Blitz et al., 2013; Sugimoto et al., 2013). There is, however, also some evidence that cartilage may play a direct role in tendon induction. An elegant experiment in chick demonstrated that induction of an ectopic digit by removal of interdigital ectoderm resulted in the formation of accompanying tendons (Hurle et al., 1989).
To address the open questions concerning the roles of muscle and skeleton in limb tendon development, we analyzed the tendon phenotype of mouse mutants that affect muscle or cartilage development. We find that limb tendons are regulated in a modular fashion, with separate and independent developmental programs for the zeugopod (arm) and autopod (paw) tendon segments, which depend on signals from muscle and cartilage, respectively. Collectively, these results establish a foundation for an integrated model of limb tendon induction, differentiation and growth.
RESULTS
The differentiation and maintenance of autopod tendon segments are independent of muscle
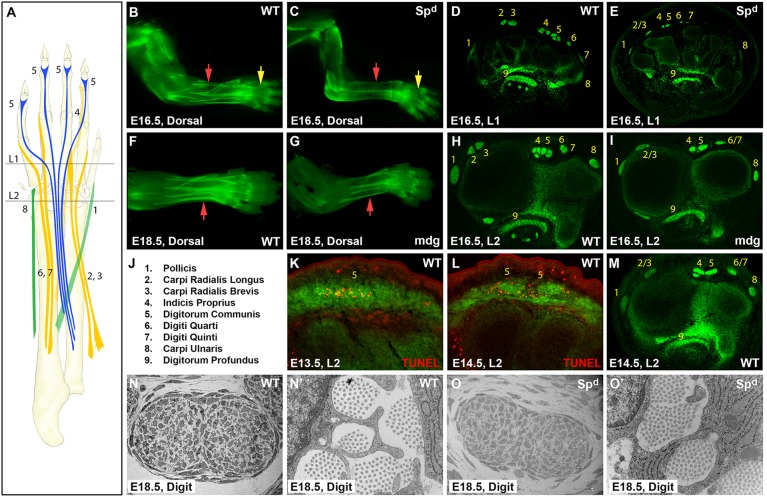
Movement of the mouse paw is largely controlled by extrinsic muscles that reside in the zeugopod. Muscle forces are transmitted by long extensor and flexor tendons that extend across the wrist and insert in the autopod (Fig. 1A) (Watson et al., 2009). To test the requirement for muscle in limb tendon formation, we used Splotch delayed (Spd) mice (Vogan et al., 1993). In contrast to the embryonic lethality before E14.5 of Pax3 loss-of-function mutations (Sp) that were used in previous studies of muscle dependency for tendons (Bober et al., 1994), Spd is a point mutation in Pax3 that also disrupts myoblast migration but mutant embryos are viable through the end of embryogenesis (Vogan et al., 1993). Using the scleraxis-GFP (ScxGFP) reporter (Pryce et al., 2007) to visualize tendons, we could not identify any tendons within the zeugopod of Spd limbs at E16.5, but found distinct tendons in the autopod, the most prominent of which were the major tendons that insert at the distal digits, namely the extensor digitorum communis (EDC) and flexor digitorum profundus (FDP) tendons (Fig. 1A-C).
Fig. 1.
Developmental modularity of limb tendons is revealed by muscle-independent autopod tendon development and muscle-dependent zeugopod tendon development. (A) Schematic of long extensor tendons from their muscle origins to skeletal insertions. Levels of transverse section are indicated (L1, L2). (B,C) Whole-mount and (D,E) transverse section images of ScxGFP WT and Spd mouse limbs at E16.5. (F,G) Whole-mount and (H,I) transverse sections of WT and mdg limbs at E16.5 and E18.5. (J) Numerical tendon assignments. (K,L) TUNEL staining of WT EDC tendon near the carpals at E13.5 and E14.5. (M) ScxGFP WT limb at E14.5. (N-O′) TEM of WT and Spd FDP tendon at digit level. Yellow and red arrows highlight autopod and zeugopod tendons, respectively.
To determine whether other tendons are also present in the autopod of Spd embryos, we analyzed transverse limb sections from E16.5 embryos. In sections through the wrist, the tendon pattern in the Spd limb was considerably different from that of wild type (WT) (Fig. 1D,E), but systematic analysis of serial sections through the autopod facilitated identification of individual tendons in the Spd mutant. In addition to the EDC and FDP, other extensor tendons were identified within the autopod, including the extensor carpi radialis longus/brevis (EL/B) and the extensor digiti quarti/quinti (EQ) (supplementary material Fig. S1). However, the tendons that insert at or near the wrist, such as the flexor carpi ulnaris, were completely absent in Spd embryos. Surprisingly, a close inspection of the zeugopod in whole-mount and transverse sections of Spd limbs also revealed thin dorsal and ventral trails of residual ScxGFP-positive cells that extended from the wrist to the elbow (Fig. 1C). The pattern and organization of these cells were uncharacteristic of any tendon and they are likely to represent residual progenitors that failed to differentiate in Spd limbs.
The distinctive appearance of autopod tendon segments in Spd embryos led us to examine whether these tendons persisted through embryogenesis, since in muscle-less chick limbs the distal tendons eventually degenerate (Kardon, 1998). We found, however, that autopod tendon segments in Spd mutants not only persisted to the end of embryogenesis (E18.5), but also generated an aligned collagen matrix that was indistinguishable at the ultrastructural level from that of WT embryos (Fig. 1N-O′).
Muscle activity is required for individuation and robustness of limb tendons
Although autopod tendon segments were formed in Spd embryos, tendon size was dramatically smaller than in WT. Analysis of transverse sections through the autopod further demonstrated that several tendons were fused near the wrist, notably the EDC, EL/B and EQ tendons (Fig. 1D,E; supplementary material Fig. S1). To distinguish a direct requirement for the presence of muscle from an indirect requirement for muscle forces in regulating tendon size and pattern, we examined tendon development in the absence of muscle activity. Muscular dysgenesis (mdg) is a spontaneous mutation in a voltage-gated calcium channel (Cacna1s) that results in the loss of muscle excitation-contraction coupling and therefore causes muscle paralysis (Pai, 1965a,b). Whole-mount images and transverse sections through autopod levels revealed noticeably smaller tendons in mdg mutants than WT at E16.5 (Fig. 1F-I), although not to the same extent as in Spd mutants. This suggests that although muscle contraction plays a key role in the regulation of tendon growth, the physical presence of muscle is likely to provide additional independent signals that regulate tendon size.
Unlike the Spd mutants, tendon formation in the zeugopod was not perturbed in mdg embryos (Fig. 1F-I). However, similar to Spd embryos, we identified tendon fusion in mdg, including fusion of the EDC tendons, as well as the EL/B tendons, and the EQ tendons. Interestingly, these tendons were fused only near the wrist; in more proximal sections of mdg limbs, the fused tendons separated and the tendon pattern was similar to WT (not shown). To determine the relationship between muscle activity and tendon fusion, we examined WT limbs at the onset of muscle activity, and found that the same tendons were fused at the wrist of E14.5 WT embryos (Fig. 1M; supplementary material Fig. S2). These observations suggest that normal development of these extensor tendons (EDC, EL/B and EQ) consists of the initial formation of a fused tendon anlage near the wrist, and that individuation of these anlagen into distinct tendons depends on muscle activity. Notably, TUNEL assays in WT embryos identified localized cell death at the EDC anlage at E13.5 and E14.5, underscoring the separation of the fused EDC anlage at the wrist into individuated EDC tendons by E15.5 (Fig. 1K,L), but cell death was not observed during the individuation of the other fused tendons. These results demonstrate that muscle contraction regulates some aspects of tendon size and patterning, but it is not essential for tendon formation or the continued maintenance and differentiation of tendon progenitors.
Tendon induction in the autopod is dependent on skeletal cues
The observations in muscle-less limbs suggest that development of the autopod and the zeugopod segments of limb tendons are regulated by distinct and independent developmental programs. Although we found that tendon segments within the autopod develop and persist in the absence of muscle, the signals that drive their development remain largely unknown. However, the induction of tendon progenitors along the forming cartilage condensations and a few published observations (Hurle et al., 1990; Yamamoto-Shiraishi and Kuroiwa, 2013) suggest a possible role for cartilage in autopod tendon induction. In the limb, cartilage specification requires the activity of the transcription factor Sox9 (Akiyama et al., 2002), and subsequent differentiation of the cartilaginous anlagen depends on the activities of Sox5 and Sox6 (Smits et al., 2001). To examine the role of cartilage in limb tendon development, we therefore analyzed tendon development in mutants for these genes.
In Sox5−/−;Sox6−/− double mutants, in which cartilage condensations fail to differentiate (Smits et al., 2001), the majority of limb tendons appeared normal (supplementary material Fig. S3). Conversely, tendon formation was severely affected in conditional Sox9f/f;Prx1Cre mutants (Prx1 is also known as Prrx1), in which Sox9 expression is eliminated in limb bud mesenchyme, resulting in early failure of mesenchymal condensations and the development of ‘skeletal-less’ limbs (Akiyama et al., 2002). Scx expression was completely absent from the autopod of Sox9f/f;Prx1Cre embryos at E13.5, but Scx-expressing tendon structures could be detected in the zeugopod of these embryos (Fig. 2A,B).
Fig. 2.
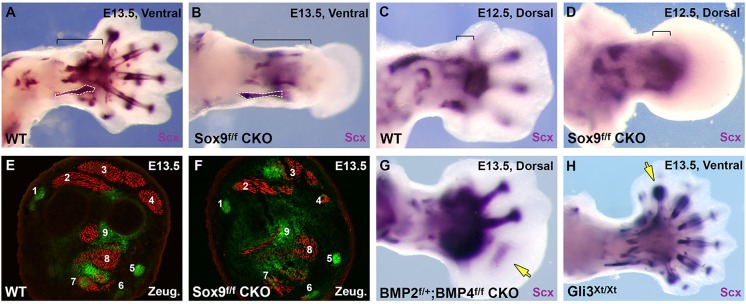
Autopod tendon development depends on cartilage. (A-D) Whole-mount in situ hybridization for Scx expression in WT and Sox9Prx1Cre limbs at E12.5 and E13.5. Analogous zeugopod tendons are outlined in A,B. Brackets delineate wrist tendon progenitors at E12.5 and zeugopod tendons derived from these progenitors at E13.5. (E,F) Transverse sections of ScxGFP WT and Sox9Prx1Cre zeugopod stained for MHC at E13.5. (G,H) Whole-mount in situ hybridization for Scx expression of conditional Bmp2f/+;Bmp4f/f;Prx1Cre limbs and Gli3Xt/Xt limbs at E13.5. Arrows (G,H) highlight missing or extra tendons.
Since apoptosis is very broad in the autopod mesenchyme of Sox9f/f;Prx1Cre embryos beginning at E13.5 (Akiyama et al., 2002), we examined Scx expression at earlier stages. At E12.5, as the WT autopod was beginning to form distal digit protrusions, the Sox9f/f;Prx1Cre autopod appeared as a smooth paddle, indicating an early arrest of autopod development (Fig. 2C,D). Although the pattern of Scx expression was normal in the stylopod and the zeugopod of mutant embryos, autopod expression was limited to a broad patch near the presumptive wrist (Fig. 2D). By E13.5, Scx expression was lost in the wrist and carpal levels of Sox9f/f;Prx1Cre mice, as the autopod failed to develop further. By contrast, the formation of zeugopod tendon segments was not disrupted in the same mutant limbs and the tendon pattern was similar to that in WT limbs. Transverse sections taken through WT and mutant limbs, in which the tendons were highlighted by ScxGFP and the muscles by MHC immunostaining, showed a striking similarity between the individual tendon and muscle groups of WT and mutant embryos, suggesting that soft tissue differentiation and patterning at zeugopod levels did not depend on skeletal signals (Fig. 2E,F).
To further test whether the induction and differentiation of zeugopod tendons are indeed independent from tendon development in the autopod, we examined other mutants in which autopod development is selectively affected. It was previously shown that autopod development depends on the combined activities of the Hoxa13 and Hoxd13 genes (Fromental-Ramain et al., 1996). In Hoxa13+/−;Hoxd13−/− embryos, a slightly milder allele combination in which autopod development is also largely arrested, we found that the autopod segments of limb tendons again failed to form, whereas zeugopod segments of the same tendons developed normally (supplementary material Fig. S4).
The failure of Scx induction in the autopod of skeletal-less limb buds suggested that prechondrogenic condensations might play a role in autopod tendon development; however, it was also possible that the effect on Scx expression was simply due to the severe disruption of autopod mesenchyme in the mutant embryos. We therefore examined the effect of a milder disruption of autopod chondrogenesis on tendon induction. Conditional targeting of Bmp2 and Bmp4 in limb mesenchyme in Bmp2f/+;Bmp4f/f;Prx1Cre compound mutants results in loss of posterior digits in the forelimb due to a posterior expansion of the limb bud and a failure of digit specification (Bandyopadhyay et al., 2006). Consistent with our previous observations, the loss of the posterior digits was accompanied by loss of their associated tendons (Fig. 2G), again highlighting the coupling between digit cartilage and tendon progenitor induction in the autopod.
To test whether cartilage is not only necessary but also sufficient for tendon induction, we next evaluated if the formation of extra digits in mouse embryos would be accompanied by the induction of an associated tendon, as was previously shown in chick embryonic experiments (Hurle et al., 1990). In the developing limb, Gli3 functions as a key regulator of digit formation and loss of Gli3 results in an array of extra digits (te Welscher et al., 2002; Vortkamp et al., 1992). Consistent with the results presented above, whole-mount in situ hybridization for Scx expression revealed distinct flexor and extensor tendons connected to each of the supernumerary digits in Gli3Xt/Xt mutant limbs at E13.5 (Fig. 2H).
Collectively, these data indicate that cartilage condensations are both necessary and sufficient for induction of autopod tendons. Moreover, when combined with the results from muscle-less limbs, these results suggest a striking modularity in the formation of limb tendons. In skeletal-less limbs, tendons develop in the zeugopod despite the absence of their distal autopod segments. Conversely, in muscle-less limbs, the autopod segments of tendons were formed but the zeugopod segments of these same tendons were missing and tendons that reside completely within the zeugopod and insert at the wrist were also completely missing (Fig. 1; supplementary material Fig. S1). These results suggest a modular construction of limb tendons, whereby independent developmental programs for the autopod and zeugopod tendon segments are combined to form contiguous functional entities.
Proliferation and lineage tracing reflect separate regulation of autopod and zeugopod tendon development
The distinct nature of the genetic programs that direct tendon formation in the autopod and zeugopod was also apparent when we analyzed proliferation and cell lineage in developing tendons. Using BrdU incorporation to label dividing cells in ScxGFP embryos, we evaluated proliferation in developing tendons between E12.5 and E15.5. Although we observed extensive proliferation in all ScxGFP-positive regions throughout the limb at E12.5, tendon progenitors in the autopod stopped proliferating by E13.5 and remained largely quiescent up to E15.5 (Fig. 3; supplementary material Fig. S5). By contrast, tendon cells in the zeugopod showed high levels of proliferation through all stages of limb development (Fig. 3; supplementary material Fig. S5). Notably, this difference in autopod and zeugopod tendon proliferation was consistent for all of the long tendons, so that within a single continuous tendon, such as the EDC and FDP, the autopod segments were non-proliferative at E14.5, while the zeugopod segments of the same tendons were highly proliferative.
Fig. 3.

Distinct regulatory patterns of tenocyte proliferation in autopod and zeugopod tendon segments. BrdU was detected using DAB staining and the images were overlaid with ScxGFP signal from an adjacent section to highlight cell proliferation in tendons. (A) Autopod and (B) zeugopod tendons at E12.5. (C) Autopod and (D) zeugopod tendons at E14.5. Enlarged views of the boxed areas in A-D are shown in A′-D′. Arrows highlight representative proliferative behavior in tendons.
Additional support for separate autopod and zeugopod tendon programs emerged from efforts to trace cell lineage in limb tendons, most distinctly when we evaluated the fate of progenitors that express the Six2 homeodomain transcription factor. Six2 was one of the first transcription factors shown to be expressed in developing tendons and it was subsequently implicated in limb muscle and tendon development (Laclef et al., 2003; Oliver et al., 1995; Yamamoto-Shiraishi and Kuroiwa, 2013). Six2 expression is first detected in the developing autopod in a pattern that is very similar to that of Scx. However, in contrast to Scx expression, which highlights all tendon progenitors (Fig. 4A-E), in situ hybridization of transverse sections taken through the autopod showed Six2 expression at E13.5 in distinct tendons and muscles, with strong expression in the FDP tendons as well as the muscle progenitors comprising the flexor digitorum superficialis (FDS). Six2 expression was also detected in the digit segments of the EDC tendons, but was hardly detectable at the carpal level in EDC tendons, whereas Six2 expression was still robust in the FDP tendons.
Fig. 4.
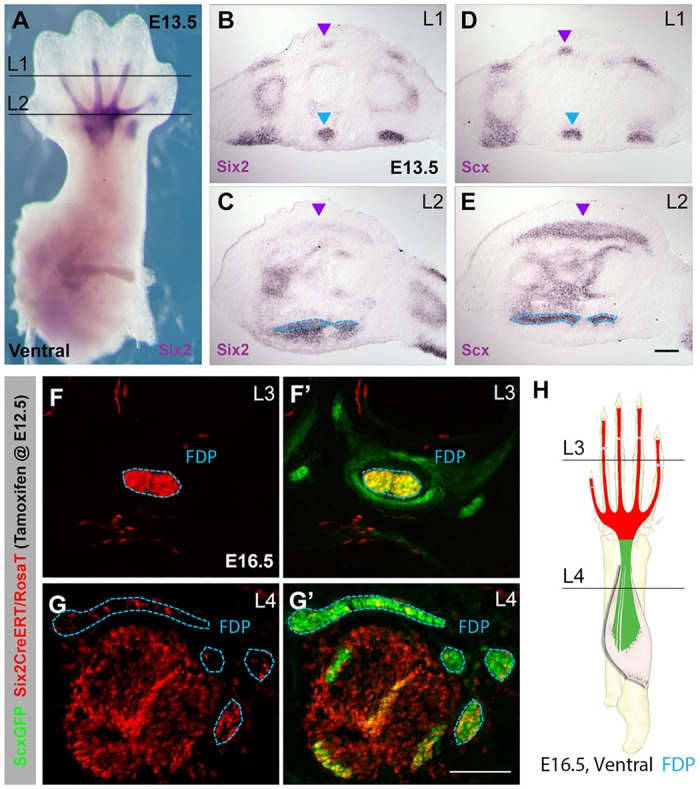
The autopod and zeugopod segments of the FDP tendons are derived from separate progenitor populations. (A) Whole-mount in situ hybridization for Six2 at E13.5. (B,C) Six2 and (D,E) Scx in situ hybridization of transverse sections (levels indicated in A). (F-G′) Transverse sections through limb of Six2CreERT2;RosaT;ScxGFP embryos at E16.5; tamoxifen was given at E12.5. The Six2 cell lineage highlighted by the RosaT signal (F,G) was overlaid with ScxGFP signal to identify the affected tendons. (H) Schematic showing lineage distribution in FDP tendons. Blue and purple arrowheads and outlines indicate FDP and EDC tendons, respectively. FDS muscles and tendons are also derived from Six2 lineage cells (non-outlined red tissues in G,G′). Scale bars: 50 μm.
Because of the distinctive expression of Six2 in FDP tendons, we used the inducible Six2CreERT2 deleter combined with the Rosa-TdTomato Cre reporter to trace the lineage of FDP tendon cells (Kobayashi et al., 2008; Madisen et al., 2010). Pregnant Six2CreERT2 females were injected with tamoxifen at E12.5, labeling early Six2-expressing cells, and the fate of Six2 lineage cells was evaluated at E16.5. Transverse sections through the forelimbs of these embryos showed a sharp demarcation in cell lineage. Whereas the autopod segments of the FDP were strongly labeled and thus derived from the early Six2-expressing cells, the zeugopod segments were mostly unlabeled (Fig. 4F-H). These results demonstrate that the autopod and zeugopod segments of the FDP are formed by different progenitor pools, again highlighting the distinct processes that regulate tendon formation in these two limb compartments.
Integration of the autopod and zeugopod developmental programs of limb tendons
Although we established that tendon development is modular and autopod or zeugopod tendon segments can develop in the absence of the other, normal tendon development does not involve the independent development and fusion of two separate segments. To determine how the independent programs for tendon development in the autopod and zeugopod are integrated, we re-examined the initial events in limb tendon development. In WT embryos, Scx expression is first detected early in limb bud development at E10.5, but a recognizable pattern reflecting tendon organization emerges only at E12.5. By E13.5, the loosely organized progenitors condense to form well-delineated functional tendons (Schweitzer et al., 2001; Murchison et al., 2007; Pryce et al., 2009) (Fig. 5A-F). Consistent with previous studies, we found that Scx induction in the forming autopod and zeugopod of Spd muscle-less embryos was not significantly affected up to E12.5 (Bonnin et al., 2005; Schweitzer et al., 2001); however, the differentiation and maintenance of the zeugopod tendon progenitors were impaired in the absence of muscles, leading to complete loss of tendons and tendon progenitors from the zeugopod by E13.5 (Fig. 5G,H) (Bonnin et al., 2005).
Fig. 5.
Zeugopod tendons develop via muscle anchoring, followed by elongation in parallel with skeletal growth. (A-D) Whole-mount in situ hybridization of WT limbs for Scx from E10.5 to E13.5. (E) Sagittal and (F) transverse sections of E12.5 limbs stained for muscle (MHC) and cartilage (collagen type II). Arrow (E) indicates integration of tendon to muscle. (G,H) Scx expression in whole-mount Spd limbs, as compared with WT (C,D), at E12.5 and E13.5. Brackets delineate wrist tendon progenitors at E12.5 and zeugopod tendons derived from these progenitors at E13.5. (I) Whole-mount MHC-stained Col2GFP limb at E12.5 and whole-mount ScxGFP limb at E12.5 (left); whole-mount ScxGFP limbs with muscle labeled by MHC staining at E12.5 and E13.5 (center); or Pax7Cre at E14.5 (right). Yellow arrows highlight the wrist. Individual tendons in I can be identified using the schematic and numerical assignments in Fig. 1.
These observations prompted us to examine the events surrounding the organization of the forming musculoskeletal system at these early stages. Interestingly, combined detection of tendon, muscle and cartilage progenitors (visualized by Scx in situ hybridization, and MHC and collagen type II immunostaining, respectively) revealed that, at E12.5, loosely packed tendon progenitors were already positioned between the forming muscles and cartilage (Fig. 5E,F), similar to previous results from the chick limb (Hurle et al., 1990; Kardon, 1998; Ros et al., 1995). The alignment and integration of the musculoskeletal system thus appears to begin at E12.5, under the influence of limb bud patterning cues, even before the condensation of tendon progenitors to form load-bearing tendon structures at E13.5.
To determine the spatial relationships between the musculoskeletal tissues in these early stages, we next examined these tissues in whole-mount preparations, using Col2GFP and ScxGFP limbs with muscles visualized by MHC staining or by Pax7Cre. Interestingly, we found that at E12.5 the muscles spanned the entire length of the short zeugopod skeleton and were attached to tendon progenitors induced in the autopod via short tendon segments near the forming wrist (Fig. 5I, arrows). Long tendons within the zeugopod only became apparent at E13.5, and tendon elongation continued in later stages concurrent with zeugopod skeletal growth.
The tendon anlagen at the wrist therefore represent a distinct population of progenitors that plays a central role in the integration of the musculoskeletal system by connecting the autopod tendon segments with their corresponding muscles in the zeugopod (Fig. 5). Although the wrist progenitors appear contiguous with the autopod tendons already at E12.5, their unique nature is highlighted by the fact that, unlike autopod progenitors, induction of these wrist tendon anlagen is independent of both skeleton and muscle since wrist progenitors can be observed at E12.5 in both skeletal-less and muscle-less mutants (brackets in Figs 5 and 2, respectively). These wrist elements are initially induced as blocks of tendon progenitors that connect groups of autopod tendons to muscles. For example, whole-mount ScxGFP limbs at E12.5 showed a broad triangular patch of progenitors comprising the future EDC tendons (Fig 5I; supplementary material Fig. S2), connecting directly to muscle. Similarly, fused wrist anlagen for the EL/B and the EQ tendons were also observed (Fig. 5I). These tendons elongate as individual tendons into the zeugopod, but the fused elements at the wrist are subsequently separated only at E15.5 depending on muscle activity (Fig 1; supplementary material Fig. S2).
These observations suggest a new model for musculoskeletal integration that combines the distinct programs for autopod and zeugopod tendon development and suggests that zeugopod tendon development occurs in two stages: at E12.5, the muscles that span the zeugopod anchor to autopod-induced tendons via tendon anlagen whose induction is independent of muscle; subsequently, these tendons elongate from the wrist into the zeugopod, in tandem with zeugopod skeletal growth. The long tendons are thus formed as continuous structures from two progenitor populations and the musculoskeletal system remains connected through this process.
Development of FDS tendons is also modular with a demarcation at the metacarpophalangeal joint
The developmental modularity that we observed for limb tendons identified the wrist as a key integration site for limb tendon development, with one exception – the FDS tendons. Like the FDP and EDC tendons, the FDS tendons extend from skeletal insertions at the interphalangeal joints to muscles in the zeugopod (Fig. 6A), but the FDS undergoes a unique development program. FDS muscles differentiate in the autopod and, starting at E14.5, translocate out of the autopod to achieve their final position in the zeugopod by E16.5 (Huang et al., 2013). Whereas most limb tendons are already formed by E14.5, the FDS tendons at that stage consist only of a narrow flattened band attached to the distal end of FDS muscles and ‘cupping’ the FDP tendons near the metacarpophalangeal (MP) joint (Fig. 6A) (Huang et al., 2013; Watson et al., 2009). Between E14.5 and E16.5, the FDS tendons grow both distally and proximally in tandem with muscle translocation to form the digit and metacarpal/zeugopod segments of the tendon, respectively (Fig. 6A). Having identified modularity as a key feature in the development of limb tendons we next examined whether the FDS tendons also follow a similar developmental program.
Fig. 6.

FDS tendons are formed via distinct cell populations. (A) Schematic of FDS tendon and muscle development. Adapted from Huang et al. (2013). (B-C″) Lineage tracing with Sox9Cre and Rosa26-TdTomato at E16.5. TdTomato (B′,C′) and ScxGFP and TdTomato (B″,C″) overlays are shown. Orange and blue arrowheads highlight FDS and FDP tendons, respectively.
The bidirectional growth of FDS tendons from the initial ‘cup’ anlage suggested a possible demarcation point for this tendon at the MP joint. Analyzing the cell lineage labeled by the Sox9Cre deleter, which labels all cartilage cells as well as a subset of tendon cells (Blitz et al., 2013; Soeda et al., 2010; Sugimoto et al., 2013), we indeed identified a cell lineage boundary at the MP level of the FDS tendons. Whereas the digit segments were largely composed of Sox9 lineage cells, the metacarpal segments of FDS tendons were nearly devoid of these cells (Fig. 6B,C). The digit and metacarpal segments of the FDS tendons thus do not share a common cellular origin.
We previously showed that the metacarpal FDS segments are formed as the FDS muscles translocate from the paw (Huang et al., 2013). In cases in which FDS muscle translocation was arrested (e.g. in paralyzed mdg mutant embryos), FDS tendon development was similarly arrested, suggesting a dependence on muscle. To investigate FDS tendon muscle dependency, we again examined the muscle-less Spd mutant. Transverse sections at E16.5 revealed that, whereas autopod FDP tendon segments were normal, the FDS tendons were completely absent in sections through the metacarpals (Fig. 7A-D). Notably, induction of digit segments of the FDS tendons was not impaired in the absence of muscle, although these tendons were significantly smaller than those of WT embryos, suggesting that although induction of the digit segments of FDS tendons was not dependent on muscle, the robustness of these tendons is likely to depend on connectivity and the application of contractile forces. This observation was reinforced by analysis of FDS tendons in paralyzed mdg embryos and in Scx−/− embryos, in which the FDS muscles are not connected to the digit tendon segments (Huang et al., 2013) and the FDS digit tendon segments were much reduced in size, although correctly patterned (Fig. 7).
Fig. 7.
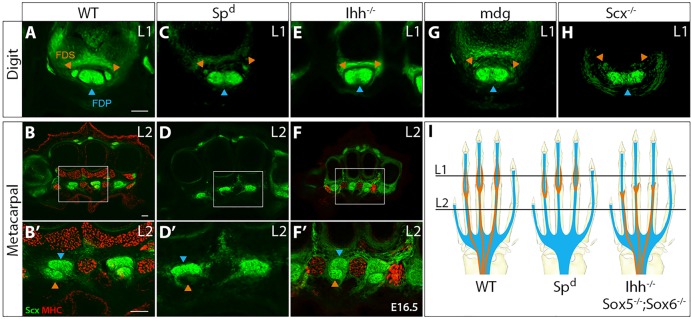
FDS tendon development is also modular and depends on muscle and cartilage. Transverse sections stained for MHC from (A,B) WT, (C,D) Spd and (E,F) Ihh−/− embryos at E16.5. Enlarged views of the boxed areas in B,D,F are shown in B′,D′,F′. Digit FDS tendons in (G) paralyzed mdg mutants and (H) Scx−/− mutants. (I) Schematic indicates digit and metacarpal levels and depicts the modular development of FDS tendons. Orange and blue arrowheads highlight FDS and FDP tendons, respectively. Scale bars: 50 μm.
The dependence of proximal but not distal segments of FDS tendons on muscle suggested an inherent similarity with the modular construction of other limb tendons. To test a possible requirement for cartilage, we examined FDS tendon development in the absence of crucial genes that govern later aspects of skeletal development, since the severe autopod phenotype in Sox9 mutants precludes analysis of the FDS tendons (Fig. 5) (Akiyama et al., 2002). In Runx2−/− embryos, in which bone formation is disrupted (Ducy et al., 1997; Komori et al., 1997; Otto et al., 1997), FDS tendons appeared normal (not shown). Conversely, Sox5−/−;Sox6−/− double-null mutants, in which cartilage differentiation is disrupted (Smits et al., 2001), revealed a phenotype consistent with modular development of the FDS tendons; in transverse sections of Sox5−/−;Sox6−/− E16.5 forelimbs, the digit segments of the FDS tendons were completely missing, but the metacarpal and zeugopod segments of the FDS tendons were normal (supplementary material Fig. S3). Surprisingly, Indian hedgehog null embryos (Ihh−/−) in which endochondral ossification is impaired (St-Jacques et al., 1999; Vortkamp et al., 1996) harbored an identical FDS tendon phenotype, despite the very different skeletal phenotype (Fig. 7).
Taken together, these results demonstrate that development of the FDS tendons is also modular, with a developmental boundary at the MP joint instead of at the wrist. Induction of the distal (digit) tendon segment depends on a signal from the cartilage, whereas development of the proximal (metacarpal and zeugopod) segment depends on the interaction with muscle. We therefore conclude that although certain features of FDS tendon development are unique, the key aspects of modular development with distinct dependence on muscle and cartilage cues are universal for all limb tendons.
DISCUSSION
This study elucidates the principles that govern limb tendon development, establishing a novel conceptual framework for the analysis of limb tendon phenotypes. We found that the development of the long autopod tendons is strikingly modular, in that independent programs regulate formation of the autopod and zeugopod segments of each tendon. We also identified a unique population of wrist tendon progenitors that mediate musculoskeletal integration by connecting the muscles in the zeugopod to the tendon progenitors induced at the autopod. Whereas autopod tendon segments are formed by the cartilage-dependent induction of tendon progenitors, the zeugopod segments are formed in two stages (Fig. 8). First, integration occurs at E12.5, when differentiating muscles that span the length of the very short zeugopod connect via tendon progenitor anlagen at the wrist to newly induced tendon progenitors in the autopod. In the second stage of elongation, muscle-dependent elongation of tendons occurs from the wrist anlagen, in tandem with zeugopod skeletal growth. Although tendon elongation into the zeugopod occurs with an individual tendon for each muscle, the tendons are initially fused at wrist level, and complete individuation of the tendons is dependent on muscle activity and manifests only by E15.5.
Fig. 8.

Model of zeugopod tendon formation as a distinctly regulated developmental process. Muscle-independent tendon induction of wrist progenitors occurs at E12.5, thus integrating the musculoskeletal tissues (muscle-tendon-cartilage). Whereas autopod tendon development requires signals from cartilage, zeugopod tendons undergo a muscle-dependent elongation phase in parallel with skeletal growth, such that the extent of skeletal growth dictates the extent of tendon elongation. The requirement for muscle for elongation lies in early attachment to the wrist tendon anlagen, and subsequent individuation and robustness of tendon depend on muscle forces. Blue highlights autopod tendon progenitors and tendons; purple highlights the wrist anlagen and the wrist-derived zeugopod tendons. Although the EDC tendons are shown here, this model of tendon formation applies to all autopod tendons with the exception of the FDS tendons, which are highlighted separately in Fig. 9.
Wrist tendon progenitor anlagen coordinate musculoskeletal integration
Tendons function as contiguous structures that transmit the forces generated by muscle contraction from early embryonic stages. Integration of the system begins at E12.5, and structural continuity from zeugopod to autopod is enabled by the wrist tendon anlagen. Unlike autopod tendon progenitors, induction of the wrist anlagen is not dependent on cartilage or muscle; they function as short-range connectors between the autopod tendon elements and their respective muscles. However, nothing is known about the molecular and cellular mechanisms that mediate these events.
Autonomous induction of tendon progenitors that is not dependent on muscle was previously demonstrated in a number of studies (Bonnin et al., 2005; Edom-Vovard et al., 2002; Kardon, 1998; Schweitzer et al., 2001). Consistent with these studies, we showed that wrist tendon anlagen were induced in the absence of muscle but subsequently degenerated. The signals that regulate the initial induction of wrist tendon progenitors remain unknown, but it is likely that TGFβ signaling is involved. We previously found that TGFβ signaling is a potent inducer of tendon progenitors and loss of TGFβ signaling resulted in complete loss of tendons (Pryce et al., 2009). Tendon loss is first manifested at E12.5 and wrist tendon anlagen are never detected in these mutants. It is therefore possible that induction of the wrist tendon anlagen is dependent on TGFβ signaling. Interestingly, it was recently demonstrated that induction of a progenitor population for the cartilage and tendon components of the entheses depends on TGFβ signaling (Blitz et al., 2013). The wrist tendon anlagen might therefore represent a unique version of such a progenitor population that, instead of mediating connection of muscle to cartilage, mediates attachment of the muscles to the autopod tendon segments.
Multiple roles for muscle and muscle activity in limb tendon development
Surprisingly, autopod tendon progenitors for the long tendons were not only maintained in Spd mutants but also differentiated and persisted through the end of embryogenesis, unlike the previous reports of tendon development in muscle-less limbs in chick (Kardon, 1998). The autopod tendons that developed in muscle-less embryos were, however, substantially smaller than those of WT, indicating that muscle forces may play a role in regulating tendon size during embryogenesis. Indeed, analysis of paralyzed embryos revealed thinner tendons at all levels of the limb. Muscles thus play a dual role in tendon development. On the one hand, muscle activity is required for lateral tendon growth, but, on the other hand, the very presence of muscle is required for maintenance of the wrist tendon anlagen, and for tendon elongation into the zeugopod from wrist progenitors, since zeugopod tendons were formed in paralyzed mutants but missing in muscle-less mutants. The specific role of the muscles in these processes is yet to be determined; the muscles might simply function as anchoring elements that allow the connected tendon progenitors to experience the mechanical strain imparted by skeletal growth. However, the requirement for muscle might also be molecular or cellular in nature; elucidating the parameters that regulate tendon growth will therefore be the focus of future studies.
Cartilage is necessary and sufficient for the induction of autopod tendon progenitors
In recent years, a number of studies have posited the existence of a chondro-tendo progenitor population (co-expressing Scx and Sox9) that gives rise to either chondrocytes or tenocytes during early development (Blitz et al., 2013; Sugimoto et al., 2013). The failure of cartilage differentiation in Sox5−/−;Sox6−/− double mutants has also been associated with an expansion of axial tendon progenitors, suggesting a conversion of chondroprogenitors to a tenogenic cell fate in the absence of these cartilage-specific transcription factors (Brent et al., 2005). It was therefore surprising that we did not find a comparable expansion of limb tendons or Scx-expressing tendon progenitors in response to the disruption of chondrocyte differentiation in Sox9Prx1Cre, Sox5−/−;Sox6−/−, Bmp2f/+;Bmp4f/f;Prx1Cre or Ihh−/− mutants. Conversely, we found that induction of autopod tendons was entirely dependent on cartilage, and tendon progenitors were not induced in the absence of cartilage in either Sox9Prx1Cre mutants or Bmp2f/+;Bmp4f/f;Prx1Cre double mutants, whereas tendons were induced in all of the supernumerary digits in Gli3Xt/Xt mutant limbs.
These results suggest that, in the autopod, cartilage might be the source of inductive factors for tendon progenitors. We previously showed that the condensing autopod cartilage expresses TGFβ2 ligand and that TGFβ signaling is a potent inducer of Scx, suggesting a model whereby TGFβ factors secreted from cartilage may induce tendon progenitors (Pryce et al., 2009). However, in the E12.5 autopod, early Scx expression is only detectable in subectodermal mesenchyme, and Scx is not expressed by mesenchymal cells closer to the cartilage condensation. An alternative model was recently suggested based on evidence that Scx expression can be induced by Wnt signaling in the autopod (Yamamoto-Shiraishi and Kuroiwa, 2013) and that BMP signaling is antagonistic to Scx expression (Schweitzer et al., 2001). In this context, Scx may be broadly induced by ectodermal Wnt factors and the restriction of Scx expression to the regions near the autopod cartilage condensations may be achieved by interdigital BMP expression (Knosp et al., 2004; Zou and Niswander, 1996). Since the cartilage condensations also express the BMP antagonist noggin, the role of cartilage in this process might be in the maintenance of Scx expression by local repression of BMP signaling (Yamamoto-Shiraishi and Kuroiwa, 2013).
The unique development of FDS tendons highlights general aspects of tendon modularity
The FDS tendon is a useful model for tendon modularity since its development is delayed relative to other tendons and there are genetic perturbations that only affect this specific tendon. Consistent with other limb tendons, however, formation of the distal and proximal FDS segments is modular and each segment can differentiate in the absence of the other (Fig. 9).
Fig. 9.

Model of FDS tendon formation and its unique features. Like the other autopod tendons, FDS tendons form first by induction of a short-range anlage attached to muscle, followed by the modular formation of independent distal and proximal tendon segments that depend on signals from cartilage and muscle, respectively. Unlike the other tendons, FDS tendon development is delayed (relative to all other tendons), the developmental boundary for modularity is the MP joint instead of the wrist, and proximal tendon elongation is regulated by a unique process of active muscle translocation from the paw into the arm (described by Huang et al., 2013). Overall, the development of FDS tendons is consistent with our general conceptual framework for limb tendon development, yet its unique features highlight these tendons as a useful model with which to test modularity and tendon elongation.
In a previous study, we found that proximal FDS tendon formation was tightly coupled to FDS muscle translocation. In mutants in which FDS muscle translocation was partially arrested, proximal FDS tendon growth was likewise arrested, such that the extent of muscle translocation dictated the extent of FDS tendon elongation (Huang et al., 2013). Although active FDS muscle translocation is a unique process specific to this muscle, the mechanical signal imparted to the FDS tendon anlage is likely to be analogous to the mechanical signals imparted to the wrist tendon anlagen of the other limb tendons as the zeugopod skeleton elongates, further supporting our hypothesis that the main function of muscle in the process of tendon elongation is as a stable anchoring element.
Likewise, the requirement for skeletal signals in distal tendon induction was conserved in FDS tendon development. In Sox5−/−;Sox6−/− and Ihh−/− mutants, the digit segment of the FDS tendon was never induced. Since other tendons were largely unaffected, whereas skeletal development was severely affected, these results suggest that Ihh and Sox5/6 do not play direct molecular roles in tendon differentiation, but that secondary signals from the skeleton might regulate FDS tendon development. Interestingly, although the skeletal phenotypes of these mutants are significantly different, a phenotypic feature common to both mutants is the loss of all phalangeal joints (Dy et al., 2010; St-Jacques et al., 1999). The possibility that induction of the FDS digit segment is dependent on cues from the forming joint is further supported by the fact that formation of the MP joint is concurrent with the initial stages of FDS digit tendon induction (Li et al., 2010). Moreover, the bilateral symmetry of the MP joint also mirrors the bifurcated structure of the FDS digit tendons, suggesting that the cues governing joint symmetry might also regulate FDS digit tendon induction.
Tendon modularity: an evolutionary perspective
The independent and modular development of autopod and zeugopod tendon segments is intriguing and likely to reflect the evolutionary history of these tendons. In mammals, muscles are largely restricted to a single limb segment and do not cross the wrist or elbow (Diogo et al., 2009). In amphibians, however, extrinsic muscles of the hand extend across the wrist and connect to tendons that are all contained within the autopod (Ashley-Ross, 1992; Diogo et al., 2009; Walthall and Ashley-Ross, 2006). The basic mechanisms that regulate autopod tendon formation are therefore likely to be conserved. For example, in regenerating newt limbs, tendons are formed in the autopod even when the regenerating limb does not include muscles (Holder, 1989), similar to the muscle-independent development of autopod tendon segments in the mouse. Since amphibians possess mostly autopod tendons, the mechanism of muscle-dependent tendon elongation, which is characteristic of the zeugopod tendon segments, is either not significantly utilized in amphibians or might be a later evolutionary addition.
The absence of significant tendon elongation in amphibians is further emphasized by the differences in tail anatomy between mammals and amphibians. Whereas the mouse tail contains a large number of long tendons that traverse long distances between muscles at the base of the tail and skeletal insertions in each of the tail vertebra (Shinohara, 1999), amphibian tails are largely muscular, connected to short local tendons. Notably, long tendons that extend across limb and tail segments can be found both in birds and lizards (Kardon, 1998; Proctor and Lynch, 1993; Zippel et al., 1999), suggesting that this simple mechanism, in which soft tissue growth is dictated by skeletal growth, exists in most tetrapod clades.
The ability of tendons to accommodate skeletal growth by elongation provides an elegant solution for the coordinated growth of musculoskeletal tissues. This simple rule of musculoskeletal growth implies that the dramatic changes in body size during growth of the individual, or changes in the length of individual bones through evolutionary mechanisms, do not require coordinated modifications in the developmental programs of all musculoskeletal tissues. Instead, a change in skeletal growth would indirectly co-opt the soft tissues, and thus ensure coordinated growth. The implied simplicity of morphological change might be fundamental to the remarkable diversity of size and form in terrestrial vertebrates.
MATERIALS AND METHODS
Mice
Existing mouse lines used in these studies were described previously: ScxGFP tendon reporter (Pryce et al., 2007), Col2GFP cartilage reporter (Grant et al., 2000), Six2CreERT2 (Kobayashi et al., 2008), Spd (Vogan et al., 1993), mdg (Pai, 1965a,b), Sox9f/f (Akiyama et al., 2002), Prx1Cre (Logan et al., 2002), Gli3Xt/Xt (Vortkamp et al., 1992), Sox9Cre (Akiyama et al., 2005), Ihh−/− (St-Jacques et al., 1999), Sox5−/−;Sox6−/− (Lefebvre et al., 2001), Hoxa13+/−;Hoxd13−/− (Davis and Capecchi, 1996; Stadler et al., 2001), Bmp2f/+;Bmp4f/f (Bandyopadhyay et al., 2006; Selever et al., 2004), Scx−/− (Murchison et al., 2007) and Ai14 Rosa26-TdTomato reporter (RosaT) (Madisen et al., 2010). All animal procedures were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University and are consistent with animal care guidelines.
Histology
Standard protocols for whole-mount and section in situ hybridization, immunostaining, BrdU and TUNEL labeling were performed as previously described (Murchison et al., 2007). Patterning of limb tendons and muscles was acquired in serial transverse sections (Watson et al., 2009). A monoclonal antibody for My32 (Sigma, M4276) was used to detect muscle-specific type II myosin heavy chain (MHC).
Transmission electron microscopy (TEM)
E18.5 mouse limbs were fixed in 1.5% glutaraldehyde/1.5% paraformaldehyde (Tousimis Research Corporation) in Dulbecco's serum-free media (SFM) containing 0.05% tannic acid, followed by an extensive rinse in SFM, then post-fixation in 1% OsO4. Samples were washed in SFM then dehydrated in a graded series of ethanol to 100%, rinsed in propylene oxide, and infiltrated in Spurrs epoxy. Samples were polymerized at 70°C over 18 h.
Whole-mount confocal microscopy
Mouse forelimbs from E12.5-E14.5 were fixed in 4% paraformaldehyde overnight at 4°C. Following fixation, whole-mount immunostaining for MHC was carried out for E12.5 and E13.5 limbs as previously described (DeLaurier et al., 2006). Limbs were then incubated in Sca/e2 solution at 4°C until cleared (Hama et al., 2011). A Zeiss LSM780 laser scanning confocal microscope was used to acquire 10× tiled z-stack images. Image processing was carried out using Zeiss Zen software to stitch tiles and obtain maximum intensity projection images.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health [AR055640, AR055973] and Shriners Hospital [85410-POR-14] grants to R.S. and a postdoctoral fellowship from the Arthritis Foundation to A.H.H. Deposited in PMC for release after 12 months.
Author contributions
A.H.H., T.J.R., B.P., J.L.W. and S.S.W. contributed to experiments and data collection. A.H.H. and R.S. contributed to conception of experiments, data analysis and interpretation. S.F.T. and D.R.K. contributed to TEM data collection and analysis. F.L., V.L., B.D.H., H.S.S. and H.A. contributed mutant embryos generated by their labs for the project. A.H.H. and R.S. prepared and edited the manuscript prior to submission.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.122374/-/DC1
References
- Akiyama H., Chaboissier M. C., Martin J. F., Schedl A. and de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813-2828. 10.1101/gad.1017802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Kim J. E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T. et al. (2005). Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665-14670. 10.1073/pnas.0504750102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley-Ross M. A. (1992). The comparative myology of the thigh and crus in the salamanders ambystoma tigrinum and dicamptodon tenebrosus. J. Morphol. 211, 147-163. 10.1002/jmor.1052110204 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A., Tsuji K., Cox K., Harfe B. D., Rosen V. and Tabin C. J. (2006). Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2, e216 10.1371/journal.pgen.0020216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M. and Ralphs J. R. (2000). The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 196, 85-130. 10.1016/s0074-7696(00)96003-0 [DOI] [PubMed] [Google Scholar]
- Blitz E., Sharir A., Akiyama H. and Zelzer E. (2013). Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680-2690. 10.1242/dev.093906 [DOI] [PubMed] [Google Scholar]
- Bober E., Franz T., Arnold H., Gruss P. and Tremblay P. (1994). Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120, 603-612. [DOI] [PubMed] [Google Scholar]
- Bonnin M.-A., Laclef C., Blaise R., Eloy-Trinquet S., Relaix F., Maire P. and Duprez D. (2005). Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech. Dev. 122, 573-585. 10.1016/j.mod.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Brand B., Christ B. and Jacob H. J. (1985). An experimental analysis of the developmental capacities of distal parts of avian leg buds. Am. J. Anat. 173, 321-340. 10.1002/aja.1001730408 [DOI] [PubMed] [Google Scholar]
- Brent A. E. and Tabin C. J. (2004). FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885-3896. 10.1242/dev.01275 [DOI] [PubMed] [Google Scholar]
- Brent A., Schweitzer R. and Tabin C. J. (2003). A somitic compartment of tendon progenitors. Cell 113, 235-248. 10.1016/S0092-8674(03)00268-X [DOI] [PubMed] [Google Scholar]
- Brent A. E., Braun T. and Tabin C. J. (2005). Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132, 515-528. 10.1242/dev.01605 [DOI] [PubMed] [Google Scholar]
- Chen J. W. and Galloway J. L. (2014). The development of zebrafish tendon and ligament progenitors. Development 141, 2035-2045. 10.1242/dev.104067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A., Kieny M. and Mauger A. (1977). Limb-somite relationship: origin of the limb musculature. J. Embryol. Exp. Morphol. 41, 245-258. [PubMed] [Google Scholar]
- Davis A. P. and Capecchi M. R. (1996). A mutational analysis of the 5′ HoxD genes: dissection of genetic interactions during limb development in the mouse. Development 122, 1175-1185. [DOI] [PubMed] [Google Scholar]
- DeLaurier A., Schweitzer R. and Logan M. (2006). Pitx1 determines the morphology of muscle, tendon, and bones of the hindlimb. Dev. Biol. 299, 22-34. 10.1016/j.ydbio.2006.06.055 [DOI] [PubMed] [Google Scholar]
- Diogo R., Abdala V., Aziz M. A., Lonergan N. and Wood B. A. (2009). From fish to modern humans - comparative anatomy, homologies and evolution of the pectoral and forelimb musculature. J. Anat. 214, 694-716. 10.1111/j.1469-7580.2009.01067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffroy V., Ridall A. L. and Karsenty G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747-754. 10.1016/S0092-8674(00)80257-3 [DOI] [PubMed] [Google Scholar]
- Dy P., Smits P., Silvester A., Penzo-Méndez A., Dumitriu B., Han Y., de la Motte C. A., Kingsley D. M. and Lefebvre V. (2010). Synovial joint morphogenesis requires the chondrogenic action of Sox5 and Sox6 in growth plate and articular cartilage. Dev. Biol. 341, 346-359. 10.1016/j.ydbio.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F., Schuler B., Bonnin M.-A., Teillet M.-A. and Duprez D. (2002). Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351-366. 10.1006/dbio.2002.0707 [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C., Warot X., Messadecq N., LeMeur M., Dolle P. and Chambon P. (1996). Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development 122, 2997-3011. [DOI] [PubMed] [Google Scholar]
- Grant T. D., Cho J., Ariail K. S., Weksler N. B., Smith R. W. and Horton W. A. (2000). Col2-GFP reporter marks chondrocyte lineage and chondrogenesis during mouse skeletal development. Dev. Dyn. 218, 394-400. [DOI] [PubMed] [Google Scholar]
- Grenier J., Teillet M.-A., Grifone R., Kelly R. and Duprez D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 4, e4381 10.1371/journal.pone.0004381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifone R., Jarry T., Dandonneau M., Grenier J., Duprez D. and Kelly R. G. (2008). Properties of branchiomeric and somite-derived muscle development in Tbx1 mutant embryos. Dev. Dyn. 237, 3071-3078. 10.1002/dvdy.21718 [DOI] [PubMed] [Google Scholar]
- Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H., Fukami K., Sakaue-Sawano A. and Miyawaki A. (2011). Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 14, 1481-1488. 10.1038/nn.2928 [DOI] [PubMed] [Google Scholar]
- Holder N. (1989). Organization of connective tissue patterns by dermal fibroblasts in the regenerating axolotl limb. Development 105, 585-593. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Riordan T. J., Wang L., Eyal S., Zelzer E., Brigande J. V. and Schweitzer R. (2013). Repositioning forelimb superficialis muscles: tendon attachment and muscle activity enable active relocation of functional myofibers. Dev. Cell 26, 544-551. 10.1016/j.devcel.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle J. M., Gañan Y. and Macias D. (1989). Experimental analysis of the in vivo chondrogenic potential of the interdigital mesenchyme of the chick leg bud subjected to local ectodermal removal. Dev. Biol. 132, 368-374. 10.1016/0012-1606(89)90233-9 [DOI] [PubMed] [Google Scholar]
- Hurle J. M., Ros M. A., Gañan Y., Macias D., Critchlow M. and Hinchliffe J. R. (1990). Experimental analysis of the role of ECM in the patterning of the distal tendons of the developing limb bud. Cell Differ. Dev. 30, 97-108. 10.1016/0922-3371(90)90078-B [DOI] [PubMed] [Google Scholar]
- Kardon G. (1998). Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019-4032. [DOI] [PubMed] [Google Scholar]
- Knosp W. M., Scott V., Bächinger H. P. and Stadler H. S. (2004). HOXA13 regulates the expression of bone morphogenetic proteins 2 and 7 to control distal limb morphogenesis. Development 131, 4581-4592. 10.1242/dev.01327 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Valerius M. T., Mugford J. W., Carroll T. J., Self M., Oliver G. and McMahon A. P. (2008). Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169-181. 10.1016/j.stem.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y.-H., Inada M. et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755-764. 10.1016/S0092-8674(00)80258-5 [DOI] [PubMed] [Google Scholar]
- Laclef C., Hamard G., Demignon J., Souil E., Houbron C. and Maire P. (2003). Altered myogenesis in Six1-deficient mice. Development 130, 2239-2252. 10.1242/dev.00440 [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Behringer R. R. and de Crombrugghe B. (2001). L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage 9 Suppl. A, S69-S75. 10.1053/joca.2001.0447 [DOI] [PubMed] [Google Scholar]
- Li Y., Qiu Q., Watson S. S., Schweitzer R. and Johnson R. L. (2010). Uncoupling skeletal and connective tissue patterning: conditional deletion in cartilage progenitors reveals cell-autonomous requirements for Lmx1b in dorsal-ventral limb patterning. Development 137, 1181-1188. 10.1242/dev.045237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M., Martin J. F., Nagy A., Lobe C., Olson E. N. and Tabin C. J. (2002). Expression of Cre Recombinase in the developing mouse limb bud driven by aPrxl enhancer. Genesis 33, 77-80. 10.1002/gene.10092 [DOI] [PubMed] [Google Scholar]
- Lorda-Diez C. I., Montero J. A., Martinez-Cue C., Garcia-Porrero J. A. and Hurle J. M. (2009). Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J. Biol. Chem. 284, 29988-29996. 10.1074/jbc.M109.014811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T., Sunkin S., Oh S. M., Zariwala H., Gu H., Ng L., Palmiter R., Hawrylycz M., Jones A. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison N., Price B., Conner D. A., Keene D. R., Olson E. N., Tabin C. J. and Schweitzer R. (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697-2708. 10.1242/dev.001933 [DOI] [PubMed] [Google Scholar]
- Oliver G., Wehr R., Jenkins N. A., Copeland N. G., Cheyette B., Hartenstein V., Zipursky S. L. and Gruss P. (1995). Homeobox genes and connective tissue patterning. Development 121, 693-705. [DOI] [PubMed] [Google Scholar]
- Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W. H., Beddington R. S. P., Mundlos S., Olsen B. R. et al. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765-771. 10.1016/S0092-8674(00)80259-7 [DOI] [PubMed] [Google Scholar]
- Pai A. C. (1965a). Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. I. Genetic analysis and gross morphology. Dev. Biol. 11, 82-92. 10.1016/0012-1606(65)90038-2 [DOI] [PubMed] [Google Scholar]
- Pai A. C. (1965b). Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. Ii. Developmental analysis. Dev. Biol. 11, 93-109. 10.1016/0012-1606(65)90039-4 [DOI] [PubMed] [Google Scholar]
- Proctor N. S. and Lynch P. J. (1993). Manual of Ornithology: Avian Structure & Function, p. 340 New Haven: Yale University Press. [Google Scholar]
- Pryce B. A., Brent A. E., Murchison N. D., Tabin C. J. and Schweitzer R. (2007). Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 236, 1677-1682. 10.1002/dvdy.21179 [DOI] [PubMed] [Google Scholar]
- Pryce B. A., Watson S. S., Murchison N. D., Staverosky J. A., Dünker N. and Schweitzer R. (2009). Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136, 1351-1361. 10.1242/dev.027342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros M. A., Rivero F. B., Hinchliffe J. R. and Hurle J. M. (1995). Immunohistological and ultrastructural study of the developing tendons of the avian foot. Anat. Embryol. 192, 483-496. 10.1007/BF00187179 [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A. and Tabin C. J. (2001). Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855-3866. [DOI] [PubMed] [Google Scholar]
- Selever J., Liu W., Lu M.-F., Behringer R. R. and Martin J. F. (2004). Bmp4 in limb bud mesoderm regulates digit pattern by controlling AER development. Dev. Biol. 276, 268-279. 10.1016/j.ydbio.2004.08.024 [DOI] [PubMed] [Google Scholar]
- Shellswell G. B. and Wolpert L. (1977). The pattern of muscle and tendon development in the chick wing. In Vertebrate Limb and Somite Morphogenesis (ed. Ede D. A., Hinchliffe J. R. and Balls M.), pp. 71-86. Cambridge: Cambridge University Press. [Google Scholar]
- Shinohara H. (1999). The size and position of the sacral hiatus in man. Okajimas Folia Anat. Jpn. 76, 89-93. 10.2535/ofaj1936.76.2-3_89 [DOI] [PubMed] [Google Scholar]
- Smits P., Li P., Mandel J., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. and Lefebvre V. (2001). The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 1, 277-290. 10.1016/S1534-5807(01)00003-X [DOI] [PubMed] [Google Scholar]
- Soeda T., Deng J. M., de Crombrugghe B., Behringer R. R., Nakamura T. and Akiyama H. (2010). Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 48, 635-644. 10.1002/dvg.20667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler H. S., Higgins K. M. and Capecchi M. R. (2001). Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development 128, 4177-4188. [DOI] [PubMed] [Google Scholar]
- St-Jacques B., Hammerschmidt M. and McMahon A. P. (1999). Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072-2086. 10.1101/gad.13.16.2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y. and Shukunami C. (2013). Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280-2288. 10.1242/dev.096354 [DOI] [PubMed] [Google Scholar]
- te Welscher P., Zuniga A., Kuijper S., Drenth T., Goedemans H. J., Meijlink F. and Zeller R. (2002). Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 298, 827-830. 10.1126/science.1075620 [DOI] [PubMed] [Google Scholar]
- Tozer S. and Duprez D. (2005). Tendon and ligament: development, repair and disease. Birth Defects Res. C Embryo Today 75, 226-236. 10.1002/bdrc.20049 [DOI] [PubMed] [Google Scholar]
- Vogan K. J., Epstein D. J., Trasler D. G. and Gros P. (1993). The splotch-delayed (Spd) mouse mutant carries a point mutation within the paired box of the Pax-3 gene. Genomics 17, 364-369. 10.1006/geno.1993.1333 [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Franz T., Gessler M. and Grzeschik K. H. (1992). Deletion of GLI3 supports the homology of the human Greig cephalopolysyndactyly syndrome (GCPS) and the mouse mutant extra toes (Xt). Mamm. Genome 3, 461-463. 10.1007/BF00356157 [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Lee K., Lanske B., Segre G. V., Kronenberg H. M. and Tabin C. J. (1996). Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613-622. 10.1126/science.273.5275.613 [DOI] [PubMed] [Google Scholar]
- Walthall J. C. and Ashley-Ross M. A. (2006). Postcranial myology of the California newt, Taricha torosa. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288A, 46-57. 10.1002/ar.a.20279 [DOI] [PubMed] [Google Scholar]
- Watson S., Riordan T. J., Pryce B. and Schweitzer R. (2009). Tendons and muscles of the mouse forelimb during embryonic development. Dev. Dyn. 238, 693-700. 10.1002/dvdy.21866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Shiraishi Y.-i. and Kuroiwa A. (2013). Wnt and BMP signaling cooperate with Hox in the control of Six2 expression in limb tendon precursor. Dev. Biol. 377, 363-374. 10.1016/j.ydbio.2013.02.023 [DOI] [PubMed] [Google Scholar]
- Zippel K. C., Glor R. E. and Bertram J. E. A. (1999). On caudal prehensility and phylogenetic constraint in lizards: The influence of ancestral anatomy on function in Corucia and Furcifer. J. Morphol. 239, 143-155. [DOI] [PubMed] [Google Scholar]
- Zou H. and Niswander L. (1996). Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 272, 738-741. 10.1126/science.272.5262.738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.