Abstract
Compared with other bacterial pathogens, the molecular mechanisms of mycoplasma pathogenicity are largely unknown. Several studies in the past have shown that pathogenic mycoplasmas are equipped with sophisticated genetic systems that allow them to undergo high-frequency surface antigenic variations. Although never clearly proven, these variable mycoplasma surface components are often implicated in host immune evasion and adaptation. Vpma surface lipoproteins of the ruminant pathogen Mycoplasma agalactiae are encoded on a genomic pathogenicity island–like locus and are considered as one of the well-characterized model systems of mycoplasma surface antigenic variation. The present study assesses the role of these phase-variable Vpmas in the molecular pathogenesis of M. agalactiae by testing the wild-type strain PG2 in comparison with the xer1-disrupted Vpma ‘phase-locked’ mutants in sheep infection models. The data clearly illustrate that although Xer1 recombinase is not a virulence factor of M. agalactiae and Vpma phase variation is not necessary for establishing an infection, it might critically influence the survival and persistence of the pathogen under natural field conditions, mainly due to a better capacity for dissemination and evoking systemic responses. This is the first study where mycoplasma ‘phase-locked’ mutants are tested in vivo to elucidate the role of phase variation during infection.
Keywords: Mycoplasma agalactiae, phase variation, variable surface lipoproteins, Vpma, phase-locked mutants, mastitis
Introduction
The wall-less mycoplasmas are the smallest self-replicating prokaryotes that belong to the bacterial class Mollicutes (mollis, soft; cutis, skin) (Razin et al., 1998; Razin & Hayflick, 2010) and have evolved extremely complex and unique capabilities to establish themselves as successful emerging and re-emerging pathogens of humans and animals (Rottem & Barile, 1993; Baseman & Tully, 1997; Rosengarten et al., 2000, 2001).
Mycoplasma agalactiae causes the contagious agalactia (CA) syndrome in sheep and goats associated with significant economic losses worldwide. The disease is characterized mainly by mastitis affecting milk yield and quality and often accompanied by conjunctivitis, septicaemia, arthritis and sporadic genital infections (DaMassa et al., 1992; Bergonier & Poumarat, 1996; Bergonier et al., 1997; Corrales et al., 2007). Annual losses in excess of US$ 30 million have been estimated to be caused in European countries around the Mediterranean, primarily due to losses in milk production (Nicholas, 2002).
CA is included in the list of transmissible diseases notifiable to the ‘Office International des Epizooties’ (OIE) that are considered to be of socio-economic and/or of public health importance (http://www.oie.int/en/animal-health-in-the-world/the-world-animal-health-information-system/old-classification-of-diseases-notifiable-to-the-oie-list-b/). The disease is predominant in the Mediterranean region (Corrales et al., 2007) but is also widespread in other countries of the Balkan Peninsula, Western and Central Asia, and Northern, Central and Eastern Africa, South America and Australia (Bergonier et al., 1997; Madanat et al., 2001; Azevedo et al., 2006; Al-Momani et al., 2008; McAuliffe et al., 2011).
Shedding of the pathogen by diseased or asymptomatic carriers continues for months or years after the initial infection with a potential risk of infecting susceptible animals (Bergonier et al., 1997; Corrales et al., 2007). Antibiotic therapy tends to reduce clinical signs but promotes the carrier state (Nicholas, 2002). Attempts to minimize the clinical expression of CA by antibiotic therapy, culling and/or vaccination are not always successful and, in fact, no single vaccine against CA has been universally adopted (http://www.oie.int/doc/ged/D6448.PDF).
Despite its agronomical significance, M. agalactiae’s mechanisms of infection and persistence are completely unknown. However, like many successful pathogens, M. agalactiae shows surface antigenic diversity via high-frequency switching of immunodominant surface lipoproteins called Vpmas (Variable proteins of M. agalactiae). A family of six related but distinct Vpma proteins was identified in a clonal variant 55-5 of the M. agalactiae type strain PG2 (Glew et al., 2002). Xer1 recombinase encoded in the vpma pathogenicity island–like locus causes site-specific vpma gene inversions that place a previously silent gene in front of the single vpma promoter and thus cause Vpma switching (Glew et al., 2002; Chopra-Dewasthaly et al., 2008). Site-specific cleavage and strand exchange occur within a minimal region of 21 bp located within the 5′ untranslated region of all six vpma genes (Czurda et al., 2010). Interestingly, this region is also highly conserved in the vsp lipoprotein genes that form a similar site-specific phase-variable system in Mycoplasma bovis, a very close phylogenetic relative of M. agalactiae (Askaa & Erno, 1976; Pettersson et al., 1996) that causes even more serious economic losses to the cattle and dairy industry (Nicholas & Ayling, 2003; Fox et al., 2005) owing to mastitis and calf pneumonia. An estimated loss of US$ 450 is incurred in lost milk value alone per case of clinical mycoplasma mastitis (Kauf et al., 2007). This phylogenetic proximity, and the fact that M. bovis also induces very similar clinical signs in cattle, led to the speculation that the Vpma and Vsp systems might play a similar role in disease progression (Glew et al., 2000).
Like the other phase-variable lipoproteins from several different Mycoplasma species, the in vivo significance of Vpma oscillations is not well understood. It is believed, but never proven, that Vpma antigenic variation plays a very important role in M. agalactiae’s pathogenicity, possibly via immunomodulation and nonimmune interactions with the host (Glew et al., 2000, 2002; Flitman-Tene et al., 2003; Nouvel et al., 2009). Previously, we accomplished the targeted disruption of xer1 recombinase gene via homologous recombination resulting in Vpma phase-locked mutants (PLMs) that could no longer switch to alternate Vpma phenotypes and exhibited stable expression of single Vpma products for several in vitro generations (Chopra-Dewasthaly et al., 2008). This was an important breakthrough setting up the basis for assessing the role of phase-variable mycoplasma lipoproteins, more specifically of Vpmas, in molecular pathogenesis. In the present study, we have used an equimolar mixture of phase-invariable mutants PLMY and PLMU, expressing single stable antigens VpmaY and VpmaU, respectively (Chopra-Dewasthaly et al., 2008), together with the wild-type PG2 strain capable of Vpma phase variation, and compared their infection traits in intramammary and conjunctival experimental sheep infections to investigate the role of Vpma antigenic switching in M. agalactiae pathogenicity.
Materials and methods
Animals
Fifteen lambs aged 4–5 months and weighing between 24 and 29 kg were used for the conjunctival route infection, whereas the intramammary infection study group comprised 15 adult lactating ewes weighing around 50–70 kg (Table 1). Although the physical age of the lactating ewes varied between 2 and 4 years, they constituted a homogeneous group in terms of their lactating status as they had the same lambing time. For both conjunctival and intramammary infections, 15 clinically healthy lambs/sheep of the local mountain breed, negative for major sheep pathogens (attested by routine bacteriological and PCR diagnostics) and also confirmed to be seronegative for M. agalactiae by a commercial EIA kit (Cypress Diagnostics, Langdorp, Belgium), were identified and randomly assigned to one of the three groups of five animals each (Table 1). In addition, prior to the intramammary route infection, the possibility of an existing subclinical mastitis was excluded in the lactating ewes by checking their milk via general bacteriological examination and for somatic cell counts (Foss Electric, Hillerôd, Denmark), which is reported to be a good indicator for the existence of subclinical mastitis of dairy sheep (González-Rodríguez et al., 1995). Absence of M. agalactiae was further confirmed in the lactating ewes prior to intramammary infection by testing their milk samples in a specific PCR (Chavez Gonzalez et al., 1995).
Table 1.
Set-up of the experimental Mycoplasma agalactiae conjunctival and intramammary route infection
| Groups | Positive control | Negative control | Phase-locked mutants |
|---|---|---|---|
| Antigen | M. agalactiae PG2 | Pyrogen-free saline | M. agalactiae Vpma phase-locked mutants: PLMU & PLMY |
| Inoculum | 109 | – | 5 × 108 each mutant (total 109) |
| Volume of Inoculum | 120 μL – conjunctival or 5 mL – intramammary | 120 μL – conjunctival or 5 mL – intramammary | 120 μL – conjunctival or 5 mL – intramammary |
| Animals | 5 lambs – conjunctival or 5 lactating ewes – intramammary | 5 lambs – conjunctival or 5 lactating ewes – intramammary | 5 lambs – conjunctival or 5 lactating ewes – intramammary |
| Necropsy Day 20 (conjunctival) and Day 28 (intramammary) postinoculation |
Each group was housed in a separate experimental stable 1 week before inoculation. Regular clinical and serological examinations were made during the course of the experiment. Excretion of bacteria in milk (in lactating ewes) was checked by quantitative bacteriological examination, and colonization of the eye, nose and genital mucosa (in conjunctivally infected lambs) was checked qualitatively from the respective swabs. All infected animals were killed and necropsied at Day 20 pi (conjunctival route infection) or Day 28 pi (intramammary inoculation) to quantify M. agalactiae in various lymph nodes (LN), organs and bronchoalveolar lavage fluids (BALF). All procedures related to the animal experiments were performed with the approval of the Austrian Federal Ministry for Education, Science and Culture (BMBWK-68.205/0145-BrGT/2006) and Austrian Federal Ministry for Science and Research (GZ/BMWF-68.205/0092-C/GT/2007), respectively.
Inocula
The details of the M. agalactiae inocula for the two experimental infections are provided in Table 1. For the preparation of inocula, M. agalactiae was cultured at 37 °C in standard Aluotto medium (Aluotto et al., 1970) containing Penicillin G, sodium pyruvate and phenol red as described previously (Chopra-Dewasthaly et al., 2005). At the time of inocula preparation, M. agalactiae type strain PG2 had undergone about seven in vitro passages in Aluotto media, whereas the PLMs derived from the parent PG2 strain had undergone additional nine passages during the process of mutant construction and isolation. For growing PLMU and PLMY inocula, tetracycline was added to the Aluotto broth at a concentration of 2 μg mL−1. The cultures were centrifuged at 4 °C for 45 min at 10900 g. and the supernatant was thoroughly decanted. The cell pellets were resuspended in pyrogen-free saline (PFS) procured from Mayrhofer Pharmazeutika, Austria, in a volume of 1/200 of the original cultures. The cell suspensions were aliquoted into sterile tubes and stored at −80 °C. Prior to inoculation, one aliquot of each culture (PG2, PLMY and PLMU) was thawed and plated as 10-fold serial dilutions on Aluotto medium containing 1% Difco™ Agar Noble to determine the titre of viable mycoplasma cells. Accordingly, fresh aliquots of the cultures were thawed on the inoculation day and diluted in PFS to obtain the desired concentrations. For both PLMs and PG2, inocula were prepared for five sheep as one common pool. The remaining sample material that was not inoculated was used for serial 10-fold dilutions and plated on Aluotto plates to reconfirm the actual number of viable mycoplasmas inoculated per sheep.
The possibility of any bacterial contamination was refuted in both PG2 and PLM inocula by thorough bacteriological quality control, including Gram staining of mycoplasma pellets. Also, because any contamination of the PLM inoculum with the parental strain PG2 would have important consequences, we negated the possibility of such contamination by several prior analyses, including PCR, Southern blot and immunoblotting.
Clinical examination
Clinical examination was performed once a day for all sheep as described previously (Baumgartner, 2002) starting from Day −5 until the end with three additional time points for Day 0 (inoculation), that is, 2 h, 4 h, and 8 h pi. During examination, general condition and appetite, rectal temperatures, ruminal activity and results of auscultation of heart and lungs were recorded. Rate of respiration and femoral arterial pulse was monitored for rhythm, rate and quality. Sheep were also examined for the presence of any ocular or nasal discharge, and the conjunctiva was additionally inspected for vascularization.
For the intramammary route infection, the appearance of milk and clinical status of the udders were evaluated daily by palpation of the mammary glands and supramammary LN and by inspection from the rear and side ends to notice any differences in the size, skin colour and asymmetry in the two udder halves. A clinical index and udder score was calculated every day for each animal to quantify the clinical signs and to estimate the time course of clinical development and severity of mastitis. The udder scores were based on several clinical factors, including appearance of mammary glands, California Mastitis Test (CMT) and quality and quantity of milk secretions.
Sample collection and analyses
A strict hygiene protocol was used for collecting the samples, always starting first with the negative control animal group, followed by the PLM group and the PG2-positive control group at the end. Disposable sterile overalls, gloves, head caps and masks were used throughout the routine sample collections.
Swabs
Ocular and nasal swabs were collected once a week for bacteriological examination of lambs.
Blood
Blood samples for haematology (blood status and differential blood count) and serology were collected from the vena jugularis on Day −5, Day 0 (2, 4 and 8 h), Day 1, Day 2, Day 5, Day 8, Day 12, Day 15, Day 19, Day 22 and Day 26 pi. For haematological studies, blood was collected in vacutainer tubes with ethylene diamine tetra-acetic acid and transported to the central laboratory of Veterinary Medicine University of Vienna for analysis. Sera were obtained by centrifugation at 1070 g for 10 min and stored at −80 °C until further testing.
Milk
Sheep were milked by hand-stripping twice a day using fresh sterile gloves for each animal and always starting first with the left uninoculated udder before moving to the right inoculated udder half. Milk yield was quantitated twice a day for all animals. Somatic cell counts were determined twice a week for milk samples from both the right and the left udder halves of all sheep using a Fossomatic method (Foss Electric) within 24 h of collection by the previously described method (Gonzalo et al., 1993). Examination of milk samples for the presence of mycoplasmas was made twice a week as described ahead. For this, milk samples were collected in sterile vials on Day − 5, Day 0 (2, 4 and 8 h), Day 1, Day 2, Day 5, Day 8, Day 12, Day 15, Day 19, Day 22 and Day 26 pi. The first streams of milk were used for the CMT, which was performed once every day.
Lymph nodes, organs and BALF
At necropsy, various organ samples (spleen, lungs, kidneys and udders) and LN (mandibular, parotideal, medial and lateral retropharyngeal, superficial cervical, mediastinal, jejunal, mesenterial, medial iliac, supramammary/scrotal) were taken from the respective animals (as mentioned in Supporting information, Table S1 and Table S3) and stored at −80 °C in individual sterile vials for bacteriological examination and in 10% neutral buffered formalin for pathological examination.
Additionally, lungs were also examined by testing BALF for the presence of mycoplasmas. For this, prior to removing tissue probes, a bronchoalveolar lavage was performed by inoculating 25 mL of sterile PBS into the right and the left bronchus and BALF was recovered by aspiration. A 2-mL aliquot was removed for bacteriological analysis, and the rest was frozen at −80 °C. A 2-mL fresh sample of the BALF was diluted 10-fold by adding to 18 mL of Aluotto broth and incubated at 37 °C for 7 days or until metabolic colour change. Samples showing signs of growth were plated as 10-fold serial dilutions on Aluotto agar to analyse any growing mycoplasma colonies.
Reisolation of M. agalactiae from samples by cultivation
All examinations were made in Aluotto broth containing sodium pyruvate and phenol red as colour indicator for growth and supplemented with thallium acetate and Penicillin G to prevent bacterial and fungal contaminations (Aluotto et al., 1970; Chopra-Dewasthaly et al., 2005).
Swabs
Swabs were incubated in 3–4 mL of Aluotto broth for 3 h at room temperature and then vortexed before removing the swab. From this, 1-mL aliquots were frozen as original specimens at −80 °C for quantitative analyses, whereas the remaining sample was incubated at 37 °C as undiluted (1 mL), as well as 1: 10 diluted culture for a maximum of 7 days. The samples showing a positive colour change were plated on Aluotto agar plates, and individual colonies with different morphologies were randomly picked and analysed further by physiological/serological/genetic methods to confirm species identity (Chavez Gonzalez et al., 1995; Poveda, 1998; Poveda & Nicholas, 1998). The specimen was considered negative if no colour change was observed within 7 days of incubation of the diluted and direct cultures. Frozen samples corresponding to the specimens found positive for M. agalactiae were grown and tested further for quantitative mycoplasma loads.
Milk samples
Milk samples from right and left udder halves, MR and ML, respectively, were plated the same day for each animal for qualitative and quantitative mycoplasma estimation. An aliquot of each sample was plated directly (0.02–0.1 mL) and also as 100-μL aliquots of 10-fold serial dilutions ranging from 10−1 to 10−9. Remaining 10-fold diluted samples were stored at −80 °C as reserve stocks for any future analysis. The plates were incubated at 37 °C for a maximum of 7 days before examining them for colony-forming units (cfu) counts under a Nikon SMZ-U stereomicroscope. The titres were expressed as viable mycoplasmas per mL of milk sample. Further confirmation and identification of M. agalactiae was made by PCR (Chavez Gonzalez et al., 1995) or by the indirect epi-immunofluorescence test using M. agalactiae-specific hyperimmune serum (Bradbury, 1998).
Organs and LN samples
Organs and LN samples were processed further on the day of necropsy to test for the presence or absence of mycoplasmas. A small part of each sample was removed and finely chopped into small pieces with a sterile blade, added to 3–5 mL of Aluotto broth and vortexed thoroughly. The remaining specimens were packed in aluminium sheets for snap-freezing in liquid nitrogen and then stored at −80 °C. The vortexed suspension was diluted 1: 10 in 3 mL Aluotto and incubated at 37 °C along with the undiluted sample and further processed the same way as described for the swabs. Specimens found positive were further re-examined for quantitative mycoplasma loads by excising an estimated small part (about 1 cm3) from the frozen −80 °C sample, weighing it and then finely chopping before incubation in Aluotto broth for 15–30 min at room temperature and then plating serial 10-fold dilutions to count viable mycoplasma cells as cfu per gram of organ or LN.
Routine confirmation of M. agalactiae was made by standard biochemical, serological and PCR methods as described earlier (Chavez Gonzalez et al., 1995; Bradbury, 1998; Poveda, 1998; Poveda & Nicholas, 1998).
Somatic cell counts
After bacteriological plating, somatic cell counts were determined for each milk sample using a Fossomatic method (Foss Electric) within 24 h postcollection by the previously described method (Gonzalo et al., 1993).
Statistics
Data were analysed using analysis of variance (anova) for repeated measurements. Differences between groups at specific time points were analysed using least significant difference (LSD) procedure for multiple comparisons. P-values < 0.05 were considered significant.
Results and discussion
Growth and Vpma profiles of M. agalactiae strains used for experimental infection
Mycoplasma agalactiae type strain PG2 was originally isolated from an infected sheep in Spain (Edward & Freundt, 1973) and shows high-frequency phase variation in Vpma surface lipoproteins because of the Xer1 recombinase-mediated vpma gene inversions (Glew et al., 2002). Unlike PG2, the xer1-disrupted PLMs do not show Vpma phase variation and continue to express single stable Vpma phenotypes, which are VpmaU for PLMU and VpmaY for PLMY, for several in vitro passages (Chopra-Dewasthaly et al., 2008). Because Glew et al. (2002) had divided the 6 vpma genes into two homology groups based on their N-terminal sequences and other shared sequences, we used one Vpma representative of each of these two groups for our infection trials with PLMs, namely VpmaY and VpmaU. Both PG2 and the Vpma PLMs, PLMU and PLMY, have been previously described (Solsona et al., 1996; Chopra-Dewasthaly et al., 2008). However, the two inocula comprising PG2 as positive control and the PLMs (equimolar mixture of PLMU and PLMY) were thoroughly characterized as a population in terms of their Vpma profiles and growth rates before injecting them into the sheep. Colony immunoblot and Western blot analysis using the six Vpma-monospecific hyperimmune antisera (Chopra-Dewasthaly et al., 2008) confirmed the expression of all six Vpma proteins in the PG2 inoculum, whereas the PLM inoculum was positive only for VpmaU and VpmaY expression in equal ratios.
The growth profiles of PG2 and PLMs were confirmed to be similar. Also, the latter inoculum continued to show equimolar ratios of VpmaY and VpmaU expressors at different stages of growth curve carried out for 70 h and analysed via colony immunoblotting using the VpmaY- and VpmaU-specific hyperimmune antisera (data not shown). This clearly demonstrated that PLMU and PLMY did not show any relative growth deficits during growth as mixed culture in vitro. The net growth rate in vivo is reported to be an important factor in the virulence of bacterial pathogens (Smith, 1998), and although the in vitro growth rates were comparable for PG2 and PLMs, as well for PLMU and PLMY, differences within the animals, especially in niches with limitations of specific nutrients, is a possibility (Ikeda et al., 2001) and could be an important factor contributing to their pathogenicity.
Challenge of sheep with M. agalactiae wild-type strain PG2 and Vpma phase-invariable PLMs
The sheep were challenged via the conjunctival and the intramammary route in two different sets of experimental infections (as shown in Table 1). 109 cfu of PLMs/PG2, suspended in 120 μL (conjunctival route) or 5 mL (intramammary route) of PFS, were inoculated in each of the five sheep via the right eye or right teat canal, respectively. The negative control group of five sheep each received sterile PFS.
The conjunctival route of infection involves the local immune system, which enables the study of the first steps of infection occurring at the mucosal level (Sanchis et al., 1998). As this infection was reported to lead to systemic spread and has also been suggested as a simple reproducible model for comparing the pathogenicity of different M. agalactiae strains, this infection route was selected to test any differences in the lymphogenic and systemic spread of PLMs as compared to the wild-type PG2 strain. The conjunctival route of infection did not lead to any major clinical signs except for follicular conjunctivitis that was milder in the PG2 group, and also, all the tested organs such as kidneys, spleen and lungs were negative for the isolation of M. agalactiae in both PLM and PG2 groups when necropsied at Day 20 pi. BALF was also negative for M. agalactiae for all animals but a single sheep (No.12) belonging to the PG2 group was found positive for M. ovipneumoniae.
Although mycoplasmas were not isolated from any of the tested organs during the conjunctival route of infection, limited lymphogenic spread of M. agalactiae to some body sites was noticed (Table S1). But this did not show any reproducible or noticeable trends for significant differences between the PLM and PG2 groups, except for the right mandibular LN, which was positive for four of five sheep of the PLM group but negative for all five PG2-infected animals. Lymphogenic spread to the left side was evident in only one sheep (No. 9) belonging to the PLM group, and it showed positive isolation of M. agalactiae from the left scrotal, superficial cervical and medial retropharyngeal LNs. In fact, this was the only animal that was positive for the reisolation of M. agalactiae from superficial cervical (left and right) and scrotal (left) LNs. Except for a few references (Hasso et al., 1993; Gil et al., 2003; Fe et al., 2009), M. agalactiae reisolation and association with male genital lesions is not very common. This has been recently confirmed by Amores et al. (2011) who indicated the presence of M. agalactiae and other CA agents in asymptomatic animals, mostly as auricular carriers. One sheep of the PG2 group (No. 15) also showed a single positive reisolation of M. agalactiae from the left side, namely from the medial retropharyngeal (left) LN. Jejunal and mediastinal LNs were negative for M. agalactiae isolation in animals of both groups. The only LN that was positive for all 10 animals was the right parotideal LN that drains the inoculation site (Freeman & Trout, 1985), and highest mycoplasma counts (average = 2.2 ± 1.4 E + 5 cfu g−1) were observed here (Table S1) in accordance with earlier published results (Sanchis et al., 1998). Throughout the infection, M. agalactiae was frequently isolated from the eye and nasal swabs corresponding to the right side, although in few cases the infection could spread to the left nasal side, as noticed mostly on Day 1 and Day 2 pi (Table S2) in four of five animals of the PLM group only and never for any PG2-infected animals. All eye swabs from the left side were negative for all animals on all days. One animal (No. 15) was especially interesting as it never showed any M. agalactiae isolation from the eyes but only from nasal swabs on four different days (Table S2). Preputial swabs were negative for all lambs on all days except for a single sporadic isolation from a single animal (No. 8) from the PLM group (data not shown). The PLM group of animals showed a total of 63 positive reisolations from the nasal and eye swab samples as compared to the 30 positive cases of the PG2-infected group. Although a common inoculum pool was prepared for the five sheep of one group such that each sheep receives about 109 cfu, upon replating the residual inoculum it was observed that the PLM sheep received almost double inoculum compared to the PG2 group. Whether this difference actually played any role in the higher persistence of PLMs over PG2 at the inoculation site and its direct vicinity (conjunctiva, nose and mandibular LN) is difficult to conclude considering the varied multiple factors involved in infection kinetics, including the local and innate immune response.
Under the given scenario, the more frequent reisolation of PLMs limited to the inoculation site and its direct vicinity cannot be safely attributed to the differential multiplicity, spread or persistence of PLMs over PG2 and needs additional studies. This is especially because apart from a general lack of reproducible trends for significant differences between the PLM and PG2 groups, the conjunctival infection failed in eliciting its expected goal of systemic spread to various organs and pronounced dissemination to extended LNs as observed by Sanchis et al. (1998). This could be due to strain differences and/or due to the delayed necropsy at Day 20 pi rather than Day 7 or 14 pi as performed by Sanchis et al. (1998) who noticed a gradual decrease in the frequency and levels of infection with prolonged necropsy times while using the same inoculum size of 109 cfu. On the other hand, the intramammary inoculation of M. agalactiae did not only cause the expected clinical signs but also led to a better lymphogenic spread and more consistent M. agalactiae reisolations. This report will therefore mainly concentrate on the details of the intramammary route infection.
Differences in clinical manifestation during infection by M. agalactiae wild-type strain PG2 and Vpma PLMs
Both infected groups, namely the PG2- and PLM-infected sheep, showed normal animal behaviour throughout the study without any signs of pulmonary distress or arthritis. Appetite, respiration and pulse rates were within the normal range, and heart and lung auscultation revealed no abnormalities.
Although follicular conjunctivitis was the only clinical manifestation observed during the M. agalactiae conjunctival route of infection, it is worth mentioning here that PG2-infected lambs showed a milder follicular conjunctivitis as compared to those infected with PLMs (data not shown).
With regard to the intramammary infection, in general M. agalactiae failed to elicit a sustained febrile response as also reported for other pathogenic bacteria causing chronic mastitis, including M. bovis (Riollet et al., 2000; Kauf et al., 2007). However, Kizil & Ozdemir (2006) have reported a statistically significant increase in body temperature of M. agalactiae-infected goats compared to the uninfected control group. Except for the two peaks at 4 h and Day 2 pi, observed on the temperature chart of PG2-infected sheep (Fig. S1), the rectal temperatures of all sheep fluctuated within the normal physiological temperature range at all tested time points. Hence, the PG2-infected sheep exhibited fever at two different time points, and especially at 4 h pi, there was a significant difference between the body temperature of the PG2 group compared to the PLM and the control group sheep (P < 0.05). Onset of a transient febrile response has been earlier associated with mycoplasmaemia preceding the colonization of the mucosal surfaces (Bar-Mosche et al., 1984; Kwantes & Harby, 1995; Kizil & Ozdemir, 2006) and can vary with the heterogeneity of the immune status of animals, which cannot be completely ruled in this study as well.
All sheep infected with PG2 or PLMs via the intramammary route developed severe clinical mastitis. This was also in accordance with the very high somatic cell counts of their milk secretions (González-Rodríguez et al., 1995). There was a remarkable and imminent reduction in the quality and yield of milk from the infected udder halves. The latter was so prominent that between Day 5–Day 8 pi, somatic cell counts could not be evaluated at all, either because of the complete absence or because of the poor quality of milk secretions, and on none of the following days, the situation was such that all five sheep of the two infected groups could be checked for somatic cell counts in milk. Daily examination of the udders by palpation gave inconsistent results in both infected groups. They were slightly painful to touch but were neither hot nor reddened. The degree of mastitis is reflected in the daily clinical udder score, which was recorded for each sheep based on its udder consistency, pain, CMT result, lymph node enlargement and milk quality and quantity. Although based on statistical analysis (anova), the differences in the average clinical udder indices of the PLM and the PG2 groups were adjudged to be insignificant, while considering a P value of < 0.05 as significant, some very important differences were observed between the two groups. A high-grade enlargement of supramammary LNs was observed in all five sheep infected with PG2, whereas this was seen only in one sheep from the PLM group and the others just showed a middle-grade to low-grade enlargement. Inspection of the udders from the rear end revealed a typical asymmetry caused by atrophy or swelling of the infected udder halves. Atrophy of the udder (Fig. 1) was more conspicuous in the PG2 group compared to the PLM group, firstly, because all animals of the PG2 group showed right udder atrophy, whereas this was evident only in three animals of the PLM group; the other two animals showed a slight swelling of the infected udder. Secondly, for the PG2 group, this atrophy set in relatively early during infection (Day 4 pi) compared to the PLM group where it had set in at Day 9 pi. Besides, one sheep of the PG2 group also exhibited left udder atrophy at Day 10 pi.
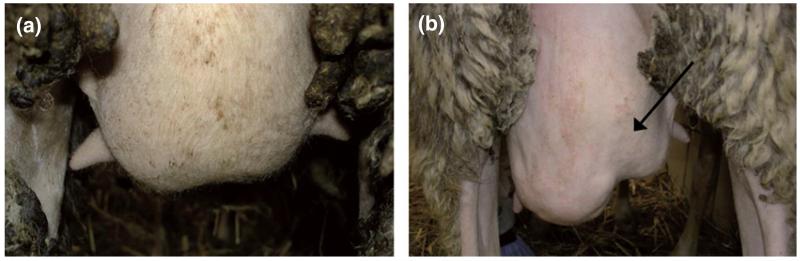
Fig. 1.
Marked atrophy of the inoculated right udder half (b) as observed at Day 4 pi onwards in all sheep infected intramammarily with Mycoplasma agalactiae PG2 type strain. Shown in comparison is a healthy PFS-inoculated control udder (a).
Wild-type strain PG2 shows a stronger M. agalactiae-specific antibody response
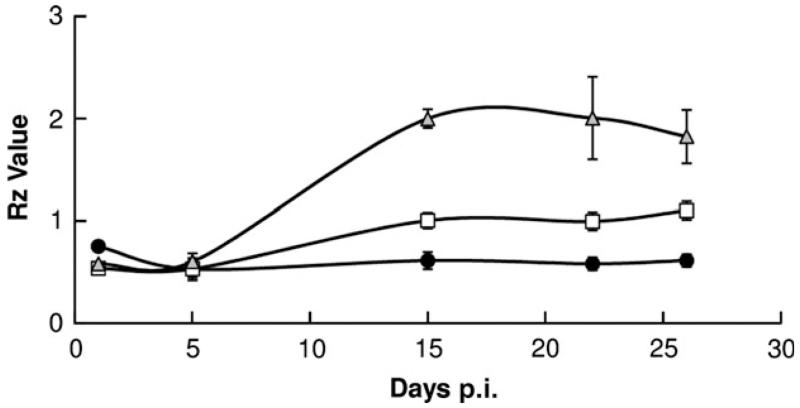
All the lambs inoculated via the conjunctival route failed to show any M. agalactiae-specific antibody response until the end of the experiment on Day 19 pi. In case of the intramammary infection, the highest antibody titres were observed for most of the ewes about 2 weeks pi, and all animals were seronegative till Day 5 pi. Considering the instructions of the commercial M. agalactiae EIA Kit (Cypress Diagnostics, Langdorp, Belgium), the PG2-infected sheep were the only ones that mounted a clear M. agalactiae-specific antibody response (Rz values above 1.5) about 2 weeks pi. This was in significant contrast to both the PLM and the negative control group, which did not show titres that could qualify for a valid positive response (Fig. 2). The titres were positive and fairly constant for the PG2-infected sheep except for a slight decrease towards the end of the study. This is in accordance with other studies where a persistent humoral immune response has been reported for mycoplasma infections of ruminants (Bergonier et al., 1997; Byrne et al., 2005). However, this was in contrast to the PLM-infected sheep that remained seronegative almost throughout the study with a slight rise in titres visible at Day 26 pi although still much lower than in the PG2-infected sheep (Fig. 2). Whether this difference is attributable to the inability of PLMs to switch Vpma phenotypes or simply because of the reduced immunogenicity of VpmaU and VpmaY lipoproteins (expressed in PLMs) as compared to the additional Vpmas (VpmaZ, VpmaV, VpmaW and VpmaX) present in the PG2 population needs to be examined, especially because mycoplasma lipoproteins have been earlier shown to be potent immunogens and preferential targets of the humoral immune response in many different studies (Jan et al., 1995; Chambaud et al., 1999; Neyrolles et al., 1999). The M. agalactiae serological response of ewes infected with PLM and PG2 strains was further confirmed by rechecking the sera using another commercial kit based on the single p48 membrane lipoprotein (IDEXX M. agalactiae Ab test kit). The results obtained with this kit again proved that the PG2-infected animals mounted the highest M. agalactiae-specific response, whereas the PLM-infected animals showed titres that were mostly near the borderline of a valid positive response as per the manufacturer’s instructions (Fig. S2).
Fig. 2.
Time course of Mycoplasma agalactiae-specific serological antibody response (arithmetical mean ± standard error of the mean represented by error bars) in sheep infected with PG2 (▲), PLMs (□) and PFS (●) via the intramammary route. Rz values (mean OD450 value of sample divided by twice the mean of two repetitions of negative control provided in the commercial EIA kit, Cypress Diagnostics, Langdorp, Belgium) > 1.5 are considered positive.
Coincidental with the higher antibody titres of the PG2-infected sheep, the average mycoplasma load in milk of these sheep was observed to be significantly lower as compared to that of the PLM-infected sheep at Day 12 pi (Fig. S3) and thereafter remained always low compared to PLM sheep at all the tested time points till the end of the study. A specific immune response against M. agalactiae has been previously correlated with decreased mycoplasma loads and vice versa in M. agalactiae-infected goat milk (Castro-Alonso et al., 2009, 2010). Increased levels of specific antibodies were reported to be concurrent with the main lymphoplasmocytic inflammatory reaction, suggesting an interaction between the humoral and cellular immune response against M. agalactiae that leads to lower levels of viable mycoplasmas in milk (Castro-Alonso et al., 2009). At the subacute stage (Day 15 pi) of a recent infection study with goats, all cell subsets of specific immune response, including IgG+ and IgA+ cells, and specific serum antibody levels were enhanced simultaneous to the increase in numbers of macrophages that were immunohistochemically positive for the M. agalactiae antigen, thereby suggesting an interaction between the host’s humoral and cellular immune responses leading to a drop in the intramammary mycoplasma load at this time point (Castro-Alonso et al., 2010). In compliance, the PG2 group not only showed higher M. agalactiae-specific antibody titres but also showed earlier and/or stronger neutropenia and lymphopenia (as discussed in the section ahead) when compared to the PLM group. However, this immune response was incapable of fully eliminating the pathogen during this acute phase of infection. Castro-Alonso et al. (2009) reported that neutrophils and macrophages fail to show frequent positive intracytoplasmic immune reaction with the specific antibody. Compromised phagocytic activity during mycoplasma infections has also been correlated with the induction of downregulating cytokines (Castro-Alonso et al., 2010). A very recent study reports the activation of M. agalactiae-specific IFN-γ positive lymphocytes in infected sheep at Day 15 pi by CD4+ T cells concomitant with the appearance of specific IgG, followed by CD8+ T cells that disappear at Day 60 pi, supporting the hypothesis that the anti-M. agalactiae immune response is mainly sustained by immunoglobulin secretion (La Manna et al., 2011). In some other studies, high levels of mycoplasma antibodies have been associated with the induction of clinical symptoms and/or high levels of infection (Sanchis et al., 1998, 2000). However, we failed to see such a correlation when comparing these parameters for the two infected groups or for individual sheep in these groups, and most importantly, the conjunctival route of infection totally failed to yield any M. agalactiae-specific antibody response throughout the study.
Inflammatory response and pathological features following intramammary M. agalactiae infection
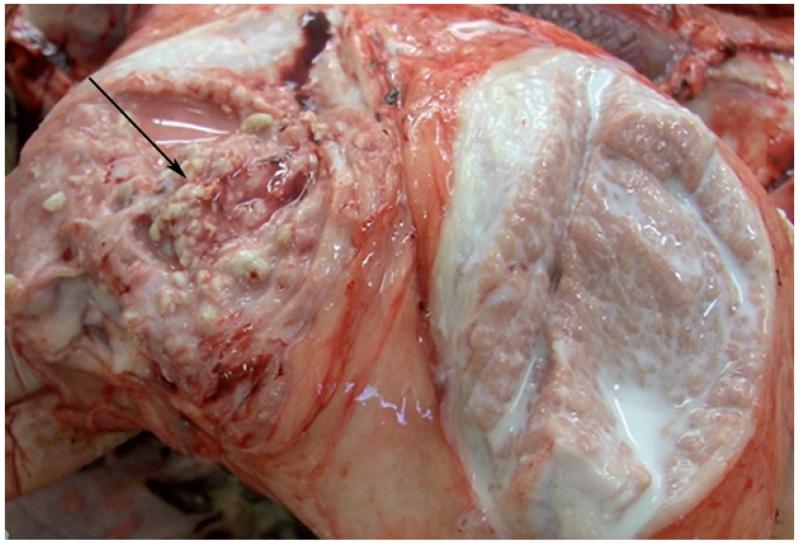
Mycoplasma lipoproteins are potent immunomodulins that induce pro-inflammatory cytokines with pathological consequences (Chambaud et al., 1999). Potentially important differences in the kinetics and levels of cytokine production were noticed in the milk samples obtained from the two infected groups. The inoculated right udder halves of all 10 infected animals reflected a typical pathomorphological picture of mycoplasma mastitis unlike the left udder halves, which lacked any signs of infection (Fig. 3). The right udder halves of all PG2-infected animals showed vacuolar degeneration and proliferation of the epithelium that was absent in all but one animal of the PLM group. In a similar experimental goat infection study, M. agalactiae-specific antigen was detected on the membrane and in the cytoplasma of degenerated epithelial cells, implying that this pathogen might be capable of invading these cells to protect itself from the host’s immune defence (Castro-Alonso et al., 2009, 2010). Moderate to severe multifocal lymphoplasmocytic infiltrates that partially also contained histiocytes were seen in almost all animals as a clear evidence of massive interstitial mastitis. Figure 3 depicts unilateral mastitis in the right udder half of a representative sheep which also shows acute purulent to apostematous mastitis lesions. Multifocal accumulations of numerous polymorphonuclear neutrophils were observed at many places in the milk ducts and dilated alveoli of the right udder in three of five animals infected with PLMs. A varying degree of interstitial fibrosis was a common observation for all infected animals with many showing atrophy of the udder tissue, which in case of some PLM-infected animals was also marked by abscesses demarcated by histiocytes and connective tissue. Aggregation of inflammatory cells into well-organized lymphoid follicles is a characteristic feature of mycoplasma lesions linked to the persistence of mycoplasmas at the mucosal surfaces (Rodríguez et al., 2000; Castro-Alonso et al., 2009). Additionally, two PLM-infected animals also showed blocked accumulation of mammary secretions with yellow flocks. Whether the histopathological differences between the PLM and the wild-type PG2 strain truly reflect their differential pathological manifestations due to differences in Vpma phase variation, as also observed for the V-1 surface antigen of M. pulmonis (Chambaud et al., 1999), or are merely due to the different immune status of the animals, needs further confirmations. Castro-Alonso et al. (2009) found two different patterns in necropsied goats experimentally infected with the same strain of M. agalactiae, one with intense inflammatory signs and the other with markedly reduced inflammation and extreme atrophy. The control animals in our study did not show any noticeable signs of infection in the udders.
Fig. 3.
A representative pathological picture depicting unilateral mastitis as seen in all except one infected animal. The right udder half (marked by a black arrow) shows several purulent mastitic lesions compared to the unaffected left udder half with normal tissue and milk texture.
Effect of Vpma ‘phase-locking’ on the quality and quantity of milk production during M. agalactiae infection
All infected sheep belonging to both the PG2 and the PLM group developed a severe mastitis affecting milk yield and quality from the infected right udder halves beginning Day 1 pi. In accordance with earlier studies of mycoplasma mastitis (Hasso et al., 1994; Gil et al., 1999; Sanchis et al., 2000), there was a significant decrease in milk production ranging between 2 and 60 mL day−1 besides cases of transient agalactia with complete absence of any milk secretions on some days of infection for some animals. A very severe transient agalactia where the infected udders were absolutely dry and failed to yield even a single drop of any secretion for mycoplasma analysis was observed for 5/45 tested time points for three of five sheep infected with PLMs, starting at Day 2 pi. In comparison, just one sheep of the PG2 group showed such severe agalactia for only one of the 45 tested time points, and that too almost towards the end of the experimental infection, that is, on Day 26 pi (Table 2). However, the rapid and total spread of infection from one udder half to the other, which is a typical characteristic of mycoplasma mastitis (Sanchis et al., 2000), was not so evident in this experimental trial except in a single sheep (No.11) of the PG2 group that showed typical signs of continuous bilateral mastitis and continuous shedding of the pathogen in significant amounts throughout the study (Table 2). This difference could be attributed to the differential virulence of field strains compared to the PG2 type strain or to the strict hygiene measures employed for milking during this experimental trial, whereby sterile gloves were used for milking by hand, and the order was always from the uninfected udder half to the infected one. Corrales et al. (2007) have reported that such a bilateral spread of infection in the field is many times caused by suboptimal milking and hygiene measures.
Table 2.
Qualitative bacteriological examination and frequency of ‘severe agalactia’ in lactating ewes inoculated intramammarily with 109 viable cfu of Mycoplasma agalactiae
| Inoculum/Sheep No. | Day 1 | Day 2 | Day 5 | Day 8 | Day 12 | Day 15 | Day 19 | Day 22 | Day 26 |
|---|---|---|---|---|---|---|---|---|---|
| PLM | |||||||||
| 6 | + | SA | + | + | + | + | + | + | + |
| 7 | + | + | + | + | + | + | + | + | + |
| 8 | + | SA | + | + | + | ++ | + | + | + |
| 9 | + | + | + | + | ++ | + | + | + | + |
| 10 | + | SA/+* | + | + | − † | + | + | SA | SA |
| PG2 | |||||||||
| 11 | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 12 | + | + | + | + | ++ | ++ | + | + | SA/+* |
| 13 | + | + | + | +‡ | + | + | ++ | + | + |
| 14 | + | ++ | + | + | ++ | + | ++ | + | + |
| 15 | + | ++ | + | + | + | + | + | + | ++ |
+, positive for right udder; ++, positive for right and left udder; SA, severe agalactia referring to the complete loss of any secretion from right udder.
Positive for left udder.
The presence of M. agalactiae could not be assessed because of bacterial contamination in the right and udder milk samples of these two ewes, respectively.
The presence of M. agalactiae could not be assessed because of bacterial contamination in the left and udder milk samples of these two ewes, respectively
When present, the milk secretions from the right udder halves were often abnormal and discoloured, varying between a watery grey or yellow discharge to a colos-trum-like consistency with clots and flakes. Some sheep also showed a red discharge because of the presence of red blood cells. Interestingly, one of the PG2-infected sheep (No.11) also showed abnormally altered milk secretions from the left udder starting at Day 12 pi. These alterations correlated very well with the results of the CMT, which demonstrated significant high-grade gel and clot formation in milk obtained from the right udder halves of all infected sheep, and also for one sheep for the milk obtained from the left udder half. Mycoplasma infections are often characterized by recruitment of polymorphonuclear leucocytes, followed by the infiltration of macrophages and lymphocytes, and the extent of this recruitment seems to directly correlate with the severity of disease (Chambaud et al., 1999). Consistent with this, the somatic cell counts in milk from infected sheep showed a significant increase within 4 h pi (7.026 × 106 ± 510 × 103 in the PLM group and 7.046 × 106 ± 338 × 103 in the PG2 group) compared to the negative control group and remained significantly increased throughout the study. On an average, the somatic cell counts of milk samples from infected udder halves were significantly high and ranged above 6 million mL−1 in the first week and above 3 million mL−1 in the third week of infection, and at no point of the study, they were below 2 million mL−1. However, because of the transient agalactia or poor quality of milk secretions, sometimes milk from none of the sheep (Day 5–Day 8 pi) or just one to four sheep per group (Day 8 pi onwards) could be tested for evaluating somatic cell counts. For this reason, a sufficient number of time points to analyse for any statistically significant difference between the PLM and the PG2 group for somatic cell counts were not available. Because mycoplasma lipoproteins are known to be potent immunomodulins capable of inducing cytokine synthesis with pathological consequences (Chambaud et al., 1999), differences in the Vpma profiles of the PG2 and PLM group are likely to cause differences in cytokine production at the local infection site, which in turn could influence the milk quality and the immune and pathological status of the infected udders. Hence, there is interest in examining the cytokine responses as important differences between the two groups have been potentially detected in the milk samples.
Comparison of mycoplasma loads in PG2- and PLM-infected animals
Milk
Right from Day 1 pi, M. agalactiae was recovered in significant amounts from the infected right udder halves of all ewes, ranging between 2.9 × 105 and 3.5 × 1010 cfu mL−1 for the PLM-infected animals and between 2.1 × 104 and 5.2 × 1010 cfu mL−1 for the PG2-infected group. This recovery was much earlier as compared to similar M. agalactiae and M. bovis intramammary infections, where comparable recovery from all inoculated udder halves was evident only after Day 3 to Day 4 pi (Sanchis et al., 2000; Kauf et al., 2007; Castro-Alonso et al., 2009). However, apart from strain and species variability, this might be a direct consequence of the size of inoculum that varied in these studies between 3 × 104 and 1010 cfu (Sanchis et al., 2000; Kauf et al., 2007; Castro-Alonso et al., 2009). The average mycoplasma load for each of the two groups on the tested days is depicted in Fig. S3. In contrast, milk from the left udder halves was not always positive for all sheep on all days tested (Table 3). Except for one sheep (No.11) belonging to the PG2 group, which showed consistent positive reisolations from the milk of the left udder half throughout the infection, all other sheep showed sporadic reisolations varying between Day 1 to Day 26 pi (Table 2). Importantly, the frequency of M. agalactiae reisolations from left udder halves was more than five-fold higher in PG2-infected sheep as compared to those infected with PLMs (Table 3). The excretion was always significantly less in the milk samples collected from these uninoculated left udder halves (0.5 × 102–1.5 × 104 cfu mL−1) except for sheep No.11, for which the counts were comparable to the milk from the inoculated right udder halves, with the highest load of 1.1 × 1010 cfu mL−1 on Day 15 pi.
Table 3.
Frequency of isolation of Mycoplasma agalactiae from milk samples obtained from the uninoculated left udder halves
| Day p.i. | 1 | 2 | 5 | 8 | 12 | 15 | 19 | 22 | 26 | Total isolations |
|---|---|---|---|---|---|---|---|---|---|---|
| PLM | 0/5* | 1/5 | 0/5 | 0/5 | 1/5 | 1/5 | 0/5 | 0/5 | 0/5 | 3/45 |
| PG2 | 1/5 | 2/5 | 1/5 | 1/4† | 3/5 | 2/5 | 3/5 | 1/5 | 3/5 | 17/45‡ |
Number of ewes from which M. agalactiae was isolated from left udder/total number of sheep in the group.
From four animals, as the fifth sample got contaminated.
The frequency of isolation of M. agalactiae from milk samples from left udder halves of ewes infected with PG2 is significantly higher than in ewes infected with PLMs.
The excretion in milk did not show a progressive decline with time as noticed by Sanchis et al. (2000) during similar intramammary infections using a comparable inoculum size of 108 viable bacteria per ewe. In our study, we observed alternate high and low phases of mycoplasma recovery, which was much more pronounced for PG2 (Fig. S3) that showed maximal numbers of M. agalactiae counts (2.16 ± 0.87 × 1010 cfu mL−1) on Day 2 pi that reduced to a significant low of 0.05 ± 0.02 × 1010 cfu mL−1 as compared to PLMs (0.67 ± 0.58 × 1010 cfu mL−1) on Day 12 pi, and thereafter never showed an increase over PLM recovery (Fig. S3) until the end of study.
Swabs
Mycoplasma analysis of nasal, ocular and preputial swabs obtained during conjunctival route infection is described in Challenge of sheep with M. agalactiae wild-type strain PG2 and Vpma phase-invariable PLMs and illustrated in Table S2. Recovery of M. agalactiae from the ears, as observed during a recent experimental goat M. agalactiae infection (Fe et al., 2010), was not tested during our conjunctival and intramammary infection studies.
Lymph nodes and organs
Qualitative and quantitative mycoplasma analyses of LNs and organs are provided in Table S1 and Table S3. During intramammary infection, although enlargement of the right supramammary LN was seen for all 10 infected animals at necropsy, only four of them showed positive reisolation of M. agalactiae from this LN ranging between 102 and 104 cfu g−1 (Table S3). Of these four, three belonged to the PG2 group and only one was from the PLM group. Interestingly, the latter PLM-infected sheep (No. 7) was the only one that was positive for mycoplasma reisolations from the left supramammary, left superficial cervical and both left and right parotideal LNs. Medial Iliac (left and right) and mesenterial were other commonly infected LNs with mycoplasma loads varying between 10 and 105 cfu g−1. Noticeably, eight of ten medial iliac LNs were positive in the PG2 group compared to the five of ten reisolations in the PLM group. This was similar to the less frequent positive reisolations in the PLM group (1/5) as compared to the PG2 group (3/5) for the right supramammary LNs, which were another common site of infection. As expected, samples from the right udder halves showed the highest mycoplasma load, as high as 108 cfu g−1, whereas the level of infection of the left udder halves was lower (102–105 cfu g−1). However, there was no significant difference in the mean cfu counts of the two infected groups. Results pertaining to the conjunctival route of infection are discussed under Challenge of sheep with M. agalactiae wild-type strain PG2 and Vpma phase-invariable PLMs.
Neutropenia following intramammary M. agalactiae infection
Important differences were observed between the PLM- and the PG2-infected animals with respect to the differential white blood cell counts. Circulating neutrophil granulocytes reached a nadir of about 1000 cells μL−1 earlier in the PG2 group, namely within 8 h of intramammary inoculation of M. agalactiae, as compared to the lowest neutrophil counts of the PLM group observed only at Day 1 pi (Fig. S4a). Also, the PG2 group showed a stronger lymphopenia as compared to the PLM group at Day 2 pi (Fig. S4b). Relative to control (time 0) lymphocyte counts, the counts remained significantly low for the PG2 group until the end of the study, whereas for the PLM group, the lymphocyte counts returned to almost normal levels by Day 5 pi. Neutropenia, although observed earlier in the PG2 group, sustained throughout the study in both infected groups. This can be correlated with the high somatic cell counts of milk throughout the study starting from 4 h pi. Extrapolating the results of an earlier study where more than 90% of milk somatic cells during acute mastitis were shown to be neutrophils (Saad & Ostensson, 1990), it can be safely concluded that neutrophil migration from the blood to the infected udders is a host endeavour to control the pathogen at the local infection site. Interestingly, in addition to neutropenia and lymphopenia, Kauf et al. (2007) have also reported a persistent state of thrombocytopenia during experimental M. bovis intramammary infections in cows. All these point towards an incessant ‘tug of war’ between the host and the pathogen, where the latter seems to be fully equipped to neutralize all immune effector cells and thus persists in the infected udders and milk throughout the study, as also seen during natural infections where M. agalactiae is shed in milk and body secretions for as long as 4 years after initial infection (Bergonier et al., 1997).
Wild-type strain PG2 is more invasive and better disseminating to the uninoculated udder halves
All five sheep infected with the PG2 strain showed bilateral transfer of M. agalactiae, whereas only three of five sheep of the PLM group showed positive isolations from milk samples of the uninoculated left udder (Table 2). Moreover, for the latter group, isolations were obtained only once per sheep throughout the experimental infection. In case of the PG2-infected sheep, however, there was an average of 3.4 isolations per sheep (Tables 2 and 3), with sheep No. 11 showing the highest frequency of isolation of M. agalactiae for 8 of the 9 days tested, with mycoplasma loads highly comparable to those in the inoculated right udder halves of other sheep (as discussed above). Interestingly, this sheep also showed signs of mild conjunctivitis and positive isolation of mycoplasma from the eye swabs, indicating a systemic spread of infection. Because the frequency of isolation of M. agalactiae from milk samples from the left udder halves of ewes infected with PG2 is significantly higher than in ewes infected with PLMs (Table 3), Vpma phase variation might be playing an important role in the dissemination, colonization and persistence of the infection in the uninoculated left udders. This could be an indirect consequence of better immune evasion by virtue of Vpma phase variation, as Denison et al. (2005) have also previously reported that lipoprotein phase variation in M. pulmonis helps the pathogen to avoid the host immune response. As shown in other studies, surface antigenic variation is not always involved in all stages of infection but becomes important for certain stages, mostly towards the later phases of infection, for better persistence, spread and transmission (Cotter & Miller, 1994; Ikeda et al., 2001). The PLM-infected animals in this study (Effect of Vpma ‘phase-locking’ on the quality and quantity of milk production during M. agalactiae infection) showed earlier and/or higher peak levels of the measured cytokines in milk, which in turn leads to greater influx of leucocytes and macrophages (Chambaud et al., 1999) that are capable of fighting the pathogens to prevent their further spread. Alternatively, it is also possible that Vpmas other than VpmaY and VpmaU, namely VpmaW, VpmaX, VpmaZ and VpmaV present in the PG2 population but absent in the PLMs, are more suited and provide a selective advantage in disseminating and invading the uninoculated udder halves and other body sites. It has been earlier seen in M. hominis that antigenic variation does not seem to be a response to the immune response but more a tool for adaptation (Jensen et al., 1998). This is in line with the widely accepted view that surface antigenic variation is a general mechanism that allows microorganisms to explore and cope with fluctuating environments and help them spread to new niches, both outside and within their natural hosts (van der Woude, 2006; Bayliss, 2009).
Summary
Although a number of pathogenic mycoplasmas are known to exhibit surface lipoprotein antigen variations, their biological significance is yet to be understood. This is the first attempt where the in vivo significance of such mycoplasma lipoprotein antigenic variation systems is tested based on the sheep and goat pathogen M. agalactiae as model organism by constructing ‘phase-locked’ mutants (PLMs) and using them in experimental sheep infection in comparison with the wild-type phase-variable type strain (PG2). The data clearly illustrate that although the Xer1 recombinase, per se, is not a virulence factor of M. agalactiae and Vpma phase variation is not necessary for establishing infection, subtle important differences were observed between the PLM and the PG2 animal groups during the short duration of the study. There are certain limitations of the study, including the heterogeneity in the immune status of animals and the lack of statistically significant differences because of small sample size. Yet the results tend to indicate that PG2 strain is more effective in evoking systemic responses and is more invasive and better disseminated to the uninoculated udder halves compared to the phase-invariable PLMs. Taken together, these differences indicate a better survival and persistence of the pathogen in its natural ‘wild-type’ form under field conditions, thereby leading to chronicity and better dissemination.
Supplementary Material
Acknowledgements
This work was supported by grant P18668-B05 (to W.J., J.S. and R.R.) of the Austrian Science Fund (FWF). The authors thank Sylvia Wildmann and Barbara Iser for their technical help and Michael Steinbrecher for his assistance with the animals.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Mean body temperatures (°C) monitored via the rect al route shown as a function of time in the three groups of five ewes each, infected intramammarily with M. agalactiae wild type strain PG2 (⋯),Vpma phase invariable PLMs (- - -) and PFS (—), respectively.
Fig. S2. Time-course of M. agalactiae specific serological antibody response (measured by the IDEXX M. agalactiae Ab test kit) in sheep infected with PG2 ( ), PLMs (□) and PFS (●) via the intramammary route.
), PLMs (□) and PFS (●) via the intramammary route.
Fig. S3. Time-course of M. agalactiae excretion in milk obtained from the inoculated right udder halves of sheep experimentally infected with 109 viable cfu of type strain PG2 ( ) and PLMs (□).
) and PLMs (□).
Fig. S4. Systemic responses to intramammary M. agalactiae infection.
Table S1. Bacteriological examination of lymph nodes and organs from sheep inoculated by conjunctival route with 109 viable cfu of M. agalactiae wild type strain (PG2) or Vpma phase locked mutants (PLMs) PLMU and PLMY and necropsied at Day 20 p.i.
Table S2. Bacteriological examination of nasal and eye swabs from lambs inoculated with 109 viable cfu of M. agalactiae
Table S3. Quantitative bacteriological examination of lymph nodes and organs from sheep inoculated by intramammary route with 109 viable cfu of M. agalactiae wild type strain (PG2) or Vpma phase locked mutants (PLMs) PLMU and PLMY and necropsied at Day 28 p.i.
Publisher's Disclaimer: Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Al-Momani W, Nicholas RA, Abo-Shehada MN. Risk factors associated with Mycoplasma agalactiae infection of small ruminants in northern Jordan. Prev Vet Med. 2008;83:1–10. doi: 10.1016/j.prevetmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Aluotto B, Wittler RG, Williams CO, Faber JE. Standardized bacteriologic techniques for the characterization of mycoplasma species. Int J Syst Bacteriol. 1970;20:35–38. [Google Scholar]
- Amores J, Gómez-Martín A, Corrales JC, Sánchez A, Contreras A, De la Fe C. Presence of contagious agalactia causing mycoplasmas in Spanish goat artificial insemination centres. Theriogenology. 2011;75:1265–1270. doi: 10.1016/j.theriogenology.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Askaa G, Erno H. Elevation of Mycoplasma agalactiae subsp. bovis to species rank: Mycoplasma bovis (Hale et al.) comb. nov. Int J Syst Bacteriol. 1976;26:323–325. [Google Scholar]
- Azevedo EO, Alcantara MDB, Nascimento ER, Tabosa IM, Barreto ML, Almeida JF, Araujo MO, Rodrigues ARO, Riet-Correa F, Castro RS. Contagious agalactia by Mycoplasma agalactaie in small ruminants in Brazil: first report. Braz J Microbiol. 2006;37:576–581. [Google Scholar]
- Bar-Mosche B, Rapapport E, Brenner J. Vaccination trials against Mycoplasma mycoides subsp. mycoides (large colony type) infection in goats. Isr J Med Sci. 1984;20:972–974. [PubMed] [Google Scholar]
- Baseman JB, Tully JG. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3:21–32. doi: 10.3201/eid0301.970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner W. Clinical Propaedeutics of Internal and Skin Diseases of Domestic Animals. 5th edn Parey Buchverlag; Berlin, Germany: 2002. [Google Scholar]
- Bayliss CD. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol Rev. 2009;33:504–520. doi: 10.1111/j.1574-6976.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- Bergonier D, Poumarat F. Contagious agalactia of small ruminants: epidemiology, diagnosis and control. Rev Sci Tech. 1996;15:1431–1475. [PubMed] [Google Scholar]
- Bergonier D, Berthelot X, Poumarat F. Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control. Rev Sci Tech. 1997;16:848–873. doi: 10.20506/rst.16.3.1062. [DOI] [PubMed] [Google Scholar]
- Bradbury JB. Identification of mycoplasmas by immunofluorescence. Methods Mol Biol. 1998;104:119–125. doi: 10.1385/0-89603-525-5:119. [DOI] [PubMed] [Google Scholar]
- Byrne W, Mekey B, McCormack R, Egan J, Ball H, Sachse K. Persistence of Mycoplasma bovis infection in the mammary glands of lactating cows inoculated experimentally. Vet Rec. 2005;156:767–771. doi: 10.1136/vr.156.24.767. [DOI] [PubMed] [Google Scholar]
- Castro-Alonso A, Rodriguez F, De la Fe C, Espinosa de Los Monteros A, Poveda JB, Andrada M, Herraez P. Correlating the immune response with the clinical-pathological course of persistent mastitis experimentally induced by Mycoplasma agalactiae in dairy goats. Res Vet Sci. 2009;86:274–280. doi: 10.1016/j.rvsc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Castro-Alonso A, De la Fe C, Espinosa de Los Monteros A, Rodriguez F, Andrada M, Poveda JB, Herráez P. Chronological and immunohistochemical characterization of the mammary immunoinflammatory response in experimental caprine contagious agalactia. Vet Immunol Immunopathol. 2010;136:43–54. doi: 10.1016/j.vetimm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Chambaud I, Wrobblewski H, Blanchard A. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 1999;7:493–499. doi: 10.1016/s0966-842x(99)01641-8. [DOI] [PubMed] [Google Scholar]
- Chavez Gonzalez YR, Ros Bascunana C, Bölske G, Mattsson JG, Fernandez Molina C, Johansson KE. In vitro amplification of the 16S rRNA genes from Mycoplasma bovis and Mycoplasma agalactiae by PCR. Vet Microbiol. 1995;47:183–190. doi: 10.1016/0378-1135(95)00058-i. [DOI] [PubMed] [Google Scholar]
- Chopra-Dewasthaly R, Zimmermann M, Rosengarten R, Citti C. First steps towards the genetic manipulation of Mycoplasma agalactiae and Mycoplasma bovis using the transposon Tn4001mod. Int J Med Microbiol. 2005;294:447–453. doi: 10.1016/j.ijmm.2004.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra-Dewasthaly R, Citti C, Glew MD, Zimmermann M, Rosengarten R, Jechlinger W. Phase-locked mutants of Mycoplasma agalactiae: defining the molecular switch of high-frequency Vpma antigenic variation. Mol Microbiol. 2008;67:1196–1210. doi: 10.1111/j.1365-2958.2007.06103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales JC, Esnal A, de la Fe C, Sanchez A, Assuncao P, Poveda JB, Contreras A. Contagious agalactia in small ruminants. Small Rumin Res. 2007;68:154–166. [Google Scholar]
- Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czurda S, Jechlinger W, Rosengarten R, Chopra-Dewasthaly R. Xer1-mediated site-specific DNA inversions and excisions in Mycoplasma agalactiae. J Bacteriol. 2010;192:4462–4473. doi: 10.1128/JB.01537-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaMassa AJ, Wakenell PS, Brooks DL. Mycoplasmas of goats and sheep. J Vet Diagn Invest. 1992;4:101–113. doi: 10.1177/104063879200400126. [DOI] [PubMed] [Google Scholar]
- Denison AM, Clapper B, Dybvig K. Avoidance of the host immune system through phase variation in Mycoplasma pulmonis. Infect Immun. 2005;73:2033–2039. doi: 10.1128/IAI.73.4.2033-2039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward DF, Freundt EA. Type-strains of species of the order Mycoplasmatales; including designation of neotypes for Mycoplasma mycoides subsp. mycoides; Mycoplasma agalactiae subsp. agalactiae and Mycoplasma arthritidis. Int J Syst Bacteriol. 1973;23:55–61. [Google Scholar]
- Fe CD, Amores J, Martín AG, Sánchez A, Contreras A, Corrales JC. Mycoplasma agalactiae detected in the semen of goat bucks. Theriogenology. 2009;72:1278–1281. doi: 10.1016/j.theriogenology.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Fe CD, Castro-Alonso A, Herráez P, Poveda JB. Recovery of Mycoplasma agalactiae from the ears of goats experimentally infected by the intramammary route. Vet J. 2010;190:94–97. doi: 10.1016/j.tvjl.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Flitman-Tene R, Mudahi-Orenstein S, Levisohn S, Yogev D. Variable lipoprotein genes of Mycoplasma agalactiae are activated in vivo by promoter addition via site-specific DNA inversions. Infect Immun. 2003;71:3821–3830. doi: 10.1128/IAI.71.7.3821-3830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LK, Kirk JH, Britten A. Mycoplasma mastitis: a review of transmission and control. J Vet Med B Infect Dis Vet Public Health. 2005;52:153–160. doi: 10.1111/j.1439-0450.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- Freeman LE, Trout HF. Lymph drainage of the conjunctiva: topographic anatomic study in calves. Am J Vet Res. 1985;9:1967–1970. [PubMed] [Google Scholar]
- Gil MC, Hermoso de Mendoza M, Alonso JM, Rey J, Poveda JB, Hermoso de Mendoza J. Mastitis caused by Mycoplasma mycoides subspecies mycoides (large colony type) in goat flocks in Spain. Zentralbl Veterinarmed B. 1999;46:741–743. doi: 10.1046/j.1439-0450.1999.00303.x. [DOI] [PubMed] [Google Scholar]
- Gil MC, Peña FJ, Hermoso De Mendoza J, Gomez L. Genital lesions in an outbreak of caprine contagious agalactia caused by Mycoplasma agalactiae and Mycoplasma putrefaciens. J Vet Med B Infect Dis Vet Public Health. 2003;50:484–487. doi: 10.1046/j.0931-1793.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- Glew MD, Papazisi L, Poumarat F, Bergonier D, Rosengarten R, Citti C. Characterization of a multigene family undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins in Mycoplasma agalactiae. Infect Immun. 2000;68:4539–4548. doi: 10.1128/iai.68.8.4539-4548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew MD, Marenda M, Rosengarten R, Citti C. Surface diversity in Mycoplasma agalactiae is driven by site-specific DNA inversions within the vpma multigene locus. J Bacteriol. 2002;184:5987–5998. doi: 10.1128/JB.184.21.5987-5998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rodríguez MC, Gonzalo C, San Primitivo F, Cármenes P. Relationship between somatic cell count and intramammary infection of the half udder in dairy ewes. J Dairy Sci. 1995;78:2753–2759. doi: 10.3168/jds.s0022-0302(95)76906-5. [DOI] [PubMed] [Google Scholar]
- Gonzalo C, Baro JA, Carriedo JA, Primitivo FS. Use of the Fossomatic method to determine somatic cell counts in sheep milk. J Dairy Sci. 1993;76:115–119. doi: 10.3168/jds.S0022-0302(93)77330-0. [DOI] [PubMed] [Google Scholar]
- Hasso SA, Al-Alubaidi JM, Al-Darraji AM. Contagious agalactia in goats: its severity as related to the route of infection and pregnancy. Small Rumin Res. 1993;10:263–275. [Google Scholar]
- Hasso SA, Al-Darraji AM, Al-Alubaidi JM. Pathology of experimentally-induced contagious agalactia in goats. Small Rumin Res. 1994;13:79–84. [Google Scholar]
- Ikeda JS, Schmitt CK, Darnell SC, et al. Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect Immun. 2001;69:3021–3030. doi: 10.1128/IAI.69.5.3021-3030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan G, Fontenelle C, Le Henaff M, Wroblewski H. Acylation and immunological properties of Mycoplasma gallisepticum membrane proteins. Res Microbiol. 1995;146:739–750. doi: 10.1016/0923-2508(96)81070-9. [DOI] [PubMed] [Google Scholar]
- Jensen LT, Thorsen P, Moller B, Birkelund S, Christiansen G. Antigenic and genomic homogeneity of successive Mycoplasma hominis isolates. J Med Microbiol. 1998;47:659–666. doi: 10.1099/00222615-47-8-659. [DOI] [PubMed] [Google Scholar]
- Kauf AC, Rosenbusch RF, Paape MJ, Bannerman DD. Innate immune response to intramammary Mycoplasma bovis infection. J Dairy Sci. 2007;90:3336–3348. doi: 10.3168/jds.2007-0058. [DOI] [PubMed] [Google Scholar]
- Kizil O, Ozdemir H. Clinical, haematological and biochemical studies in goats naturally infected with Mycoplasma agalactiae. Bull Vet Inst Pulawy. 2006;50:325–328. [Google Scholar]
- Kwantes LJ, Harby HAM. Caprine mycoplasmal arthritis in the Sultanate of Oman. Small Rumin Res. 1995;16:287–289. [Google Scholar]
- La Manna MP, Agnone A, Villari S, Puleio R, Vitale M, Nicholas R, Sireci G, Dieli F, Loria GR. Expansion of intracellular IFN-γ positive lymphocytes during Mycoplasma agalactiae infection in sheep. Res Vet Sci. 2011;91:e64–e67. doi: 10.1016/j.rvsc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Madanat A, Zendulkova D, Pospisil Z. Contagious agalactia of sheep and goats. A review. Acta Vet Brno. 2001;70:403–412. [Google Scholar]
- McAuliffe L, Gosney F, Hlusek M, de Garnica ML, Spergser J, Kargl M, Rosengarten R, Ayling RD, Nicholas RA, Ellis RJ. Multilocus sequence typing of Mycoplasma agalactiae. J Med Microbiol. 2011;60:803–811. doi: 10.1099/jmm.0.028159-0. [DOI] [PubMed] [Google Scholar]
- Neyrolles O, Chambaud I, Ferris S, Prevost MC, Sasaki T, Montagnier L, Blanchard A. Phase variations of the Mycoplasma penetrans main surface lipoprotein increase antigenic diversity. Infect Immun. 1999;67:1569–1578. doi: 10.1128/iai.67.4.1569-1578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RAJ. Improvements in the diagnosis and control of diseases of small ruminants caused by mycoplasmas. Small Rumin Res. 2002;45:145–149. [Google Scholar]
- Nicholas RAJ, Ayling RD. Mycoplasma bovis: disease, diagnosis, and control. Res Vet Sci. 2003;74:105–112. doi: 10.1016/s0034-5288(02)00155-8. [DOI] [PubMed] [Google Scholar]
- Nouvel LX, Marenda M, Sirand-Pugnet P, Sagne E, Glew M, Mangenot S, Barbe V, Barre A, Claverol S, Citti C. Occurrence, plasticity, and evolution of the vpma gene family, a genetic system devoted to high-frequency surface variation in Mycoplasma agalactiae. J Bacteriol. 2009;191:4111–4121. doi: 10.1128/JB.00251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson B, Uhlen M, Johansson KE. Phylogeny of some mycoplasmas from ruminants based on 16S rRNA sequences and definition of a new cluster within the Hominis group. Int J Syst Bacteriol. 1996;46:1093–1098. doi: 10.1099/00207713-46-4-1093. [DOI] [PubMed] [Google Scholar]
- Poveda JB. Biochemical characteristics in mycoplasma identification. In: Miles R, Nicholas RAJ, editors. Methods in Molecular Biology: Mycoplasma Protocols. Humana Press Inc; Totowa, NJ: 1998. pp. 69–78. [DOI] [PubMed] [Google Scholar]
- Poveda JB, Nicholas R. Serological identification of mycoplasmas by growth and metabolic inhibition tests. In: Miles R, Nicholas RAJ, editors. Methods in Molecular Biology: Mycoplasma Protocols. Humana Press Inc.; Totowa, NJ: 1998. pp. 105–111. [DOI] [PubMed] [Google Scholar]
- Razin S, Hayflick L. Highlights of mycoplasma research-an historical perspective. Molecular biology and pathogenicity of mycoplasmas. Biologicals. 2010;38:183–190. doi: 10.1016/j.biologicals.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riollet C, Rainard P, Poutrel B. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin Diagn Lab Immunol. 2000;7:161–167. doi: 10.1128/cdli.7.2.161-167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F, Sarradell J, Poveda JB, Ball HJ, Fernández A. Immunohistochemical characterization of lung lesions induced experimentally by Mycoplasma agalactiae and Mycoplasma bovis in goats. J Comp Pathol. 2000;123:285–293. doi: 10.1053/jcpa.2000.0418. [DOI] [PubMed] [Google Scholar]
- Rosengarten R, Citti C, Glew M, Lischewski A, Droesse M, Much P, Winner F, Brank M, Spergser J. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int J Med Microbiol. 2000;290:15–25. doi: 10.1016/S1438-4221(00)80099-5. [DOI] [PubMed] [Google Scholar]
- Rosengarten R, Citti C, Much P, Spergser J, Droesse M, Hewicker-Trautwein M. The changing image of mycoplasmas: from innocent bystanders to emerging and reemerging pathogens in human and animal diseases. Contrib Microbiol. 2001;8:166–185. doi: 10.1159/000060409. [DOI] [PubMed] [Google Scholar]
- Rottem S, Barile MF. Beware of mycoplasmas. Trends Biotechnol. 1993;11:143–151. doi: 10.1016/0167-7799(93)90089-R. [DOI] [PubMed] [Google Scholar]
- Saad AM, Ostensson K. Flow cytofluorometric studies on the alteration of leukocyte populations in blood and milk during endotoxin-induced mastitis in cows. Am J Vet Res. 1990;51:1603–1607. [PubMed] [Google Scholar]
- Sanchis R, Abadie G, Lambert M, Cabasse E, Guibert JM, Calamel M, Dufour P, Vitu C, Vigoni M, Pepin M. Experimental conjunctival-route infection with Mycoplasma agalactiae in lambs. Small Rumin Res. 1998;27:31–39. [Google Scholar]
- Sanchis R, Abadie G, Lambert M, Cabasse E, Dufour P, Guibert JM, Pepin M. Inoculation of lactating ewes by the intramammary route with Mycoplasma agalactiae: comparative pathogenicity of six field strains. Vet Res. 2000;31:329–337. doi: 10.1051/vetres:2000104. [DOI] [PubMed] [Google Scholar]
- Smith H. What happens to bacterial pathogens in vivo? Trends Microbiol. 1998;6:239–243. doi: 10.1016/s0966-842x(98)01250-5. [DOI] [PubMed] [Google Scholar]
- Solsona M, Lambert M, Poumarat F. Genomic, protein homogeneity and antigenic variability of Mycoplasma agalactiae. Vet Microbiol. 1996;50:45–58. doi: 10.1016/0378-1135(95)00200-6. [DOI] [PubMed] [Google Scholar]
- van der Woude MW. Re-examining the role and random nature of phase variation. FEMS Microbiol Lett. 2006;254:190–197. doi: 10.1111/j.1574-6968.2005.00038.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.