Abstract
Purpose
Anodal transcranial direct current stimulation (tDCS) can transiently increase corticomotor excitability of intrinsic hand muscles and improve upper limb function in patients with chronic stroke. As a preliminary study, we tested whether increased corticomotor excitability would be similarly observed in muscles acting about the wrist, and remain present during robotic training involving active wrist movements, in six chronic stroke patients with residual motor deficit.
Methods
Transcranial magnetic stimulation (TMS) generated motor evoked potentials (MEP) in the flexor carpi radialis (FCR) and provided a measure of corticomotor excitability and short-interval cortical inhibition (SICI) before and immediately after a period of tDCS (1 mA, 20 min, anode and TMS applied to the lesioned hemisphere), and robotic wrist training (1hr).
Results
Following tDCS, the same TMS current strength evoked an increased MEP amplitude (mean 168 ± 22%SEM; p < 0.05), that remained increased after robot training (166 ± 23%; p < 0.05). Conditioned MEPs were of significantly lower amplitude relative to unconditioned MEPs prior to tDCS (62 ± 6%, p < 0.05), but not after tDCS (89 ± 14%, p = 0.40), or robot training (91 ± 8%, p = 0.28), suggesting that the increased corticomotor excitability is associated with reduced intracortical inhibition.
Conclusion
The persistence of these effects after robotic motor training, indicates that a motor learning and retraining program can co-exist with tDCS-induced changes in cortical motor excitability, and supports the concept of combining brain stimulation with physical therapy to promote recovery after brain injury.
1. Introduction
Despite the best rehabilitation efforts, stroke survivors are often left with significant and permanent residual motor impairments (Cramer 2004; Cramer 2008), secondary health implications from the sedentary lifestyle (Hankey et al., 2002; Ivey et al., 2006), as well as wider economic burden (American-Heart-Association, 2007). Yet recovery of motor function following stroke is known to be enhanced with motor training aimed at reducing impairments and relearning motor skills (Butefisch 2006; Fasoli et al., 2003; Krebs & Hogan 2006; Platz et al, 2005; Richards et al.,; Richards et al, 2007; Van Peppen et al, 2004; Volpe et al., 2005). Emerging technologies such as robot controlled and guided motor training represent an exciting and important advance on existing therapies, which are labour intensive (Colombo et al., 2008; Kwakkel et al., 2007; Lum et al., 2002; Prangeet al., 2006; Volpe et al., 2000), and may serve as a useful complementary therapy (Masiero et al., 2007; Riener 2007). It remains to be determined if non-invasive brain stimulation could be used to further enhance the effects of behavioural training such as robotic therapy. Anodal transcranial direct current stimulation (tDCS), applied at rest over primary motor cortex can raise corticomotor excitability and transiently improve motor function in healthy participants (Boggio et al., 2006), and chronic stroke patients (Hummel et al., 2005; Hummel et al., 2006). Few studies of tDCS have investigated the application of tDCS in temporal proximity to motor training. One study in a small group of sub-acute stroke patients, showed that tDCS and robotic arm therapy can be safely applied and was well tolerated, however the functional results were unclear (Hesse et al., 2007). For motor training to be augmented after tDCS, the tDCS-effects need to be sustained through the training period, yet there are few studies of the interaction of tDCS and motor training. The aim of this preliminary study was to test if raised corticomotor excitability of the forearm area might be observed after 20 minutes anodal stimulation in chronic stroke patients, and whether the after-effects of tDCS on corticospinal excitability and short-interval cortical inhibition (SICI) are modulated or reset by robotic wrist training.
2. Methods
2.1. Participants and study design
Six right-handed participants, two with right hemi-paresis and four with left hemiparesis, volunteered for the study (3/3 Male/Female, mean 4.7 yrs since stroke; 67. 7± 12.7 years of age; upper distal extremity mean Fugl-Meyer wrist/hand = 16.8 (out of 30), range 8 to 25; mean MRC (wrist) Motor Power= 19.5(out of 30), range 12 to 25; further details Fig. 1). These subjects were not naïve to robotic training, and had completed between 6 and 12 weeks of robotic training aimed at measuring the effects of training on distal and proximal limb segment recovery. Volunteers were included in these studies if they met the following criteria: a) a first single focal unilateral lesion with diagnosis verified by brain imaging (MRI or CT scans) that occurred at least 6 months prior; b) cognitive function sufficient to understand the experiments and follow instructions (Mini-Mental Status Score of 22 or higher or interview for aphasic subjects); and c) Motor Power score ≥1/5 and < 4/5 (neither hemiplegic nor fully recovered motor function in the muscles of the shoulder and elbow and wrist).
Fig. 1.
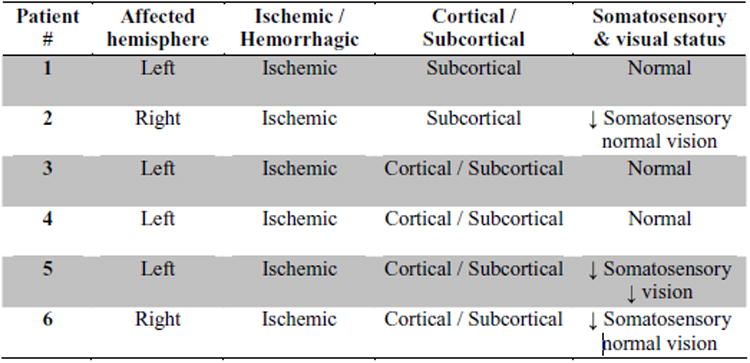
Supplementary clinical information for the study participants.
Informed consent was obtained prior to the study, which had approval of the Ethics Committee of Burke Rehabilitation Hospital, White Plains, NY, MIT-COUHES (Committee on the Use of Humans as Experimental Subjects), and Beth Israel Deaconess Medical Center, Boston, MA. Subjects received tDCS at rest, and then performed the robot training. Transcranial magnetic stimulation (TMS) was used to assess corticospinal excitability and intracortical inhibition before tDCS, immediately after tDCS and again immediately after 1hr robotic training.
2.2. Transcranial direct current stimulation
A 1mA current was delivered using surface rubber-carbon electrodes (35cm2) with surrounding saline soaked sponges (0.9% NaCl) (Dundas et al., 2007) by a specially developed battery driven, constant current stimulator (maximum output 10 mA). Participants received stimulation for 20 minutes while seated at rest, with the anode over the optimal site for flexor carpi radialis (FCR) as identified using TMS, and the cathode on the contralateral supraorbital area (Nitsche & Paulus 2001).
2.3. Robot training
The wrist robot affords active-assistance in all three-degrees of freedom of the wrist: flexion/extension, abduction/adduction, and pronation/supination.
The patient was seated with the robot on the right side and secured at the hand, wrist, and above the elbow. The workstation is designed to fit the patient comfortably with around 20° of shoulder abduction and 30° of shoulder flexion. The forearm rests comfortably on a curved rest so that the ulnar styloid sits barely beyond the distal edge. The hand is secured to the handle in a comfortable composite flexion grip with the least restrictive support provided. Humeral and forearm supports are supplied to maintain snug support. A four-point seatbelt is tightened to minimize movement of the torso. The wrist protocol consists of 3 batches of 320 “assisted-as-needed” point-to-point movements. Each assisted batch is preceded and followed by an unassisted game and the assistance is tuned based on patient's performance (Krebs et al., 2003). The first two assisted batches exercise wrist flexion/extension, abduction/adduction, and combination of these movements with 8 outbound targets distributed around an ellipse with major axis of 60 degrees (30 degrees for flexion-extension each) and minor axis of 30 degrees (15 degrees for adduction/abduction each). The last assisted batch exercises exclusively pronation and supination (30 degrees for pronation/supination each).
2.4. Transcranial magnetic stimulation
2.4.1. Positioning and set-up
The shoulder-and-elbow MIT-MANUS has been described in detail in several publications (Krebs et al., 1998b; Krebs et al., 2007a), and was used in the present study for subject positioning and force measurements. Each participant was comfortably seated with the right arm abducted, elbow supported, and hand lightly grasping the shoulder-and-elbow robot handle approximately 30 cm in front of the sternum. A custom-made hand-holder maintained the subject's wrist in a neutral position. Participants were given standard instructions prior to the commencement of the study to look directly forward at the computer screen and to focus on maintaining constant force and direction of tension according to real-time visual biofeedback.
2.4.2. Motor cortex stimulation
A lycra cap was positioned over the head and the vertex marked by measuring the mid-point intersection of the nasioninion and interaural lines. Stimulus sites were then marked on the cap using the vertex as a reference point, in 1-cm steps in the coronal and sagittal planes, over the region of the primary motor cortex in the affected hemisphere. TMS was delivered using a MAGSTIM 200 stimulator with a 5 cm diameter figure-of-eight coil, held tangential to the skull and aligned in the para-sagittal plane with the handle rotated 45° lateral.
2.4.3. Motor evoked potential recordings
Surface electromyographic (EMG) recordings were made from electrodes positioned over the muscle belly of the right flexor carpi radialis (FCR) muscle. EMG signals were amplified (×1000) and band-pass filtered between 3 and 1000 Hz, before being digitised at 2000 Hz for 100 ms following each stimulation, using a Powerlab 8/30 acquisition and analysis system (ADIn-stuments). The optimal site of stimulation for FCR was determined from initial exploration using the reference cap (1 × 1 cm grid), and was defined as the site corresponding to the largest amplitude MEPs (mean of four MEPs at each site) at a consistent suprathreshold stimulus intensity. The selected site was used throughout the experiment. Active motor threshold (AMT) was determined during a sustained 10% maximal voluntary isometric contraction (MVIC) of wrist flexion, using four stimuli starting at 30% of stimulator output and increasing at 5% increments until three of the four stimuli produced a MEP. A short rest period was included between each increment. During the study, stimuli were delivered at an intensity 120% AMT whilst holding a 10%MVIC. MVIC was taken as the highest value of three five-second attempts each separated by two minutes, and assessed pre-tDCS, post-tDCS and post-robot training. 10%MVIC was re-calculated for each individual if MVIC varied across conditions.
2.4.4. Short-interval cortical inhibition
Short-interval cortical inhibition (SICI) was evaluated using paired-pulse TMS with a subthreshold conditioning stimulus (95% of AMT) followed by a suprathreshold test stimulus (120% AMT), at an inter-stimulus interval (ISI) of 3 ms (Kujirai et al., 1993). 10 serial MEPs at > 5 second intervals were recorded for single pulse and paired conditions, with the condition presentation order randomised between subjects.
2.5. Data analysis
To determine changes in MEP amplitude following tDCS and robot conditions, the mean peak-to-peak amplitude was established for each time point (pre-tDCS, post-tDCS, post-robot) and averaged across participants. Significant differences were tested using the Friedman repeated measures analysis of variance with Dunn's post hoc multiple comparisons. At each time point, conditioned MEP amplitude was expressed as a percentage of unconditioned MEP amplitude, to provide an index of SICI, and the group mean tested against a value of 100% by a Z-test. MVIC peak force expressed in Newtons, was averaged across the group, then post-tDCS and post-robot time points were tested separately against pre-tDCS using a two-tailed paired t-test. Significant results were those achieving an alpha level of 0.05.
3. Results
Following tDCS, MEP amplitude was significantly increased to 168 ± 22%SEM of baseline (mean pre-tDCS =0.77± 0.18 mV, post-tDCS=1.36±0.42mV, p < 0.05, Fig. 3), and remained significantly increased at 166 ± 23% after robot training (post-robot = 1.36 ± 0.44 mV, p < 0.05). Figure 4 shows sample MEP waveforms from one subject, illustrating the rise in MEP amplitude after tDCS, and the comparable amplitude following the robotic wrist therapy. In four subjects, the effect of robot treatment alone was examined on a separate occasion, and no significant change in mean MEP amplitude was observed (pre-robot 1.23 ± 0.2 mV, post-robot 1.47 ± 0.5 mV, post-robot normalised to pre-robot = 117 ± 29%, p = 0.44). Prior to tDCS, conditioned MEPs were significantly reduced, to 62 ± 6% (Z = 5.96, p < 0.05, Fig. 5) of unconditioned MEP amplitude, in five patients (mean pre-tDCS; test = 1.35 ± 0.5 mV, conditioned= 0.78± 0.2 mV). Conditioned MEPs were not significantly reduced from unconditioned following tDCS (89± 14%, Z = 0.83,p = 0.40; unconditioned; = 1.63 ± 0.6 mV, conditioned = 1.46 ± 0.5 mV) or robot training (91 ± 8%, Z = 1.08, p = 0.28; test = 1.87 ± 0.8 mV, conditioned = 1.66 ± 0.6mV). MVIC force did not significantly vary from baseline following tDCS or robotic training (pre-tDCS 121.25 ± 12N; post-tDCS 114.33 ± 8N, p = 0.33; post-robot 111.75 ± 12N, p = 0.47). All patients tolerated the tDCS and robotic therapy well, with no adverse effects.
Fig. 3.
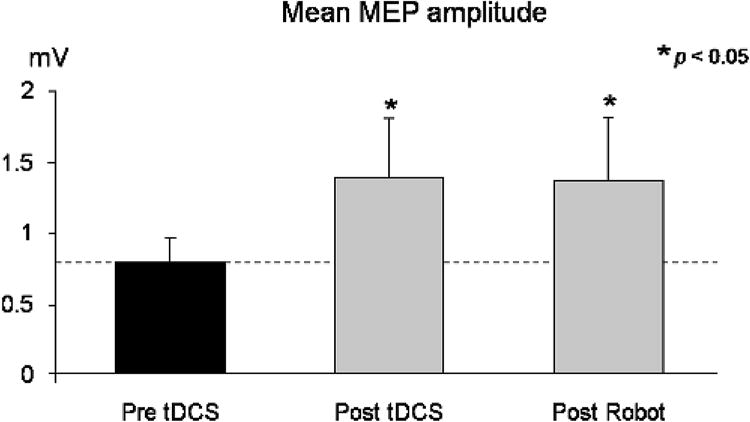
Mean (± SEM) MEP amplitude from across subjects. MEPs were recorded from the FCR muscle during a low-level isometric wrist flexion, before and immediately following 20min anodal brain stimulation (tDCS), then again after 1hr of robotic wrist therapy. Following tDCS, MEP amplitude was significantly elevated, and remained significantly elevated after robotic therapy.
Fig. 4.
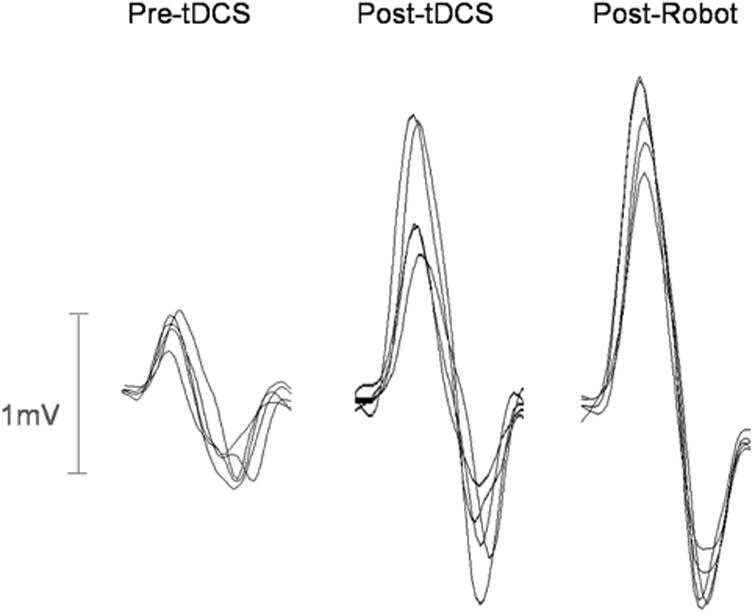
Sample overlaid MEPs from one subject, showing the marked increase in MEP amplitude following 20min anodal brain stimulation, which remains present following the 1hr robotic therapy.
Fig. 5.
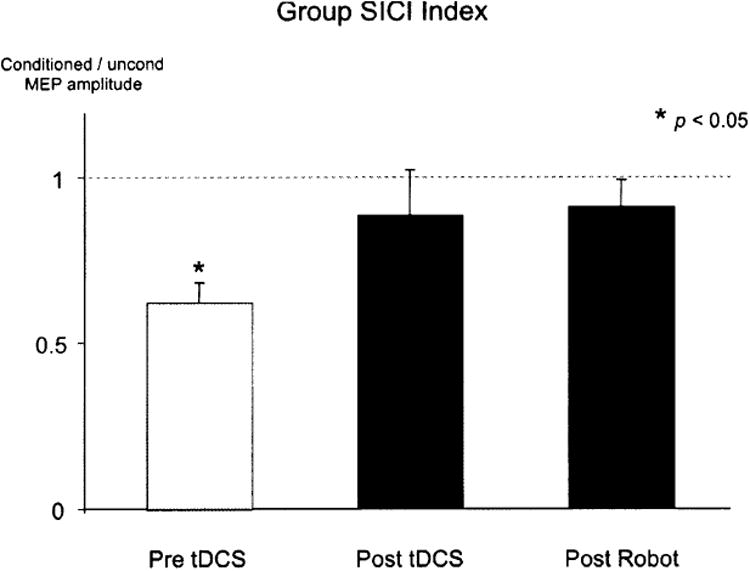
Percentage reduction in the conditioned compared to the unconditioned MEP amplitude before and after tDCS, then again after robot therapy, showing the significant reduction in conditioned MEP amplitude before intervention, is not observed after tDCS or robot therapy. This demonstrates reduced short-interval cortical inhibition resulting from tDCS, that is sustained during robotic therapy and may be implicated in the corresponding increase in corticomotor excitability.
4. Discussion
The present study shows that raised corticospinal excitability accompanied by reduced cortical inhibition following anodal tDCS, can occur in forearm muscles of chronic stroke patients, and persists during task specific robotic wrist training.
Primary motor cortex can reorganize during recovery from lesion and motor skill acquisition (Classen et al., 1998; Kleim et al., 2007; Matsuzaka et al., 2007; Nudo et al., 1996a; Nudo et al., 1996b; Pascual-Leone et al., 1995; Sanes & Donoghue 2000), through unmasking of latent synapses (Sanes & Donoghue 2000) and modification of synaptic strength, including long-term potentiation (Hess & Donoghue 1996). These mechanisms are strongly promoted by reduced GABAergic activity (Hess & Donoghue 1994; Jacobs & Donoghue 1991), and functional plasticity in human motor cortex preferentially occurs in areas of reduced inhibition (Liepert et al., 2006). Ziemann and colleagues demonstrated that practice-dependent plasticity in human motor cortex, could be enhanced during cortical disinhibition, following ischemic nerve block (Ziemann et al., 2001), and suggest that interventions designed to promote practice-dependent plasticity should aim to reduce intracortical inhibition. In the present study, a reduction of cortical inhibition, potentially involving decreased GABAergic activity (Ilic et al., 2002), was observed following anodal tDCS. This is consistent with earlier studies (Hummel et al., 2005; Nitsche et al., 2005), and supports the concept that tDCS may be useful to promote robotic practice-dependent plasticity in stroke patients.
The significance of the present findings is that the cortical excitability shifts observed following tDCS, were not modified by robotic motor training. While our robotic protocol did not lead to sustained excitability shifts after a single session, when performed over multiple sessions, this training is known to induce lasting improvements in motor function in chronic stroke patients (Krebs et al., 2007b). Repetitive motor skill practice (but not passive training), transiently increases motor cortex excitability (Perez et al., 2004a) and reduces cortical inhibition (Liepert et al., 1998; Perez et al., 2004b). These transient changes in excitability can lead to sustained, cumulative changes, and are associated with motor learning (Pascual-Leone et al., 1995). Interventions such as tDCS that enhance motor cortex excitability and reduce cortical inhibition are therefore appealing for augmenting motor learning in behavioural therapies. However, sustained cortical excitability increases following tDCS may not necessarily be additive, to transient excitability shifts during motor training. Homeostatic mechanisms acting to maintain stability in cortical networks prevent excessive increases in excitability (Abraham & Bear 1996). For example, experimental protocols that induced LTP-like increases in synaptic efficacy and raise cortical excitability, can lead to LTD-like decreases in synaptic efficacy and reduce cortical excitability if delivered during anodal tDCS when cortical excitability is already elevated (Nitsche et al., 2007). Furthermore, despite reduced corticospinal excitability in the affected hemisphere of stroke patients (Pennisi et al., 1999; Traversa et al., 1997; Tsai et al., 1992), intracortical inhibition can be already reduced (Swayne et al., 2008), thus potentially decreasing the range of tolerance for homeostatic influence. Thus, motor training in stroke patients could interfere with tDCS after-effects. Yet we have demonstrated that anodal tDCS excitability shifts, as well as muscle force production, can be maintained across a period of motor training. TDCS after-effects can therefore remain unaffected by cortical excitability changes during task-specific muscle activation.
Our findings support the plausibility of combined tDCS and motor training to enhance practice-induced plasticity in stroke. While it was not expected that a single session of combined therapy would lead to significant functional improvement, the rationale for this novel combined therapy, is that the effect across multiple practice sessions may translate into enhanced and sustained behavioural changes. Such experiments are now underway in our laboratory. Further work is also warranted to investigate the optimal parameters of stimulation. For example, Hesse and colleagues showed functional improvement in only 3 of 10 sub-acute stroke patients, with anodal, ipsilesional tDCS during robotic motor training, applied over repeated sessions (Hesse et al., 2007). It remains unclear whether a greater duration of training (Krebs et al., 1998a) or tDCS (Nitsche & Paulus 2000; Nitsche & Paulus 2001) would be beneficial. Furthermore, a critical parameter may be the time at which tDCS is applied in relation to the training. Stimulation during training can diminish learning (Rosenkranz et al., 2000), although the polarity of stimulation may be important, as cathodal tDCS, acting to reduce background cortical activity, can promote simultaneously induced LTP-like plasticity (Nitsche et al., 2007). Our findings that cortical activation during robotic motor training does not interfere with post-tDCS excitability shifts, suggests that potential simultaneous modification of cortical GABAergic inhibitory networks resulting from the current protocol, are complementary rather than antagonistic. This is in keeping with studies showing that facilitatory associative plasticity can be complementary to preceding but not simultaneous anodal tDCS (Nitsche et al., 2007). These preliminary findings suggest that anodal tDCS may be effective if preceding motor learning paradigms, yet the nature of the training or learning paradigm may be a critical factor (Kuo et al., 2008). The timing of tDCS application relative to motor training, as well as the polarity of stimulation, in relation to motor task specifics requires further investigation.
Technical considerations maybe important for future studies. Firstly, muscle facilitation is useful in chronic stroke for obtaining a suitable amplitude test MEP, however SICI can be reduced as a consequence (Ridding et al., 1995), and may therefore influence sensitivity for detecting a change in SICI; and second, SICI can be influenced by a post-tDCS increase in test MEP amplitude, however it is not thought to be influenced within the range of amplitude changes observed in the present study (Benwell et al., 2006). Our findings were reported in chronic stroke patients with moderate motor dysfunction, and it remains unclear how these findings might apply in different stages of recovery or be influenced by stroke severity. Further work is required to understand the relationship of cortical excitability changes to function during stroke recovery, and the influence of lesion location and size, and time since stroke (Koski et al., 2004; Misra & Kalita 1995; Swayne et al., 2008). Intracortical facilitation (ICF) was not measured in the present study and may be implicated in excitability changes resulting from robotic training and tDCS. Future studies may also test if tDCS improves motor learning by enhancing performance during practice tasks, through factors such as improved muscle strength and reaction time (Hummel et al., 2006), and decreased muscle fatigue (Cogiamanian et al., 2007).
5. Conclusion
We have demonstrated in chronic stroke patients with a residual motor deficit, that post-tDCS excitability shifts can persist during robotic motor practice. This supports the idea of using tDCS to prime the motor system prior to training.
Fig. 2.
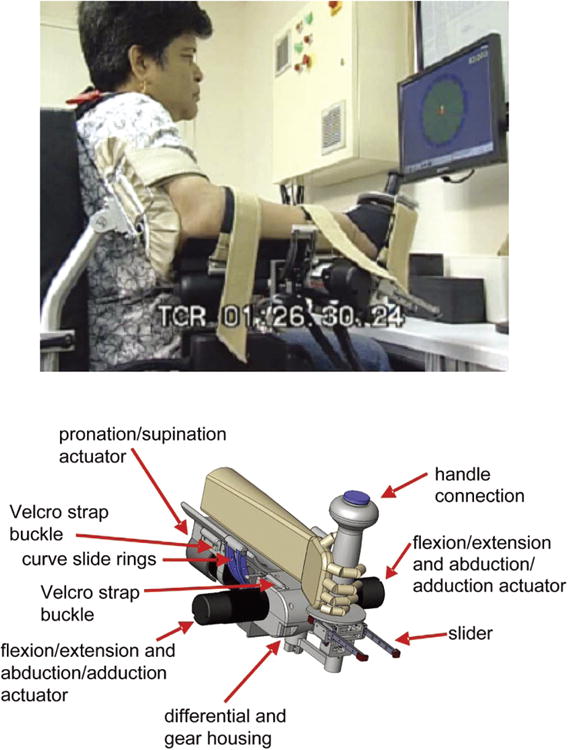
Wrist Robot. The top row shows the device during therapy at Burke Rehabilitation Hospital. The bottom row shows a solid view of the design. (Colours are visible in the online version of the article www.iospress.nl.)
Acknowledgments
This work was supported by NIH grant K24 RR018875 for APL, and RO1 HD045343 for BTV and HIK. The authors would like to thank Felipe Fregni MD PhD for insightful comments.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- American-Heart-Association. Heart Disease and Stroke Statistics — 2007 Update. Dallas, Texas: ©2007, American Heart Association; 2007. [Google Scholar]
- Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW. Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res. 2006;170:191–198. doi: 10.1007/s00221-005-0195-7. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MTA, Fregni F. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–236. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Butefisch CM. Neurobiological bases of rehabilitation. Neurol Sci. 2006;27:S18–23. doi: 10.1007/s10072-006-0540-z. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid Plasticity of Human Cortical Movement Representation Induced by Practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci. 2007;26:242–249. doi: 10.1111/j.1460-9568.2007.05633.x. [DOI] [PubMed] [Google Scholar]
- Colombo R, Pisano F, Micera S, Mazzone A, Delconte C, Carrozza MC, Dario P, Minuco G. Assessing Mechanisms of Recovery During Robot-Aided Neurorehabilitation of the Upper Limb. Neurorehab Neural Re. 2008;22:50–63. doi: 10.1177/1545968307303401. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Changes in motor system function and recovery after stroke. Restor Neurol Neuros. 2004;22:231–238. [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- Dundas JE, Thickbroom GW, h Mastaglia FL. Perception of comfort during transcranial DC stimulation: Effect of Na-Cl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007;118:1166–1170. doi: 10.1016/j.clinph.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Archives of Physical Medicine and Rehabilitation. 2003;84:477–482. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-Term Disability After First-Ever Stroke and Related Prognostic Factors in the Perth Community Stroke Study, 1989-1990. Stroke. 2002;33:1034–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars) 1996;56:397–405. doi: 10.55782/ane-1996-1143. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neuros. 2007;25:9–15. [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel F, Voller B, Celnik P, Floel A, Giraux P, Gerloff C, Cohen L. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey F, Hafer-Macko C, Macko R. Exercise rehabilitation after stroke. NeuroRx. 2006;3:439–450. doi: 10.1016/j.nurx.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the Cortical Motor Map by Unmasking Latent Intracortical Connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Kleim ED, Cramer SC. Systematic assessment of training-induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat Protoc. 2007;2:1675–1684. doi: 10.1038/nprot.2007.206. [DOI] [PubMed] [Google Scholar]
- Koski L, Mernar TJ, Dobkin BH. Immediate and Long-Term Changes in Corticomotor Output in Response to Rehabilitation: Correlation with Functional Improvements in Chronic Stroke. Neurorehabil Neural Re. 2004;18:230–249. doi: 10.1177/1545968304269210. [DOI] [PubMed] [Google Scholar]
- Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM. Robot-aided functional imaging: Application to a motor learning study. Human Brain Mapping. 1998a;6:59–72. doi: 10.1002/(SICI)1097-0193(1998)6:1<59::AID-HBM5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Hogan N. Therapeutic Robotics: A Technology Push. Proceedings of IEEE. 2006;94:1727–1173. doi: 10.1109/JPROC.2006.880721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng. 1998b;6:75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Palazzolo JJ, Dipietro L, Ferraro M, Krol J, Rannekleiv K, Volpe BT, Hogan N. Rehabilitation robotics: performance-based progressive robot-assisted therapy. Autonomous robots. 2003;15:7–20. [Google Scholar]
- Krebs HI, Volpe BT, Williams D, Celestino J, Charles SK, Lynch D, Hogan N. Robot-aided neurorehabilitation: a robot for wrist rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2007a;15:327–335. doi: 10.1109/TNSRE.2007.903899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Volpe BT, Williams D, Celestino J, Charles SK, Lynch D, Hogan N. Robot-aided neurorehabilitation: a robot for wrist rehabilitation. IEEE transactions on neural systems and rehabilitation engineering. 2007b;15:327–335. doi: 10.1109/TNSRE.2007.903899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, Nitsche MA. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologica. 2008;46(8):2122–8. doi: 10.1016/j.neuropsychologia.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Krebs HI. Effects of Robot-Assisted Therapy on Upper Limb Recovery After Stroke: A Systematic Review. Neurorehabil Neural Repair. 2007;22(2):111–21. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Haevernick K, Weiller C, Barzel A. The surround inhibition determines therapy-induced cortical reorganization. NeuroImage. 2006;32:1216–1220. doi: 10.1016/j.neuroimage.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83:952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- Masiero S, Celia A, Rosati G, Armani M. Robotic-Assisted Rehabilitation of the Upper Limb After Acute Stroke. Arch Phys Med Rehabil. 2007;88:142–149. doi: 10.1016/j.apmr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill Representation in the Primary Motor Cortex After Long-Term Practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kalita J. Motor evoked potential changes in ischaemic stroke depend on stroke location. J Neurol Sci. 1995;134:67–72. doi: 10.1016/0022-510x(95)00216-4. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, Tergau F, Paulus W. Timing-Dependent Modulation of Associative Plasticity by General Network Excitability in the Human Motor Cortex. J Neurosci. 2007;27:3807–3812. doi: 10.1523/JNEUROSCI.5348-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during & after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996a;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural Substrates for the Effects of Rehabilitative Training on Motor Recovery After Ischemic Infarct. Science. 1996b;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pennisi G, Rapisarda G, Bella R, Calabrese V, Maertens de Noordhout A, Delwaide PJ. Absence of Response to Early Transcranial Magnetic Stimulation in Ischemic Stroke Patients : Prognostic Value for Hand Motor Recovery. Stroke. 1999;30:2666–2670. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004a;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Experimental brain research. 2004b;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Platz T, Eickhof C, van Kaick S, Engel U, Pinkowski C, Kalok S, Pause M. Impairment-oriented training or Bobath therapy for severe arm paresis after stroke: a single-blind, mul-ticentre randomized controlled trial. Clinical Rehabilitation. 2005;19:714–724. doi: 10.1191/0269215505cr904oa. [DOI] [PubMed] [Google Scholar]
- Prange GB, Jannink MJ, Groothuis-Oudshoorn CG, Hermens HJ, Ijzerman MJ. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev. 2006;43:171–184. doi: 10.1682/jrrd.2005.04.0076. [DOI] [PubMed] [Google Scholar]
- Richards LG, Stewart KC, Woodbury ML, Senesac C, Cauraugh JH. Movement-dependent stroke recovery: A systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia. 46(1):3–11. doi: 10.1016/j.neuropsychologia.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riener R. Robot-aided rehabilitation of neural function in the upper extremities. Acta Neurochir Suppl. 2007;97:465–471. doi: 10.1007/978-3-211-33079-1_61. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulationinthe human. Neurosci Lett. 2000;296:61–63. doi: 10.1016/s0304-3940(00)01621-9. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and Primary Motor Cortex. Annual Review of Neuroscience. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Swayne OBC, Rothwell JC, Ward NS, Greenwood RJ. Stages of Motor Output Reorganization after Hemispheric Stroke Suggested by Longitudinal Studies of Cortical Physiology. Cereb Cortex. 2008;18(8):1909–22. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of Motor Cortical Reorganization After Stroke: A Brain Stimulation Study With Focal Magnetic Pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Tchen PH, Chen JD. The relation between motor evoked potential and clinical motor status in stroke patients. Electromyogr Clin Neurophysiol. 1992;32:615–620. [PubMed] [Google Scholar]
- Van Peppen R, Kwakkel G, Wood-Dauphinee S, Hendriks H, Van der Wees P, Dekker J. The impactofphysical therapy on functional outcomes after stroke: what's the evidence? Clin Rehabil. 2004;18:833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Ferraro M, Lynch D, Christos P, Krol J, Trudell C, Krebs HI, Hogan N. Robotics and other devices in the treatment of patients recovering from stroke. Curr Neurol Neurosci Rep. 2005;5:465–470. doi: 10.1007/s11910-005-0035-y. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N, Edelstein L, Diels C, Aisen M. Anovel approach tostroke rehabilitation: Robot-aided sensorimotor stimulation. Neurology. 2000;54:1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]


