SUMMARY
The primary goal of surgical oncology is to obtain a tumour resection with disease-free margins. Transoral robotic surgery (TORS) for surgical treatment of head-neck cancer is commensurate with standard treatments. However, the likelihood of positive margins after TORS is up to 20.2% in a recent US survey. The aim of this study is to evaluate the efficacy and the feasibility of narrow-band imaging (NBI) during TORS in order to improve the ability to achieve disease-free margins during tumour excision. The present study was conducted at the ENT, Head- Neck Surgery and Oral Surgery Unit, Department of Special Surgery, Morgagni Pierantoni Hospital, Azienda USL Romagna. From March 2008 to January 2015, 333 TORS were carried out for malignant and benign diseases. For the present study, we retrospectively evaluated 58 biopsy-proven squamous cell carcinoma patients who underwent TORS procedures. Patients were divided into 2 groups: (1) 32 who underwent TORS and intra-operative NBI evaluation (NBI-TORS); (2) 21 who underwent TORS with standard intra-operative white-light imaging (WLITORS). Frozen section analysis of margins on surgical specimens showed a higher rate of negative superficial lateral margins in the NBI-TORS group compared with the WLI-TORS group (87.9% vs. 57.9%, respectively, p = 0.02). The sensitivity and specificity of intra-operative use of NBI, respectively, were 72.5% and 66.7% with a negative predictive value of 87.9%. Tumour margin enhancement provided by NBI associated with magnification and 3-dimensional view of the surgical field might increase the capability to achieve an oncologically-safe resection in challenging anatomical areas where minimal curative resection is strongly recommended for function preservation.
KEY WORDS: Robotic surgical procedures, Narrow band imaging, Frozen section, Optical imaging, Minimally invasive surgery
RIASSUNTO
Il principio fondamentale della chirurgia oncologica è ottenere una exeresi della massa tumorale con margini di resezione liberi da malattia. Tuttavia nel corso degli anni sono state messe a punto tecniche mini invasive, quali la chirurgia robotica trans orale (TORS), che permettono di ottenere risultati comparabili alle tecniche tradizionali in termini di controllo loco-regionale della malattia e sopravvivenza. D'altronde, l'approccio chirurgico robotico prevede una resezione minimale e sufficiente della lesione tumorale al fine di ottenere una exeresi che non comporti una eccessiva demolizione dei tessuti non coinvolti dalla patologia in modo da ridurre le sequele funzionali. Il punto chiave del concetto di chirurgia mini-invasiva è la capacità di localizzare in maniera appropriata il piano di resezione al fine di minimizzare l'asportazione di tessuto normale ma allo stesso tempo di consentire un buon controllo sui margini ai fini di prevenzione della recidiva locale. Lo scopo dello studio è di analizzare se l'uso della narrow-band imaging (NBI) nella valutazione intra-operatoria dei margini superficiali di resezione possa permettere un maggior successo in termini di margini liberi da malattia durante la chirurgia robotica trans orale per tumori faringei e laringei. Nel periodo compreso da marzo 2008 e gennaio 2015, presso l'UO di Otorinolaringoiatria e Chirurgia Cervico-Facciale, Servizio di Stomatologia e Chirurgia Orale (Dipartimento di Chirurgie Specialistiche) dell'Ospedale Morgagni-Pierantoni di Forlì (AUSL della Romagna) sono state eseguite 333 procedure TORS sia per patologia benigna che maligna. In tale casistica 61 pazienti sono stati trattati con TORS per diagnosi istologica di malignità. Il carcinoma squamocellulare rappresenta l'entità istopatologica più frequente (95,1%); solo un paziente(1,6%) presentava un adenocarcinoma di tipo salivare della base lingua mentre due pazienti (3,3%) soffrivano di melanoma mucoso della tonsilla palatina. Tuttavia sono stati valutati nello studio solo 58 pazienti con carcinoma squamocellulare. I pazienti, quindi, sono stati divisi in due gruppi: 1) 37 soggetti sottoposti a TORS con valutazione intraoperatoria con l'uso di NBI; 2) 21 soggetti sottoposti a TORS valutati con luce bianca standard. L'analisi istopatologica al congelatore dei margini superficiali sul pezzo operatorio ha dimostrato una alta percentuale di negatività nelle resezioni assistite da NBI rispetto alle resezioni valutate con metodica standard (87,9% vs. 57,9%, rispettivamente, p = 0,02). La sensibilità e la specificità della NBI nel suo uso intraoperatorio raggiunge rispettivamente il 72,5% e il 66,7% con valore predittivo negativo pari al 87,9%. Concludendo l'uso della NBI durante le resezioni TORS per malignità consente di aumentare potenzialmente il successo di una resezione con margini liberi da malattia limitando la distruzione dei tessuti non coinvolti da tumore.
Introduction
The primary goal of surgical tumour removal is to obtain disease-free margins. The advent of transoral robotic surgery (TORS), using the daVinci® Surgical System (Intuitive Surgical, Sunnyvale, CA), allows for a three-dimensional view with magnification and increased freedom of mobility of surgical instruments (cautery/laser and Maryland forceps) in the surgical management of pharyngeal and laryngeal tumours. These advantages, besides avoiding mandibular splitting or neck incision, also improve surgical excision in terms of tumour-free margins, and whenever possible to spare important functional structures. In a recent US national survey, the likelihood of positive margins after TORS for oropharyngeal cancers was reported to be 20.2% 1. On the other hand, narrow band imaging (NBI) has been shown to be a useful additional tool for the early detection of small superficial cancers in the oropharynx, hypopharynx and larynx 2 3. This technique has also enabled the lateral spread of oropharyngeal, hypopharyngeal and laryngeal tumours to be visualised, allowing an accurate evaluation of lesion extension, which is essential for the best planning of the surgical margins 4. In our study, we used NBI combined with TORS to examine the superficial spread of headneck cancers during surgery to enhance tumour resection with disease-free margins. The main aim is to evaluate the feasibility and efficacy of NBI during TORS in order to achieve disease-free excision margins.
Materials and methods
The study was conducted in compliance with our Institutional Review Board and Ethics Committee requirements. Between March 2008 and January 2015 at ENT, Head-Neck and Oral Surgery Unit, Department of Special Surgery, Morgagni Pierantoni Hospital, Azienda USL Romagna, 333 TORS were carried out for malignant and benign diseases (Table I). The study was a prospective non-randomised, single-centre cohort trial. For the present study, we enrolled all patients who underwent TORS procedures for biopsy-proven squamous cell carcinoma (SCC). All cases were presented and discussed at our Head and Neck Multidisciplinary Tumour Board. Informed consent form was obtained by all patients after attending a counselling session on the alternatives to surgery and on the intraoperative use of NBI. We staged tumours in accordance with the American Joint Committee on Cancer staging criteria (AJCC, 7th) 5. A first group of cases was treated without the use of NBI until the instrumentation was acquired. In 2010, NBI was acquired by our Department and after an in-office training period, was applied intra-operatively to each cancer patient who underwent TORS.
Table I.
Forlì ENT Department TransOral Robotic Surgery procedures (May 2008-January 2015).
| 333 cases: | |||
|---|---|---|---|
| Benign diseases | Malignant diseases | ||
| 199 | OSAHS (BOT Reduction+Supraglottoplasty) | 21 | Tonsil Cancers (Radical Tonsillectomy) |
| 45 | Lingual Tonsil Hyperplasia | 18 | Tongue Base Cancers (Partial BOT resection) |
| 3 | Vallecular Fibroma | 8 | Supraglottic Cancers (Supraglottic laryngectomy) |
| 3 | Glottic-Supraglottic Obstruction/Adhesion (Lysis) | 5 | Lingual Tonsil Lymphomas |
| 2 | Velo-Pharyngeal Insufficiency (Veloplasty) | 4 | Posterior Pharyngeal Wall Cancer (Partial pharyngectomy) |
| 1 | Tongue Base Lymphangioma | 4 | Piryform Sinus Cancer (Partial Pharyngectomy) |
| 1 | Palate Pemphigoid Adhesion (Veloplasty) | 2 | Laryngeal Cancers (Total laryngectomy) |
| 1 | Lingual Thyroidectomy | 1 | Palate Cancer |
| 1 | Laryngocele | 1 | Retromolar Trigone + 1 lingual marginal cancer |
| 1 | Pyriform Sinus Fistula (closure) | 1 | Nasopharyngeal Cancer (Nasopharyngectomy) |
| 1 | Tongue Base Lymphangioma | 1 | Glottic Cancer (Cordectomy) |
| 2 | Parapharyngeal Tumours (Liposarcoma, Pleomorphic adenoma) | ||
A Fey-Kastenbauer retractor (Gyrus Medical Inc., Maple Grove, MN), Dingman retractor and Crowe Davis retractor were used to expose the operative site. The tumour margins were observed intraoperatively with a 0 or 30° 8 mm Hopkins Scopes (Karl Storz, Germany) by white light imaging (WLI) alone and then with the NBI high-definition video-endoscopy system (CV-260SL processor, CVL-260SL light source, Olympus Optical Co., Ltd., Japan) to evaluate the feasibility of the NBI system for clinical use and its ability to identify abnormal findings. The key point was the enhancement of definition of the lesion's extension and its macroscopic boundaries and maintain from them the margins established. The surgical margins were set at least 1 cm in the oral cavity/oropharynx and at least 2 mm in hypopharynx and larynx. The edges of surgical excision were inked and/or marked with monopolar (in case of difficulties to mark with ink, i.e. supraglottic/hypopharyngeal cancer) and controlled with NBI. When NBI showed a suspicious pattern 6 7 outside the inked edges, a further marking was done including that area (Figs. 1, 2). The daVinci® Surgical System surgical robot was positioned 30° angled on the right side of the patient. A 0 or 30° 8.5 mm endoscope was used with two 5-mm side arms Maryland dissectors and cautery. The operating surgeon was seated in the console, while the assistant was seated at the patient's head. An emergency tracheostomy set and head and neck operative instruments tray were opened and kept on standby in case of airway problems or uncontrolled intra-operative bleeding. Visualised vessels were clipped prior to transaction. All surgical specimens were firstly oriented and then submitted to the pathologist for assessment of the status of margins with frozen sections. Both frozen and definitive surgical specimens were analysed by the head-neck specialised pathologist of our Institution. In case of positive or close or unclear margins by frozen sections, additional resections were carried out until frozen sections were negative for residual malignancy. According to other authors, we stated that clear margin was > 5 mm for oral cavity/ pharyngeal tumours and > 1 mm for laryngeal cancers on microscopic evaluation 7 9. For the present study, only the first line superficial resection margins were included. Deep margins and secondary or tertiary superficial resection margins were excluded. All robotic procedures were performed as first surgeon by V.C. Neck dissection was either done concurrently during the "dead time" required for the frozen section examination (in case of cN+) or one week before (in case of major vessels close to the tumour showed by imaging) or 4 weeks after TORS (as elective neck dissection). According to our Tumour Board Policy, patients received adjuvant therapy if they had: (1) positive margins at definitive histologic report; (2) extra-capsular spread; (3) lymphovascular invasion; (4) perineural invasion; and/or (5) multiple positive nodes. Associations between variables and endpoints were tested with Fisher exact test or t tests, as appropriate. A 2-tailed P value < 0.05 was regarded as statistically significant. Statistical analysis was performed with STATA 12.0 software (Stata Corp., College Station, TX, USA).
Fig. 1.
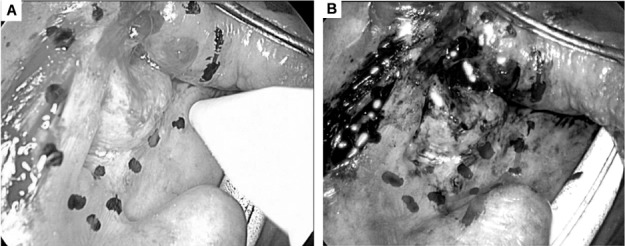
A) Marking the margins of a squamous cell carcinoma of the left tonsil under white light vision. B) Defining surgical margins with narrow-band imaging (red line showing further extension of the resection line).
Fig. 2.
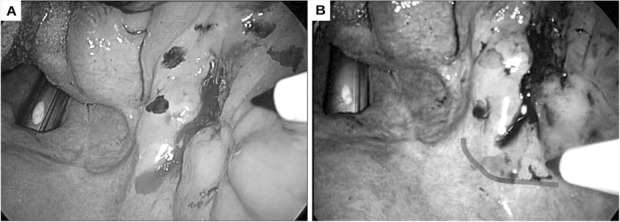
A) Marking the margins of a squamous cell carcinoma of the right retromolar trigon in white light vision. B) Defining surgical margins with narrow-band imaging (red line showing the further extension of the resection line).
Results
A total of 61 patients with head-neck biopsy proven cancers were evaluated. SCC represented the most frequent histologic finding in 58 patients (95.1%); one (1.6%) patient had a salivary adenocarcinoma and 2 (3.3%) patients suffered from mucosal melanoma. According to exclusion criteria, we excluded these cases from the analysis as they had histology different from SCC. Patients were divided into 2 groups: (1) 37 who underwent TORS and intraoperative NBI evaluation (NBI-TORS); (2) 21 who underwent TORS with standard intra-operative WLI (WLITORS). Demographic data and tumour staging for each group are summarised in Table II. Frozen section analysis of the margins on the primary surgical specimens showed a high rate of negative superficial lateral margins in the NBI-TORS group compared with the WLI-TORS group (87.9% vs. 57.9%, respectively, p=0.02). The sensitivity and specificity of the intra-operative use of NBI, respectively, was 72.5 and 66.7% with a negative predictive value of 87.9%.
Table II.
Demographic data and tumour staging for each group.
| NBI-TORS (n = 37) |
WLI-TORS (n = 21) |
p | |||
|---|---|---|---|---|---|
|
Mean Age ± SD |
66.1 ± 9.9 | 66.3 ± 8.3 | |||
| Male:Female | 24:13 | 16:5 | |||
| Tumour stage | |||||
| cT1 | 18 | 9 | 0.27 | ||
| cT2 | 16 | 8 | |||
| cT3 | 2 | 4 | |||
| cT4 | 1 | - | |||
| pT1 | 18 | 14 oropharynx | 9 | 8 oropharynx | 0.24 |
| 1 hypopharynx | 1 hypopharynx | ||||
| 2 larynx | |||||
| 1 oral cavity | |||||
| pT2 | 17 | 14 oropharynx | 8 | 8 oropharynx | |
| 2 hypopharynx | |||||
| 2 larynx | |||||
| 1 nasopharynx | |||||
| pT3 | 1 | 1 larynx | 3 | 3 larynx | |
| pT4 | 2 | 2 larynx | 1 | 1 larynx |
TORS: TransOral Robotic Surgery; NBI: Narrow Band Imaging; WLI: White Light Imaging; SD: Standard Deviation.
Discussion
In recent years, chemo-radiation (CRT) has become standard treatment in the management of most oropharyngeal cancers, and an alternative choice in laryngeal cancers for the preservation of laryngeal function 10 11. However, radiotherapy has some adverse effects, such as xerostomia and dysphagia, and can induce late complications, such as trismus, osteoradionecrosis and, not only theoretically, late induced field cancerisation. Accordingly, there is an increasing interest in transoral surgical techniques that reduce operation-related morbidity and improve the patients' quality of life, offering a low morbidity alternative to chemoradiation. Recently, Weinstein et al. 10 reported that the disease control and survival rates as well as the safety of TORS using the daVinci® Surgical System for the surgical treatment of oropharyngeal cancer were comparable to standard treatments. Since then, TORS has become part of the surgical armamentarium in the treatment of malignant and benign diseases 13. Furthermore, comparable oncologic and functional results with CRT has been shown in several trials 14-16. Nevertheless, the likelihood of positive margins after TORS ranged from 0-12% 15-19, although a recent survey in US reported positive margins up to 20.2% of TORS patients 1. These patients would require further adjuvant treatments such as CRT, which would reduce the basic advantage of TORS. Cooper et al. 20 have shown that adjuvant CRT is associated with an increased risk of severe adverse events. On the other hand, the philosophical approach by TORS requires minimal sufficient resection of the lesion in order to clear safely the tumour without excessive removal of normal tissue to obtain a real minimally-invasive resection with less risk of late functional impairments 12. The key point is to be able to properly locate the superficial resection line as close as possible to the lesion to avoid over-resection and far enough to the tumour margins in order to prevent recurrence. However, there is no universal agreement on how much normal tissue should be removed around a tumour to reduce the risk of local recurrence from incomplete resection 9. Furthermore, the thermal injury delivered to tissue by the monopolar scalpel of the robotic arm might create false positive margins and the pathologist should be aware of this surgical artefact 21. The rationale of the intra-operative use of additional optical imaging in our TORS setting is to minimise as much as possible surgical resections with a suspicious mucosal pattern close to the main tumour and reduce the rate of positive superficial margins. Concomitantly, in two different case series, Patsias et al. 22 showed that the use of high resolution microendoscopy imaging during TORS for oropharyngeal cancers might provide real time histological assessment of tumour margins, while Tateya et al. 23 evaluated the feasibility and efficacy of NBI in determining the extent of resection during TORS for oropharyngeal cancer. In previous studies, NBI was used to assess the extent of pharyngeal and oesophageal tumours, 4 and in a preoperative setting to enhance surgical margins in duodenal papilla cancers 24. The reported sensitivities and accuracies for the diagnosis of superficial cancer spread in the oropharynx and hypopharynx were 7.7 and 62.9% for WLI, respectively, compared with 100 and 86.7% for NBI, respectively 4 24. Our study has shown that the use of the NBI in controlling tumour resection might improve the chance to obtain disease-free margins without excessive removal of normal tissue (unnecessary over-resection). The sensitivity and specificity in defining tumour-free margins was 72.5 and 66.7%, respectively. Of note, the learning curve in TORS might theoretically be a potential bias in reducing the rate of positive margins over time; however, a recent study of 168 head-neck cancers treated with TORS showed no significant reduction in the rate of positive resection margins over other factors such as operative time 25. The limits of intra-operative use of this technique are highlighted during TORS sessions: (1) NBI improves visualisation of suspicious cancer patterns only on lateral superficial margins of the lesion resection; (2) the technique may be not be reliable on a surgical field after resection for assessing lateral margins because of the blood blinding the view on the same frequency spectrum; (3) NBI is not suitable to assess deep margins because it was developed to discern a specific mucosal pattern without any information on different tissues. However, in a recent study, preliminary data showed that the use of the NBI with magnifying endoscopy might predict the depth of invasion in laryngo-pharyngeal cancer 26. Nevertheless, NBI might be a reliable and safe tool in the surgical framework and does not increase either operative time or costs. On the contrary, a primary resection with tumour-free margins does not impose any time consuming additional resection (surgical and histological times).
Conclusions
The superficial enhancement of tumour margins provided by NBI associated with magnification and 3-dimensional view of the surgical field might increase the ability to achieve an oncologically safe resection in challenging anatomical areas.
Acknowledgements
The authors wish to thank the pathologist Matteo Costantini for his outstanding activity and for actively supporting the Head and Neck Multidisciplinary Tumour Board of our Institution.
References
- 1.Chen MM, Roman SA, Kraus DH, et al. Transoral robotic surgery: a population-level analysis. Otolaryngol Head Neck Surg. 2014;150:968–975. doi: 10.1177/0194599814525747. [DOI] [PubMed] [Google Scholar]
- 2.Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katada C, Nakayama M, Tanabe S, et al. Narrow band imaging for detecting metachronous superficial oropharyngeal and hypopharyngeal squamous cell carcinoma after chemoradiotherapy for head and neck cancers. Laryngoscope. 2008;118:1787–1790. doi: 10.1097/MLG.0b013e31817f4d22. [DOI] [PubMed] [Google Scholar]
- 4.Matsuba H, Katada C, Masaki T, et al. Diagnosis of the extent of advanced oropharyngeal and hypopharyngeal cancers by narrow band imaging with magnifying endoscopy. Laryngoscope. 2011;121:753–759. doi: 10.1002/lary.21553. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, et al., editors. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer; 2009. [Google Scholar]
- 6.Takano JH, Yakushiji T, Kamiyama I, et al. Detecting early oral cancer: narrow-band imaging system observation of the oral mucosa microvasculature. Int J Oral Maxillofac Surg. 2010;39:208–213. doi: 10.1016/j.ijom.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Bertino G, Cacciola S, Fernandes WB, Jr, et al. Effectiveness of narrow band imaging in the detection of premalignant and malignant lesions of the larynx: Validation of a new endoscopic clinical classification. Head Neck. 2015;37:215–222. doi: 10.1002/hed.23582. [DOI] [PubMed] [Google Scholar]
- 8.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27:952–958. doi: 10.1002/hed.20269. [DOI] [PubMed] [Google Scholar]
- 9.Hinni ML, Ferlito A, Brandwein-Gensler MS, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35:1362–1370. doi: 10.1002/hed.23110. [DOI] [PubMed] [Google Scholar]
- 10.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Machtay M, Rosenthal DI, Algazy KM, et al. Pilot study of organ preservation multimodality therapy for locally advanced resectable oropharyngeal carcinoma. Am J Clin Oncol. 2000;23:509–515. doi: 10.1097/00000421-200010000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein GS, O'Malley BW, Jr, Cohen MA, et al. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–1085. doi: 10.1001/archoto.2010.191. [DOI] [PubMed] [Google Scholar]
- 13.Pellini R, Mercante G, Ruscito P, et al. Ectopic lingual goiter treated by transoral robotic surgery. Acta Otorhinolaryngol Ital. 2013;33:343–346. [PMC free article] [PubMed] [Google Scholar]
- 14.Genden EM, Kotz T, Tong CCL, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121:1668–1674. doi: 10.1002/lary.21845. [DOI] [PubMed] [Google Scholar]
- 15.Durmus K, Apuhan T, Ozer E. Transoral robotic surgery for retromolar trigone tumours. Acta Otorhinolaryngol Ital. 2013;33:425–427. [PMC free article] [PubMed] [Google Scholar]
- 16.Mercante G, Ruscito P, Pellini R, et al. Transoral robotic surgery (TORS) for tongue base tumours. Acta Otorhinolaryngol Ital. 2013;33:230–235. [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Friedlander DF, Patel S, et al. The current status of robotic oncologic surgery. CA Cancer J Clin. 2013;63:45–56. doi: 10.3322/caac.21160. [DOI] [PubMed] [Google Scholar]
- 18.Blanco RGF, Fakry C, Ha PK, et al. Transoral robotic surgery experience in 44 cases. J Laparoendosc Adv Surg Tech A. 2013;23:800–907. doi: 10.1089/lap.2013.0261. [DOI] [PubMed] [Google Scholar]
- 19.White H, Ford S, Bush B, et al. Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg. 2013;139:773–778. doi: 10.1001/jamaoto.2013.3866. [DOI] [PubMed] [Google Scholar]
- 20.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous- cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 21.Mannelli G, Meccariello G, Deganello A, et al. Impact of lowthermal- injury devices on margin status in laryngeal cancer. An experimental ex vivo study. Oral Oncol. 2014;50:32–39. doi: 10.1016/j.oraloncology.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Patsias A, Giraldez-Rodriguez L, Polydorides A, et al. Feasibility of transoral robotic-assisted high resolution microendoscopic imaging of oropharyngeal squamous cell carcinoma. Head Neck. 2014 Oct 17; doi: 10.1002/hed.23892. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tateya I, Ishikawa S, Morita S, et al. Magnifying endoscopy with narrow band imaging to determine the extent of resection in transoral robotic surgery of oropharyngeal cancer. Case Rep Otolaryngol. 2014;2014:604737–604737. doi: 10.1155/2014/604737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoi T, Tsuji S, Sofuni A, et al. A novel approach emphasizing preoperative margin enhancement of tumour of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy. Gastrointestinal Endoscopy. 2009;69:136–144. doi: 10.1016/j.gie.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 25.White HN, Frederick J, Zimmerman T, et al. Learning curve for transoral robotic surgery: a 4-year analysis. JAMA Otolaryngol Head Neck Surg. 2013;139:564–567. doi: 10.1001/jamaoto.2013.3007. [DOI] [PubMed] [Google Scholar]
- 26.Tateya I, Morita S, Muto M, et al. Magnifying endoscope with NBI To predict the depth of invasion in laryngo-pharyngeal cancer. Laryngoscope. 2015;125:1124–1129. doi: 10.1002/lary.25035. [DOI] [PubMed] [Google Scholar]


