SUMMARY
As L-type voltage-gated calcium channels (VGCCs) control Ca2+ influx and depolarisation of cardiac and vascular smooth muscle, they represent a specific therapeutic target for calcium channel blockers (CCBs), which are approved and widely used to treat hypertension, myocardial ischaemia and arrhythmias. L-type currents also play a role in calcium entry in the sensory cells of the inner ear. In hair cells of both cochlea and labyrinth, calcium cytoplasmic influx is the first physiological process that activates complex intracellular enzymatic reactions resulting in neurotransmitter release. Excessive calcium ion entry into sensory cells, as a consequence of L-VGCCs malfunction is responsible for over-activation of phospholipase A2 and C, protein kinase II and C, nitric oxide synthase and both endonucleases and depolymerases, which can cause membrane damage and cellular death if the cytoplasmic buffering capacity is overcome. Nimodipine, a highly lipophilic 1-4 dihydropyridine that easily crosses the brain-blood barrier, is generally used to reduce the severity of neurological deficits resulting from vasospasm in patients with subarachnoid haemorrhage. Moreover, due to its selective blocking activity on L-channel calcium currents, nimodipine is also suggested to be an effective countermeasure for cochlear and vestibular dysfunctions known as channelopathies. Indeed, experimental data in amphibians and mammalians indicate that nimodipine has a stronger efficacy than other CCBs (aminopyridine, nifedipine) on voltage-dependent wholecell currents within hair cells at rest and it is the only agent that is also effective during their mechanically induced depolarisation. In humans, the efficacy of nimodipine is documented in the medical management of peripheral vestibular vertigo, sensorineural hearing loss and tinnitus, even in a pathology as complex as Ménière's disease. Nimodipine is also considered useful in the prophylaxis of damage to the facial and cochlear nerves caused by ablative surgery of cerebellopontine tumours; it has been recently hypothesised to accelerate functional recovery of recurrent nerve lesions during thyroid cancer surgery. Further trials with adequate study design are needed to test the efficacy of nimodipine in the treatment of vertigo due to cerebrovascular disease and vestibular migraine.
KEY WORDS: Vertigo, Tinnitus, Hearing loss, Vestibular migraine, Nimodipine
RIASSUNTO
I canali del calcio di tipo L sono indispensabili alla normale contrattilità del miocardio e della muscolatura liscia del sistema vascolare. In quanto tali, essi rappresentano uno specifico bersaglio terapeutico di una vasta famiglia di farmaci ad attività calcio-antagonista, che sono ampiamente usati per trattare l'ipertensione, l'ischemia miocardica e le aritmie. Tuttavia, i canali del calcio di tipo L svolgono un ruolo determinante anche nel normale funzionamento delle cellule sensoriali nell'orecchio interno. Nelle cellule cigliate, infatti, sia della coclea che del labirinto, l'ingresso del calcio all'interno del citoplasma è il primo processo fisiologico che attiva un complesso di meccanismi intracellulari, ovvero una sequenza di attivazioni enzimatiche, il cui risultato finale è il rilascio dei neurotrasmettitori a livello delle sinapsi. Al contrario, una concentrazione eccessiva di ioni calcio nelle stesse cellule sensoriali, ad esempio come conseguenza di un malfunzionamento dei canali del calcio di tipo L, è responsabile dell'attivazione delle fosfolipasi di tipo A2 e C, delle protein-chinasi II e C, dell'ossido nitrico sintetasi che possono causare un danno alla membrana plasmatica e la stessa morte cellulare qualora i limiti funzionali dei sistemi tampone del citoplasma vengano superati. La nimodipina, un agente altamente lipofilo, appartenente alla famiglia delle 1-4 diidropiridinine, che attraversa facilmente la barriera emato-encefalica, è generalmente utilizzata per ridurre la gravità dei deficit neurologici derivanti da vasospasmo nei pazienti con emorragia subaracnoidea. Inoltre, a causa della sua azione calcio-antagonista che si esplica selettivamente nei confronti dei canali del calcio di tipo L, viene suggerita come trattamento farmacologico efficace principalmente per quelle disfunzioni cocleari e vestibolari che vengono considerate delle "canalopatie". In effetti, i dati sperimentali ottenuti negli anfibi e in alcune specie di mammiferi indicano che nimodipina ha maggiore efficacia di altri calcioantagonisti (aminopiridina e nifedipina) nel bloccare le correnti di ioni calcio in ingresso nelle cellule ciliate e che è l'unico calcio-antagonista a mantenere tale efficacia anche durante la loro depolarizzazione indotta da stimolo meccanico. Negli esseri umani l'efficacia terapeutica della nimodipina è stata documentata nel trattamento della vertigine labirintica, dell'ipoacusia neurosensoriale e dell'acufene anche se riferibili ad una patologia complessa e non ancora del tutto chiarita come la malattia di Ménière. La nimodipina è inoltre considerata un valido approccio farmacologico nella profilassi dei danni neurali ai nervi facciali e cocleari causati dalla chirurgia ablativa dei tumori dell'angolo ponto-cerebellare ed è stata recentemente indicata per accelerare il recupero funzionale delle lesioni del nervo ricorrente conseguenti alla chirurgia del cancro alla tiroide. Per verificare, infine, l'efficacia della nimodipina nel trattamento delle vertigini di origine centrale, perlopiù associate ai disordini cerebrovascolari, e nel trattamento della vertigine emicranica, sono necessari ulteriori studi che confermino con maggiore rigore scientifico tali prospettive, peraltro già ampiamente riportate nella letteratura scientifica.
Introduction
Recent advances in the management of vertigo and tinnitus focus on device-related modalities that provide some patients with aural symptoms with acceptable results 1, but others with less positive ones 2. There is therefore an increasing interest for pharmacological interventions that are traditionally used to treat various inner ear diseases and that are particularly effective in controlling symptoms if prescribed at higher dosages than in the past 3 and/or as add-on medication in multi-component therapy 4. Finally, there is accumulating evidence that drugs approved for use in other diseases can be prescribed "off-label" to patients affected by inner ear dysfunctions with satisfactory results 5. For example, the 1-4 dihydropyridine calcium channel blocker nimodipine (NMDP) is approved by the Food and Drug Administration for reducing vasospasm after subarachnoid haemorrhage and neurological conditions 6. Initially developed to treat high blood pressure, NMDP was more recently suggested to be an effective countermeasure for labyrinthine dysfunction 7 8, dizziness due to central nervous system disorders 9 and migraine-related vertigo 10. It has also been shown to prevent reduction of cochlear blood flow of different origins 11 12, and represents a potential therapeutic option for at least some types of tinnitus 13 14. Recent experience has shown that NMDP reduces the calcium intracellular overload in traumatised neurons so to exert an anti-apoptotic effect and improves the rate of nerve collateral re-sprouting, axonal growth and re-myelination of injured cranial nerves 15 16. Accordingly, its use in the prophylaxis and/or reparation of nerve damage occurring during head and neck surgery has been proposed 17 18. Despite this accumulating experimental and clinical evidence, which could potentially expand the prescription of NMDP in otolaryngology, its use in clinical practice is far from widespread. This manuscript: (a) briefly summarises the nature and function of calcium channels with particular attention to their role in auditory and vestibular systems; (b) reports on the experimental findings regarding the application of NMDP in inner ear function and dysfunction; and (c) reviews the evidence that suggest further indications for NMDP both as a single strategy or as an adjunct to standard care in otolaryngology.
Calcium channels: basic concepts
Voltage-gated calcium channels (VGCCs) are a group of ion-conducting pores located in the plasma membrane of excitable cells (e.g., muscle, glial cells, neurons, etc.) with a selective permeability to the calcium ion (Ca2+). They belong to a large family of transmembrane ion channels that also include voltage-gated sodium, barium and potassium channels 19. At resting membrane potential, VGCCs are normally closed and the concentration of Ca2+ is much higher outside of the cell than inside. In response to depolarisation, VGCCs allow Ca2+ to enter the cell and promote exocytosis which, depending on the cell type, results in activation of calcium-sensitive potassium channels, neurotransmitter and hormone release, muscular contraction, excitation of neurons and gene expression. The slow decline of cytoplasmic Ca2+ concentration after its initial rise to a peak level is due to both an increase of ion extrusion through a Na+Ca2+ exchange system at the plasma membrane and to reuptake into internal organelles.
Malfunction of VGCCs can lead to an excess of intracellular calcium that over-activates enzymes such as nucleases 20 and phospholipases 21 causing DNA injury and breakdown of phospholipids, respectively, which in turn irreversibly damage the membrane causing cellular death. VGCCs are complex proteins composed of distinct subunits (α1, α2δ, β1-4, and γ) (Fig. 1). The α1 subunit with a mass of 190 to 250 kDa is the largest. It represents the primary subunit that forms the ion conducting pore necessary for channel functioning, determines most of the channel voltage-dependent opening and closing behaviours and contains the drug/toxin-binding sites. It is encoded by at least 10 different genes in mammals. It consists of four homologous (I-IV) domains containing six transmembrane α-helices each (S1-S6) 22. The other subunits only exert an auxiliary modulation of the pharmacological and electrophysiological properties of VGCCs 23.
Fig. 1.
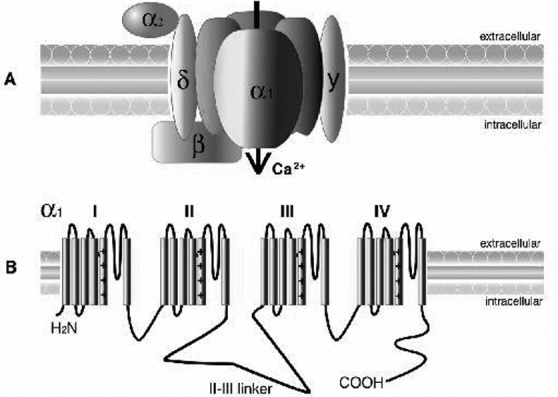
A) Calcium channel structure comprising the α1, α2δ, β, and, γ subunits. The α1 subunit, represented here with its 4-fold monomeric structure, is responsible for many of the functional characteristics of these channels, including the pore voltagedependent gating and dihydropyridine binding. The α2 is the extracellular glycosylated subunit that interacts with the α1 subunit. The δ subunit has a single transmembrane region with a short intracellular portion that serves to anchor the protein in the plasma membrane. The β subunit is the only Ca2+ channel subunit that is entirely cytoplasmic. The γ1 subunit is a glycoprotein that, for the most part, is not required to regulate the channel complex. B) The α1 subunit forms the Ca2+ selective pore, which contains voltage-sensing apparatus and drug/toxin-binding sites. This subunit contains 4 homologous domains (labelled I–IV), each containing 6 transmembrane helices (S1–S6).
Multiple types of Ca2+ channels are known and are classified according to differences in ionic conductance, gating modality and pharmacology. For instance, it was initially observed that most calcium channels need strong depolarisation for opening and are thus are defined "high-voltage activated channels"; on the other hand, only a minority are activated by weak depolarisation and are referred to as " low-voltage activated channels" 24. A further alphabetical nomenclature was proposed for different types of calcium currents 25. For instance, most VGCCs are termed L-type because they inactivate slowly ("long-lasting"), need strong depolarisation to activate and are blocked by organic L-type calcium channel antagonists, such as the dihydropyridines nifedipine and NMDP 26. They are heterotetrameric polypeptide complexes comprising the α1, α2δ, β, and, in some tissues, γ subunits. The α1 subunit is encoded by CACNA1S, CACNA1C, CACNA1D and CACNA1F genes. Deficiency in 1D subunit of L-type Ca2+ channels causes the absence of L-type Ca2+ currents in cochlear inner hair cells and leads to degeneration of both outer and inner hair cells, with subsequent congenital deafness in the mouse 27.
Recently, a mutation in CACNA1D, which encodes for the pore-forming α1 subunit of Ca(v)1.3 L-type calcium channel, has been identified in two consanguineous families with deafness. All deaf subjects also showed pronounced sinoatrial node dysfunction at rest 28. Auditory sensory hair cells of most species prevalently have L-type calcium channels.
P/Q-type calcium channels are present in Purkinje neurons in the cerebellum (and thus the name) and in cerebellar granule neurons. They are presynaptic high-voltagegated calcium channels that couple neuronal excitation to release of neurotransmitter. The α1 subunit is encoded by the CACNA1A gene and multiple splice variants exist in the central nervous system. Malfunction of P/Q channels due to mutations are probably associated with familial hemiplegic migraine type 1. In the mammalian auditory system, P/Q voltage-gated calcium channels modulate transmitter release at the olivo-cochlear efferent-inner hair cell cholinergic synapses, but their involvement in hearing defects is not known.
N-type ('N' for "Neural-Type") Ca2+ channels are highvoltage activated calcium channels present throughout the central and peripheral nervous systems at presynaptic terminals. The α1 subunit is encoded by CACNA1B gene. N-type voltage-gated calcium channels, as well the P/Q type are responsible for the synaptic activation of the inhibitory auditory efferent system.
The R-type calcium channel is a unique subtype of VGCC as its electrophysiological properties are intermediate between those of typical high-voltage-activated (P/Q-type, N-type, and L-type) or low-voltage-activated (T-type) channels. R-type channels are also typically resistant (thus the name) to antagonists of L-, N-, and P/Q-type Ca2+ channels. They have been identified in various areas of the CNS such as the cortex, hippocampus, striatum, amygdala, and interpeduncular nucleus and their role is believed to be predominantly implicated in spatial memory, fear behaviour, pain perception and morphine analgesia. The α1 subunit is encoded by CACNA1E gene. Finally, T-type calcium channels are distinguished from other VGCCs by their low voltage thresholds for activation and inactivation. "T" stands for transient, referring to the length of activation. In many neurons, cytosolic Ca2+ influx through LVA channels triggers low-threshold spikes, which in turn trigger a burst of action potentials mediated by Na+ channels. T-type calcium channels contribute to the pacemaker activity of the sinoatrial node of the heart. The α1 subunit is encoded by the CACNA1L gene.
A further classification of calcium channels was proposed in 2000 29, based on the chemical symbol of the ion (Ca), with the principal physiological regulator (voltage) indicated as a subscript (Cav). The Cav1 subfamily (Cav1.1 to Cav1.4) includes L-type calcium channels. The Cav2 (Cav2.1 to Cav2.3) includes P/Q-, R-, N-type calcium channels and the subfamily Cav3 (Cav3.1 to Cav3.3) identifies T-type calcium channels. The aforementioned main biochemical properties of VGCCs and their sensitivity to 1,4 dihydropyridine are summarised in Table I.
Table I.
Classification of voltage-gated calcium channels (VGCCs).
Classification of voltage-gated calcium channels (Cav) according to alphabetical nomenclature, electrophysiological properties (HVA: high voltage activated, LVA: low voltage activated), genetic determinants and dihydropyridine (DPH) sensitivity.
| Type | Voltage | α1 subunit (gene) Chromosome | DPH sensitivity |
|---|---|---|---|
| L-type calcium channel | HVA | Cav1.1 (CACNA1S) 1q32 Cav1.2 (CACNA1C) 12p13.3 Cav1.3 (CACNA1D) 3p14.3 Cav1.4 (CACNA1F) Xp11.23 |
Blocked |
| P/Q-type calcium channels | HVA | Cav2.1 (CACNA1A) 19p13 | Resistant |
| N-type calcium channel | HVA | Cav2.2 (CACNA1B) 9q34 | Resistant |
| R-type calcium channel | Intermediate | Cav2.3 (CACNA1E) 1q25.3 | Resistant |
| T-type calcium channel | LVA | Cav3.1 (CACNA1G) 17q22 Cav3.2 (CACNA1H) 16p13.3 Cav3.3 (CACNA1L) 22q13.1 |
Partially blocked |
Calcium channels in the auditory and vestibular systems
The free intracellular concentration of calcium (Ca2+) in hair cells is very low at rest, but it rapidly increases due to entry through clusters of VGCCs in response to acoustic stimulation, which primarily allow the entry of cations close to the stereociglia tips at the cellular apex. The calcium release from intracellular stores (endocytosis) is actually considered less relevant in this process. Calcium promotes vesicles fusion with the presynaptic membrane, leading to neurotransmitter release at the baso-lateral cellular wall 30, in order to activate auditory nerve terminals and generate action potential firing patterns. Hair cells, as retinal bipolar cells and photoceptors, contain ribbon-type active zones. The synaptic ribbons are plate-like, proteinaceous structures packed with synaptic vesicles. The "ribbon" or "dense body" anchors to the presynaptic membrane only nanometres away from the clustered VGCCs (Fig. 2). Ribbons are thought to maintain exocytosis and sustained neurotransmitter release, albeit at reduced rates, in response to prolonged sound stimuli 31. The intracellular spread of Ca2+ in hair cells is limited by the endogenous buffering activity of the cytosol. This system incorporates endogenous fixed and mobile Ca2+ buffers that limit the spread of free calcium in hair cells and carry incoming Ca2+ away from synaptic areas 32. A plasma membrane Ca2+-ATPase type 2 pump is responsible for extrusion of calcium from mammalian hair cells and its central role in intracellular calcium homeostasis is revealed by the hearing loss resulting from mutations in the plasma membrane CaATPase 33. Moreover, calcium influx into hair cells and its growing concentration opens Ca2+-activated K+ channels. The outward K+ flow that follows repolarises the cell, closing Ca2+ channels and priming the cell for another cycle of oscillation. The number, distribution and kinetics of Ca2+ channels and K+ channels determine the characteristic frequency at which a hair cell specifically responds and represents the basic process of electrical tuning in lower vertebrates' cochlea 34. The presence of L-type channels is reported in the olivary nuclei, but they express a higher proportion of P/Q-type Ca2+ channels 35 36 while T-type calcium channels are expressed in thalamic projection neurons to cortical auditory areas 37.
Fig. 2.
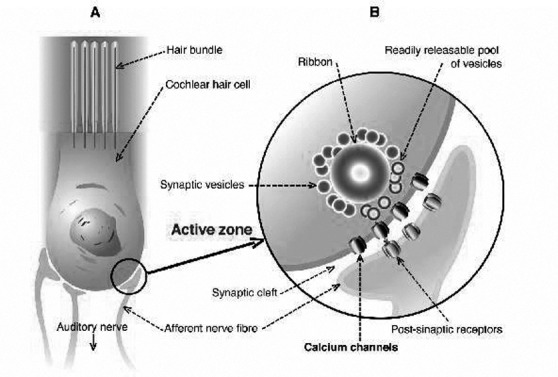
A) schematic representation of cochlear hair cell structure with baso-lateral wall cytoplasmic membrane (marked with circle) and afferent auditory nerve bottoms; and B) presynaptic "active zone" with vesicles and ribbon containing neurotransmitters with clustered voltage-gated calcium channel (VGCCs).
The role of VGCCs in the vestibular end-organ is more debated. From animal experiments, it appears that the majority of VGCCs in semi-circular canals hair cells are L-type 38, although an R-type current component has been proposed based on its resistance to L- and N-type antagonists 39. Furthermore, an antibody to N-type subunits labelled the baso-lateral membranes of type I and type II hair cells 40, but further evidence is needed. Similarly, the saccular region displayed at least two different type of calcium channels, including L-type, both inhibited by nifedipine and promoted by the calcium-channel agonist Bay K 8644 41. Besides this uncertainty, the mechanism of calcium sequestration for bio-crystallisation from endolymph through calcium pores is partially unknown, so that the formation and growth of otoconia remains to be elucidated 42. In the brainstem, within the circuitry for the vestibulo-ocular reflex (VOR), neurons in the medial vestibular nucleus show adaptive changes in firing rate responses in relation to VOR gain (the ratio of evoked eye velocity to input head velocity). The firing rate response of neurons in the medial vestibular nuclei is reduced by increasing extracellular calcium and increased either by lowering extracellular calcium or with antagonists to calcium-dependent potassium channels 43. L-, N- and Ttype calcium channels neurons are present in the medial vestibular nuclei 44 45, but their specific contribution to vestibular-ocular reflex is not fully understood. The large amount of ion-gated channels in hair cells found in these experimental studies points to their likely relevant role in highly excitable cells of both the cochlea and the labyrinth, supporting the hypothesis that inner ear dysfunction as complex as Ménière's disease could be interpreted as channelopathy, particularly in those cases where familial expression of the disease is documented 46.
Nimodipine: from experimental data to clinical evidence
NMDP is a calcium channel blocker (CCB) belonging to the dihydropyridine class and is a highly lipophilic agent that rapidly crosses the blood-brain barrier (Fig. 3). Its mechanism of action is the selective blockage of intracellular calcium ions influx through L-type VGCCs. NMDP exerts a vasoactive effect by predominantly dilating small and collateral cerebral vessels, thus improving blood supply to hypo-perfused (post-ischaemia) areas and cerebral oxygenation even in healthy subjects 47. In the inner ear, the perilymphatic perfusion of NMDP is known to suppress both spontaneous neural noise and compound action potential. These are reversible and dose-dependent effects and provide support for an exclusively presynaptic role of L-type Ca2+ channels in the regulation of both spontaneous and evoked neurotransmitter release from hair cells 48. Similarly, the guinea pig compound action potential, the summating potential and the spontaneous firing of single cochlear ganglion neurons can be substantially reduced by the direct administration of NMDP in the cochlea 49. Recent findings have shown that Deiter's cells in the cochlea not only provide hair cells with supporting activity, but also contribute to their electrical and biomechanical properties. It has been suggested that these cells modify cochlear dynamics by influencing the force produced by sound-induced motion of outer hair cells, depending on their large voltage-activated, outwardly K+ selective conductance. Even if voltage-activated Ca2+ currents are not present in Deiter's cells, NMDP interacts directly with K+ channels in a voltage dependent manner and potentially interferes with depolarisation of the organ of Corti 50. The role of L-calcium channels in exerting presynaptic control on both acoustically provoked and spontaneous excitation of auditory nerve fibres appears to be of relevant interest since their abnormal function is suspected to be a potential source of annoying symptoms such as tinnitus 51. The effect of selective Ca2+ channel agonists and antagonists on the afferent neuronal activity of isolated frog posterior semicircular canal is clearly documented 52 53; resting activity is substantially affected by all dihydropyridines, whereas only NMDP has been shown to significantly reduce the mechanically evoked activity 38. In particular, the stronger inhibitory activity of NMDP compared with nifedipine, also observed in isolated hair cells of the cochlea 54, may be due to their different biophysical and conformation-dependent interactions with the L-type calcium channel. Their overlapping actions on resting discharge could depend on different enzymatic patterns involved in rest and mechanically-evoked stimulation 55. Since the underlying pathogenic mechanism of Ménière's disease is an over-distension of the membranous structures of both the labyrinth and cochlea (endolymphatic hydrops) that modifies resting discharge of hair cells in the absence of sound stimuli and head movements, NMDP, cinnarizine and, partially, flunarizine qualify as a rational choice for this disease because of their selective activity on L-type calcium channels. The effects of mechanical over-stimulation of outer hair cells, such as that by acoustic trauma, results in excessive entry of Ca2+ in hair cells 56 and its sustained intracellular overload primarily activates complex enzymatic reactions, namely phospholipase A2, protein kinase II and nitric oxide synthase that subsequently cause degeneration of the phospholipids of the membrane, cellular lysis and death 57. VGCCs are therefore supposed to be involved in the pathogenesis of acoustic injury in the auditory system as demonstrated by the recording of auditory brainstem responses (ABR) and morphological study of the cochlea in ddY mice after acoustic over-exposure 58. Many experiments in animals have been carried out to test the hypothesis that NMDP and other L-type calcium channel blockers can protect hair cells from acoustic trauma, but the results are so far inconclusive 59-61. In guinea pigs, cochlear blood flow (CBF) significantly decreases after administration of salicylate (100 mg/kg), but increases after administration of NMDP (2 mg/kg). After simultaneous administration of both salicylate (100 mg/kg) and NMDP (2 mg/kg), CBF remains unchanged. These results suggest that NMDP prevents the decrease in CBF induced by salicylate in animals 62, but no experimental data are available for human CBF in relation to administration of VGCCs blockers alone or in association with ototoxic drugs. In order to study the efficacy of calcium antagonists in vertebrobasilar insufficiency, an animal model of VBI was developed by occluding one vertebral artery with a balloon catheter. Judging by the reversal of the nystagmic pattern, the CCBs flunarizine and NMDP were suggested to be effective drugs in this animal model 63. The protective effect of NMDP with respect to traumatic damage to the auditory nerve is documented by a recent study in rats 64. The histological examination of the temporal bones of NMDPtreated animals revealed significant preservation of spiral ganglion cells in the basal turn of the cochlea in response to experimentally provoked compression of the cerebellopontine angle portion of the eighth nerve. In humans, a prospective, randomised, double-blind study to assess the intra-operative efficacy of diltiazem (a non-dihydropyridine member of the calcium channel blocker family commonly used to treat hypertension and angina pectoris) in preventing acoustic trauma during otologic surgery was carried out in 1995 65. The results were influenced by a small postoperative sensorineural hearing loss in all cases (treated and placebo) and by the tendency for better preservation of the bone-conduction hearing threshold in the therapy group, although this did not reach statistical significance. No further trials regarding this possible indication have been reported to date. On the other hand, prophylactic treatment with NMDP is definitively shown to be effective in reducing both facial and cochlear nerve damage in humans due to vestibular schwannoma surgery 17 66-68 by improving axon regeneration and collateral sprouting. One study 17 showed that all patients who had received NMDP and hydroxyethyl starch-based prophylaxis had a significant recovery of facial nerve function (House-Brackmann Grade I-II) compared with preoperative staging (House-Brackmann Grade III or worse), whereas one third of patients with pre-operative facial nerve paresis (House-Brackmann Grade III or worse) who had not received such prophylactic treatment were unchanged at long-term follow-up post-operation. In addition, in more than 50% of patients undergoing surgical removal of vestibular schwannoma without prophylactic medication, intraoperative brainstem auditory evoked potentials monitoring showed a sudden or slowly progressive loss of potentials. Despite prompt initiation of intraoperative vasoactive treatment, preservation of hearing function could not be obtained, suggesting that prophylaxis is superior to intraoperative vasoactive treatment. In a recent experience, the resected recurrent nerve due to removal of a thyroid cancer was repaired with a nerve graft and the patient was further treated with nimodipine for 3 months. After therapy, the electromyography showed complete re-innervation of laryngeal muscles 18. This experience suggests the potential utility of NMDP as add-on therapy to prevent and/or facilitate re-innervation of both superior and recurrent laryngeal nerve lesions that may occur during thyroid surgery 69 70 or of other nerves that could be damaged during neck dissection 71. The use of CCBs in the treatment of peripheral vertigo has been reported for many years in Europe 72 and their efficacy and safety has been tested in two double-blind studies that included different types of peripheral vestibular vertigo 8 73. In the first study, the effectiveness of NMDP (30 mg three times daily) was compared to cinnarizine (150 mg per day); after 12 weeks of treatment, the former drug reduced the incidence of moderate vertigo attacks to 78.8% of cases, and of severe vertigo in 85% of cases, whereas cinnarizine reduced 65.8% of moderate vertigo episodes and 89.8% of severe ones. Both NMDP and cinnarizine exhibited a significant therapeutic effect and similar safety profile. In the second study, conventional NMDP was administrated three times daily at a dosage of 30 mg in 26 patients and its effect compared to that of an extended release formulation (90 mg, once a day) in 25 patients. After 8 weeks of extended release drug treatment, about 90% of both vertigo disability and severity was rated as decreased by 50%. In the conventional group, rating of severity was reduced by 50% in 90% of patients after 8 weeks whereas vertigo disability was decreased by 50% in 64% of cases. No significant difference was found between groups and both drug regimens were considered effective and well tolerated. While the aforementioned studies investigated the effectiveness of NMPD in the treatment of miscellaneous peripheral vestibular disorders, only one pilot study specifically explored the beneficial use of NMDP as monotherapy in medical treatment of Ménière's disease 7. NMDP was prescribed to Ménière's disease patients whose first-line medical treatment (diuretics, salt dietary restriction and vestibular suppressants) was not effective in controlling vertigo, or ameliorating or stabilising hearing loss. A recent retrospective analysis 14 of 10-year experience in the long-term management of patients affected by definite Ménière's disease, diagnosed according to the American Academy of Otolaryngology- Head and Neck Surgery Committee on Hearing and Equilibrium diagnostic guidelines, highlighted a specific effect of NMDP as an add-on therapy for cochlear dysfunction 74. This study documented that a fixed combination of NMDP (at a dose of 40 mg a day) and betahistine (at a dose of 32 mg a day) for six months was more effective than monotherapy with betahistine (at a dose of 32 mg a day) for the same period in reducing the number of vertigo attacks, subjective annoyance due to tinnitus and severity of sensorineural hearing loss. These data suggest that NMDP should be prescribed as an add-on therapy in the long-term medical management of Ménière's disease in consideration of its efficacy on cochlear symptoms. This result confirms previous evidence on the beneficial effect of NMDP on tinnitus in both humans 13 and animal models 75. Drug treatments are not commonly used for benign paroxysmal positional vertigo because different repositioning manoeuvres are now available to cure the unusual manifestations of otolith dysfunction 76 77, but it could be speculated that CCBs offer an attempt to prevent flare-ups in patients who are also affected with other peripheral vestibulopathies 78.
Fig. 3.
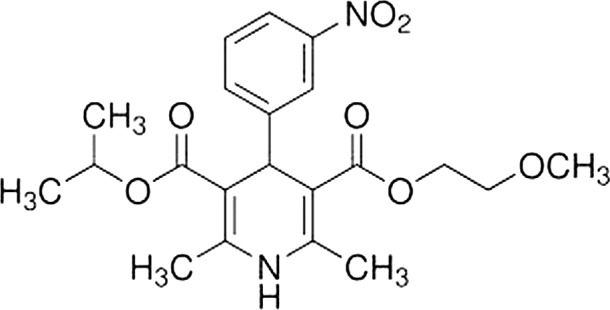
Chemical structure and formula of nimodipine. 3-(2-methoxyethyl) 5-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4- dihydropyridine-3,5-dicarboxylate C21H26N2O7.
Finally, in order to test the effect of NMDP on the haemorheology and brainstem auditory evoked potentials (BAEP) in patients with vertebrobasilar insufficiency (VBI), a pilot study on 50 cases was carried out 79. Patients were divided into a NMDP group (25 cases) and a therapy- as-usual group (25 cases). Compared with the therapyas- usual group, plasma viscosity was markedly decreased (p < 0.05) in the NMDP group, and peak latency of V wave, inter-peak latency of III-V and I-V were also improved significantly (p < 0.05). It was therefore suggested that NMPN could ameliorate central auditory pathway function through its beneficial effect on haemorheology in patients with vertebrobasilar insufficiency. This study further confirms the possible role of nimodipine in vestibular disorders since it has been shown that vertigo may also arise from cerebellar and cerebral vascular diseases 80, as reported below.
Further strategies and applications
Nimodipine and vertigo/dizziness of vascular origin
Vestibular compensation is a progressive neural process taking place in the central nervous system, which recalibrates vestibulo-oculomotor and vestibulo-spinal reflexes after peripheral vestibular-end organ failure and/or a vestibular (VIII nerve) neuritis. Its evolution is generally thought to be complete and recovery may be achieved in a relatively short time in most cases. However, some patients complain of residual symptoms such as postural instability and episodic vertigo for long periods of time.
A single cause for this delayed compensation process is seldom identifiable and a multifactorial pathogenesis is most likely. For example, persistent vertigo and dizziness are more frequently observed in patients with cerebrovascular disease affecting those specific central nervous system areas and neural pathways that are supposed to be directly involved in vestibular compensation 81. Moreover, it has been largely confirmed that patients with balance and gait disorders have a higher incidence of MRI white matter hyper-intensities due to white matter ischaemic disease and hypertension, so that cerebrovascular disorders may account for persistent vertigo per se 82. Since the beneficial effects of NMDP on age-related cerebrovascular disorders in animals 83 and in human clinical trials 84-86 are extensively documented, its use has been proposed in the treatment of vertigo in such patients. Interestingly, a study on elderly patients with chronic brain failure who received a single 30 mg oral dose of NMDP followed by two weeks treatment at a dose of 30 mg three times a day showed a significant amelioration of dizziness 87.
It should be added that both cerebrovascular diseases 88 and vertigo 89 are often associated with depression, a relevant cause of significant comorbidity. In a double-blind, randomised clinical trial 90, 101 patients diagnosed with "vascular depression" treated with fluoxetine at standard doses were randomised to placebo or NMDP administration. Treatment outcomes were assessed by the Hamilton Depression Rating Scale. Depression was decreased in both groups, but a greater improvement and a lower percentage of recurrence were seen in fluoxetine-NMDP patients. It should be added that in a previous multicentre, placebo-controlled, double-blind clinical study in 178 elderly patients with cognitive decline 91, NMDP alone seemed to exert an antidepressant effect. Unfortunately, the exact mechanism of the probable antidepressant action of NMDP is unclear.
Finally, unlike other calcium channel blockers, NMDP can also modulate other calcium-dependent processes such as acetylcholine release, which is potentially of benefit in improving vestibular compensation in the elderly affected by uncompensated peripheral vestibular disorders and mild cognitive impairment due to cerebrovascular diseases 92.
All these results clearly suggest a potential effect of NMPD in the pharmacological treatment of patients with uncompensated peripheral associated with cerebral vascular diseases presenting with persistent and recurrent dizziness. This indication seems to be most relevant in dizzy patients, and the elderly in particular, who are also affected by mood disturbance. Further investigations with appropriate study-designs are needed.
Nimodipine and vestibular migraine
Vestibular migraine (VM), also known as migraine-associated vertigo, is a common cause of dizziness in the adult population 93 94, but in a large percentage of patients with vertigo, VM is under-diagnosed. Symptoms include spontaneous and positional vertigo, light-headedness and unsteadiness of variable duration, ranging from seconds to days. Some patients report a temporal relationship with the headache episode, but most do not. The wide variability in clinical presentation of patients with VM, lack of a generally accepted pathophysiologic model linking migraine and vertigo and the absence of specific biomarkers for the disease underlie the lack of commonly used criteria for diagnosis 95. A recent classification of vestibular migraine 96 postulates that a diagnosis of VM must be formulated on the basis of the following criteria:
at least 5 episodes with vestibular symptoms of moderate or severe intensity, lasting 5 min to 72 hours;
current or previous history of migraine with or without aura according to the International Classification of Headache Disorders (ICHD);
-
one or more migraine features with at least 50% of vestibular episodes:
-
headache with at least two of the following characteristics:
one sided location, pulsating quality, moderate or severe pain intensity, aggravation by routine physical activity;
photophobia and phonophobia;
visual aura;
-
not better accounted for by another vestibular or ICHD diagnosis.
Unfortunately, no standard medical treatment for acute attacks of VM is available, whereas prophylactic medications have been proposed.
A review of the pharmacological treatments of VM showed that common drugs routinely used in migraine prophylaxis (triptans, CCBs, nortriptyline, or metoprolol) can also be successfully prescribed to patients with VM 97. This finding has been recently confirmed by a retrospective analysis of 'epigone' migraine vertigo, a type of vertigo, migrainous in origin, starting late in lifetime and replacing, as an equivalent, a pre-existing migraine headache 98. This study reported successful results in the prophylaxis of both headache and vertigo spells after the administration of flunarizine. In addition, emerging clinical evidence supports not only a strong association between migraine and Ménière's disease 99, but also clearly indicates that patients with episodic vertigo, fluctuating hearing loss and migraine respond better to CCBs than to anti-hydropic drugs 100 101.
The efficacy of NMDP in prophylaxis of migraine has been extensively investigated, but the results of placebocontrolled trials are controversial. Three of six comparative trials with placebo suggested no significant difference 102-104, while the remaining three reported relatively large and statistically significant treatment effects 105-107. This discrepancy could be due to many factors such as different types of migraine/headache included in the studies, dosages and duration of treatments and outcome measures used.
Taken together, these observations suggest an expanding indication for the use of NMDP in VM, but its potential efficacy needs to be documented in clinical trials with adequate study design.
Conclusions
The L-type calcium channel blocker NMDP has been widely utilised in numerous experimental and clinical investigations, far beyond its internationally approved indication. Primarily due to its vasoactive and neuroprotective effects on both the inner ear and the brain, it may be beneficial in many otoneurological syndromes, both peripheral, central and mixed, as monotherapy or as an add-on therapy to standard regimens. Its greater efficacy on aural symptoms such as tinnitus and hearing loss, if prescribed in association to betahistine compared to betahistine alone in the treatment of Ménière's disease, should be considered by otolaryngologists and audiologists and included in their routine pool of possible medications.
Since cerebrovascular lesions often cause gait disturbances and dizziness, and represent a possible cause of un uncompleted compensation of vestibular end-organ diseases, NMDP, by facilitating perfusion and oxygenation of human brain hypoxic areas, appears to be a rational choice in the treatment of vertigo of vascular origin. Despite existing uncertainties about the origin of vestibular migraine, it has been recently confirmed that patients suffering from this disease can be successfully treated with anti-migraine drugs so that NMDP may be an effective medical option considering both its efficacy as a migraine prophylactic drug and activity in inner ear disorders.
A more restricted field of application for NMDP is the surgery of the eighth nerve and cerebello-pontine angle lesions because of its neuroprotective effects, which are useful in preventing damage to both facial and cochlear nerves. It should be finally noted that despite increasing indications for the use of NMDP in otolaryngology, its prescription is still off-label in this field and informed consent should be obtained by patients before use.
References
- 1.Thomsen J, Sass K, Ödkvist L, et al. Local overpressure treatment reduces vestibular symptoms in patients with Ménière's Disease: a clinical, randomized, multicenter, double-blind, placebo-controlled study. Otol Neurotol. 2005;26:68–73. doi: 10.1097/00129492-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371–CD006371. doi: 10.1002/14651858.CD006371.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Strupp M, Hupert D, Frenzel C, et al. Long-term prophylactic treatment of attacks of vertigo in Menière's disease--comparison of a high with a low dosage of betahistine in an open trial. Acta Otolaryngol. 2008;128:520–524. doi: 10.1080/00016480701724912. [DOI] [PubMed] [Google Scholar]
- 4.Hahn A, Sejna I, Stefflova B, et al. A fixed combination of cinnarizine/dimenhydrinate for the treatment of patients with acute vertigo due to vestibular disorders: a randomized, reference-controlled clinical study. Clin Drug Investig. 2008;28:89–99. doi: 10.2165/00044011-200828020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ganança MM, Caovilla HH, Ganança FF, et al. Clonazepam in the pharmacological treatment of vertigo and tinnitus. Int Tinnitus J. 2002;8:50–53. [PubMed] [Google Scholar]
- 6.Agnoli A. The classification of calcium antagonists by the WHO expert committee: relevance in neurology. Cephalalgia. 1988;(Suppl 8):7–10. doi: 10.1177/03331024880080S802. [DOI] [PubMed] [Google Scholar]
- 7.Lassen LF, Hirsch BE, Kamerer DB. Use of nimodipine in the medical treatment of Ménière's disease: clinical experience. Am J Otol. 1996;17:577–580. [PubMed] [Google Scholar]
- 8.Pianese CP, Hidalgo LO, González RH, et al. New approaches to the management of peripheral vertigo: efficacy and safety of two calcium antagonists in a 12-week, multinational, double-blind study. Otol Neurotol. 2002;23:357–363. doi: 10.1097/00129492-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Liao L, Yan X, et al. Effects of Yangxue Qingnao Granules on chronic cerebral circulation insufficiency: a randomized, double-blind, double-dummy, controlled multicentre trial. Psychogeriatrics. 2013;13:29–34. doi: 10.1111/j.1479-8301.2012.00423.x. [DOI] [PubMed] [Google Scholar]
- 10.Cha YH. Migraine-associated vertigo: diagnosis and treatment. Semin Neurol. 2010;30:167–174. doi: 10.1055/s-0030-1249225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochi K, Kinoshita H, Kenmochi M, et al. Effects of nimodipine on salicylate ototoxicity. Ann Otol Rhinol Laryngol. 2002;111:1092–1096. doi: 10.1177/000348940211101206. [DOI] [PubMed] [Google Scholar]
- 12.Ma F, Li X, Zhong Z, et al. Effects of nimodipine on cochlear blood flow with normal and vertebrobasilar insufficiency status. Lin Chuang Er Bi Yah Hou Ke Za Zhi. 2002;16:174–176. [PubMed] [Google Scholar]
- 13.Davies E, Knox E, Donaldson I. The usefulness of nimodipine, an L-calcium channel antagonists, in the treatment of tinnitus. Br J Audiol. 1994;28:125–129. doi: 10.3109/03005369409086559. [DOI] [PubMed] [Google Scholar]
- 14.Monzani D, Barillari MR, Alicandri Ciufelli M, et al. Effect of a fixed combination of nimodipine and betahistine versus betahistine as monotherapy in the long-term treatment of Ménière's disease: a 10-year experience. Acta Otorhinolaryngol Ital. 2012;32:393–403. [PMC free article] [PubMed] [Google Scholar]
- 15.Angelov DN, Neiss WF, Streppel M, et al. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996;16:1041–1048. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson P, Janson AM, Alikogius H, et al. Nimodipine promotes regeneration and functional recovery after intracranial facial nerve crush. J Comp Neurol. 2001;437:106–117. doi: 10.1002/cne.1273. [DOI] [PubMed] [Google Scholar]
- 17.Scheller C, Richter HP, Engelhardt M, et al. The influence of prophylactic vasoactive treatment on cochlear and facial nerve functions after vestibular schwannoma surgery: a prospective and open-label randomized pilot study. Neurosurgery. 2007;61:92–98. doi: 10.1227/01.neu.0000279728.98273.51. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson P, Björck G, Remahl S, et al. Nimodipine and microsurgery induced recovery of the vocal cord after recurrent laryngeal nerve resection. Laryngoscope. 2005;115:1863–1865. doi: 10.1097/01.mlg.0000177034.51559.50. [DOI] [PubMed] [Google Scholar]
- 19.Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;253:p.re15–p.re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 20.Krause GS, White BC, Aust SD, et al. Brain cell death following ischemia and reperfusion: a proposed biochemical sequence. Crit Care Med. 1988;16:714–726. doi: 10.1097/00003246-198807000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Siesjo BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1:155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- 22.Catterall WA, Perez-Reyes E, Snutch TP, et al. International Union of Pharmacology. XLVIII. Nomenclature and structure- function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–411. doi: 10.1124/pr.57.4.5. 251. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 24.Carbone E, Lux HD. A low voltage-activated fully inactivating Ca channel in vertebrate sensory neurons. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- 25.Tsien RW, Lipscombe D, Madison D, et al. Reflections on Ca(2+)-channel diversity, 1988-1994. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 26.Fleckensstein A. History of calcium antagonists. Circ Res. 1983;52:13–16. [PubMed] [Google Scholar]
- 27.Platzer J, Engel J, Schrott-Fischer A, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 28.Baig SM, Koschak A, Lieb A, et al. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 29.Ertel EA, Campbell KP, Harpold MM, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 30.Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA. 1994;91:7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beutner D, Voets T, Neher E, et al. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 32.Hall JD, Betarbet S, Jaramillo F. Endogenous buffers limit the spread of free calcium in hair cells. Biophys J. 1997;73:1243–1252. doi: 10.1016/S0006-3495(97)78157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Street VA, McKee-Johnson JW, Fonseca RC, et al. Mutations in plasma membrane Ca2+-ATPase gene causes deafness in deafwaddler mice. Nat Genet. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- 34.Roberts WM, Howard J, Hudspeth AJ. Hair cells: transduction, tuning, and transmission in the inner ear. Annu Rev Cell Biol. 1988;4:63–92. doi: 10.1146/annurev.cb.04.110188.000431. [DOI] [PubMed] [Google Scholar]
- 35.Hirtz JJ, Boesen M, Braun N, et al. Cav1.3 calcium channels are required for normal development of the auditory brainstem. J Neurosci. 2011;31:8280–8294. doi: 10.1523/JNEUROSCI.5098-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes-Davies M, Owens S, Forsythe ID. Calcium channels triggering transmitter release in the rat medial superior olive. Hear Res. 2001;162:134–145. doi: 10.1016/s0378-5955(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 37.Bayazitov IT, Westmoreland JJ, Zakharenko SS. Forward suppression in the auditory cortex is caused by the Ca(v)3.1 calcium channel-mediated switch from bursting to tonic firing at thalamocortical projections. J Neurosci. 2013;33:18940–18950. doi: 10.1523/JNEUROSCI.3335-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perin P, Soto E, Vega R, et al. Calcium channels functional roles in the frog semicircular canal. Neuroreport. 2000;11:417–420. doi: 10.1097/00001756-200002070-00039. [DOI] [PubMed] [Google Scholar]
- 39.Martini M, Rossi ML, Rubbini G, et al. Calcium currents in hair cells isolated from semicircular canals of the frog. Biophys J. 2000;78:1240–1254. doi: 10.1016/S0006-3495(00)76681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez I, Ishiyama G, Ishiyama A, et al. Differential subcellular immunolocalization of voltage-gated calcium channel alpha1 subunits in the chinchilla cristae ampullaris. Neuroscience. 1992;92:773–782. doi: 10.1016/s0306-4522(99)00005-6. [DOI] [PubMed] [Google Scholar]
- 41.Su ZL, Jiang SC, Gu R, et al. Two types of calcium channels in bullfrog saccular hair cells. Hear Res. 1995;87:62–68. doi: 10.1016/0378-5955(95)00079-j. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg YW, Zhao X, Yamoah EN. Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain Res. 2006;1091:47–57. doi: 10.1016/j.brainres.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 43.Serafin M, Khateb A, Waele C, et al. Low threshold calcium spikes in medial vestibular nuclei neurons in vitro: a role in the generation of the vestibular nystagmus quick phase in vivo? Exp Brain Res. 1990;82:187–190. doi: 10.1007/BF00230850. [DOI] [PubMed] [Google Scholar]
- 44.Serafin M, Waele C, Khateb A, et al. Medial vestibular nucleus in the guinea-pig: II. Ionic basis of the intrinsic membrane properties in brainstem slices. Exp Brain Res. 1991;84:426–433. doi: 10.1007/BF00231465. [DOI] [PubMed] [Google Scholar]
- 45.Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- 46.Gates P. Hypothesis: could Meniere's disease be a channelopathy? Intern Med J. 2005;35:488–489. doi: 10.1111/j.1445-5994.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 47.Canova D, Roatta S, Micieli G, et al. Cerebral oxygenation and haemodynamic effects induced by nimodipine in healthy subjects. Funct Neurol. 2012;27:169–176. [PMC free article] [PubMed] [Google Scholar]
- 48.Sueta T, Zhang SY, Sellick PM, et al. Effects of a calcium channel blocker on spontaneous neural noise and gross action potential waveforms in the guinea pig cochlea. Hear Res. 2004;188:117–125. doi: 10.1016/S0378-5955(03)00374-5. [DOI] [PubMed] [Google Scholar]
- 49.Robertson D, Paki B. Role of L-Type Ca2+ channels in transmitter release from mammalian inner hair cells. II. Single neuron activity. J Neurophysiol. 2002;87:2734–2740. doi: 10.1152/jn.2002.87.6.2734. [DOI] [PubMed] [Google Scholar]
- 50.Nenov AP, Chen C, Bobbin RP. Outward rectifying potassium currents are the dominant voltage activated currents present in Deiters' cells. Hear Res. 1998;123:168–182. doi: 10.1016/s0378-5955(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 51.Jastreboff PJ, Brennan JF. Special effects of nimodipine on the auditory system. Ann NY Acad Sci. 1988;522:716–718. [Google Scholar]
- 52.Masetto S, Russo G, Taglietti V, et al. K+ and Ca++ currents in hair cells isolated from the semicircular canals of the frog. Boll Soc Ital Biol Sper. 1991;67:493–500. [PubMed] [Google Scholar]
- 53.Prigioni I, Masetto S, Russo G, et al. Calcium currents in solitary hair cells isolated from frog crista ampullaris. J Vestib Res. 1992;2:31–39. [PubMed] [Google Scholar]
- 54.Lin X, Hume RI, Nuttall AL. Dihydropyridines and verapamil inhibit voltage-dependent K+ current in isolated outer hair cells of the guinea pig. Hear Res. 1995;88:36–46. doi: 10.1016/0378-5955(95)00096-m. [DOI] [PubMed] [Google Scholar]
- 55.Guth PS, Aubert A, Ricci AJ, et al. Differential modulation of spontaneous and evoked neurotransmitter release from hair cells: some novel hypotheses. Hear Res. 1991;56:69–78. doi: 10.1016/0378-5955(91)90155-3. [DOI] [PubMed] [Google Scholar]
- 56.Fridberger A, Flock A, Ulfendahl M, et al. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci U S A. 1998;95:7127–7132. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trump BF, Berezesky IK. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol. 1992;4:227–232. doi: 10.1016/0955-0674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- 58.Uemaetomari I, Tabuchi K, Nakamagoe M, et al. L-type voltage-gated calcium channel is involved in the pathogenesis of acoustic injury in the cochlea. Tohoku J Exp Med. 2009;218:41–47. doi: 10.1620/tjem.218.41. [DOI] [PubMed] [Google Scholar]
- 59.Boettcher FA, Caldwell RK, Gratton MA, et al. Effects of nimodipine on noise-induced hearing loss. Hear Res. 1998;121:139–146. doi: 10.1016/s0378-5955(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 60.Ison JR, Payman GH, Palmer MJ, et al. Nimodipine at a dose that slows ABR latencies does not protect the ear against noise. Hear Res. 1997;106:179–183. doi: 10.1016/s0378-5955(96)00216-x. [DOI] [PubMed] [Google Scholar]
- 61.Kansu L, Ozkarakas H, Efendi H, et al. Protective effects of pentoxifylline and nimodipine on acoustic trauma in Guinea pig cochlea. Otol Neurotol. 2011;32:919–925. doi: 10.1097/MAO.0b013e3182267e06. [DOI] [PubMed] [Google Scholar]
- 62.Ochi K, Kinoshita H, Kenmochi M, et al. Effects of nimodipine on salicylate ototoxicity. Ann Otol Rhinol Laryngol. 2002;111:1092–1096. doi: 10.1177/000348940211101206. [DOI] [PubMed] [Google Scholar]
- 63.Hofferberth B. Calcium entry blockers in the treatment of vertebrobasilar insufficiency. Eur Neurol. 1986;25(Suppl 1):80–85. doi: 10.1159/000116102. [DOI] [PubMed] [Google Scholar]
- 64.Sekiya T, Yagihashi A, Asano K, et al. Nimodipine ameliorates trauma-induced cochlear neuronal death. Neurol Res. 2002;24:775–780. doi: 10.1179/016164102101200889. [DOI] [PubMed] [Google Scholar]
- 65.Maurer J, Riechelmann H, Amedee RG, et al. Diltiazem for prevention of acoustical trauma during otologic surgery. ORL J Otorhinolaryngol Relat Spec. 1995;57:319–324. doi: 10.1159/000276773. [DOI] [PubMed] [Google Scholar]
- 66.Strauss C, Bischoff B, Neu M, et al. Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J Neurosurg. 2001;95:771–777. doi: 10.3171/jns.2001.95.5.0771. [DOI] [PubMed] [Google Scholar]
- 67.Scheller C, Strauss C, Fahlbusch R, et al. Delayed facial nerve paresis following acoustic neuroma resection and postoperative vasoactive treatment. Zentralbl Neurochir. 2004;65:103–107. doi: 10.1055/s-2004-816268. [DOI] [PubMed] [Google Scholar]
- 68.Strauss C, Bischoff B, Romstock J, et al. Hearing preservation in medial vestibular schwannomas. J Neurosurg. 2008;109:70–76. doi: 10.3171/JNS/2008/109/7/0070. [DOI] [PubMed] [Google Scholar]
- 69.Marchese-Rangona R, Restivo DA, Mylonakys I, et al. The superior laryngeal nerve injury of a famous soprano, Amelita Galli-Curci. Acta Otorhinolaryngol Ital. 2013;33:67–71. [PMC free article] [PubMed] [Google Scholar]
- 70.Rulli F, Ambrogi V, Dionigi G. Meta-analysis of recurrent laryngeal nerve injury in thyroid surgery with or without intraoperative nerve monitoring. Acta Otorhinolaryngol Ital. 2014;34:223–229. [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CH, Huang NC, Chen HC, et al. Minimizing shoulder syndrome with intra-operative spinal accessory nerve monitoring for neck dissection. Acta Otorhinolaryngol Ital. 2013;33:93–96. [PMC free article] [PubMed] [Google Scholar]
- 72.Olesen J. Calcium entry blockers in the treatment of vertigo. Ann NY Sci. 1988;522:690–697. doi: 10.1111/j.1749-6632.1988.tb33414.x. [DOI] [PubMed] [Google Scholar]
- 73.Lisbeth M, Consuelo P, Glenda C, et al. Evaluation of the effect of nimodipine o.d. (extended release) vs nimodipine t.i.d. in the treatment of peripheral vertigo. Curr Drug Deliv. 2013;10:343–347. doi: 10.2174/1567201811310030011. [DOI] [PubMed] [Google Scholar]
- 74.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Ménière's Disease. Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Jiang S, Yang W, et al. Evaluating effects of some medicine on tinnitus with animal behavioural model in rats. Zhonohua Er Bi Yan Hou Ke Za Zhi. 2000;25:331–334. [PubMed] [Google Scholar]
- 76.Califano L, Vassallo A, Melillo MG, et al. Direction-fixed paroxysmal nystagmus lateral canal benign paroxysmal positioning vertigo (BPPV): another form of lateral canalolithiasis. Acta Otorhinolaryngol Ital. 2013;33:254–260. [PMC free article] [PubMed] [Google Scholar]
- 77.Califano L, Salafia F, Mazzone S, et al. Anterior canal BPPV and apogeotropic posterior canal BPPV: two rare forms of vertical canalolithiasis. Acta Otorhinolaryngol Ital. 2014;34:189–197. [PMC free article] [PubMed] [Google Scholar]
- 78.Balatsouras DG, Ganelis P, Aspris A, et al. Benign paroxysmal positional vertigo associated with Meniere's disease: epidemiological, pathophysiologic, clinical, and therapeutic aspects. Ann Otol Rhinol Laryngol. 2012;121:682–688. doi: 10.1177/000348941212101011. [DOI] [PubMed] [Google Scholar]
- 79.Zang Jian-hong, Fan Jian-zhong, Qi Zhi-qiang. Effect of nimodipine on hemorrheology and BAEP in the patients with vertebrobasilar insufficiency. Cin J Reab Te Prac. 2002;11:679–681. [Google Scholar]
- 80.Armato E, Ferri E, Pinzani A, et al. Cerebellar haemorrhage mimicking acute peripheral vestibulopathy: the role of the video head impulse test in differential diagnosis. Acta Otorhinolaryngol Ital. 2014;34:288–291. [PMC free article] [PubMed] [Google Scholar]
- 81.Furman JM, Balaban CD, Pollack IF. Vestibular compensation in a patient with a cerebellar infarction. Neurol. 1997;48:916–920. doi: 10.1212/wnl.48.4.916. [DOI] [PubMed] [Google Scholar]
- 82.Franch O, Calandre L, Alvarez-Linera J, et al. Gait disorders of unknown cause in the elderly: Clinical and MRI findings. J Neurol Sci. 2009;280:84–86. doi: 10.1016/j.jns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Jong GI, Traber J, Luiten PGM. Formation of cerebrovascular anomalies in the aging rats is delayed by chronic nimodipine application. Mech Aging Dev. 1992;64:255–272. doi: 10.1016/0047-6374(92)90083-p. [DOI] [PubMed] [Google Scholar]
- 84.Rossi R, Inzitari D, Pantoni L, et al. Nimodipine in subcortical vascular dementia trial. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S159–S165. [PubMed] [Google Scholar]
- 85.Pantoni L, Rossi R, Inzitari D, et al. Efficacy and safety of nimodipine in subcortical vascular dementia: a subgroup analysis of the Scandinavian Multi-Infarct Dementia Trial. J Neurol Sci. 2000;175:124–134. doi: 10.1016/s0022-510x(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 86.Pantoni L, Ser T, Soglian AG, et al. Efficacy and safety of nimodipine in subcortical vascular dementia: a randomized placebo-controlled trial. Stroke. 2005;36:619–624. doi: 10.1161/01.STR.0000155686.73908.3e. [DOI] [PubMed] [Google Scholar]
- 87.Eicher H, Hilgert D, Zeeh J, et al. Pharmacokinetics of nimodipine in multimorbid elderly patients with chronic brain failure. Arch Gerontol Geriatr. 1992;14:309–319. doi: 10.1016/0167-4943(92)90030-8. [DOI] [PubMed] [Google Scholar]
- 88.Göthe F, Enache D, Wahlund LO, et al. Cerebrovascular diseases and depression: epidemiology, mechanisms and treatment. Panminerva Med. 2012;54:161–170. [PubMed] [Google Scholar]
- 89.Chandra RK, Epstein VA, Fishman AJ. Prevalence of depression and antidepressant use in an otolaryngology patient population. Otolaryngol Head Neck Surg. 2009;141:136–138. doi: 10.1016/j.otohns.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 90.Taragano FE, Bagnatti P, Allegri RF. A double-blind, randomized clinical trial to assess the augmentation with nimodipine of antidepressant therapy in the treatment of "vascular depression". Int Psychogeriatr. 2005;17:487–498. doi: 10.1017/s1041610205001493. [DOI] [PubMed] [Google Scholar]
- 91.Ban TA, Morey L, Aguglia E, et al. Nimodipine in the treatment of old age dementias. Prg Neuropsychopharmacol Biol Psychiatry. 1990;14:525–551. doi: 10.1016/0278-5846(90)90005-2. [DOI] [PubMed] [Google Scholar]
- 92.Monzani D, Genovese E, Marrara A, et al. Stimulation of the cholinergic neurotransmissions enhances the efficacy of vestibular rehabilitation. Acta Otorhinolaryngol Ital. 2010;30:11–19. [PMC free article] [PubMed] [Google Scholar]
- 93.Neuhauser HK, Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurol. 2005;65:898–904. doi: 10.1212/01.wnl.0000175987.59991.3d. [DOI] [PubMed] [Google Scholar]
- 94.Pagnini P, Verrecchia L, Giannoni B, et al. Migraine-related vertigo (MV) Acta Otorhinolaryngol Ital. 2003;23(5 Suppl 75):19–27. [PubMed] [Google Scholar]
- 95.Savundra PA, Carroll JD, Davies RA, et al. Migraine-associated vertigo. Cephalalgia. 1997;17:505–510. doi: 10.1046/j.1468-2982.1997.1704505.x. [DOI] [PubMed] [Google Scholar]
- 96.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22:167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 97.Fotuhi M, Glaun B, Quan SY, et al. Vestibular migraine: a critical review of treatment trials. J Neurol. 2009;256:711–716. doi: 10.1007/s00415-009-5050-5. [DOI] [PubMed] [Google Scholar]
- 98.Pagnini P, Vannucchi P, Giannoni B, et al. Epigone migraine vertigo (EMV): a late migraine equivalent. Acta Otorhinolaryngol Ital. 2014;34:62–70. [PMC free article] [PubMed] [Google Scholar]
- 99.Ibekwe TS, Fasunla JA, Ibekwe PU, et al. Migraine and Menière's disease: two different phenomena with frequently observed concomitant occurrences. J Natl Med Assoc. 2008;100:334–338. [PubMed] [Google Scholar]
- 100.Goto F, Tsutsumi T, Ogawa K. Migraine-associated vertigo with hearing loss and recurrent vertigo attack. Nihon Jibiinkoka Gakkai Kaiho. 2013;116:600–605. doi: 10.3950/jibiinkoka.116.600. [DOI] [PubMed] [Google Scholar]
- 101.Teggi R, Fabiano B, Recanati P, et al. Case reports on two patients with episodic vertigo, fluctuating hearing loss and migraine responding to prophylactic drugs for migraine. Menière's disease or migraine-associated vertigo? Acta Otorhinolaryngol Ital. 2010;30:217–221. [PMC free article] [PubMed] [Google Scholar]
- 102.Ansell E, Fazzone T, Festenstein R, et al. Nimodipine in migraine prophylaxis. Cephalalgia. 1988;8:269–272. doi: 10.1046/j.1468-2982.1988.0804269.x. [DOI] [PubMed] [Google Scholar]
- 103. Migraine-Nimodipine European Study Group (MINES) , author. European multicenter trial of nimodipine in the prophylaxis of classic migraine (migraine with aura) Headache. 1989;29:639–642. [PubMed] [Google Scholar]
- 104. Migraine-Nimodipine European Study Group (MINES) , author. European multicenter trial of nimodipine in the prophylaxis of common migraine (migraine without aura) Headache. 1989;29:633–638. [PubMed] [Google Scholar]
- 105.Gelmers HJ. Nimodipine, a new calcium antagonist, in the prophylactic treatment of migraine. Headache. 1983;23:106–135. doi: 10.1111/j.1526-4610.1983.hed2303106.x. [DOI] [PubMed] [Google Scholar]
- 106.Havanka-Kanniainen H, Hokkanen E, Myllylä VV. Efficacy of nimodipine in the prophylaxis of migraine. Cephalalgia. 1985;5:39–43. doi: 10.1046/j.1468-2982.1985.0501039.x. [DOI] [PubMed] [Google Scholar]
- 107.Bussone G, Baldini S, D'Andrea G, et al. Nimodipine versus flunarizine in common migraine: a controlled pilot trial. Headache. 1987;27:76–79. doi: 10.1111/j.1526-4610.1987.hed2702076.x. [DOI] [PubMed] [Google Scholar]


