Abstract
Purpose
This study assessed the efficacy of methylphenidate versus placebo for cancer-related fatigue reduction. Other objectives were to analyze cytokine levels and to determine the effects of methylphenidate on other symptoms, cognitive function, work yield, and patients’ perceptions, and preferences.
Methods
Patients were randomly assigned (1:1) to receive methylphenidate-placebo or placebo-methylphenidate for 4 weeks. Patients crossed over after 2 weeks. Wilcoxon signed-rank tests and McNemar tests were used to assess continuous and categorical variables. The primary efficacy end point was change in the level of worst fatigue on the Brief Fatigue Inventory at the end of each 2-week period.
Results
The mean baseline Brief Fatigue Inventory was moderate (5.7). Methylphenidate treatment did not affect patients’ worst level of fatigue or other symptoms. Results from the Wechsler Adult Intelligence Scale Digit Symbol Test and the Hopkins Verbal Learning Test with Brief Fatigue Inventory interference questions and Brief Fatigue Inventory activity questions showed significant improvement in the methylphenidate-treated patients’ verbal learning, memory, visual perception, analysis, and scanning speed. Patients treated with methylphenidate missed significantly fewer work hours owing to health reasons and worked significantly more hours. After 4 weeks, 64% of patients reported that methylphenidate improved their cancer-related fatigue, and 58% wanted to continue treatment. Significant difference in IL-6R (positive), IL-10 (negative) and TNFα (positive) was noted between the methylphenidate and the placebo group.
Discussion
Low-dose methylphenidate did not improve cancer-related fatigue. Patients taking methylphenidate had better cognition and were able to work more hours. Patients tolerated methylphenidate well, and a majority felt better and wanted to continue treatment.
Keywords: Cancer-related fatigue, sustained-release methylphenidate, methylphenidate, cancer, stimulant
INTRODUCTION
Cancer-related fatigue (CRF) is the most prevalent cancer symptom, reported by 50% to 90% of patients, and can severely affect patients’ quality of life and functional capacity.1-5 Pharmacologic therapies such as stimulants may be helpful, although studies have shown conflicting results. Because of varying populations, dosing regimens, stimulants used, and few well-designed trials, we still do not know whether stimulants effectively treat CRF. Currently, no standard pharmacologic treatment is available for CRF. One of the most frequently used stimulants for managing CRF is methylphenidate.
This study's primary objective was to compare the efficacy of sustained-release methylphenidate (18 mg/day) with a placebo to treat CRF in patients with lymphoma, myeloma, or breast, gastrointestinal, or lung cancers. Secondary objectives included assessing the effects of methylphenidate on other symptoms (depression, sleep, and mood), quality of life, work productivity, and cognitive function as determined by neuropsychological testing. We also analyzed cytokine levels in relation to CRF. After the study, we recorded patients’ perceptions and preferences regarding methylphenidate. We hypothesized that patients receiving methylphenidate would improve on average one unit on the worst level of fatigue on the BFI from the level with placebo.
METHODS
We conducted a randomized, double-blind, two-period, placebo-controlled, crossover trial of methylphenidate as treatment for CRF. This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board (MDACC IRB). Study participants were approached and consented by the Principle Investigator or a delegated study investigator after the informed consent was approved by The MDACC IRB. All participants signed the informed consent and received a copy of the consent document. The informed consent and documentation of the consenting process have been placed in the patients’ medical records.
To be eligible, patients had to be at least 18 years old, use acceptable birth control, and have a life expectancy of at least 6 months. Patients also had to score ≤ 2 on the Eastern Cooperative Oncology Group (ECOG)6 performance status at enrollment, score ≥ 4 on the Brief Fatigue Inventory (BFI)7 at enrollment, and speak and understand English. Patients had to be diagnosed with lymphoma, myeloma, or breast, gastrointestinal, or lung cancers, and either undergoing chemotherapy or hormonal treatment or completed treatment in the previous 12 months.
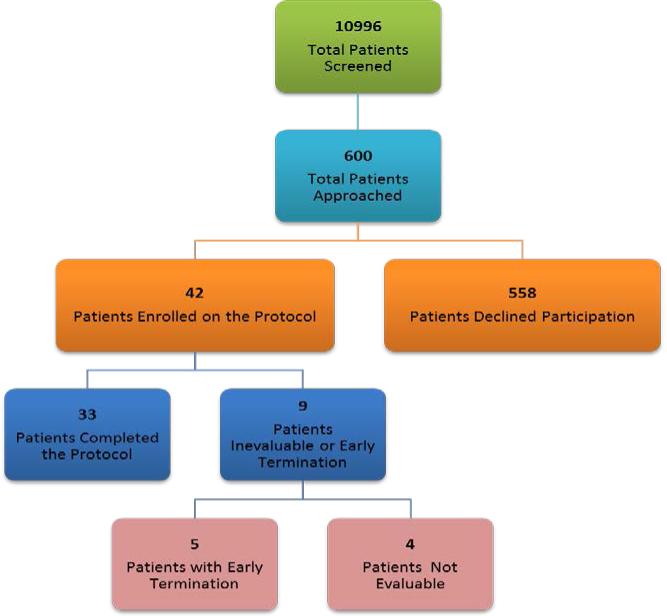
Forty-two patients were enrolled between 3/11/2005 and 3/23/2011. However, only 33 completed the study visits; five did not complete all study visits, and four were excluded for various reasons (Fig 1a). Tables 1 and 2 describe the demographic and clinical characteristics of the 38 eligible patients who began the study. All had breast cancer, and the mean baseline BFI was 5.7 (range, 4.1–8.6) at enrollment. All patients were receiving cancer treatment. Twenty (53%) were employed, and 26 (68%) had more than a high school education. Of the 38, 45% were on pain medicines and none on antidepressants.
Fig 1a.
Study Enrollment
Table 1.
Patient Characteristics (n = 38)
| Demographics | n (%) |
|---|---|
| Age (years) | |
| 57; 32–79 (mean; range) | |
| 30–39 | 2 (5) |
| 40–49 | 5 (13) |
| 50–59 | 16 (42) |
| 60–69 | 12 (32) |
| 70–79 | 3 (8) |
| Sex | |
| Female | 38 (100) |
| Race and Ethnicity | |
| White | 28 (74) |
| Black | 6 (16) |
| Hispanic | 4 (11) |
| Education (years) | |
| ≤ 12 | 12 (32) |
| 13–16 | 19 (50) |
| > 17 | 7 (18) |
| Employment Status | |
| Full time | 13 (34) |
| Part time | 7 (18) |
| Homemaker | 7 (18) |
| Retired | 5 (13) |
| Medical leave | 3 (8) |
| Other | 2 (5) |
| Unemployed | 1 (3) |
| Marital Status | |
| Married | 28 (74) |
| Single | 4 (11) |
| Widowed | 3 (8) |
| Divorced | 3 (8) |
Table 2.
Clinical Parameters (n = 38)
| Extent of disease | n (%) |
|---|---|
| Local | 28 (74) |
| Metastatic | 10 (26) |
| Current Cancer Therapy | |
| Chemotherapy | 32 (84) |
| Hormonal Therapy | 3 (8) |
| Chemotherapy and Hormonal Therapy | 3 (8) |
| ECOG Performance Status | |
| 0 | 13 (34) |
| 1 | 22 (58) |
| 2 | 3 (8) |
| Symptom Inventories Baseline | Mean (Range) |
| Brief Fatigue Inventory | 5.7 (4.1–8.6) |
| Beck Depression Index-II | 11.2 (2.0–19.0) |
| Brief Sleep Disturbance Scale | 21.1 (5.0–40.0) |
| Profile of Moods States | |
| Tension | 4.4 (0–15.0) |
| Depression | 2.9 (0–15.0) |
| Anger | 3.5 (0–15.0) |
| Vigor | 4.3 (0–18.0) |
| Fatigue | 12.5 (1.0–20.0) |
| Confusion | 3.9 (0–10.0) |
| MD Anderson Symptom Inventory | |
| Severity | 3.1 (0.6–5.9) |
| Interference | 3.9 (0.5–7.0) |
| Hemoglobin (g/dL) Mean; (range) | 11.7 (9.8–13.8) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group.
The study duration was 4 weeks. Patients were randomized into one of two arms: methylphenidate (18 mg/day) for 2 weeks followed by placebo for 2 weeks (Arm A) or placebo for 2 weeks followed by methylphenidate (18 mg/day) for 2 weeks (Arm B). Because of the drug's short half-life, we did not include a washout period. All of the research team was blinded to treatment throughout the study.
Prior to treatment, all patients underwent examination, including ECOG performance status.6 A battery of tests assessing symptoms (fatigue, depression, sleep, mood), cognitive function, and work productivity was completed (Table 3). These psychometric instruments were selected because they have been widely used and have shown sensitivity to the neurotoxic effects of cancer treatment in other trials: Wechsler Adult Intelligence Scale (WAIS) Digit Span Test and Digit Symbol Test8, the Hopkins Verbal Learning Test9, the Controlled Oral Word Association10, the Trail-Making Test parts A and B11, and the Grooved Pegboard Test11. Cancer diagnosis and stage, current cancer treatment, concurrent medications, and caffeine consumption were recorded at baseline, crossover, and final visit. Demographic information was collected and adverse events were recorded.
Table 3.
Clinical Assessment Tools
| Instrument | For assessment of | # of items | Response scale | Score interpretation |
|---|---|---|---|---|
| Brief Fatigue Inventory (BFI) | Fatigue | 9 | 10 points | Mild: < 4 Moderate: 4–6 Severe: ≥ 7 |
| Beck Depression Inventory-II (BDI-II) | Depression | 21 | 4 points | Minimal: 0–13 Mild: 14–19 Moderate: 20–28 Severe: ≥ 29 |
| Brief Sleep Disturbance Scale (BSDS)* | Sleep | 5 | Yes/no; 11 points | Mild: 22–29 Moderate: 30–34 Severe: ≥ 35 |
| Profile of Mood States (POMS) | Mood (anxiety, depression, anger, vigor, fatigue, and confusion) | 30 | 4 points | Higher scores > mood disturbance |
| Eastern Cooperative Oncology Group (ECOG) | Functional Status | 1 | 5 points | Mild-Moderate: 0–2, Poor: 3–4 |
| Work Productivity and Activity Impairment Questionnaire (WPAI) | Work Productivity and Activity | 6 | Yes/no, Hours |
Developed by the Symptom Research Group at The University of Texas MD Anderson Cancer Center
Patients were asked to record their fatigue level and its interference with general activity in a daily fatigue diary. Research staff contacted patients twice a week to check on side effects and study compliance. The patients were also contacted at the end of weeks 1 and 3 to complete a BFI, report two blood pressure readings taken 5 minutes apart, any adverse events and their compliance with the regimen.
Blood was drawn to test cytokine levels at the beginning, crossover, and study completion. To study the effects of methylphenidate, levels of angiogenic factors, inflammatory cytokines (IL-10, IL-1β, IL-6, TNFα, IL-12p70, and C-reactive protein [CRP]), soluble cytokine receptor sIL-6R, and soluble TNF receptors (sTNF-RI and sTNF-RII) were measured at baseline, crossover, and the end of the study. We obtained 108 samples from patients who consented for optional studies. The serum was separated and stored frozen at −80°C for batch analysis at a later date. On the day of analysis, the frozen serum samples were rapidly thawed to room temperature and assayed for the presence of cytokines and soluble cytokine receptors using Millipore Milliplex kits for Luminex. (EMD Millipore Corporation, Billerica, MA), following the manufacturer's instructions and as previously described. High-sensitivity kits were used to measure IL-1β, IL-6, IL-8, IL-10, TNFα, and IL-12p70.
At the end of the trial, patients were unblinded and asked if they could identify which 2 weeks they were on methylphenidate. Patients who subjectively experienced an improvement in their fatigue levels while on methylphenidate, without medical contraindications, and who wished to continue it were given a prescription after the study and followed in our CRF Clinic. Adverse events were graded by the Common Terminology Criteria for Adverse Events version 3.0.12
The primary end point was to improve the worst level of fatigue on the BFI at the end of 14 days of treatment. This variable is the most clinically sensitive in the BFI. The secondary measures of response include reductions in scores of the BFI, the Beck Depression Inventory (BDI)-II,13 the Brief Sleep Disturbance Scale (BSDS),14 the Profile of Mood States (POMS),15 the MD Anderson Symptom Inventory (MDASI),16 and improvement in quality of life and cognitive function as determined by neuropsychological testing at the end of 2 weeks of treatment. Other secondary objectives included an exploratory analysis of cytokines, comparison of Work Productivity and Impairment (WPAI)17 scores, and a description of patient perceptions and preferences after the study.
The study was designed as a crossover in which patients were randomized into two groups: (1) methylphenidate for 14 days (Arm A) then a placebo for 14 days (Arm B) or (2) a placebo for 14 days (Arm B) then methylphenidate for 14 days (Arm A). Since each patient received both methylphenidate and placebo, each served as a self-control; therefore, the inclusion of patients with various types of cancer was reasonable, although we only enrolled breast cancer patients. Responses were assessed at the end of the 2-week period for methylphenidate and placebo.
The sample size was estimated by assuming a crossover analysis of variance, in which the null hypothesis is rejected if the F-test for no interaction between groups and time is rejected. The groups correspond to the two treatment sequences. We planned for each arm to have 25 patients for 50 evaluable patients (crossover design). This sample size will detect a difference of 1.0 on the BFI 0–10 scale. It was assumed that the type 1 error would be 0.05 and the power would be 0.8. Unfortunately, the study was terminated before reaching our target enrollment; only 42 patients were enrolled, with 33 eligible and evaluable.
The distribution of continuous variable was summarized by mean, standard deviation, median, and range. The distribution of each categorical variable was summarized in terms of frequencies and percentages. A Wilcoxon signed-rank test was used to compare differences between the two groups. The Pearson correlations between the primary end points (including the worst level of fatigue score and the compound scores based on the BFI) and the cytokine and neuropsychological data were examined. The McNemar test was conducted to assess the correlation between the fatigue levels and treatment and placebo. Subgroup analyses were performed to evaluate whether patients’ fatigue level improved. All computations were carried out in Statistical Analysis Software version 9.1.
RESULTS
The primary end point was the worst level of fatigue score (range, 0–10) on the BFI at the end of the 2-week treatment. Since this was a crossover study, the potential carryover effect was examined. If a significant carryover effect existed we planned to use only period 1 (the first 2 weeks) data for treatment effect analysis. Otherwise, pooled data (period 1 and period 2) were used to assess the treatment effect (A versus B). The analysis results demonstrated no carryover effect (P = 0.4). Therefore, the data were pooled (n = 33) and showed no significant difference between arm A (methylphenidate) and B (placebo) by the worst level of fatigue, the primary end point (P = 0.54, based on a Wilcoxon signed-rank test). We also compared arms for worst level of fatigue and overall BFI score (mild, BFI score < 4; moderate, BFI score 4–6.9; and severe, BFI score 7–10), according to the level of CRF. Again, treatment arms did not significantly differ (McNemar test P = 0.6 and P = 0.5, respectively).
The average worst fatigue and interference with activity in patients’ diaries did not significantly differ. This comparison was also performed by level of CRF (mild, moderate, and severe). However, the two arms did not significantly differ according to Wilcoxon signed-rank test and McNemar test (P = .18 and P = .6, respectively, for worst fatigue; P = 0.13 interference with activity). The symptom inventories of the two arms did not significantly differ; however, the subscale of confusion in the POMS was lower in the methylphenidate group than placebo (P = 0.05).
The WAIS-III Digit Span Test demonstrated improved cognitive-processing speed in the treatment versus placebo arm (P = 0.01). Other neuropsychological testing did not show significant differences. The Pearson correlation between the neuropsychological difference (treatment versus placebo) and the worst level of fatigue on the BFI showed no significant differences. The compound score (severity: Questions 1–3; interference: Questions 3a–3f; activity: Questions 4a, 4c, and 4d; and mood: Questions 4b, 4e, and 4f) also showed no difference when correlated with the worst level of fatigue on the BFI and BFI severity. However, the Hopkins Verbal Learning Test showed significant correlations between BFI interference and activity level, demonstrating declining memory with higher levels of fatigue (−0.38, P = 0.04; −0.4, P = 0.03; respectively). Also, the WAIS-III Digit Span Test showed significant difference when correlated with BFI activity (0.36, P = 0.05).
Patients receiving methylphenidate missed significantly fewer hours of work owing to health (5.3 hours, P = 0.03) and worked significantly more hours (3.2 hours, P = 0.04) than those taking placebo. Other aspects of the WPAI showed no significant results.
Baseline cytokine levels did not significantly differ regardless of the randomization during the first 2 weeks of the study, according to a Wilcoxon signed-rank test. Two weeks of treatment did not produce significant change in cytokine levels. At the end of the study, IL-6R, IL-10, and TNFα levels significantly differed between methylphenidate and placebo groups (P = 0.03, 0.0004, 0.02, respectively). The median paired difference between methylphenidate and placebo was 4,928.1 (range, −68,440.1 to 75,388.4) increasing; −2.16 (−68.5 to 14.4) decreasing; and −0.48 (−4.6 to 29.6) increasing for IL-6R, IL-10, and TNFα, respectively. Results for the Pearson correlation between the cytokine difference (treatment-placebo) and BFI worst level of fatigue and compound score (severity, interference, activity, and mood) were not significant.
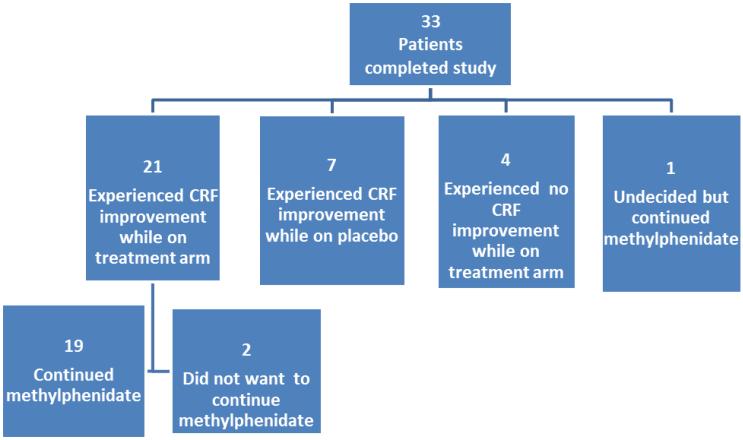
Figure 2 shows patients’ perceptions of improvement on methylphenidate and whether they wanted to continue treatment at trial's end. Twenty patients (61%) continued methylphenidate following the termination of the study.
Fig 2.
Post-Study Patient Perceptions and Treatment Status. Twenty patients continued methylphenidate (19 patients experienced CRF improvement, and one was uncertain but chose to continue treatment).
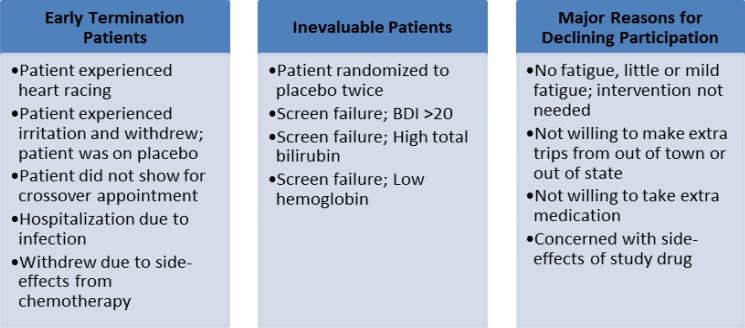
There were no serious adverse events related to methylphenidate. One patient was hospitalized for neutropenic fever (unrelated) and removed from the study. We compared patients’ blood pressure readings at baseline and after methylphenidate and found no differences. Table 4 outlines adverse events experienced. Twelve patients (36%) experienced 23 adverse events (grade 1 to 2, majority grade 1) while on methylphenidate. While taking placebo, 11 patients (33%) reported 18 symptoms. One patient experiencing irritability while taking placebo withdrew from the study.
Table 4.
Adverse Events
| Adverse Events (n) | Methylphenidate Arm | Grade 1/2 | Placebo Arm | Total N |
|---|---|---|---|---|
| Pulmonary (16) | ||||
| Dyspnea | 1 | 1 | ||
| Sinus congestion | 2 | 1 | 2 | |
| Sore throat | 1 | 1 | ||
| Cough | 2 | 1 | 2 | 4 |
| Upper respiratory infection | 3 | Grade 1:2, grade 2:1 | 3 | |
| Rhinitis | 2 | 1 | 2 | 4 |
| Dry mouth | 1 | 1 | 1 | |
| Coagulation (1) | ||||
| Nosebleed | 1 | 1 | 1 | |
| Neurologic (10) | ||||
| Irritability | 1 | 1 | ||
| Anxiety | 1 | 1 | 1 | |
| Agitation | 1 | 1 | ||
| Insomnia | 1 | 2 | 2 | 3 |
| Headache | 1 | 1 | 1 | |
| Depression | 1 | 1 | ||
| Neuropathy | 2 | 2 | ||
| Cardiac(2) | ||||
| Palpitations | 1 | 1 | 1 | |
| Tachycardia | 1 | 1 | 1 | |
| Gastrointestinal (7) | ||||
| Abdominal pain | 2 | 1 | 2 | |
| Vomiting | 1 | 1 | 1 | |
| Diarrhea | 1 | 1 | 1 | 2 |
| Nausea | 1 | 1 | 1 | 2 |
| Dermatologic (1) | ||||
| Facial rash | 1 | 1 | 1 | |
| Renal (2) | ||||
| Urinary tract infection | 1 | 1 | ||
| Urinary frequency | 1 | 1 | ||
| Constitutional (2) | ||||
| Weakness | 1 | 1 | 1 | |
| Hot/cold intolerance | 1 | 1 | ||
DISCUSSION
The findings of this study do not support our hypothesis; patients receiving methylphenidate (18 mg/day) did not score on average one unit less on the BFI worst level of fatigue compared with their score while receiving placebo. Our finding is similar to the findings reported by Moraska's18 study of 148 patients taking 54 mg of methylphenidate daily for 4 weeks. This trial included several cancer types, and a majority had advanced disease. In contrast, Lower 19 reported improved fatigue in a study of 152 patients post-chemotherapy. This population also included several types of cancer patients taking dexmethylphenidate over 8 weeks. Roth20 recently found that short-acting methylphenidate (maximum of 30 mg daily in two doses) effectively treated fatigue in elderly men (70 years, average) with advanced prostate cancer over a 6-week period. However, the study was discontinued early with only 16 patients in each of the arms (methylphenidate and placebo). Our study population consisted of women (57 years, average) with breast cancer treated with methylphenidate for 2 weeks. Only about one-third had advanced disease. Perhaps some or all of these differences contributed to our dissimilar findings, which only reinforces the need to continue to seek treatments for CRF. We also used a low dose of methylphenidate without a titration schedule. Perhaps a titration schedule with the ability to increase doses may have produced different results. Also, although this drug acts relatively quickly, a longer treatment period may have changed our findings. Despite limitations, our study includes unique aspects not previously considered in a randomized clinical trial of methylphenidate, such as the effect of fatigue on neurocognitive abilities and on work productivity and descriptions of patient perceptions and preferences.
Although our study was stopped before adequate power was reached, some findings support methylphenidate as a treatment for CRF. Further study may be warranted. Methylphenidate improved patients’ cognitive-processing speed and recall. Cognitive improvement with stimulants has previously been noted, supporting our results.21 Our results also show that methylphenidate significantly reduced patients’ absences from work owing to their illness and increased the number of hours they were able to work. Of the 20 employed patients (61%), 13 (65%) were working full time (40 hours or more per week). The ability to continue working is extremely important to the majority of cancer patients—not only to maintain financial and family stability and health insurance coverage but also to stabilize patients’ mood, personal perceptions of self-worth, and satisfaction.
Our documentation of patient perceptions and preferences show that the majority of patients subjectively experienced improvement, as they chose to continue methylphenidate following study completion. This shows some patients are willing to take a stimulant for longer periods of time if they feel it will help CRF. They had minimal adverse events, and all were mild. The number and severity of adverse events were similar on both study arms. The stimulant did not induce hypertension in our patients. This contrasts to the recent study of short-acting methylphenidate published by Roth20, whereas 38% (6/16) had increased blood pressure or tachycardia and discontinued treatment. Our decreased rates of these events may be due to a younger and potentially healthier cohort than those of Roth.
Our exploratory cytokine analysis, the first study of cytokines in a clinical trial of a stimulant for CRF, showed changes in some cytokines between arms. Meyers et al22 showed a significant relationship between levels of IL-6, IL-1RA, and TNFα and fatigue and overall quality-of-life. They found high levels of IL-6 were associated with worst performance in executive function, whereas higher IL-8 levels were related to improved memory performance. Other preliminary evidence suggests that fatigue is associated with elevations in proinflammatory cytokines ( IL-1, IL-6, TNFα), and interferons.23 Because these cytokines may be affected by many factors, including cancer treatment, the significance of our findings is unclear, and continued work is necessary. It is difficult to interpret and correlate our findings with others, as patients enrolled in our study did not receive the same cancer treatments, did not have the same extent of disease, and were not on matching time points during their cancer treatments. All of these factors may influence cytokine levels and thus our results.
We encountered several challenges in recruiting and subsequently enrolling patients in this trial. As noted in Fig 1b, over 10,000 patients were screened. Of these, approximately 600 (6%) were eligible and approached. Over 90% declined participation for various reasons. Subsequently, 7% of those approached were enrolled. The Roth trial20 was also stopped prior to reaching their target enrollment, though they approached and screened over 1000 patients. The Moraska trial18 recruited 148 patients from multiple sites through a cancer trial group network. Multi-site studies frequently require greater resources and financial support; however, this expense may be necessary for success in these trials.
Fig 1b.
Study Screening
Other limitations to consider in interpreting our results include the cohort studied. Our patients were studied at a comprehensive cancer center and may be dissimilar to other populations. There were no men, and the population was moderately well educated and over half were employed.
In summary, we believe our findings have made progress toward a better understanding of CRF. Employed patients had fewer work absences and were able to work more when taking methylphenidate. Our study is the first randomized trial to report patients’ perceptions and preferences, incorporating the desires of our patients into treatment plans. A majority of patients experienced improved fatigue and desired to continue methylphenidate following the trial. There were no serious adverse events and minimal other adverse events, demonstrating the tolerability and safety of methylphenidate in the study population. Our study is also the first clinical trial of a stimulant for CRF to investigate cytokine levels. Further investigations of cytokines’ role in CRF may aid in understanding this cancer treatment-related toxicity. Additional studies with higher dosages and/or longer treatment periods and varying cancer populations may help to clarify the efficacy of methylphenidate for CRF. Including work productivity and patient preferences may add an important metric to our efforts to improve CRF in our patients.
Acknowledgments
Grant funding was provided by Ortho-McNeil Janssen Scientific Affairs, LLC.
Footnotes
All participants signed the IRB approved study informed consent.
Conflicts of Interest and Source of Funding: Carmen Escalante has received funding from Teva Pharmaceuticals. Phuong Khanh Morrow has a leadership position and stock ownership at Amgen. Charles Cleeland is a consultant at Amgen, and has received remuneration from Amgen. For the remaining authors none were declared.
This work was presented as a poster at the 2012 Annual American Society of Clinical Oncology meeting in Chicago, IL.
REFERENCES
- 1.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34:4–12. [PubMed] [Google Scholar]
- 3.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 4.Flechtner H, Bottomley A. Fatigue and quality of life: lessons from the real world. Oncologist. 2003;8(Suppl 1):5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- 5.Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:1319–1328. doi: 10.1200/JCO.2002.20.5.1319. [DOI] [PubMed] [Google Scholar]
- 6.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 7.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Wechsler D. Wechsler Adult Intelligence Scale. Rev. ed. Psychological Corporation; New York: 1981. [Google Scholar]
- 9.Brandt J. The hopkins verbal learning test: Development of a new memory test with six equivalents forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- 10.Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. AJA Associates: Inc; Iowa City, IA: 1989. [Google Scholar]
- 11.Lezak M, Howieson D, Loring D, Hannay H, Fischer J. Neurophysiological assessment. J Clin Psychol. 1995;50:596–600. [Google Scholar]
- 12.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25:307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 15.Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. J Chronic Dis. 1987;40:939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28:3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38:650–662. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Roth AJ, Nelson C, Rosenfeld B, et al. Methylphenidate for fatigue in ambulatory men with prostate cancer. Cancer. 2010;116:5102–5110. doi: 10.1002/cncr.25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci. 2005;30:100–107. [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 23.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92:1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]