Abstract
Background
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has been widely used in the diagnosis of mediastinal lymphadenopathies. Here, we performed a systematic review and meta-analysis to explore the diagnostic value of EBUS-TBNA in mediastinal tuberculous lymphadenopathy (TBLA).
Material/Methods
PubMed, EMBASE, and Sinoced were systematically searched for articles published in English or Chinese that reported the diagnostic yield of EBUS-TBNA in mediastinal TBLA. The quality of studies was assessed using the QualSyst tool. Using 95% confidence intervals (CI), the diagnostic yields of EBUS-TBNA were calculated for the individual studies, and the results were then pooled using a random-effects model. Heterogeneity and publication bias were also assessed.
Results
A total of 14 studies, consisting of 684 patients with mediastinal TBLA, were finally included. The pooled diagnostic yield of EBUS-TBNA for mediastinal TBLA was 80% (95% CI: 74–86%). Significant heterogeneity (I2=77.9%) and significant publication bias were detected (Begg’s test p=0.05 and Egger’s test p=0.02). From subgroup analyses, significant differences in the diagnostic yield of EBUS-TBNA were associated with Asian vs. European (UK) studies, retrospective vs. prospective studies, those employing rapid on-site cytological evaluation vs. not, those employing different anesthetic types, and those employing smear vs. culture. However, microbiological examination and the number of lymph node passes did not have a significant effect on the diagnostic yield of EBUS-TBNA. Fifteen minor complications for EBUS-TBNA were reported.
Conclusions
EBUS-TBNA appears to be an efficacious and safe procedure and should be used as an initial diagnostic tool for mediastinal TBLA.
Keywords: Bronchial Provocation Tests; Meta-Analysis; Tuberculosis, Cutaneous
Background
According to the World Health Organization (WHO), tuberculosis (TB) remains a global public health concern [1]. In China in particular, the morbidity associated with active TB has doubled while TB-associated mortality has increased 7-fold over the past decade [2,3]. Nearly all primary TB patients present with pulmonary infiltrates along with tubercular lymphadenitis (TBLA), and a substantial proportion of patients with other types of pulmonary TB also present with TBLA [4,5].
Although pulmonary TB can be easily diagnosed through sputum examination for acid-fast bacilli (AFB), by smear, or by culturing for Mycobacterium tuberculosis, such methods are not effective for the diagnosis of TBLA. Owing to its non-specific clinical and radiographic presentation, the diagnosis of mediastinal and hilar TBLA can be challenging [6]. Moreover, imaging by chest computed tomography (CT) scanning often cannot detect changes soon after TBLA treatment, leading to uncertainty regarding treatment strategy. To address this challenge, the development of alternative diagnostic approaches to TBLA is essential.
Since its introduction in 2004, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has emerged as a minimally-invasive approach to sampling mediastinal and hilar lymph nodes [7]. Although EBUS-TBNA was primarily developed to diagnose and stage lung cancer [8], it has since been applied to the diagnosis of lymphoma [9], TB [10], and sarcoidosis [11]. Previous meta-analyses have demonstrated the diagnostic efficacy of EBUS-TBNA in lung cancer and sarcoidosis [12,13]; however, we have found no previously published systematic reviews or meta-analyses concerning the diagnostic efficacy of EBUS-TBNA in mediastinal TBLA. Therefore, we performed a systematic review and meta-analysis to explore the diagnostic value of EBUS-TBNA in patients with mediastinal TBLA.
Material and Methods
Ethics Statement
This study was conducted in compliance with the Ethics Statement of the First Affiliated Hospital of Bengbu Medical College (Bengbu, China).
Search strategy
A comprehensive search strategy was independently applied by 2 authors (WL and TZ) to search 3 computer databases (PubMed, EMBASE, and Sinoced) for relevant studies published between January 1, 2002 and April 1, 2014 using the following search term combinations: (i) (EBUS OR “EBUS TBNA” OR TBNA OR “endobronchial ultrasound” OR “endobronchial ultrasonography” OR “endobronchial ultrasound-guided” OR “endoscopic ultrasound” OR “transbronchial needle aspiration”) AND tuberculosis; and (ii) (EBUS OR “endobronchial ultrasound” OR “endobronchial ultrasonography” OR “endobronchial ultrasound-guided” OR “endoscopic ultrasound”) AND (TBNA OR “transbronchial needle aspiration”).
Study selection
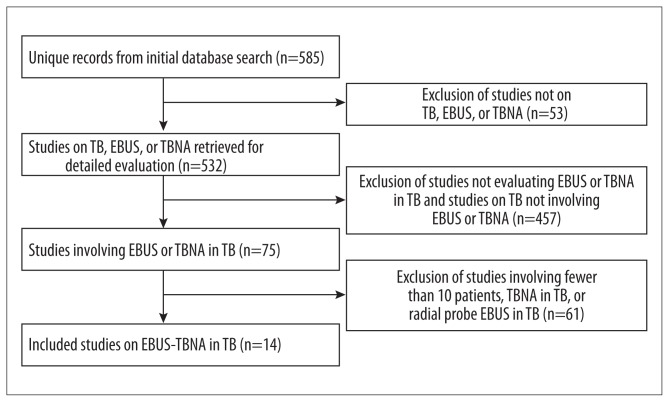
All records from the database search were imported into Endnote to eliminate duplicate records. The remaining records were screened by title and abstract to capture all relevant studies. Studies were eligible for inclusion if they reported the diagnostic yield of convex probe EBUS-TBNA in patients with clinical suspicion of mediastinal TBLA. The full text of each article meeting the inclusion criteria was obtained and reviewed. We excluded the following studies: (i) abstracts, editorials, reviews, letters, and case reports; (ii) studies reporting the diagnostic value of TBNA or endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or radial probe EBUS-TBNA in TB; (iii) studies describing EBUS-TBNA in fewer than 10 patients with mediastinal TBLA; and (iv) studies in which the number of patients with a final diagnosis of mediastinal TBLA (i.e., pathology displaying granulomatous inflammation with clinical manifestations of TB) was not reported. Any disagreement was resolved by discussion between the authors.
Data extraction
We extracted and recorded the following data from all eligible studies: publication details (authors, publication year, geographic location, and other citation details); study design (prospective or retrospective); size of lymph nodes by chest CT; type of sedation; diameter of EBUS-TBNA needle; stations sampled; size of the lymph nodes on EBUS and number of lymph nodes passed made through EBUS; availability of rapid on-site evaluation (ROSE); evidence of microbiology; diagnostic yield of EBUS-TBNA in mediastinal TBLA as the percentage of the diagnosis of mediastinal TBLA by EBUS-TBNA in the patients with confirmed mediastinal TBLA; and complications associated with the procedure.
Assessment of study quality
The QualSyst tool [14] was used to assess the quality and validity of each article included in the meta-analysis. This tool consists of 10 questions scored from zero to 2 with a maximum total score of 20. Each study was independently evaluated by the 2 authors for the stated criteria.
Statistical analysis
All calculations were performed using STATA version 9.0 (Stata Corp., College Station, TX, USA). We calculated the 95% CI for each study in order to calculate the diagnostic yield of EBUS-TBNA in mediastinal TBLA and used the data to derive a pooled 95% CI through back-transformation of the weighted mean of the transformed proportions using DerSimonian weights for the random-effects model [15] in the presence of significant heterogeneity. Chi-square and I2 testing were used to assess heterogeneity of study outcomes. An I2 value of greater than 50% or a p-value of less than 0.10 was deemed to indicate the presence of significant statistical heterogeneity [16,17]. We also performed several subgroup analyses: geographic location (Asian vs. European), research design (prospective vs. retrospective), employing ROSE vs. not, anesthetic type (conscious sedation vs. intravenous), the use of microbiological assessment, the use of smear vs. culture, and by number of lymph node passes (<3 vs. ≥3). The presence of publication bias was evaluated using funnel plots [18], Egger’s test [19], and Begg’s test [20] with a p-value of less than 0.05 indicating significant publication bias.
Results
Characteristics of included studies
The database search yielded 14 studies consisting of 684 patients with mediastinal TBLA [10,21–33]. The full details of these included studies are shown in Table 1. The quality of the included studies was generally good with the median (IQR) score of 18 (Table 2). Of the 14 included studies, 7 were prospective [10,21,23,26,27,29,33], and 7 were retrospective [22,24,25,28,30–32]. A total of 11 patients presented with isoniazid-resistant TB [25,26,31], and 25 patients presented with human immunodeficiency virus (HIV)-related TB [25,33]. Five studies used conscious sedation with midazolam [10,21,23,28,29], while 8 studies used intravenous anesthesia [22,24–27,30,31,33] with fentanyl or propofol to assist the anesthesia and lidocaine as the topical throat anesthetic (Figure 1). The 1 remaining study could not provide this information [32].
Table 1.
Characteristics of included studies.
| Author (year) | Country | Study design | Age (in years) | Participants (n) | TB diagnosis (n) | HIV+ |
|---|---|---|---|---|---|---|
| Caglayan (2011) | Turkey | Prospective | 19–81 (range) | 19 | 16 | NA |
| Hu (2011) | China | Retrospective | 24–84 (range) | 10 | 5 | NA |
| Cetinkaya (2011) | Turkey | Prospective | 50.2 (mean) | 48 | 38 | NA |
| Zhao (2012) | China | Retrospective | 60.4 (mean) | 11 | 10 | NA |
| Navani (2012) | UK | Retrospective | 18–86 (range) | 156 | 146 | 17 |
| Navani (2012) | UK | Prospective | 42 (median) | 28 | 26 | NA |
| Gu (2012) | China | Prospective | 16–82 (range) | 124 | 105 | NA |
| Luo (2013) | China | Retrospective | 51.7 (mean) | 13 | 9 | NA |
| Sun (2013) | China | Prospective | 49 (median) | 36 | 35 | NA |
| Kuo (2013) | China | Prospective | 25–91 (range) | 10 | 7 | NA |
| Xie (2013) | China | Retrospective | 47.7 (mean) | 38 | 34 | NA |
| Ren (2013) | China | Retrospective | >18 | 65 | 48 | NO |
| Kaur (2013) | India | Retrospective | NA | 27 | 13 | NO |
| Dhasmana (2014) | UK | Prospective | 65.5 (median) | 99 | 85 | 8 |
Table 2.
QualSyst quality assessment of the included studies.
| Item | Caglayan (2011) | Hu (2011) | Cetinkaya (2011) | Zhao (2012) | Navani (2012) | Navani (2012) | Gu (2012) | Luo (2013) | Sun (2013) | Kuo (2013) | Xie (2013) | Ren (2013) | Kaur (2013) | Dhasmana (2014) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Question/objective sufficiently described? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Study design evident and appropriate? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Context for the study clear? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Connection to a wider body of knowledge? | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Sampling strategy described, relevant, and justified? | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Data collection methods clearly described? | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
| Data analysis clearly described? | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 |
| Use of verification procedure(s) to establish credibility? | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 |
| Conclusions supported by the results? | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Reflexivity of the account? | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 2 |
| Total score | 18 | 18 | 18 | 19 | 18 | 19 | 17 | 17 | 19 | 18 | 18 | 19 | 17 | 18 |
Figure 1.
Flowchart of study selection.
The majority of studies sampled the paratracheal, subcarinal, hilar, and interlobar lymph nodes (stations 2, 4, 7, 10, and 11). Findings on lymph node size, number of lymph nodes aspirated, number of passes of lymph nodes, the use of ROSE, and the availability of microbiological data are shown on Table 3. Eight studies employed additional ROSE [22,24,25,27,30–33] (with only a portion of patients employed in 1 study [25]), and 9 studies employed microbiologic evidence for diagnosis by either culture or smear [10,23,25,26,29–33]. However, in 2 studies [23,29], the culture/smear data provided unclear findings. By the random-effects model, the overall culture positive rate was 54% [10,25,26,31–33], and the sample smear positive rate was 30% [10,25,30–33]. The positive rate of culture was significantly higher than that of the smear (p<0.05).
Table 3.
EBUS-TBNA details for included studies.
| Author (year) | Nodal size by CT (mm) | Anesthesia | Stations examined | Nodal short axis on EBUS (mm) | Aspirations | Roes | Microbiology (smear or culture) | PCR | BAL | Needle gauge |
|---|---|---|---|---|---|---|---|---|---|---|
| Caglayan (2011) | >10 | Conscious sedation | 2,4,7,10,11 | 19.6 (mean) | 1.71 (mean) | No | No | No | No | 22G |
| Hu (2011) | NA | General anesthesia | NA | NA | NA | Yes | NA | No | No | 22G |
| Cetinkaya (2011) | >10 | Conscious sedation | 4,7,10 | NA | 2.6 (mean) | No | 5 (unknown Species) | No | No | 22G |
| Zhao (2012) | >10 | General anesthesia | NA | NA | ≥3 | Yes | No | No | No | 22G |
| Navani (2012) | NA | General anesthesia | 2,4,7,10,11 | 22 (mean) | 1.28 (mean) | Yes (only portion) | 74/156 | No | No | 22G or 21G |
| Navani (2012) | NA | General anesthesia | 2,4,7 | 23 (mean) | 4 | No | unknown, 11/26 | No | No | 22G or 21G |
| Gu (2012) | >10 | General anesthesia | 2,3,4,7,10,11,12 | NA | 1.95 | Yes | No | No | No | 22G |
| Luo (2013) | NA | Conscious sedation | 2,3,4,7,10,11,12 | 22.1 (mean) | 3.5 | No | No | No | No | 22G |
| Sun (2013) | >10 | Conscious sedation | 2,4,7,10,11,12 | 20.1 (mean) | 2.91 | No | 8/35, 17/32 | No | No | 22G |
| Kuo (2013) | >10 | Conscious sedation | 2,4,7,10,11 | Symptomatic (23.8±6.4); asymptomatic (18.9±8.3) | ≥3 | No | 4 (unknown species) | No | Yes | 22G |
| Xie (2013) | ≥10 | General anesthesia | 2,4,7,10,11 | 18.7 (mean) | 3.5 | Yes | 21/34 | Yes (only 9) | No | 22G |
| Ren (2013) | >10 | General anesthesia | 7,4R | 15 (median) | NA | Yes | 11/20,17/20 | No | Yes | 22G |
| Kaur (2013) | NA | NA | NA | NA | NA | Yes | 8/13,5/36 | No | Yes | NA |
| Dhasmana (2014) | NA | General anesthesia | 2,4,7,10,11 | NA | 4–14 (range) | Yes | 14/85,84/85 | Yes | Yes (only 2) | 22G |
Diagnostic yield
In our results, the pooled diagnostic yield of EBUS-TBNA for mediastinal TBLA was 80% (95% CI, 74–86%) as calculated by the random-effects model (Figure 2). There was evidence of significant heterogeneity (I2=77.9%) and significant publication bias (Begg’s test p=0.05 and Egger’s test p=0.02).
Figure 2.
Meta-analysis on the diagnostic yield of EBUS-TBNA for mediastinal TBLA. Forest plot depicting the effect sizes (ES) and 95% confidence intervals (CI) for the included studies. Due to the presence of significant heterogeneity, studies were pooled by a random-effects mode.
According to our subgroup analyses (Table 4), the diagnostic yield was significantly different in Asian vs. European (UK) studies, retrospective vs. prospective studies, those employing ROSE vs. not, those employing different anesthetic types, and those employing smear vs. culture. However, the use of microbiological assessment and the number of lymph node passes did not have a significant effect on the diagnostic yield.
Table 4.
Subgroup analysis of included studies.
| Item | Studies (n) | Participants (n) | Diagnostic yield | Lower 95% CI limit | Upper 95% CI limit | P-value | I2 (%) |
|---|---|---|---|---|---|---|---|
| Geography | |||||||
| Asian | 11 | 401 | 75 | 68 | 83 | <0.05 | 68.3 |
| European (UK) | 3 | 283 | 91 | 86 | 96 | 46.8 | |
| Research design | |||||||
| Prospective | 7 | 364 | 82 | 75 | 89 | <0.05 | 60.0 |
| Retrospective | 7 | 320 | 77 | 65 | 89 | 84.9 | |
| Employing ROSE | |||||||
| Yes | 7 | 374 | 79 | 70 | 87 | <0.05 | 74.7 |
| No | 6 | 154 | 77 | 69 | 89 | 67.1 | |
| Anesthesia | |||||||
| Conscious sedation | 5 | 126 | 73 | 64 | 83 | <0.05 | 34.6 |
| Intravenous anesthesia | 8 | 531 | 86 | 81 | 92 | 69.0 | |
| Employing microbiology | |||||||
| Yes | 8 | 459 | 79 | 70 | 88 | >0.05 | 85.4 |
| No | 5 | 177 | 81 | 72 | 91 | 39.4 | |
| Microbiology | |||||||
| Smear | 6 | 377 | 30 | 18 | 42 | <0.05 | 86.1 |
| Culture | 6 | 361 | 54 | 20 | 89 | 98.9 | |
| No. of lymph nodal passes | |||||||
| <3 | 5 | 383 | 82 | 72 | 91 | >0.05 | 83.6 |
| ≥3 | 6 | 199 | 88 | 83 | 92 | 4.5 | |
Complications
Only 15 minor complications (1 case of bronchospasm, 1 panic attack, 1 case of bacteremia, 6 cases of transient hypoxia, 4 occurrences of self-limiting bleeding, and 2 occurrences of fever) were reported in the 684 patients.
Discussion
Mediastinoscopy remains the criterion standard in the diagnosis and staging of enlarged mediastinal lymph nodes and has been preferred over the more invasive conventional TBNA and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) (which cannot access the right paratracheal and hilar nodes [34]) for the diagnosis of mediastinal lymphadenopathy. However, mediastinoscopy is invasive, costly, requires general anesthesia, and is associated with a 2% morbidity risk and 0.08% mortality risk [35,36]. Moreover, mediastinoscopic sampling of mediastinal lesions can be difficult owing to their adjacency to many vital structures, and posterior-lower carinal and hilar nodes stations are typically inaccessible by mediastinoscopy [37].
Although conventional TBNA can produce superior diagnostic yields over mediastinoscopy, complication rates for conventional TBNA are high since imaging of needle placement is not possible [38]. Therefore, the utility and safety of conventional TBNA can be improved under EBUS guidance. This EBUS-TBNA technique allows for the performance of needle aspiration biopsy under real-time ultrasound monitoring, which can clearly show the relationship between blood vessels, lymph nodes, and space-occupying lesions in the mediastinum [39]. As a result, EBUS-TBNA provides an efficacious and safe alternative in patients with mediastinal TBLA [11–13] and can be performed under conscious intravenous sedation [40]. EBUS-TBNA has been shown to have significantly better safety and accuracy compared to conventional TBNA [41,42]. In agreement with these previous findings, this meta-analysis reveals a strong overall diagnostic yield (80%) for EBUS-TBNA in detecting mediastinal TBLA. These findings suggest that EBUS-TBNA can be used as an initial diagnostic tool for mediastinal TBLA.
Although tuberculin skin testing, IFN-γ release assays with M. tuberculosis antigens, and recombinant M. tuberculosis early secreted antigenic target 6-kDa protein (ESAT-6) skin testing can be used to detect the presence of a TB infection [43–45], a definitive TB diagnosis can only be established with a M. tuberculosis-positive culture or smear. Although sputum examination has been useful in diagnosing active pulmonary TB, about half of suspected patients are unable to produce sputum, and even if sputum is produced, acid-fast bacilli (AFB) are often not found by repeated examination of direct smears [46]. In particular, TB patients with isolated mediastinal lymphadenopathy display particularly low culture yields by sputum culture and bronchoscopy [47]. With respect to EBUS-TBNA, Navani et al. [25] reported a culture rate of 47% in 146 TBLA patients by EBUS-TBNA, which was similar to culture rates for mediastinoscopy [48], TBNA without EBUS [49], and EUS-FNA [50]. The poor culture rates in mediastinal TBLA may be due to the scarcity of acid-fast bacilli in the lymph node, lack of suitable cellular material in samples obtained from necrotic tissue, and/or technical challenges in culturing M. tuberculosis. In this meta-analysis, we found that the culture positive rate (54%) was significantly higher than the sample smear positive rate (30%). Our microbiological findings support the same conclusions as previous studies [51,52], but 1 included study [33] reported a culture positivity rate approaching 99% (84/85), which may be attributable to differing operator experience/skill, lymph node size, lymph node location, sampling capacity, and/or bacillary loads in the sampled lymph nodes.
We conducted several subgroup analyses to determine factors that may affect the diagnostic yield for EBUS-TBNA in detecting mediastinal TBLA. Notably, our subgroup analyses revealed that neither microbiological assessment nor the use of more or less than 3 lymph node passes significantly affected the diagnostic yield of EBUS-TBNA in detecting mediastinal TBLA. This finding contradicts the conclusion of Yarmus et al. [53], which may be attributable to differences in operator experience and skill levels, the heterogeneity in bacillary load of M. tuberculosis in aspirated lymph nodes, and the skills for culturing the M. tuberculosis.
The rising incidence of HIV-related TB poses a clinical challenge, as it is associated with smear-negative pulmonary TB [54,55]. Smear-negative HIV-related TB displays an increased mortality compared to smear-positive TB [54,56], which may be related to misdiagnoses and treatment delays [57]. For instance, a recent study [58] in culture-confirmed TB patients reported that the positive predictive value of an AFB smear-positive respiratory specimen is significantly inferior in HIV-seropositive patients compared to HIV-uninfected patients (p<0.001). In this meta-analysis, 25 patients were infected with HIV – the culture was positive in 6 of these patients, while only 1 smear was positive. Therefore, further studies are required to assess the diagnostic utility of EBUS-TBNA in HIV-infected individuals.
The major limitation of this meta-analysis is the significant heterogeneity between the included studies. This can be broadly segregated into clinical heterogeneity (i.e., variability in the participants, interventions, and outcomes), methodological heterogeneity (i.e., variations in trial design and quality), and statistical heterogeneity (i.e., variability in the treatment effects being evaluated in different trials). Since heterogeneity is a source of variability among the included studies, a random-effects model was used here to minimize these effects. Moreover, several subgroup analyses were performed to explore the sources of heterogeneity. These analyses revealed significant differences in Asian vs. European (UK) studies, retrospective vs. prospective studies, those employing ROSE vs. not, those employing different anesthetic types, and those employing smear vs. culture – these factors may contribute to the observed statistical heterogeneity. In addition, we detected significant publication bias, which may have also contributed to the observed heterogeneity. The inclusion criteria, which may have limited certain article types and languages, may have contributed to the observed publication bias.
Conclusions
EBUS-TBNA shows a strong overall diagnostic yield (80%) for EBUS-TBNA in detecting mediastinal TBLA. These findings suggest that EBUS-TBNA can be used as an initial diagnostic tool for mediastinal TBLA. Based on our subgroup analyses, the use of ROSE, culturing, and intravenous anesthesia in prospective studies should result in superior diagnostic yields for EBUS-TBNA in detecting mediastinal TBLA.
Footnotes
Source of support: Self financing
Conflicts of interests
None.
References
- 1.World Health Organization. Global tuberculosis control: WHO report 2010. http://whqlibdocwhoint/publications/2010/9789241564069_engpdf.
- 2.Siu GK, Zhang Y, Lau TC, et al. Mutations outside the rifampicin resistance-determining region associated with rifampicin resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2011;66:730–33. doi: 10.1093/jac/dkq519. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Jiang S, Mei J, et al. Predictors on delay of initial health-seeking in new pulmonary tuberculosis cases among migrants population in East China. PloS One. 2012;7:e31995. doi: 10.1371/journal.pone.0031995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodring JH, Vandiviere HM, Fried AM, et al. Update: the radiographic features of pulmonary tuberculosis. Am J Roentgenol. 1986;146:497–506. doi: 10.2214/ajr.146.3.497. [DOI] [PubMed] [Google Scholar]
- 5.McAdams H, Erasmus J, Winter J. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am. 1995;33:655–78. [PubMed] [Google Scholar]
- 6.Chang SC, Lee PY, Perng RP. Clinical role of bronchoscopy in adults with intrathoracic tuberculous lymphadenopathy. Chest. 1988;93:314–17. doi: 10.1378/chest.93.2.314. [DOI] [PubMed] [Google Scholar]
- 7.Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–28. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 8.Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393–400.e1. doi: 10.1016/j.jtcvs.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax. 2008;63:360–65. doi: 10.1136/thx.2007.084079. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Teng J, Yang H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in diagnosing intrathoracic tuberculosis. Ann Thorac Surg. 2013;96:2021–27. doi: 10.1016/j.athoracsur.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med. 2012;106:883–92. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–96. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Lee HS, Lee GK, Kim MS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134:368–74. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 14.Kmet L, Lee R, Cook L. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Heritage Foundation for Medical Research. 2004 [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials. 1986;7:267–75. doi: 10.1016/0197-2456(86)90034-6. [DOI] [PubMed] [Google Scholar]
- 18.Dear KB, Begg CB. An approach for assessing publication bias prior to performing a meta-analysis. Statistical Science. 1992;7:237–45. [Google Scholar]
- 19.Egger M, Davey Smith G, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 21.Caglayan B, Salepci B, Fidan A, et al. Sensitivity of convex probe endobronchial sonographically guided transbronchial needle aspiration in the diagnosis of granulomatous mediastinal lymphadenitis. J Ultrasound Med. 2011;30:1683–89. doi: 10.7863/jum.2011.30.12.1683. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, Chen HQ, Chen Y, et al. Evaluation of the value of EBUS-TBNA in diagnosis of mediastinal lesions. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2011;33:787–90. [in Chinese] [PubMed] [Google Scholar]
- 23.Cetinkaya E, Gunluoglu G, Ozgul A, et al. Value of real-time endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Med. 2011;6:77–81. doi: 10.4103/1817-1737.78422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Wang J, Zhou ZL, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of isolated mediastinal lesions. Beijing da xue xue bao Yi xue ban [Journal of Peking University Health Sciences] 2012;44:147–50. [in Chinese] [PubMed] [Google Scholar]
- 25.Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax. 2011;66:889–93. doi: 10.1136/thoraxjnl-2011-200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012;186:255–60. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Y, Jiang GN, Zhou X, et al. Evaluation of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of hilar and mediastinal tumors. Zhonghua jie he he hu xi za zhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2013;36:22–26. [in Chinese] [PubMed] [Google Scholar]
- 28.Luo GY, Cai PQ, He JH, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration in the management of mediastinal and hilar lymphadenopathy without intrapulmonary mass: experience from the largest cancer center of southern China. Cell Biochem Biophys. 2013;67:1533–38. doi: 10.1007/s12013-013-9657-x. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CH, Lin SM, Lee KY, et al. Algorithmic approach by endobronchial ultrasound-guided transbronchial needle aspiration for isolated intrathoracic lymphadenopathy: a study in a tuberculosis-endemic country. J Formos Med Assoc. 2014;113:527–34. doi: 10.1016/j.jfma.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Zhao H, Zheng HF, Shen DH, JW Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of thoracic tuberculosis. Chin J Thorac Cardiovasc Surg. 2013;29:739–42. [Google Scholar]
- 31.Ren S, Zhang Z, Jiang H, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques enhanced the diagnosis yields of pulmonary tuberculosis patients with lymphadenopathy. Panminerva Med. 2013;55:363–70. [PubMed] [Google Scholar]
- 32.Kaur G, Dhamija A, Augustine J, et al. Can cytomorphology of granulomas distinguish sarcoidosis from tuberculosis? Retrospective study of endobronchial ultrasound guided transbronchial needle aspirate of 49 granulomatous lymph nodes. Cytojournal. 2013;10:19. doi: 10.4103/1742-6413.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhasmana DJ, Ross C, Bradley CJ, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc. 2014;11:392–96. doi: 10.1513/AnnalsATS.201308-250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri R, Vilmann P, Sud R, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462–67. doi: 10.1055/s-0029-1244133. [DOI] [PubMed] [Google Scholar]
- 35.Sihoe AD, Yim AP. Lung cancer staging. J Surg Res. 2004;117:92–106. doi: 10.1016/j.jss.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:202S–20S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 37.Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg. 1999;118:894–99. doi: 10.1016/s0022-5223(99)70059-0. [DOI] [PubMed] [Google Scholar]
- 38.Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50:347–54. doi: 10.1016/j.lungcan.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Herth F, Becker HD, Ernst A. Conventional vs. endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322–25. doi: 10.1378/chest.125.1.322. [DOI] [PubMed] [Google Scholar]
- 40.Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care. 2010;55:702–6. [PubMed] [Google Scholar]
- 41.Zhu T, Zhang X, Xu J, et al. Endobronchial ultrasound guided-transbronchial needle aspiration vs. conventional transbronchial needle aspiration in the diagnosis of mediastinal masses: A meta-analysis. Mol Clin Oncol. 2014;2:151–55. doi: 10.3892/mco.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cetinkaya E, Ozgul MA, Tutar N, et al. The diagnostic utility of real-time EBUS-TBNA for hilar and mediastinal lymph nodes in conventional TBNA negative patients. Ann Thoracic Cardiovasc Surg. 2014;20:106–12. doi: 10.5761/atcs.oa.12.02072. [DOI] [PubMed] [Google Scholar]
- 43.Babayigit C, Ozer B, Inandi T, et al. Performance of QuantiFERON-TB Gold In-Tube test and Tuberculin Skin Test for diagnosis of latent tuberculosis infection in BCG vaccinated health care workers. Med Sci Monit. 2014;20:521–29. doi: 10.12659/MSM.889943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du W-X, Chen B-W, Lu J-B, et al. Preclinical study and phase I clinical safety evaluation of recombinant Mycobacterium tuberculosis ESAT6 protein. Med Sci Monit Basic Res. 2013;19:146–52. doi: 10.12659/MSMBR.883912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Q-F, Xu M, Wu J-G, et al. Efficacy and safety of recombinant Mycobacterium tuberculosis ESAT-6 protein for diagnosis of pulmonary tuberculosis: A phase II trial. Med Sci Monit. 2013;19:969–77. doi: 10.12659/MSM.889425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chawla R, Pant K, Jaggi OP, et al. Fibreoptic bronchoscopy in smear-negative pulmonary tuberculosis. Eur Respir J. 1988;1:804–6. [PubMed] [Google Scholar]
- 47.Ayed AK, Behbehani NA. Diagnosis and treatment of isolated tuberculous mediastinal lymphadenopathy in adults. Eur J Surg. 2001;167:334–38. doi: 10.1080/110241501750215186. [DOI] [PubMed] [Google Scholar]
- 48.Farrow PR, Jones DA, Stanley PJ, et al. Thoracic lymphadenopathy in Asians resident in the United Kingdom: role of mediastinoscopy in initial diagnosis. Thorax. 1985;40:121–24. doi: 10.1136/thx.40.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilaceroglu S, Gunel O, Eris N, et al. Transbronchial needle aspiration in diagnosing intrathoracic tuberculous lymphadenitis. Chest. 2004;126:259–67. doi: 10.1378/chest.126.1.259. [DOI] [PubMed] [Google Scholar]
- 50.Song HJ, Park YS, Seo DW, et al. Diagnosis of mediastinal tuberculosis by using EUS-guided needle sampling in a geographic region with an intermediate tuberculosis burden. Gastrointest Endosc. 2010;71:1307–13. doi: 10.1016/j.gie.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 51.Radhika S, Gupta SK, Chakrabarti A, et al. Role of culture for mycobacteria in fine-needle aspiration diagnosis of tuberculous lymphadenitis. Diagn Cytopathol. 1989;5:260–62. doi: 10.1002/dc.2840050306. [DOI] [PubMed] [Google Scholar]
- 52.Gupta SK, Chugh TD, Sheikh ZA, al-Rubah NA. Cytodiagnosis of tuberculous lymphadenitis. A correlative study with microbiologic examination. Acta Cytol. 1993;37:329–32. [PubMed] [Google Scholar]
- 53.Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc. 2013;10:636–43. doi: 10.1513/AnnalsATS.201305-130OC. [DOI] [PubMed] [Google Scholar]
- 54.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:97–107. [PubMed] [Google Scholar]
- 55.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–49. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 56.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–52. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 57.Whitehorn J, Ayles H, Godfrey-Faussett P. Extra-pulmonary and smear-negative forms of tuberculosis are associated with treatment delay and hospitalisation. Int J Tuberc Lung Dis. 2010;14:741–44. [PubMed] [Google Scholar]
- 58.Adelman MW, Kurbatova E, Wang YF, et al. Cost analysis of a nucleic acid amplification test in the diagnosis of pulmonary tuberculosis at an urban hospital with a high prevalence of TB/HIV. PloS One. 2014;9:e100649. doi: 10.1371/journal.pone.0100649. [DOI] [PMC free article] [PubMed] [Google Scholar]