Abstract
Platelets are anucleate blood cells, long known to be critically involved in hemostasis and thrombosis. In addition to their role in blood clots, increasing evidence reveals significant roles for platelets in inflammation and immunity. However, the notion that platelets represent immune cells is not broadly recognized in the field of Physiology. This manuscript reviews the role of platelets in inflammation and immune responses, and highlights their interactions with other immune cells, including examples of major functional consequences of these interactions.
Introduction
Platelets are small anucleate blood elements, known since the late 19th century to participate in blood clot formation (17). They are critical mediators of the physiologic response of cessation of bleeding following blood vessel injury (hemostasis) as well as pathologic formation of blood clots (thrombosis) (175). Platelets are traditionally viewed in the context of hemostasis and thrombosis, while leukocytes are regarded as primary immune cells mediating the body’s response to pathogens. In recent years, increasing evidence supports the notion that platelets participate in immune responses, and interactions between platelets and leukocytes contribute to both thrombosis and inflammation. Despite these data, much general teaching of the physiologic roles of platelets and leukocytes is limited to the original descriptions. In this manuscript, we will focus on platelets from a perspective of immunity and inflammation, including the mechanisms of their interactions with leukocytes and the functional consequences of these interactions. We will devote little attention to the role of platelets in hemostasis and thrombosis and instead refer readers to available reviews on these traditional functions of platelets (25, 125). We will highlight numerous examples that support the premise implied in the title of this manuscript, that platelets should be regarded as immune cells.
Overview of Platelets
Human platelets are the smallest blood cells with ~2–5 μm in diameter, 0.5 μm in thickness and ~6–10 femtoliters in mean cell volume. They are derived as fragments from megakaryocytes and are released into the circulation with an average life span of ~7–10 days (reviewed in (221)). A primary and critical function of platelets is to sustain hemostasis. This is achieved by forming a stable platelet plug at the site of vascular injury via adhesion and aggregation to the exposed sub-endothelial matrix proteins. However, similar processes at the site of rupture of an atherosclerotic plaque can lead to occlusive platelet thrombi and cause thrombosis. Whereas the normal count for human platelets range from 150,000 to 400,000/μl, hemostasis can be achieved with platelet counts more than 10,000/μl (187). This observation suggests that platelets likely have roles beyond hemostasis and thrombosis, a theme that is addressed in this review. Despite their small size and limited life span, platelets display an elaborate structure that provides clues to their biological role.
Platelet structure
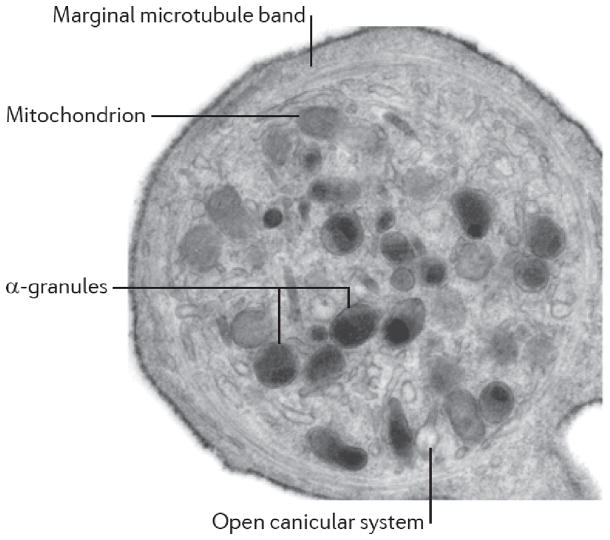
Resting platelets reveal a unique discoid shape cell, in part, due to the robust cytoskeletal structure encompassed by several loops of the microtubular coils (marginal microtubule bands) (106). However, platelet activation is associated with major shape change due to cytoskeletal changes that enable filopodial and lamellopodial extensions to occur (Fig. 1). The phospholipid bilayer of the platelet membrane embeds cell surface receptors that engage soluble ligands or fixed ligands on other immune cells and the endothelium. The functional consequences of receptor-ligand engagement include activation of platelets and complexes of activated platelets with leukocytes, erythrocytes, or endothelial cells and contribute to inflammation. Also present on the plasma membrane are numerous openings or pores that lead to several invaginations in platelets called the open canalicular system (OCS), which provide the small-sized platelets with a much greater surface area (218, 219). Distinct from the plasma membrane-associated OCS, platelets also display a channel system called dense tubular system (DTS). The DTS is believed to be a remnant of megakaryocyte smooth endoplasmic reticulum and stores calcium and enzymes that support the activation of platelets (42, 169). Most importantly, electron microscopy images of platelets disclose the notable absence of nucleus and a chockfull presence of organelles including mitochondria, glycosomes and secretory granules (Fig. 2).
Figure 1.
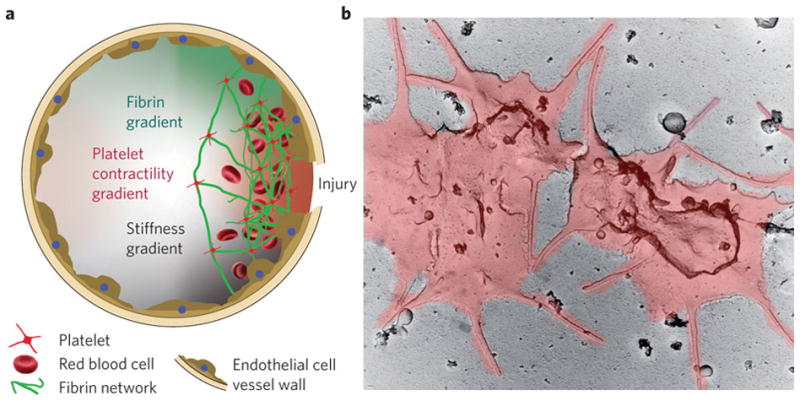
(A) Platelets localize to the site of injury, binding to fibrin, and forming a hemostatic plug. (B) Electron micrograph of activated platelets, which spread out over an injured area and extend filopodia. Reprinted by permission from Macmillan Publishers Ltd: Nature Materials (59), 2010.
Figure 2.
Ultrastructural features of a discoid platelet showing α granules, mitochondrion, the marginal microtubule band, and open canicular system. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Immunology (182), 2011.
Platelet granules
The anuclear feature of platelets, including the inability to replicate does not impede their ability to respond effectively to the external stimuli. Platelets are endowed with presynthesized proteins within their granules, which can be secreted to the extracellular milieu or expressed on the platelet surface following their activation. Certain proteins like platelet factor 4 (PF4) are synthesized by the megakaryocytes and carried over to platelet granules, while immunoglobulins (IgG) are endocytosed from the plasma by platelets. One recent proteomic study concluded the presence of eight hundred and twenty seven proteins in the granules (234). This suggests that secretion events can facilitate the cross talk of platelets with a variety of cell types, including the immune and endothelial cells, and thus influence a wide range of physiological functions. Platelets possess three types of granules: α granules, dense granules, and lysozymes.
α granules
α granules are the most abundant granules (~50–80/platelet), measuring 200–500 nm in diameter (18). Their contents include proteins that support platelet adhesion, aggregation and coagulation, which are required for hemostasis and thrombosis function. Some examples include fibrinogen, von Willebrand factor (VWF), vitronectin, fibronectin, thrombospondin, factor V, factor VIII, and cell adhesion molecules like integrins αIIbβ3 (GPIIb/IIIa) and αvβ3. α granules also contain proteins and peptides that recruit, localize, or activate immune cells and thus modulate inflammatory or immune function. One of these granular proteins is P-selectin, which is expressed on the platelet surface following activation of platelets (93). As discussed in detail below, P-selectin on activated platelets can engage its ligand P-selectin glycoprotein ligand-1 (PSGL-1) expressed on neutrophils and monocytes and activate these leukocytes (140, 208, 230). Chemokines (PF4 or CXCL4) and β-thromboglobulin, [neutrophil activating peptide (NAP), CXCL7] secreted from the granules can recruit and activate neutrophils, suppress neutrophil apoptosis (24, 79, 177) and participate in the homing of endothelial progenitor cells (EPC) (90). Chemokine CCL3 [Macrophage inhibitory protein-1 α (MIP-1α)], CCL5 (RANTES) and CXCL1 (GRO α) can recruit monocytes (73, 92), while CXCL-5 can modulate chemokine scavenging and neutrophil chemotaxis (137, 165). α granules also contain proteins that exhibit anti-microbial effects and thus influence innate immune function. Examples include thrombocidin 1 and 2, which have anti-bacterial and anti-fungal actions (115). α granules also contain proteins that possess mitogenic and angiogenic abilities and thus regulate wound healing and angiogenesis function. Platelet derived growth factor (PDGF), transforming growth factor beta (TGF-β), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) could influence the effector cells like monocytes macrophages, and endothelial cells (10, 61, 129). α granules contain hundreds of distinct proteins, including substances with opposing physiological roles (e.g., pro- and anti-angiogenic, pro- and anti-coagulant (18)). Recent studies demonstrate that platelets possess the ability to store α granule contents differentially, which enables differential release of α granule content in response to various stimuli (180). Differential storage and release of platelet alpha granule proteins has important implications for the role of platelets as immune cells; the mechanisms responsible for these complex processes remain to be fully characterized.
Dense granules
These granules appear as dense bodies on electron microscopy due to the elevated contents of calcium and phosphate. They are ~10 fold less abundant than α granules and about ~150 nm in diameter (70, 88). The contents of these granules are also released into the extracellular environment upon activation of platelets. These granules store nucleotides (ATP and ADP) (169). ATP can modulate inflammatory pathways by activating dendritic cells, while ADP provides a feedback mechanism that activates platelets. Serotonin can mediate vascular tone and also recruit neutrophils at sites of inflammation (14, 58). Other constituents include cations, like calcium and magnesium that can support signal transduction processes.
Lysosomes
These granules are approximately 200–250 nm in diameter and can be microscopically identified by staining for lysosomal enzymes, like acid phosphatase or arylsulfatases (12). Lysosomes contain the lysosomal-associated membrane proteins, LAMP1, LAMP2 and LAMP-3 (CD63) (183). CD63 is translocated to the platelet surface following stimulation with strong stimuli. Lysosomes are home to proteases like carboxylpeptidases, cathespin D and E that contribute to the inflammatory potential of platelets (169, 186, 213) and enzymes that remodel the extracellular matrix (37).
Platelet membrane receptors
Receptors on platelets enable them to sense the external environment and respond to biological changes. For the purpose of this review, we broadly classified platelet receptors into receptors that support hemostasis and receptors that sustain the interaction with other immune cells or participate in immune function. Several receptors are constitutively expressed on the platelet surface, although some receptors are stored in the granules and surface expressed only after activation.
Receptors involved in hemostasis
Given the prominent role of platelets in hemostasis, the major receptors on platelets have a direct role in their activation and/or supporting the adhesive interaction of platelets with adhesive proteins at the site of injury.
Some platelet receptors involved in hemostasis primarily mediate platelet activation. These include receptors that engage platelet agonists (agents that activate platelets). At the site of vascular injury, activation of coagulation pathways generates a serine protease thrombin. Platelets express two thrombin receptors, namely protease-activated receptor 1 (PAR1) and PAR4 (101). PAR1 responds to lower levels of thrombin ~1 nM while PAR4 is sensitive to at least 10 times higher concentration of thrombin. Interestingly, differential expression of PAR is noticed among species. Mouse platelets express PAR3, instead of PAR1, along with PAR4 (94). Thrombin is a potent activator of platelets, and activated platelets degranulate their contents including ADP and metabolize arachidonic acid to generate thromboxane A2. Platelets express two ADP receptors, P2Y1 and P2Y12, and a thromboxane receptor, TP (8, 76, 145). Binding of ADP and thromboxane A2 to their respective receptors enables critical autocrine platelet activation processes to occur. Exposed collagen at the site of injury can be recognized by integrin α2β1 and an immunoglobulin type receptor glycoprotein VI (GPVI) on platelets (4, 141, 192).
Other platelet receptors are involved in hemostasis by mediating stable platelet adhesion. These include receptors that engage in platelet-extracellular matrix or platelet-platelet interactions. Given that platelets often have to accomplish hemostasis in the context of a high shear stress environment, it is imperative that they possess a shear responsive receptor. A leucine–rich repeat family member, glycoprotein GP Ib-IX-V complex that includes GPIα (~145 kD) as well as GPIbβ (~22 kD), GPIX (~17 kD), and GPV (~82kD), endows platelets to adhere von Willebrand factor (VWF) exposed at the site of injury under high shear stress (3). The interaction of GPIbα with VWF transduces signals, which activate another class of cell adhesion molecules on platelets or integrins, specifically αIIbβ3 (75). Platelets express the integrin family of receptors, namely αIIbβ3, αvβ3, α5β1 and α6β1. Integrin αIIbβ3 is the most abundant receptor with 50,000–80,000 copies on the platelet surface with an additional pool in the α granules (150, 207). αIIbβ3 is in an inactive state on platelets, and agonist stimulation leads to integrin activation via a conformational change in the structure. This enables the binding of αIIbβ3 to soluble plasma fibrinogen and the sustain platelet–platelet aggregation. Integrin αvβ3 integrin primarily supports adhesiveness to vitronectin (45, 166), while α5β1 and α6β1 have supplementary roles in adhesion to fibronectin and laminin at the injury site (162, 189). Integrins are often in complex with four membrane-spanning receptors called tetraspanins, which include CD9 and CD63, which may support signal transduction and stable integrin adhesion (81, 95).
Receptors that enable platelet interaction with immune cells, the endothelial cells, or participate in immune function
Emerging evidence indicates that platelets also possess receptors that enable stable interaction of platelets with other immune cells or complexes in the circulation as well as adhesion to the vascular endothelium. In some cases, these receptors are only expressed on the surface of activated platelets, such as P-selectin, which can bind PSGL-1 on leukocytes (78, 120). Some receptors are expressed constitutively, such as GPIbα, which can bind with β2 integrin Mac 1 (CD11b/CD18; αMβ2 integrin) on neutrophils (184). Intercellular adhesion molecule-2 (ICAM-2) expressed on platelets (52) can support leukocyte tethering via leukocyte function antigen 1 (LFA1) (212). In other cases as described above for αIIbβ3, receptors can be expressed in an inactive form and upon activation they become active; αIIbβ3 can support adhesion to leukocytes via fibrinogen (212). Platelets may also interact with endothelial cells under inflammatory conditions; for example, GPIbα expressed on platelets can interact with activated endothelial cells that express P-selectin and VWF (170). Activated platelets express a 39 kD membrane glycoprotein of the tumor necrosis factor (TNF) family called CD40L (CD154), which can interact with CD40 on endothelial cells and trigger an inflammatory response (83). Platelets also express a C-type lectin receptor CLEC-2 (197) that interacts with podoplanin on lymphatic endothelial cells and participates in blood-lymphatic separation, via lymphovenous hemostasis during development (16). Platelet CLEC-2 can engage with tumor cells expressing podoplanin, contributing to cancer metastasis (104). Interaction of platelet CLEC-2 with podoplanin expressing inflammatory macrophages can activate platelets (107). Consistent with an immune role for platelets, CLEC-2 on platelets can also capture human immunodeficiency virus type 1 (34). Please see Table 1 and associated text for details of adhesive mechanisms mediating platelet adhesive interactions with immune cells and endothelium.
Table 1.
Selected examples of soluble element-mediated interactions between platelets and leukocytes
| Platelet Element | Leukocyte Element | Effect (References) |
|---|---|---|
| sCD40L | CD40 | Atherosclerosis (28) Inflammatory bowel disease (206) |
| Platelet factor 4 | Chondroitin sulfate (neutrophils) | Activation of neutrophils (159) |
| CXCR3B (microvascular endothelial cells) | Regulating angiogenesis (121, 203) | |
| Fibrinogen | Mac-1 (neutrophils) | Neutrophil adhesion to fibrin deposits (114, 232) |
| TLR4 | Histones (neutrophils) | Platelet activation and thrombosis (68, 117, 223) |
| ? | DNA (neutrophils) | Thrombosis (50, 69) |
| RANTES (CCL5) | CCR5 (monocytes, endothelium) | Monocyte arrest on HMVEC (9) Development of atherosclerosis (77) |
| CCR5 (endothelium, leukocytes) | Cerebral microvascular dysfunction and inflammation in I/R (199) | |
| LIGHT (TNFSF14) | Herpes virus entry mediator (HVEM; neutrophils, monocytes) | Enhances phagocytosis and bactericidal activity (84) |
| HVEM (endothelial cells) Lymphotoxin beta receptor (LTβR; endothelial cells) |
Platelet-endothelial interactions; Levels elevated in STEMI (33) | |
| ? | Myeloperoxidase (MPO; neutrophils) Hydrogen peroxide (H2O2) |
MPO and H2O2 induce platelet secretion of serotonin and adenine (39) |
| LPS-stimulated platelet | ??? (neutrophils) | Release of neutrophil extracellular traps (40) |
| Platelet activating factor | CR1 (monocytes) | Enhances phagocytosis (31) |
Certain receptors on platelets mediate platelet-immunoglobulin (Ig) or platelet-complement interaction and thus participate in modulating immune complexes and/or function. These include FcγRIIA, the low affinity receptor for the IgG Fc domain (171), FcεRI high affinity receptor for IgE (99) and FcαRIA receptor for IgA (167). FcγRIIA engaged on platelets may provide immunological defense against bacteria, while activation of platelets via FcεR1 trigger release of RANTES (regulated on activation, normal T cells expressed and secreted) and serotonin, mediators that promote pro-inflammatory response in other immune cells (80). Crosslinking of platelet FcαRI led to production of prothrombotic mediators like tissue factor and interleukin-1β via pre-mRNA splicing and protein synthesis (167); a topic that will be discussed in the next section. Platelets also possess receptors for complement components including a 33kD receptor to complement protein C1q (C1qR) (156) that can facilitate the classical complement activation (158). Platelet C1qR can also interact with protein A from Staphylococcus aureus enabling direct interaction of platelets with bacteria (147). Decay accelerating factor (DAF or CD55) is another glycosyl phosphatidylinositol (GPI) anchored receptor on platelets (109), which likely protects platelets from complement attack during defense against bacteria. Thus, platelets share some common receptors with primary phagocytes like neutrophils and monocytes and participate in immune functions.
Protein synthesis by platelets
In addition to containing proteins pre-stored in their granules or on their membrane, platelets have the ability to synthesize proteins, despite lacking a nucleus. This concept was first described in the late 1960s (211); however, it was not widely accepted until several decades later (217). Further, the notion that anucleate platelets have the capability to synthesize proteins is rarely addressed in traditional physiology textbooks. Platelets possess messenger RNA (mRNA) and can synthesize proteins upon activation, including proteins involved in inflammatory responses (152, 179, 214). For example, activated platelets can synthesize interleukin-1β, which can promote inflammatory responses in other cell types including leukocytes and endothelial cells (89, 128). For a broader review of protein synthesis by platelets, including a historical perspective, please refer to the following recent review (217).
Other mediators in platelets
Platelets also possess mediators that are not specifically located on the membrane or the three granules. These mediators can also regulate the interaction of platelets with other immune cells and influence inflammatory process. For example, β defensin 1 was reported to be located in an extragranular compartment in platelets, and upon release by activated platelets, it induces the release of neutrophil extracellular traps (NET) from neutrophils and limit bacterial growth (112). High mobility group box 1 (HMGB1) expressed on activated platelets causes neutrophil extracellular trap (NET) formation in neutrophils and commit them to autophagy (173). These features represent additional characteristics in support of the premise that platelets represent immune cells.
Platelets as Immune Cells
As exemplified in this review, there is abundant evidence that platelets have important roles in a broad range of immune responses, in addition to their well-established role in hemostasis and thrombosis. There is a gradually increasing awareness that platelets can function as immune cells (as the most abundant immune cells in blood), although this concept is not uniformly considered in traditional physiology teaching (57). Herein, we review some mechanisms by which platelets participate in immune responses.
Release of chemokines/cytokines
Chemokines are small (8–10 kD) proteins with four cysteine residues in the conserved positions and are known to induce chemotactic response on effector cells by engaging the chemokine receptors. Depending on the spacing of the N-terminal cysteine residues, chemokines are grouped into four categories: CC, CXC, CX3C, and XC. CC chemokines have two adjacent conserved cysteines, while CXC and CX3C have either one or three residues between the two conserved cysteines. The latter, XC, is a variant chemokine with only one N-terminal cysteine and a second cysteine downstream (123). Platelets store chemokines of the CC and CXC class within the α granules, and these mediators are released upon platelet activation (20, 23). CC chemokines such as MIP-1 α, monocyte chemotactic protein-3 and RANTES recruit and activate leukocytes (73, 92, 102, 178, 215, 216). CXC chemokines such as PF4 and β-thromboglobulin can recruit neutrophils and suppress neutrophil apoptosis (24, 79, 177). CXCL-5 can modulate neutrophil chemotaxis (137). Platelets also release other growth factors with immune modulating activities such as PDGF (134) and TGF β (7) and immune mediators such as histamine (134) and serotonin (14). Interleukin-1 β from platelets can promote inflammatory responses in leukocytes and endothelial cells (89, 128). Platelets can activate peripheral B cells and potentiate IgG production probably through sCD40L and RANTES (44). Thus, platelets contribute to the immune function by releasing the mediators that recruit, localize, or activate immune cells and thus modulate inflammatory or immune function chemokines.
Toll-like receptors
Toll-like receptors (TLRs) are transmembrane pattern recognition receptors responsible for a variety of responses against microbial cell wall components and are critically involved in innate immune responses (72, 196). Ten TLRs (i.e., TLR-1, through TLR-10) have been identified in humans; they are expressed on a broad range of cell types, though their sub-cellular localization and function has been best characterized on “traditional” immune cells (36, 72, 196). Platelets have also been shown to express various TLRs with evidence of a functional role; these include TLR-2, TLR-4, TLR-7, and TLR-9 (2, 19, 43, 111). Stimulation of platelet TLR-2 and TLR-9 has been reported to induce platelet aggregation responses to various agonists (19, 105, 154, 181). Similarly, platelet TLR-4 has been shown to participate in a number of responses to bacterial endotoxin, including platelet recruitment to inflamed or injured vascular walls (2, 193) and platelet release of IL-1β-containing-microvesicles (27). Further, platelet TLR-4 was shown to promote release of neutrophil extracellular traps (NETs) capable of trapping bacteria during bacterial infection (40). Recently, platelets were shown to express TLR-7 and to mediate host responses to viral infections without detectable effects on platelet aggregation (111). The evidence of functional toll-like-receptors on platelets provides further support to the notion that platelets are important immune cells.
Release of microvesicles
Another mechanism by which platelets may participate in immune responses is the release of microvesicles, also known as microparticles. They are sub-micrometer particles released not only by platelets, but also endothelial cells, leukocytes, and tumor cells (151). A functional role for platelet microvesicles in hemostasis was proposed by the observation that patients with Scott syndrome, a hemostatic disorder, had defective platelet microvesiculation (185). Microvesicles of platelet origin represent a major fraction of circulating microvesicles (48, 149). Based on proteomic analyses, microvesicles derived from human platelets contain nearly 600 distinct proteins (71), raising the intriguing possibility that microvesicles may recapitulate many of the known functional roles of platelets. Despite technical challenges and variability in methods to quantify microvesicles (29), there is considerable clinical research interest in quantification of circulating microvesicles. Elevated platelet-derived microvesicles have been demonstrated in the circulation of patients with a variety of thrombotic and inflammatory disorders (130, 144, 148, 149, 190). Release of microvesicles is another mechanism by which platelets contribute to immune responses; as mentioned earlier, platelets were shown to release IL-1β-enriched microvesicles following stimulation by bacterial (27). Interleukin 1 enriched microvesicles from platelets activated by collagen amplify inflammation in a model of rheumatoid arthritis (21).
Expression and transfer of microRNA
Since the 1960s, platelets were known to contain mRNA (22); however, limited information was available of the mechanisms for regulation of mRNA and consequently protein synthesis in platelets. Recently, human platelets were shown to contain a broad range of functional microRNA (miRNA, reference (118)). MiRNA are short non-coding RNAs that regulate mRNA translation by a variety of mechanisms reviewed in detail elsewhere (176). Human platelets have been described to contain >200 distinct miRNA despite using stringent criteria for miRNA identification (38, 146, 195). Platelet miRNA may regulate protein translation on platelets (146) and can be secreted or delivered via microvesicles and influence gene expression in endothelial cells (74, 146, 153). Whether platelet miRNA mediates gene expression in other cell types, including other immune cells, remains to be defined.
Direct interactions with microorganisms
Aside from their ability to modulate the immune response, several studies have examined how platelets directly interact with microorganisms, specifically bacteria and fungi. Early studies on the antimicrobial properties of platelets focused on the presence of microbicidal proteins. One of these is β-lysin, a small (6 kDa), cationic, thermostable protein whose main function is to kill gram-positive bacteria, namely Staphylococcus aureus and Bacillus spp (55). Processes that stimulate release of β-lysin include coagulation, inflammation, antigen-antibody complex formation, the generalized Shwartzman reaction, and bacteremia. Once released, β-lysin induces plasma membrane disruption of Gram-positive bacteria and eventual cell lysis. This process is synergistic with lysozyme, complement and antibody, as removal of β-lysin decreases the bacteriocidal effect of serum (54, 62, 87).
Platelet microbicidal proteins are another class of bacteriocidal peptides, similar to β-lysin. They are small, cationic peptides that are released from platelet α granules by thrombin stimulation and have the ability to kill Staphylococcus aureus through the disruption of the plasma membrane (225, 227). Studies in infective endocarditis suggest that platelet microbicidal proteins are also important in fighting viridans Streptococci infections (47), whereas the development of resistance to platelet microbicidal protein by S. aureus may lead to persistent bacteremia (64). Not only are platelet microbicidal proteins antibacterial, they also possess antifungal properties (226, 228). Included within the class of platelet microbicidal proteins are thrombocidin-1 and -2, CXC chemokines stored within platelet α granules. These proteins are related to neutrophil-activating peptide-2 (NAP-2) and connective tissue-activating peptide III (CTAP-III), respectively, and are able to kill both Gram-positive (Bacillus subtilis, S. aureus) and Gram-negative (Escherichia coli) organisms and are effective against some species of Candida (113).
In addition to microbicidal proteins, platelets also have direct cell-cell interactions with microbes. It has been well described that bacteria can induce platelet aggregation, leading to sequestration of the bacteria. Additionally, studies have shown that platelets are also able to engulf bacteria and the human immunodeficiency virus (HIV) (229, 233). However, the consequences of these interactions have been debated. Although sequestration of bacteria by platelets may assist removal of bacteria by the reticuloendothelial system (202), sequestration may also protect bacteria from bacteriocidal agents. This concept has been suggested by the retrieval of viable organisms from platelet-bacterial aggregates in vitro (41). Additionally, there are data suggesting that platelet-bacterial aggregation propagates and protects the development of vegetative lesions in bacterial endocarditis (86, 100, 198). The engulfment of bacteria by platelets appears distinct and less effective than the phagocytic capability of leukocytes (220); the functional consequences of pathogen engulfment by platelets remain to be clearly delineated.
Interactions with complement
Platelets have also been shown to interact with the complement system to enhance innate immunity. One method is through platelet activating factor, which enhances phagocytosis of complement-bound erythrocytes (RBC) by monocytes through complement receptor 1 (31). Another method is through phosphorylation of complement C3 and C3b; activated platelets promote activation of the complement system via release of protein kinases and ATP and phosphorylation of C3 and C3b (60). In addition to phosphorylation of C3b, platelets are also able to bind to C3b via P-selectin; platelet activation induces activation and propagation of the complement system via P-selectin (51). Platelets have also been shown to bind C1q and activate the classical pathway of complement (158). The interactions between platelets and the complement system are complex; platelets can activate complement and vice-versa; the interactions between platelets and the complement system remain to be fully characterized (157).
Interactions between Platelets and Leukocytes
In addition to platelets’ innate ability to interact with microorganisms, complement, and endothelium; platelets also modulate inflammation through their direct interactions with leukocytes in circulation and tissue. In this review, we will discuss the molecules involved in platelet-leukocyte interactions and the prototypical functional consequences.
Mechanisms of platelet-leukocyte interactions
A variety of mechanisms has been described to participate in platelet-leukocyte interactions and can be divided into two broad categories: adhesive and soluble mechanisms. Although we will discuss these mechanisms separately, many of them work in unison to activate platelets and leukocytes. And while a multitude of mechanistic interactions have been identified between platelets and leukocytes (1, 132, 135, 230), this review will emphasize selected interactions that have been most actively studied.
Adhesive mechanisms of platelet-leukocyte interactions
There have been several adhesive molecules described to participate in platelet-leukocyte interactions (Figs. 3 & 4) (85). We will discuss the main adhesive interactions in this section.
Figure 3.
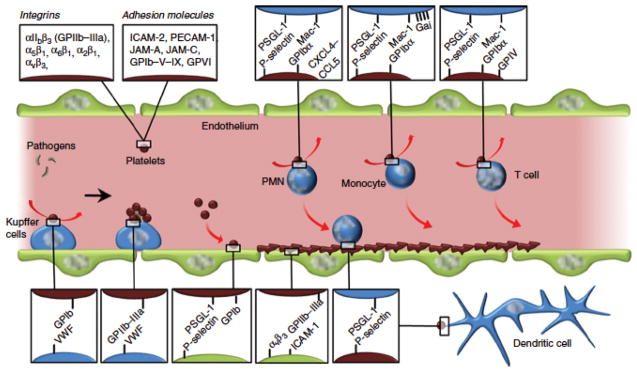
Examples of adhesive interactions between platelets (red) and leukocytes (blue). Platelets contain a number of integrins and cell adhesion molecules on their surface which bind to both leukocytes and endothelial cells (green). Major cell adhesion molecule interactions include (platelet-leukocyte) P-selectin-PSGL-1 and GP1bα-Mac-1. Platelets also adhere to endothelial cells and help capture flowing leukocytes from the circulation. Reprinted from reference (85) with permission from Wiley.
Figure 4.
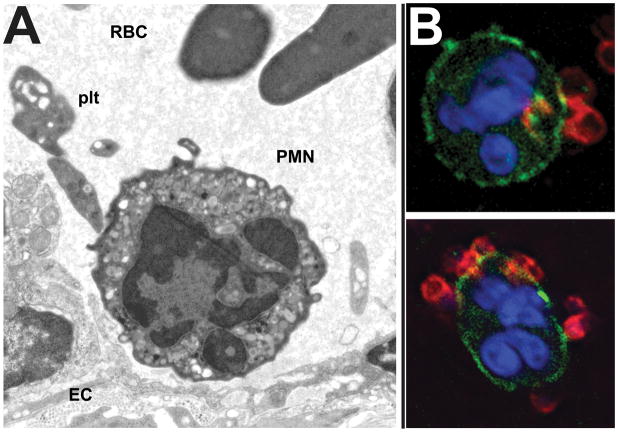
Adhesive interactions between activated platelets and neutrophils. (A) EM image of an inflamed mouse cremaster venule, demonstrating platelet-neutrophil-endothelial cell interaction. Image courtesy of Dr. Alan Burns, University of Houston College of Optometry. (B) Platelet (red)-neutrophil (green/blue) interactions in suspension after platelet activation.
One adhesive mechanism involves the interaction between P-selectin on platelets to P-selectin glycoprotein ligand-1 (PSGL-1) on leukocytes. P-selectin (also known as granule membrane protein-140 (GMP-140), PADGEM protein, and CD62) is a membrane glycoprotein found in secretory granules of platelets (α-granules) (15, 136) and endothelial cells (Weibel-Palade bodies) (82) that is upregulated on the platelet surface after activation. The counter-ligand for P-selectin is PSGL-1, a homodimeric mucin composed of two ~120 kDa subunits (140) and expressed on microvilli on most leukocytes (122, 139). PSGL-1 is important for mediating neutrophil rolling on platelets and endothelial cells in the microcirculation (30, 139). When bound to P-selectin, PSGL-1 induces a signaling pathway to activate leukocytes by inducing a conformational change of the β2 integrin, Mac-1, to an active state (133, 209) as well as clustering of Mac-1 on the leukocyte surface (224). In addition to their effect on leukocyte capture and rolling, P-selectin-PSGL-1 interactions between platelets and neutrophils promote neutrophil transendothelial migration (116) independent of effects on neutrophil adhesion to endothelial cells.
Another adhesive mechanism involves the interaction between platelet glycoprotein Ibα (GPIbα; CD42b) and Mac-1 (CD11b/CD18; αMβ2 integrin). GPIbα is the α-subunit of the heterodimeric transmembrane protein GPIb located on platelets (65). It is part of the GPIb-IX-V complex and binds to von Willebrand factor for thrombus formation (138). In addition to its role in thrombosis, GPIbα has recently been described to bind to the β2 integrin, Mac-1, on leukocytes to promote microvascular inflammation and thrombosis (210). Mac-1 is a heterodimer that consists of an alpha (CD11b; αM) and beta subunit (CD18; β2). It is found on moderate levels on the surface of leukocytes with preformed stores expressed on the surface after stimulation (98). Mac-1 serves as both a complement receptor to iC3b (11) as well as for firm adhesion and transmigration by binding to ICAM-1 on endothelial cells (188). In addition to GPIbα, platelets also bind to Mac-1 (as well as LFA-1) via ICAM-2 (52, 222). ICAM-2 is found on the membrane surface of resting platelets and does not change after activation. Binding of platelets to ICAM-2 may assist in leukocyte transendothelial migration (96).
Adhesive interactions between platelets and leukocytes may also be mediated by CD40L (CD154; gp39) on platelets and CD40 on leukocytes. CD40L was first described on the surface of activated T cells (5), but has also been described on platelets (83). CD40L is a member of the tumor necrosis factor (TNF) family and is the ligand for the TNR receptor, CD40. CD40 is found on a variety of cells, including B cells and endothelial cells, and the interaction between CD40 and CD40L is important in the regulation of the adaptive immunity (reviewed in (66)). CD40 has also been found on neutrophils, possibly contributing to the pro-inflammatory effects of platelets (204). However, recent data have demonstrated its importance in the formation of atherosclerosis (28) as well as of acute inflammatory conditions, such as inflammatory bowel disease (206). In addition to its surface-bound state, CD40L is also cleaved by metalloproteinases and can be found in solution as sCD40L and able to exert its effects (108).
Depending on the molecules involved, the attachment of platelets onto leukocytes may alter the behavior of the latter; the functional consequences of these interactions are summarized in Figure 5. As described previously, P-selectin-PSGL-1 interactions lead to activation and clustering of Mac-1 on leukocytes. Neutrophils may actively scan the vasculature for activated platelets in order to promote inflammation via clustering of PSGL-1. Ex vivo observations in the late 1990s showed that activated neutrophils demonstrated clustering of P-selectin ligand at the uropods, resulting in polarized neutrophils with platelets bound to the uropod. (56). Similar findings were recently described in vivo, with evidence that neutrophil polarization in activated venules induced organization of a protruding domain that bound platelets and resulted in a PSGL-1-mediated signal transduction that drove neutrophil migration; neutrophils from platelet-depleted mice were less motile than those from untreated mice (191). Similar to observations by others, survival in acute lung injury in mice is improved after platelet depletion, suggesting their importance in propagating the proinflammatory response after injury (191, 231).
Figure 5.
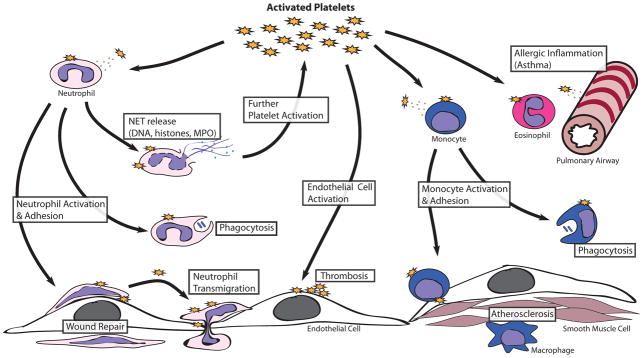
Selected examples of pro-inflammatory effects of platelets resulting in functional consequences on leukocytes. Platelets interact with leukocytes through both adhesive mechanisms as well as release of cytokines/chemokines. This results in leukocyte activation and enhanced leukocyte-endothelial adhesion. Through these mechanisms, platelets participate in several normal and pathologic immune functions including microbial killing, leukocyte homing, wound healing, allergic inflammation, and atherosclerosis, among others.
Soluble mechanisms of platelet-leukocyte interactions
In addition to the adhesive elements described above, platelets can release soluble factors that activate, modulate, or assist with capture of circulating leukocytes. A large number of the hundreds of biologically active mediators contained in platelet granules (see Table 1) are capable of inducing these effects. In this section, we will highlight a few selected examples:
Platelet factor 4 (PF4; CXCL4) is a protein belonging to the CXC-subfamily of chemokines and is released from α-granules after platelet activation. PF4 activates neutrophils by binding to the proteoglycan chondroitin sulfate on the surface of neutrophils as tetrameric PF4 (159). In isolated neutrophils, the addition of tumor necrosis factor-α (TNF-α) appears to be necessary for PF4-induced activation (160), however, TNF-α co-stimulation is not required in the presence of endothelial cells (159). This leads to enhanced neutrophil adherence to endothelial cells and secondary granule exocytosis (measured by lactoferrin release) without an increase in intracellular calcium levels or chemotaxis. A second receptor for PF4, CXCR3B, has been described, although it has been primarily described in microvascular endothelial cells and important for regulating angiogenesis (121, 203).
Fibrinogen, released from platelets and endothelial cells, is traditionally thought of as a hemostatic element. However, neutrophils are also able to adhere to bind fibrinogen via the CD11b/CD18 integrin (114), and neutrophils binding to fibrinogen is regulated in a shear stress-dependent manner (232). These studies suggest that fibrinogen-CD18 interactions may be a way for leukocytes to adhere to both platelets and endothelium at sites of vascular injury.
Neutrophil extracellular traps (NETs) have recently been described as important effectors of innate immunity. NETs are primarily composed of both nuclear (histones and DNA) and granular products (e.g. neutrophil elastase, myeloperoxidase, matrix metalloproteinases) and are released predominantly by neutrophils, although other leukocytes are able to release them (26). Neutrophils release NETs through an NADPH oxidase-dependent mechanism, as neutrophils from patients with deficiencies in this enzyme (chronic granulomatous disease) are unable to form NETs (67). In addition to the oxidative burst, decondensation of chromatin by peptidylarginine deiminase-4 (PAD4) is required for proper NET formation (124). Once released, NETs play an important role in the capture and clearance of bacteria and fungi (155, 161, 201), but may also promote thrombosis and coagulation. Histones, as part of NETs, may be particularly thrombogenic as they have been shown to promote platelet aggregation and thrombosis (68, 117); the toll-like receptors TLR2 and TLR4 appear to mediate these effects of histones on platelets (181, 223). In addition to histones, DNA also appears to be prothrombotic (69) whereas degradation of DNA by recombinant DNAse I is protective against stroke in mice (50).
Functional consequences of platelet-leukocyte interactions
Interactions between platelets and leukocytes have been shown to contribute to a broad range of physiologic and pathologic conditions. In this review, we will highlight some prototypical examples of these interactions, resulting in both beneficial and detrimental responses.
Acute lung injury
Acute lung injury (ALI) is a potentially fatal condition in which the body is unable to oxygenate or ventilate appropriately to support the metabolic demands of the body. ALI is a consequence of a variety of different diseases, including infection, chemical pneumonitis, and trauma (110, 174). On histology, it is characterized by leukocytic infiltrates into the lung parenchyma and airspaces with edema formation. Animal studies have demonstrated that platelets contribute to the development of ALI, since depletion of platelets resulted in improved organ function and reduced leukocyte infiltration in experimental ALI (6, 231); P-selectin (231) and sCD40L (168) have been implicated in these responses. Further, platelets and their released products have been associated with the development of (blood) transfusion-related ALI (TRALI) via sCD40L (108). These effects may be through platelet-induced NET formation, as suggested by an improvement in lung injury and NET formation after the use of GPIIb/IIIa inhibitors or aspirin in experimental models of TRALI (32).
Infection
As described above, interaction of platelets with bacteria can induce neutrophils to release neutrophil extracellular traps (NET). The formation of NETs appears to be another mechanism by which the innate immunity combats infections. Recent data demonstrate that platelets, via toll-like receptor 4, promote trapping of Escherichia coli by neutrophils in the liver sinusoids (40). This suggests a role of platelets as an integral component of the innate immunity.
Sterile inflammation
Platelets and leukocytes are often recruited to sites of inflammation, particularly in the microcirculation, with close spatial and temporal correlation. Recruitment of platelets and leukocytes to sites of inflammation can be inter-dependent. For example, in a model of corneal abrasion, depletion of platelets reduced leukocyte recruitment while depletion of leukocytes reduced platelet recruitment (126). Efficient recruitment of both platelets and leukocytes was necessary for effective wound healing (126). These findings exemplify a closely coordinated effort by leukocytes and platelets in response to sterile inflammation. Similarly, platelet-dependent leukocyte recruitment was shown in experimental autoimmune encephalomyelitis, a model of a demyelinating disease; recruitment of platelets and leukocytes mediate inflammation in that model (119). Platelet-leukocyte interdependence has been described in a variety of other models; these interactions have been reviewed elsewhere (175, 194).
Allergic inflammation
Platelets have been studied in the context of allergic asthma; platelets contain a number of mediators that can lead to bronchial smooth muscle hyperreactivity and mucus production, such as platelet activating factor (35) and histamine (13). Further, there are some reports that platelet factor 4 and β-thromboglobulin are increased in patients with asthma, particularly those with most airway hyperreactivity (97, 143, 200), though these data have been questioned (103, 131). In mouse models of allergic lung inflammation, platelet depletion reduced measures of lung inflammation, including eosinophil and lymphocyte recruitment (164) as well as epithelial and smooth muscle thickening (163). However, platelet depletion did not affect the degree of airway hyperresponsiveness (163). These data suggest that platelets may contribute to allergic inflammation, though much remains to be clarified about these processes.
Atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the blood vessels in which platelet-leukocyte interactions play a central role, in addition to the role of platelets in thrombosis associated with atherosclerosis (reviewed in (49, 172)). The interaction between activated platelets and both circulating leukocytes as well as the vascular endothelium propagates vascular inflammation, leading to plaque formation. Several platelet-dependent mechanisms have been suggested to mediate these responses. One of the key molecules in this interaction is platelet CD40L (cell-bound and soluble). Platelet CD40L binds to endothelial CD40, inducing surface expression of a number of key adhesion molecules (E-selectin, VCAM-1, and ICAM-1) as well as cytokine release (IL-8, MCP-1). This activation of the endothelium then attracts circulating neutrophils, T-cells, and monocytes into adhering to the inflamed endothelium, leading to vascular inflammation and plaque formation (83, 127). Platelet P-selectin also plays an important role in the pathogenesis of atherosclerosis by binding to monocyte PSGL-1 to help capture monocytes, form platelet-monocyte aggregates, and to induce inflammation via a cyclooxygenase-2 (COX-2) pathway (53, 63). A variety of platelet-derived chemokines have also been suggested to participate in atherogenic responses (reviewed in (91)). These represent important pathways through which platelets promote the inflammation seen in the development of atherosclerosis; a greater understanding of these mechanisms may impact prevention and management of this common clinical entity.
Inflammatory bowel disease
The inflammatory bowel diseases (IBD; ulcerative colitis and Crohn’s disease) are a set of chronic inflammatory disorders that encompasses complex interactions between genetic, microbiotic, and environmental factors; increasing evidence supports the notion that platelets play an important role in the inflammatory responses associated with IBD. Increased blood platelet counts in humans with IBD was shown in the 1960s (142). Subsequently, a variety of qualitative changes in platelets have been described in IBD (205), including elevated levels of platelet CD40L and a higher number of CD40L-positive platelets in inflamed intestinal mucosa, as compared to control subjects (46). In a mouse model of colonic inflammation, blockade of the CD40-CD40L pathway or deficiency of either CD40 or CD40L led to decreased inflammation and damage of the colonic mucosa in experimental colitis. Furthermore, absence of CD40 or CD40L resulted in less platelet and leukocyte adhesion to inflamed colonic venules (206). A number of platelet-derived mediators have been suggested as possible mediators of inflammatory responses in IBD (reviewed in (205)); however, the mechanisms involved remain to be clearly delineated.
Conclusion
In addition to their role in hemostasis and thrombosis, platelets are key mediators of inflammation and warrant to be recognized as immune cells. They can interact with pathogens, regulate the function of other immune cells, and mediate a broad range of physiologic processes. Interactions between platelets and other immune cells contribute to a broad range of important human diseases and may provide targets for future therapies in these conditions. Greater awareness of the role of platelets as key components of the immune system is warranted.
Acknowledgments
Supported in part by NHLBI HL116524, HL081613, NIGMS GM112806, and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
References
- 1.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- 2.Andonegui G, Kerfoot SM, McNagny K, Ebbert KVJ, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 3.Andrews RK, Gardiner EE, Shen Y, Whisstock JC, Berndt MC. Glycoprotein Ib-IX-V. Int J Biochem Cell Biol. 2003;35:1170–1174. doi: 10.1016/s1357-2725(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 4.Arai M, Yamamoto N, Moroi M, Akamatsu N, Fukutake K, Tanoue K. Platelets with 10% of the normal amount of glycoprotein VI have an impaired response to collagen that results in a mild bleeding tendency. Br J Haematol. 1995;89:124–130. doi: 10.1111/j.1365-2141.1995.tb08900.x. [DOI] [PubMed] [Google Scholar]
- 5.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 6.Asaduzzaman M, Lavasani S, Rahman M, Zhang S, Braun OÖ, Jeppsson B, Thorlacius H. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Critical Care Medicine. 2009;37:1389–1396. doi: 10.1097/CCM.0b013e31819ceb71. [DOI] [PubMed] [Google Scholar]
- 7.Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102:1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayyanathan K, Webbs TE, Sandhu AK, Athwal RS, Barnard EA, Kunapuli SP. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem Biophys Res Commun. 1996;218:783–788. doi: 10.1006/bbrc.1996.0139. [DOI] [PubMed] [Google Scholar]
- 9.Baltus T, Hundelshausen von P, Mause SF, Buhre W, Rossaint R, Weber C. Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. Journal of Leukocyte Biology. 2005;78:435–441. doi: 10.1189/jlb.0305141. [DOI] [PubMed] [Google Scholar]
- 10.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. Journal of Experimental Medicine. 1982;156:1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentfeld-Barker ME, Bainton DF. Identification of primary lysosomes in human megakaryocytes and platelets. Blood. 1982;59:472–481. [PubMed] [Google Scholar]
- 13.Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. Journal of Experimental Medicine. 1972;136:1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986;78:130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen C-Y, Xu B, Lu M-M, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizzozero G. Sur un nouvel élément morphologique du sang chez les mammifères et sur son importance dans la thrombose et dans la coagulation. Archives Italiennes de Biologie. 1882;1:1–4. [Google Scholar]
- 18.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circulation Research. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–417. doi: 10.1046/j.1365-3148.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 21.Boilard E, Nigrovic PA, Larabee K, Watts GFM, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booyse FM, Rafelson ME. Stable messenger RNA in the synthesis of contractile protein in human platelets. Biochim Biophys Acta. 1967;145:188–190. doi: 10.1016/0005-2787(67)90673-9. [DOI] [PubMed] [Google Scholar]
- 23.Brandt E, Ludwig A, Petersen F, Flad HD. Platelet-derived CXC chemokines: old players in new games. Immunol Rev. 2000;177:204–216. doi: 10.1034/j.1600-065x.2000.17705.x. [DOI] [PubMed] [Google Scholar]
- 24.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. Journal of Leukocyte Biology. 2000;67:471–478. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- 25.Brass LF, Tomaiuolo M, Stalker TJ. Harnessing the platelet signaling network to produce an optimal hemostatic response. Hematol Oncol Clin North Am. 2013;27:381–409. doi: 10.1016/j.hoc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 27.Brown GT, McIntyre TM. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. The Journal of Immunology. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruemmer D, Riggers U, Holzmeister J, Grill M, Lippek F, Settmacher U, Regitz-Zagrosek V, Fleck E, Graf K. Expression of CD40 in vascular smooth muscle cells and macrophages is associated with early development of human atherosclerotic lesions. Am J Cardiol. 2001;87:21–27. doi: 10.1016/s0002-9149(00)01266-2. [DOI] [PubMed] [Google Scholar]
- 29.Burnouf T, Goubran HA, Chou M-L, Devos D, Radosevic M. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014;28:155–166. doi: 10.1016/j.blre.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. Journal of Leukocyte Biology. 1999;65:299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- 31.Bussolino F, Fischer E, Turrini F, Kazatchkine MD, Arese P. Platelet-activating factor enhances complement-dependent phagocytosis of diamide-treated erythrocytes by human monocytes through activation of protein kinase C and phosphorylation of complement receptor type one (CR1) J Biol Chem. 1989;264:21711–21719. [PubMed] [Google Scholar]
- 32.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celik S, Langer H, Stellos K, May AE, Shankar V, Kurz K, Katus HA, Gawaz MP, Dengler TJ. Platelet-associated LIGHT (TNFSF14) mediates adhesion of platelets to human vascular endothelium. Thromb Haemost. 2007;98:798–805. [PubMed] [Google Scholar]
- 34.Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A, Suzuki-Inoue K, Fuller GL, Pearce AC, Watson SP, Hoxie JA, Baribaud F, Pöhlmann S. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan-Yeung M, Lam S, Chan H, Tse KS, Salari H. The release of platelet-activating factor into plasma during allergen-induced bronchoconstriction. J Allergy Clin Immunol. 1991;87:667–673. doi: 10.1016/0091-6749(91)90386-3. [DOI] [PubMed] [Google Scholar]
- 36.Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791–808. doi: 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- 37.Chesney CM, Harper E, Colman RW. Human platelet collagenase. J Clin Invest. 1974;53:1647–1654. doi: 10.1172/JCI107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clancy L, Freedman JE. New paradigms in thrombosis: novel mediators and biomarkers platelet RNA transfer. J Thromb Thrombolysis. 2014;37:12–16. doi: 10.1007/s11239-013-1001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark RA, Klebanoff SJ. Neutrophil-platelet interaction mediated by myeloperoxidase and hydrogen peroxide. J Immunol. 1980;124:399–405. [PubMed] [Google Scholar]
- 40.Clark SR, Ma AC, Tavener SA, Mcdonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, Mcavoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FHY, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 41.Clawson CC, White JG. Platelet interaction with bacteria. II. Fate of the bacteria. The American Journal of Pathology. 1971;65:381–397. [PMC free article] [PubMed] [Google Scholar]
- 42.CLAY CB, JACKSON G. Anaphylactoid reaction with death following the injection treatment of varicose veins with sodium morrhuate. J Med Assoc Ga. 1955;44:25. [PubMed] [Google Scholar]
- 43.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 44.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogné M, Richard Y, Garraud O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35:1376–1387. doi: 10.1016/j.exphem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Coller BS, Seligsohn U, West SM, Scudder LE, Norton KJ. Platelet fibrinogen and vitronectin in Glanzmann thrombasthenia: evidence consistent with specific roles for glycoprotein IIb/IIIA and alpha v beta 3 integrins in platelet protein trafficking. Blood. 1991;78:2603–2610. [PubMed] [Google Scholar]
- 46.Danese S, la Motte de C, Sturm A, Vogel JD, West GA, Strong SA, Katz JA, Fiocchi C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 47.Dankert J, Krijgsveld J, van der Werff J, Joldersma W, Zaat SA. Platelet microbicidal activity is an important defense factor against viridans streptococcal endocarditis. J Infect Dis. 2001;184:597–605. doi: 10.1086/322802. [DOI] [PubMed] [Google Scholar]
- 48.Dasgupta SK, Abdel-Monem H, Niravath P, Le A, Bellera RV, Langlois K, Nagata S, Rumbaut RE, Thiagarajan P. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 50.De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD. Extracellular Chromatin Is an Important Mediator of Ischemic Stroke in Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012 May 24; doi: 10.1161/ATVBAHA.112.250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Conde I, Cruz MA, Zhang H, López JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. Journal of Experimental Medicine. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon DA, Tolley ND, Bemis-Standoli K, Martinez ML, Weyrich AS, Morrow JD, Prescott SM, Zimmerman GA. Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Invest. 2006;116:2727–2738. doi: 10.1172/JCI27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donaldson DM, Roberts RR, Larsen HS, Tew JG. Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect Immun. 1974;10:657–666. doi: 10.1128/iai.10.3.657-666.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donaldson DM, Tew JG. beta-Lysin of platelet origin. Bacteriol Rev. 1977;41:501–513. doi: 10.1128/br.41.2.501-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dore M, Burns AR, Hughes BJ, Entman ML, Smith CW. Chemoattractant-induced changes in surface expression and redistribution of a functional ligand for P-selectin on neutrophils. Blood. 1996;87:2029–2037. [PubMed] [Google Scholar]
- 57.Duerschmied D, Bode C, Ahrens I. Immune functions of platelets. Thromb Haemost. 2014;112:678–691. doi: 10.1160/TH14-02-0146. [DOI] [PubMed] [Google Scholar]
- 58.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C, Wagner DD. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121:1008–1015. doi: 10.1182/blood-2012-06-437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrlicher A, Hartwig JH. Cell mechanics: Contracting to stiffness. Nat Mater. 2011;10:12–13. doi: 10.1038/nmat2928. [DOI] [PubMed] [Google Scholar]
- 60.Ekdahl KN, Nilsson B. Phosphorylation of complement component C3 and C3 fragments by a human platelet protein kinase. Inhibition of factor I-mediated cleavage of C3b. J Immunol. 1995;154:6502–6510. [PubMed] [Google Scholar]
- 61.Fava RA, Casey TT, Wilcox J, Pelton RW, Moses HL, Nanney LB. Synthesis of transforming growth factor-beta 1 by megakaryocytes and its localization to megakaryocyte and platelet alpha-granules. Blood. 1990;76:1946–1955. [PubMed] [Google Scholar]
- 62.Feingold DS, Goldman JN, Kuritz HM. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968;96:2118–2126. doi: 10.1128/jb.96.6.2118-2126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandes LS, Conde ID, Wayne Smith C, Kansas GS, Snapp KR, Bennet N, Ballantyne C, McIntire LV, O’Brian Smith E, Klem JA, Mathew S, Frangogiannis N, Turner NA, Maresh KJ, Kleiman NS. Platelet-monocyte complex formation: effect of blocking PSGL-1 alone, and in combination with alphaIIbbeta3 and alphaMbeta2, in coronary stenting. Thromb Res. 2003;111:171–177. doi: 10.1016/j.thromres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Fowler VG, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190:1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 65.Fox JE, Aggerbeck LP, Berndt MC. Structure of the glycoprotein Ib.IX complex from platelet membranes. J Biol Chem. 1988;263:4882–4890. [PubMed] [Google Scholar]
- 66.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 67.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukami MH. Isolation of dense granules from human platelets. Meth Enzymol. 1992;215:36–42. doi: 10.1016/0076-6879(92)15051-d. [DOI] [PubMed] [Google Scholar]
- 71.Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–1521. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- 72.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14:546–558. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- 73.Gear ARL, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 74.Gidlöf O, van der Brug M, Ohman J, Gilje P, Olde B, Wahlestedt C, Erlinge D. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood. 2013;121:3908–17. S1–26. doi: 10.1182/blood-2012-10-461798. [DOI] [PubMed] [Google Scholar]
- 75.Gu M, Xi X, Englund GD, Berndt MC, Du X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3) using a reconstituted mammalian cell expression model. J Cell Biol. 1999;147:1085–1096. doi: 10.1083/jcb.147.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Habib A, FitzGerald GA, Maclouf J. Phosphorylation of the thromboxane receptor alpha, the predominant isoform expressed in human platelets. J Biol Chem. 1999;274:2645–2651. doi: 10.1074/jbc.274.5.2645. [DOI] [PubMed] [Google Scholar]
- 77.Halvorsen B, Smedbakken LM, Michelsen AE, Skjelland M, Bjerkeli V, Sagen EL, Taskén K, Bendz B, Gullestad L, Holm S, Biessen EA, Aukrust P. Activated platelets promote increased monocyte expression of CXCR5 through prostaglandin E2-related mechanisms and enhance the anti-inflammatory effects of CXCL13. Atherosclerosis. 2014;234:352–359. doi: 10.1016/j.atherosclerosis.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Hamburger SA, McEver RP. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990;75:550–554. [PubMed] [Google Scholar]
- 79.Hartwig H, Drechsler M, Lievens D, Kramp B, Hundelshausen von P, Lutgens E, Weber C, Döring Y, Soehnlein O. Platelet-derived PF4 reduces neutrophil apoptosis following arterial occlusion. Thromb Haemost. 2014;111:562–564. doi: 10.1160/TH13-08-0699. [DOI] [PubMed] [Google Scholar]
- 80.Hasegawa S, Pawankar R, Suzuki K, Nakahata T, Furukawa S, Okumura K, Ra C. Functional expression of the high affinity receptor for IgE (FcepsilonRI) in human platelets and its’ intracellular expression in human megakaryocytes. Blood. 1999;93:2543–2551. [PubMed] [Google Scholar]
- 81.Hastings JW, Sailer M, Johnson K, Roy KL, Vederas JC, Stiles ME. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991;173:7491–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 83.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 84.Heo S-K, Ju S-A, Lee S-C, Park S-M, Choe S-Y, Kwon B, Kwon BS, Kim B-S. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. Journal of Leukocyte Biology. 2006;79:330–338. doi: 10.1189/jlb.1104694. [DOI] [PubMed] [Google Scholar]
- 85.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12:1764–1775. doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 86.Herzberg MC, Brintzenhofe KL, Clawson CC. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983;39:1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.HIRSCH JG. Comparative bactericidal activities of blood serum and plasma serum. Journal of Experimental Medicine. 1960;112:15–22. doi: 10.1084/jem.112.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holmsen H, Weiss HJ. Secretable storage pools in platelets. Annu Rev Med. 1979;30:119–134. doi: 10.1146/annurev.me.30.020179.001003. [DOI] [PubMed] [Google Scholar]
- 89.Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122:3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circulation Research. 2007;100:590–597. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 91.Hundelshausen von P, Schmitt MMN. Platelets and their chemokines in atherosclerosis-clinical applications. Front Physiol. 2014;5:294. doi: 10.3389/fphys.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hundelshausen von P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 93.Isenberg WM, McEver RP, Shuman MA, Bainton DF. Topographic distribution of a granule membrane protein (GMP-140) that is expressed on the platelet surface after activation: an immunogold-surface replica study. Blood Cells. 1986;12:191–204. [PubMed] [Google Scholar]
- 94.Ishihara H, Zeng D, Connolly AJ, Tam C, Coughlin SR. Antibodies to protease-activated receptor 3 inhibit activation of mouse platelets by thrombin. Blood. 1998;91:4152–4157. [PubMed] [Google Scholar]
- 95.Israels SJ, McMillan-Ward EM. CD63 modulates spreading and tyrosine phosphorylation of platelets on immobilized fibrinogen. Thromb Haemost. 2005;93:311–318. doi: 10.1160/TH04-08-0503. [DOI] [PubMed] [Google Scholar]
- 96.Issekutz AC, Rowter D, Springer TA. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. Journal of Leukocyte Biology. 1999;65:117–126. doi: 10.1002/jlb.65.1.117. [DOI] [PubMed] [Google Scholar]
- 97.Johnson CE, Belfield PW, Davis S, Cooke NJ, Spencer A, Davies JA. Platelet activation during exercise induced asthma: effect of prophylaxis with cromoglycate and salbutamol. Thorax. 1986;41:290–294. doi: 10.1136/thx.41.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones DH, Anderson DC, Burr BL, Rudloff HE, Smith CW, Krater SS, Schmalstieg FC. Quantitation of intracellular Mac-1 (CD11b/CD18) pools in human neutrophils. Journal of Leukocyte Biology. 1988;44:535–544. doi: 10.1002/jlb.44.6.535. [DOI] [PubMed] [Google Scholar]
- 99.Joseph M, Auriault C, Capron A, Vorng H, Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983;303:810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- 100.Jung C-J, Yeh C-Y, Shun C-T, Hsu R-B, Cheng H-W, Lin C-S, Chia J-S. Platelets Enhance Biofilm Formation and Resistance of Endocarditis-Inducing Streptococci on the Injured Heart Valve. J Infect Dis. 2012;205:1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 101.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kameyoshi Y, Schröder JM, Christophers E, Yamamoto S. Identification of the cytokine RANTES released from platelets as an eosinophil chemotactic factor. Int Arch Allergy Immunol. 1994;104 (Suppl 1):49–51. doi: 10.1159/000236751. [DOI] [PubMed] [Google Scholar]
- 103.Kasperska-Zajac A, Rogala B. Markers of platelet activation in plasma of patients suffering from persistent allergic rhinitis with or without asthma symptoms. Clin Exp Allergy. 2005;35:1462–1465. doi: 10.1111/j.1365-2222.2005.02357.x. [DOI] [PubMed] [Google Scholar]
- 104.Kato Y, Kaneko MK, Kunita A, Ito H, Kameyama A, Ogasawara S, Matsuura N, Hasegawa Y, Suzuki-Inoue K, Inoue O, Ozaki Y, Narimatsu H. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99:54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keane C, Tilley D, Cunningham A, Smolenski A, Kadioglu A, Cox D, Jenkinson HF, Kerrigan SW. Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. J Thromb Haemost. 2010;8:2757–2765. doi: 10.1111/j.1538-7836.2010.04093.x. [DOI] [PubMed] [Google Scholar]
- 106.Kenney DM, Linck RW. The cystoskeleton of unstimulated blood platelets: structure and composition of the isolated marginal microtubular band. Journal of Cell Science. 1985;78:1–22. doi: 10.1242/jcs.78.1.1. [DOI] [PubMed] [Google Scholar]
- 107.Kerrigan AM, Navarro-Nuñez L, Pyz E, Finney BA, Willment JA, Watson SP, Brown GD. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. J Thromb Haemost. 2012;10:484–486. doi: 10.1111/j.1538-7836.2011.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. Journal of Experimental Medicine. 1985;162:75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995;332:27–37. doi: 10.1056/NEJM199501053320106. [DOI] [PubMed] [Google Scholar]
- 111.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WHA, Lindemann S, Seizer P, Yost CC, Zimmerman GA, Weyrich AS. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krijgsveld J, Zaat SA, Meeldijk J, van Veelen PA, Fang G, Poolman B, Brandt E, Ehlert JE, Kuijpers AJ, Engbers GH, Feijen J, Dankert J. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem. 2000;275:20374–20381. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 114.Kuijper PH, Gallardo Torres HI, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood. 1997;89:166–175. [PubMed] [Google Scholar]
- 115.Kwakman PHS, Krijgsveld J, de Boer L, Nguyen LT, Boszhard L, Vreede J, Dekker HL, Speijer D, Drijfhout JW, Velde te AA, Crielaard W, Vogel HJ, Vandenbroucke-Grauls CMJE, Zaat SAJ. Native thrombocidin-1 and unfolded thrombocidin-1 exert antimicrobial activity via distinct structural elements. Journal of Biological Chemistry. 2011;286:43506–43514. doi: 10.1074/jbc.M111.248641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lam FW, Burns AR, Smith CW, Rumbaut RE. Platelets enhance neutrophil transendothelial migration via P-selectin glycoprotein ligand-1. AJP: Heart and Circulatory Physiology. 2011;300:H468–75. doi: 10.1152/ajpheart.00491.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lam FW, Cruz MA, Leung H-CE, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132:69–76. doi: 10.1016/j.thromres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 118.Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16:961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Langer HF, Choi EY, Zhou H, Schleicher R, Chung K-J, Tang Z, Göbel K, Bdeir K, Chatzigeorgiou A, Wong C, Bhatia S, Kruhlak MJ, Rose JW, Burns JB, Hill KE, Qu H, Zhang Y, Lehrmann E, Becker KG, Wang Y, Simon DI, Nieswandt B, Lambris JD, Li X, Meuth SG, Kubes P, Chavakis T. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circulation Research. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 121.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. Journal of Experimental Medicine. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. 1996;88:3010–3021. [PubMed] [Google Scholar]
- 123.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 124.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. Journal of Experimental Medicine. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Z, Rumbaut RE, Burns AR, Smith CW. Platelet response to corneal abrasion is necessary for acute inflammation and efficient re-epithelialization. Investigative Ophthalmology & Visual Science. 2006;47:4794–4802. doi: 10.1167/iovs.06-0381. [DOI] [PubMed] [Google Scholar]
- 127.Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix ICA, Wijnands E, Goossens P, van Kruchten R, Thevissen L, Boon L, Flavell RA, Noelle RJ, Gerdes N, Biessen EA, Daemen MJAP, Heemskerk JWM, Weber C, Lutgens E. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Linder BL, Chernoff A, Kaplan KL, Goodman DS. Release of platelet-derived growth factor from human platelets by arachidonic acid. Proc Natl Acad Sci USA. 1979;76:4107–4111. doi: 10.1073/pnas.76.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lukasik M, Rozalski M, Luzak B, Michalak M, Ambrosius W, Watala C, Kozubski W. Enhanced platelet-derived microparticle formation is associated with carotid atherosclerosis in convalescent stroke patients. Platelets. 2013;24:63–70. doi: 10.3109/09537104.2011.654292. [DOI] [PubMed] [Google Scholar]
- 131.Luzzatto G, Cella G, Boschetto P, Fabbri L. Lack of increase or reduction in circulating platelet factor 4 and heparin-releasable platelet factor 4 in symptomatic patients with asthma. J Lipid Mediat. 1993;7:151–154. [PubMed] [Google Scholar]
- 132.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 133.Ma Y-Q, Plow EF, Geng J-G. P-selectin binding to P-selectin glycoprotein ligand-1 induces an intermediate state of alphaMbeta2 activation and acts cooperatively with extracellular stimuli to support maximal adhesion of human neutrophils. Blood. 2004;104:2549–2556. doi: 10.1182/blood-2004-03-1108. [DOI] [PubMed] [Google Scholar]
- 134.Mannaioni PF, Di Bello MG, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res. 1997;46:4–18. doi: 10.1007/PL00000158. [DOI] [PubMed] [Google Scholar]
- 135.May AE, Langer H, Seizer P, Bigalke B, Lindemann S, Gawaz M. Platelet-leukocyte interactions in inflammation and atherothrombosis. Semin Thromb Hemost. 2007;33:123–127. doi: 10.1055/s-2007-969023. [DOI] [PubMed] [Google Scholar]
- 136.McEver RP, Martin MN. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984;259:9799–9804. [PubMed] [Google Scholar]
- 137.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity. 2010;33:106–117. doi: 10.1016/j.immuni.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Michelson AD, Loscalzo J, Melnick B, Coller BS, Handin RI. Partial characterization of a binding site for von Willebrand factor on glycocalicin. Blood. 1986;67:19–26. [PubMed] [Google Scholar]
- 139.Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest. 1989;84:1440–1445. doi: 10.1172/JCI114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morowitz DA, Allen LW, Kirsner JB. Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med. 1968;68:1013–1021. doi: 10.7326/0003-4819-68-5-1013. [DOI] [PubMed] [Google Scholar]
- 143.Morrison JF, Pearson SB, Dean HG, Craig IR, Bramley PN. Platelet activation in nocturnal asthma. Thorax. 1991;46:197–200. doi: 10.1136/thx.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R. Circulating microparticles from patients with septic shock exert protective role in vascular function. American Journal of Respiratory and Critical Care Medicine. 2008;178:1148–1155. doi: 10.1164/rccm.200712-1835OC. [DOI] [PubMed] [Google Scholar]
- 145.Murugappan S, Shankar H, Kunapuli SP. Platelet receptors for adenine nucleotides and thromboxane A2. Semin Thromb Hemost. 2004;30:411–418. doi: 10.1055/s-2004-833476. [DOI] [PubMed] [Google Scholar]
- 146.Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, Chen J, McKnight GS, López JA, Yang L, Jin Y, Bray MS, Leal SM, Dong J-F, Bray PF. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117:5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nguyen T, Ghebrehiwet B, Peerschke EI. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect Immun. 2000;68:2061–2068. doi: 10.1128/iai.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 149.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, Have ten K, Eijsman L, Hack CE, Sturk A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 150.Niiya K, Hodson E, Bader R, Byers-Ward V, Koziol JA, Plow EF, Ruggeri ZM. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987;70:475–483. [PubMed] [Google Scholar]
- 151.Owens AP, Mackman N. Microparticles in hemostasis and thrombosis. Circulation Research. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pabla R, Weyrich AS, Dixon DA, Bray PF, McIntyre TM, Prescott SM, Zimmerman GA. Integrin-dependent control of translation: engagement of integrin alphaIIbbeta3 regulates synthesis of proteins in activated human platelets. J Cell Biol. 1999;144:175–184. doi: 10.1083/jcb.144.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang C-Y, Zen K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. The Journal of Immunology. 2014;192:437–446. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- 154.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circulation Research. 2013;112:103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Peerschke EI, Reid KB, Ghebrehiwet B. Identification of a novel 33-kDa C1q-binding site on human blood platelets. J Immunol. 1994;152:5896–5901. [PubMed] [Google Scholar]
- 157.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peerschke EIB, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- 159.Petersen F, Bock L, Flad HD, Brandt E. A chondroitin sulfate proteoglycan on human neutrophils specifically binds platelet factor 4 and is involved in cell activation. J Immunol. 1998;161:4347–4355. [PubMed] [Google Scholar]
- 160.Petersen F, Ludwig A, Flad HD, Brandt E. TNF-alpha renders human neutrophils responsive to platelet factor 4. Comparison of PF-4 and IL-8 reveals different activity profiles of the two chemokines. J Immunol. 1996;156:1954–1962. [PubMed] [Google Scholar]
- 161.Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FHY, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 162.Piotrowicz RS, Orchekowski RP, Nugent DJ, Yamada KY, Kunicki TJ. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J Cell Biol. 1988;106:1359–1364. doi: 10.1083/jcb.106.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pitchford SC, Riffo-Vasquez Y, Sousa A, Momi S, Gresele P, Spina D, Page CP. Platelets are necessary for airway wall remodeling in a murine model of chronic allergic inflammation. Blood. 2004;103:639–647. doi: 10.1182/blood-2003-05-1707. [DOI] [PubMed] [Google Scholar]
- 164.Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, Rose MJ, Giannini S, Momi S, Spina D, O’connor B, Gresele P, Page CP. Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol. 2003;112:109–118. doi: 10.1067/mai.2003.1514. [DOI] [PubMed] [Google Scholar]
- 165.Power CA, Furness RB, Brawand C, Wells TN. Cloning of a full-length cDNA encoding the neutrophil-activating peptide ENA-78 from human platelets. Gene. 1994;151:333–334. doi: 10.1016/0378-1119(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 166.Pytela R, Pierschbacher MD, Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci USA. 1985;82:5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qian K, Xie F, Gibson AW, Edberg JC, Kimberly RP, Wu J. Functional expression of IgA receptor FcalphaRI on human platelets. Journal of Leukocyte Biology. 2008;84:1492–1500. doi: 10.1189/jlb.0508327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rahman M, Roller J, Zhang S, Syk I, Menger MD, Jeppsson B, Thorlacius H. Metalloproteinases regulate CD40L shedding from platelets and pulmonary recruitment of neutrophils in abdominal sepsis. Inflamm Res. 2012;61:571–579. doi: 10.1007/s00011-012-0446-6. [DOI] [PubMed] [Google Scholar]
- 169.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 170.Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, López JA. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. Journal of Experimental Medicine. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosenfeld SI, Looney RJ, Leddy JP, Phipps DC, Abraham GN, Anderson CL. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J Clin Invest. 1985;76:2317–2322. doi: 10.1172/JCI112242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 173.Rouhiainen A, Imai S, Rauvala H, Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb Haemost. 2000;84:1087–1094. [PubMed] [Google Scholar]
- 174.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 175.Rumbaut RE, Thiagarajan P. Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 176.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiological Reviews. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 177.Scheuerer B, Ernst M, Dürrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 178.Schober A, Manka D, Hundelshausen von P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 179.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. Journal of Experimental Medicine. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost. 2007;5:2009–2016. doi: 10.1111/j.1538-7836.2007.02698.x. [DOI] [PubMed] [Google Scholar]
- 181.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and 4. Blood. 2011 Jun 14; doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 183.Silverstein RL, Febbraio M. Identification of lysosome-associated membrane protein-2 as an activation-dependent platelet surface glycoprotein. Blood. 1992;80:1470–1475. [PubMed] [Google Scholar]
- 184.Simon DI, Chen Z, Xu H, Li CQ, Dong JF, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, López JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989;264:17049–17057. [PubMed] [Google Scholar]
- 186.Sixma JJ, van den Berg A, Hasilik A, Figura von K, Geuze HJ. Immuno-electron microscopical demonstration of lysosomes in human blood platelets and megakaryocytes using anti-cathepsin D. Blood. 1985;65:1287–1291. [PubMed] [Google Scholar]
- 187.Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18:153–167. doi: 10.1016/j.tmrv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 188.Smith CW, Rothlein R, Hughes BJ, Mariscalco MM, Rudloff HE, Schmalstieg FC, Anderson DC. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988;82:1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 190.Soriano AO, Jy W, Chirinos JA, Valdivia MA, Velasquez HS, Jimenez JJ, Horstman LL, Kett DH, Schein RMH, Ahn YS. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Critical Care Medicine. 2005;33:2540–2546. doi: 10.1097/01.ccm.0000186414.86162.03. [DOI] [PubMed] [Google Scholar]
- 191.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nácher M, Pitaval C, Radovanovic I, Fukui Y, McEver RP, Filippi M-D, Lizasoain I, Ruiz-Cabello J, Zarbock A, Moro MA, Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Staatz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989;108:1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stark RJ, Aghakasiri N, Rumbaut RE. Platelet-derived Toll-like receptor 4 (Tlr-4) is sufficient to promote microvascular thrombosis in endotoxemia. PLoS ONE. 2012;7:e41254. doi: 10.1371/journal.pone.0041254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol (Lond) 2012;590:1023–1034. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Stratz C, Nührenberg TG, Binder H, Valina CM, Trenk D, Hochholzer W, Neumann FJ, Fiebich BL. Micro-array profiling exhibits remarkable intra-individual stability of human platelet micro-RNA. Thromb Haemost. 2012;107:634–641. doi: 10.1160/TH11-10-0742. [DOI] [PubMed] [Google Scholar]
- 196.Sukhithasri V, Nisha N, Biswas L, Anil Kumar V, Biswas R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res. 2013;168:396–406. doi: 10.1016/j.micres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 197.Suzuki-Inoue K, Fuller GLJ, García A, Eble JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RDG, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VLJ, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 198.Svensson L, Baumgarten M, Mörgelin M, Shannon O. Platelet activation by Streptococcus pyogenes leads to entrapment in platelet aggregates, from which bacteria subsequently escape. Infect Immun. 2014;82:4307–4314. doi: 10.1128/IAI.02020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tutluoglu B, Gurel CB, Ozdas SB, Musellim B, Erturan S, Anakkaya AN, Kilinc G, Ulutin T. Platelet function and fibrinolytic activity in patients with bronchial asthma. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2005;11:77–81. doi: 10.1177/107602960501100109. [DOI] [PubMed] [Google Scholar]
- 201.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 202.Van Aken WG, Vreeken J. Accumulaon of macromolecular particles in the reticuloendothelial system (RES) mediated by platelet aggregation and disaggregation. Thromb Diath Haemorrh. 1969;22:496–507. [PubMed] [Google Scholar]
- 203.Van Raemdonck K, Gouwy M, Lepers SA, Van Damme J, Struyf S. CXCL4L1 and CXCL4 signaling in human lymphatic and microvascular endothelial cells and activated lymphocytes: involvement of mitogen-activated protein (MAP) kinases, Src and p70S6 kinase. Angiogenesis. 2014;17:631–640. doi: 10.1007/s10456-014-9417-6. [DOI] [PubMed] [Google Scholar]
- 204.Vanichakarn P, Blair P, Wu C, Freedman JE, Chakrabarti S. Neutrophil CD40 enhances platelet-mediated inflammation. Thromb Res. 2008;122:346–358. doi: 10.1016/j.thromres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 205.Voudoukis E. Multipotent role of platelets in inflammatory bowel diseases: A clinical approach. WJG. 2014;20:3180. doi: 10.3748/wjg.v20.i12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vowinkel T, Anthoni C, Wood KC, Stokes KY, Russell J, Gray L, Bharwani S, Senninger N, Alexander JS, Krieglstein CF, Grisham MB, Granger DN. CD40-CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 2007;132:955–965. doi: 10.1053/j.gastro.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 207.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88:907–914. [PubMed] [Google Scholar]
- 208.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang H-B, Wang J-T, Zhang L, Geng ZH, Xu W-L, Xu T, Huo Y, Zhu X, Plow EF, Chen M, Geng J-G. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8:882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- 210.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, Zago AC, Lopez J, Andre P, Plow E, Simon DI. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 211.Warshaw AL, Laster L, Shulman NR. Protein synthesis by human platelets. J Biol Chem. 1967;242:2094–2097. [PubMed] [Google Scholar]
- 212.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest. 1997;100:2085–2093. doi: 10.1172/JCI119742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- 214.Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci USA. 1998;95:5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA. Protein synthesis by platelets: historical and new perspectives. J Thromb Haemost. 2009;7:241–246. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.White JG, Clawson CC. The surface-connected canalicular system of blood platelets--a fenestrated membrane system. The American Journal of Pathology. 1980;101:353–364. [PMC free article] [PubMed] [Google Scholar]
- 219.White JG, Krumwiede MD, Escolar G. Glycoprotein Ib is homogeneously distributed on external and internal membranes of resting platelets. The American Journal of Pathology. 1999;155:2127–2134. doi: 10.1016/S0002-9440(10)65530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.White JG. Why human platelets fail to kill bacteria. Platelets. 2006;17:191–200. doi: 10.1080/09537100500441234. [DOI] [PubMed] [Google Scholar]
- 221.White JG. Platelet structure. In: Michelson AD, editor. Platelets. Academic Press; 2007. pp. 45–73. [Google Scholar]
- 222.Xie J, Li R, Kotovuori P, Vermot-Desroches C, Wijdenes J, Arnaout MA, Nortamo P, Gahmberg CG. Intercellular adhesion molecule-2 (CD102) binds to the leukocyte integrin CD11b/CD18 through the A domain. J Immunol. 1995;155:3619–3628. [PubMed] [Google Scholar]
- 223.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular Histones Are Mediators of Death through TLR2 and TLR4 in Mouse Fatal Liver Injury. J Immunol. 2011 Jul 22; doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xu T, Zhang L, Geng ZH, Wang H-B, Wang J-T, Chen M, Geng J-G. P-selectin cross-links PSGL-1 and enhances neutrophil adhesion to fibrinogen and ICAM-1 in a Src kinase-dependent, but GPCR-independent mechanism. Cell Adh Migr. 2007;1:115–123. doi: 10.4161/cam.1.3.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yeaman MR, Bayer AS, Koo SP, Foss W, Sullam PM. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Invest. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yeaman MR, Ibrahim AS, Edwards JE, Bayer AS, Ghannoum MA. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yeaman MR, Puentes SM, Norman DC, Bayer AS. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yeaman MR, Soldan SS, Ghannoum MA, Edwards JE, Filler SG, Bayer AS. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect Immun. 1996;64:1379–1384. doi: 10.1128/iai.64.4.1379-1384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Youssefian T, Drouin A, Massé J-M, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 230.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 231.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang X, Zhan D, Shin HY. Integrin subtype-dependent CD18 cleavage under shear and its influence on leukocyte-platelet binding. Journal of Leukocyte Biology. 2013;93:251–258. doi: 10.1189/jlb.0612302. [DOI] [PubMed] [Google Scholar]
- 233.Zucker-Franklin D, Seremetis S, Zheng ZY. Internalization of human immunodeficiency virus type I and other retroviruses by megakaryocytes and platelets. Blood. 1990;75:1920–1923. [PubMed] [Google Scholar]
- 234.Zufferey A, Schvartz D, Nolli S, Reny J-L, Sanchez J-C, Fontana P. Characterization of the platelet granule proteome: evidence of the presence of MHC1 in alpha-granules. J Proteomics. 2014;101:130–140. doi: 10.1016/j.jprot.2014.02.008. [DOI] [PubMed] [Google Scholar]