Abstract
The shaping of the vertebrate embryonic body plan depends heavily on the narrowing and lengthening (convergence and extension) of embryonic tissues by cell intercalation, a process by which cells actively crawl between one another along the axis of convergence to produce a narrower, longer array. We discuss recent evidence that the vertebrate non-canonical Wnt/Planar Cell Polarity (PCP) pathway, known to directly function in polarizing the movements of intercalating cells, is also involved in the localized assembly of extracellular matrix (ECM). These cell-ECM interactions, in turn, are necessary for expression of the oriented, polarized cell intercalation. The mechanism of PCP/ECM interactions, their molecular signaling, and their mechanical consequences for morphogenesis are discussed with the goal of identifying important unsolved issues.
Introduction
Shaping the body plan of chordates depends on the narrowing and lengthening, or convergent extension (CE), of the major axial and paraxial tissues of notochord and somitic mesoderm, and the neural plate. CE occurs as a result of intercalation of cells transverse to the axis of tissue extension to form a narrower (convergence) but longer (extension) array [1]. This intercalation is driven by oriented, bipolar, mediolaterally polarized protrusive activity, and in some cases, monopolar variants of this behavior, which pulls the cells between one another. This process depends on a tissue level signal for its expression, and for alignment of polarized motility transverse to the presumptive anterior-posterior body axis, the axis of extension. It also depends on the non-canonical Wnt/PCP pathway for expression of this cell polarization. Recent evidence shows that in addition to the direct role of the PCP pathway in cell polarization, it has a role in assembly of extracellular matrix (ECM), notably fibronectin (FN) and fibrillin (Fib), on the surfaces of tissues. In turn, integrin-mediated interactions with this surfaceassembled matrix have essential roles in PCP-mediated cell polarization. Moreover, these matrix surfaces also regulate radial intercalation, oriented orthogonally to mediolateral intercalation, and the genetically regulated balance of radial and mediolateral forces plays a major role in tissue shaping by mass cell movements. We identify major issues resulting from this complex integration of reciprocal non-canonical Wnt/PCP and ECM signaling, and its cell biological and biomechanical consequences for morphogenesis.
Polarized cell intercalation behavior requires non-canonical Wnt/PCP and integrin- FN signaling
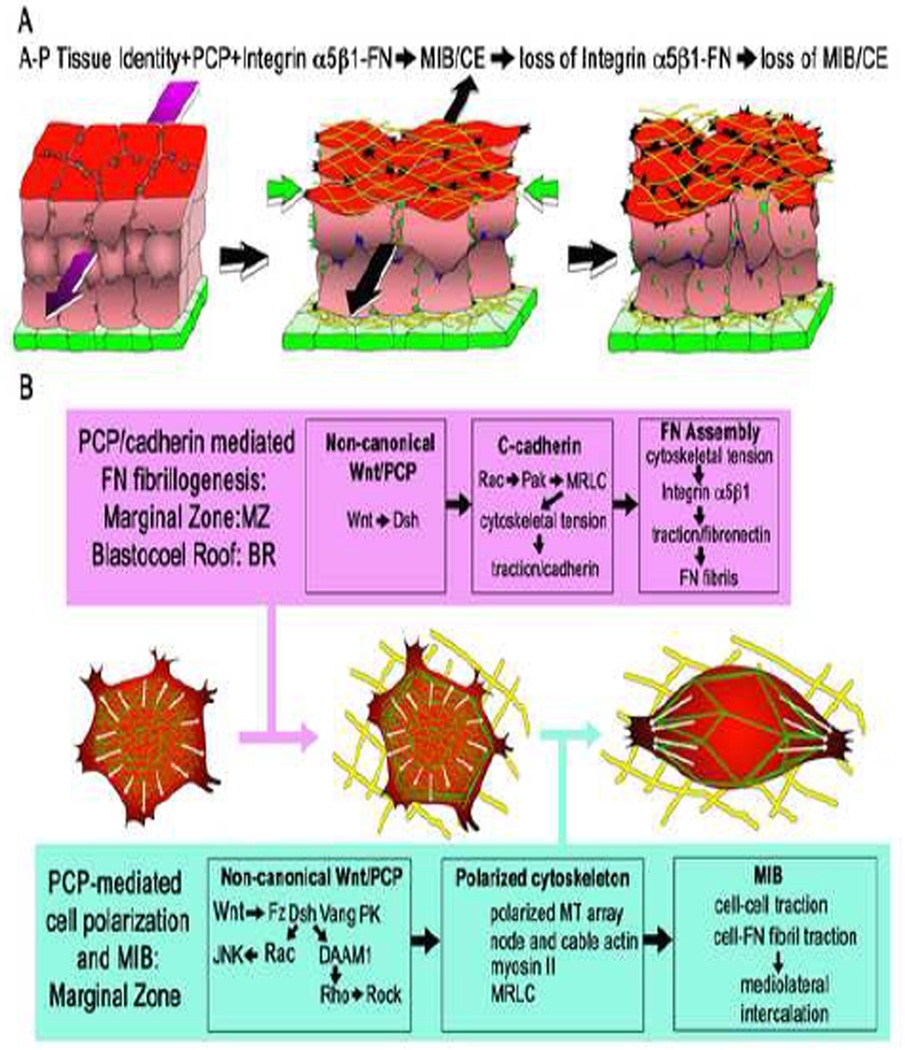
In Xenopus, CE begins as the dorsal mesodermal cells change from a multipolar, unoriented protrusive activity to a bipolar, mediolaterally-directed filo-lamelliform protrusive activity (Fig. 1A,B) [2]. A myosin IIB-dependent cortical actin cytoskeleton is organized, and it generates mediolaterally oriented tractional forces that pull the cells between one another to form a narrower, longer tissue [3] (Fig. 1A,B). These events, which comprise a stereotyped mediolateral intercalation behavior (MIB), are dependent on a tissue-level, anterior-posterior positional identity signal of unknown nature [4] and the non-canonical Wnt/PCP pathway [5–7] (Fig. 1A). MIB is also dependent on integrin α5β1-mediated interactions with fibrillar FN initially assembled on the dorsal, and later on the ventral (endodermal facing) surfaces of the intercalating mesodermal cells in frogs and fish [8–10] (Fig.1A). Integrin activation allows suppression of protrusive activity on the anterior and posterior sides, leaving it at the ends and thus polarizing the cell, whereas blocking integrin-FN interaction expands protrusive activity to the normally quiescent anterior and posterior sides, making them multipolar (Fig. 1B) [8]. The rope-like, flexible FN fibrils may function as mechanical tethers that transmit tensile forces from cell-tocell, thus supplementing direct cell-on-cell traction to bring about intercalation [9]. However, expression of a FN fragment that supports FN-integrin signaling but blocks FN-fibril formation only minimally affects CE [11], so if fibrils normally transmit tractional forces during intercalation, the embryo nevertheless can do most of CE with only cell-on-cell traction. The failure of CE and formation of short, wide embryos following perturbation of FN-integrin interactions in frog, fish, and mouse embryos [8, 10, 12, 13] are due in large part to this dependence of MIB, and thus CE, on integrin interactions with these tissue surface-assemblies of FN.
Figure 1.
Diagrams show the deep mesodermal tissue of the marginal zone (red) in the process of undergoing convergence (green arrows) and extension (black arrow) by mediolateral cell intercalation (A). (The mediolateral axis is oriented across the page and the radial axis is vertical.) Initially unpolarized mesodermal cells (left) become polarized mediolaterally, in the plane of the tissue, and the resulting mediolaterally oriented traction drives the intercalation of cells along this axis (middle). Mediolaterally polarized protrusions occur at tissue surfaces bounding fibrillar FN (yellow), which are indicated as black protrusions, and internally, indicated by green protrusions. Polarization is dependent on anterior-posterior (A-P) tissue positional identity cues (magenta arrow), which aligns polarized behavior mediolateral to the A-P axis (see ref. 4). The expression of the polarized motility requires PCP signaling (see B, and refs. 5–7, 22) and FN-integrin α5β1 signaling (see B and refs. 8, 16). Absence of PCP signaling, or loss of FN-integrin signaling, results in loss of polarized behavior and failure of CE (right). The superficial endodermal epithelium (green) bounds the outside of marginal zone and reduces mesodermal surface tension, a necessary factor in allowing CE (26). The non-canonical Wnt/PCP pathway has dual roles in regulating polarized cell protrusive activity (B). PCP functions in a C-cadherin-mediated assembly of FN fibrils (yellow) in planarly unpolarized cells of blastocoel roof and in the planarly polarizing cells of the marginal zone (B, magenta box) (see refs. 9, 16, 21). This involves localization of actin (green) and development of adherens-like junctions at the cell periphery, and development of tensile forces at the cell periphery, which are necessary for fibrillogenesis (16). Expression of the polarized mediolateral intercalation behavior (MIB) (2–7, 31), which occurs only in the presumptive dorsal mesoderm of the marginal zone, is also dependent on integrin α5β1-FN signaling (B, blue box; see ref. 8). This involves mediolateral polarization the cytoskeleton, including formation of a mediolaterally oriented node-and-cable array of actin microfilaments, (green) (3), polarized microtubule growth (white arrows) (35), and mediolaterally polarized traction in the form of mediolaterally-directed protrusive activity (2).
The PCP pathway also has role in assembly of FN fibrils
The PCP pathway has dual roles in regulating MIB, a direct one in regulating cell aspects of polarity and one indirect in regulating the assembly of FN matrix, which is a permissive requirement for MIB. Assembly of FN depends on integrin (mainly α5β1) ligation of FN dimers, combined with a cytoskeletally generated tension that causes a conformational change in FN dimers, which in turn allows higher order fibril assembly [14–16]. In Xenopus, FN fibrils are assembled on the surfaces of tissues throughout the embryo, including the inner surface of the blastocoel roof and marginal zone, and later around major tissue masses (notochordal and somitic mesoderm and neural plate/tube) [9, 17].
On the blastocoel roof of Xenopus, FN fibril assembly involves interaction of PCP- and C-cadherin-mediated signaling. The PCP protein, Disheveled, is translocated to the plasma membrane [17] by a FN-dependent interaction of syndecan-4 with frizzled7 [18]. Wnt11 signaling through the non-canonical Wnt/PCP pathway up-regulates Ccadherin- mediated traction of blastocoel roof cells on cadherin substrates, an increased traction that is dependent on Rac and Pak, and results in activation of myosin regulatory light chain (MRLC) [16] (Fig. 1B). It is proposed that the tensile forces implied by this result are somehow transferred to an integrin-linked cytoskeleton that then applies tension to FN dimers and results in multimerization into fibrils [16]. On the one hand, PCP regulates cytoskeletal polarization through Dsh, DAAM1, Rho, and ROCK to modulate actin organization through Profilins 1 and 2, as well through activation of MRLC [19, 20]. On the other hand, it regulates FN fibrillogenesis through cadherin-Rac- Pak-MRLC-dependent activation of the actomyosin cytoskeleton (Fig. 1B). There are many potential mechanical and signaling cross-over points between these pathways, and how these two activities of actomyosin networks are coordinated remains a major, unsolved problem.
How is matrix assembly targeted to tissue surfaces/boundaries?
Normal FN assembly on the blastocoel roof surface, as well as that stimulated by PCP and C-cadherin over-expression, is correlated with increased staining for cadherin and actin cytoskeleton along cell boundaries, which become polygonal in profile, compared to their earlier, rounded profile. Dzamba and others argue that this indicates self-organization of adherens-like contacts and an associated tensile cytoskeleton at the inner ends of the blastocoel roof cells, which would account for tissue-surface FN assembly originating there [16]. PCP signaling has a role in superficial FN assembly; inhibition of PCP blocks FN assembly on tissue surfaces [16], whereas over-expression of PCP components results in FN assembly throughout the tissues rather than primarily at their surfaces [21]. With Rho, ROCK, and MRLC downstream of PCP (Fig. 1B), this could be due to excess, myosin II-mediated tension on FN, which over-rides normal polarity cues, spreads into deeper surfaces, and even internal cells of the tissue, where it assembles FN fibrils and is consistent with a simple relationship between PCP signaling, the actomyosin cytoskeleton, and matrix deposition. However, inhibition of PCP does not affect initial assembly of another matrix component, laminin (LN), in ascidians, but eventually the loss of polarity does result in abnormal, internal assembly of LN in the notochord [22]. In the zebrafish somite, reciprocal non-cell autonomous inhibition of FN assembly occurs wherever two cell membranes that express integrin α5 are apposed [23], which then allows FN assembly at free tissue surfaces, or separations that are recognized as boundaries. How this mechanism, which may involve ephrin signaling, works, and if PCP signaling is integrated with it, are issues that should be explored.
Tissue surface arrays of FN fibrils regulate proportions of tissue shape change
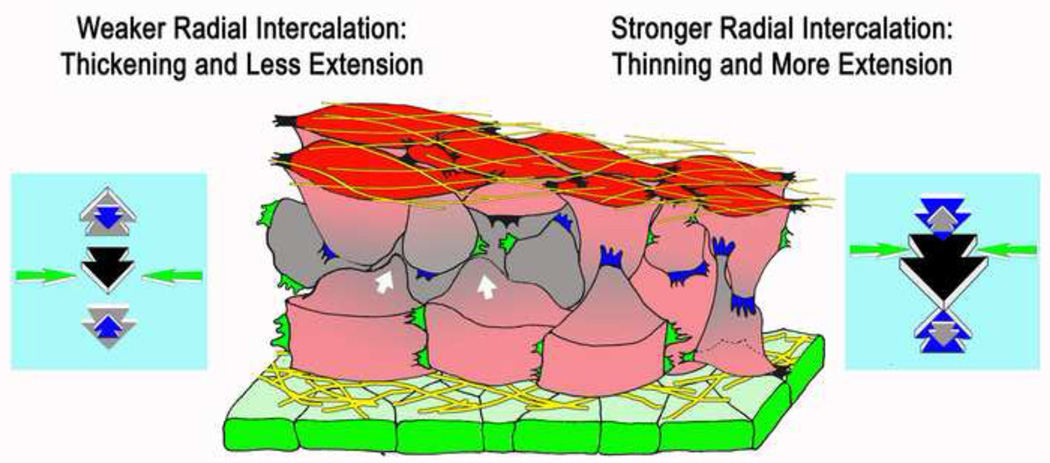
As cells in Xenopus converge by mediolateral intercalation, they generate extension forces of about 0.5μN, and come under compression along the axis of extension. Then they tend to leave the surface, form internal layers, and thicken the tissue (left side, Fig. 2). This "convergent thickening" is resisted by countering forces due to active, radial intercalation of cells from the deeper layers into surface layers of the tissue; when they contact the free surface of the tissue, or boundary with another tissue, they spread on this surface and only rarely detach. This "boundary capture" biases movement of cells into the surface layers and thereby produces thinning of the tissue [24, 25] (right side, Fig. 2). In the marginal zone, both the inner, free surface and the surface bounding the endodermal epithelium of the converging, extending mesoderm are covered with fibrillar FN matrix [8, 9] (Fig. 1A, 2). As deep, internal cells contact these surface layers of FN, an integrin α5β1-mediated interaction with the FN stimulates them to spread on and remain in contact with this layer, a process inhibited by expression of dominant negative integrin [17]. The surface tension-reducing property of adhesion or affinity of the outer endodermal epithelium to the underlying mesodermal cells play a major role in reducing surface tension-driven rounding up (thickening) during CE [26]. In addition to adhesion, the epithelial layer could have complex signaling roles in radial polarization of the marginal zone and in matrix deposition, perhaps through PCP signaling, all issues that remain unexplored.
Figure 2.
A diagram shows a model of how cell-FN matrix interaction could regulate the amount of extension and thickening that results from convergence by regulating the forces generated by radial intercalation (blue arrows) and radial thickening forces (grey arrows) generated by compression of the tissue due to convergence (mediolateral intercalation, yellow arrows). Mediolaterally polarized protrusive activity at the tissue surfaces (black protrusions) and internally (green protrusions), drive mediolateral intercalation and generate convergence forces (yellow arrows), which brings the cells under compression and tends to bring about multi-layering and thickening (white arrows, grey cells). Radially polarized protrusive activity (blue protrusions) generates radial intercalation forces (blue arrows), which oppose the tendency to thicken. The balance of thickening forces and radial thinning forces determine the amount of thickening versus extension will result from convergence (see text, and refs. 17, 24–26,29).
This behavior forms the basis of a general molecular and mechanical model of matrix/boundary-mediated tissue shaping. Mediolateral intercalation produces convergence forces of up to 1.5–2 μN during gastrulation and higher thereafter (Shook D. and Keller, R., unpublished data) (green arrows, Fig. 2), and as the cells wedge between one another along the anterior-posterior axis, and come under compression in this axis, they generate anterior-posterior extension forces of 0.5μN or more [27] (black arrows, Fig. 2). But we also believe that this anterior-posterior compression tends to push the cells above and below one another, in the radial direction, and thus generates radial, thickening forces (grey arrows, Fig. 2c), which have not been measured. We view the convergence (intercalation) as being the primary force-generating process. How much of the resulting compression forces are channeled into producing anterior-posterior extension and how much into producing the radial thickening could be influenced by geometric factors, such as cell shape and connectivity, but the radial thinning force, generated by radial intercalation, is probably the primary factor determining the resulting tissue shape. In the presence of strong integrin α5β1-FN interactions (right side, Fig. 2), strong radial intercalation produces thinning forces (blue arrows) that are larger than the thickening forces (grey arrows), and more convergence force is channeled into extension (black arrows) than into thickening. With weaker integrin α5β1-FN interactions (left side, Fig. 2), radial intercalation forces are weaker (blue arrows) and offer less resistance to the larger thickening forces (grey arrows), and more convergent thickening and less convergent extension occurs. The proportions of convergence going into extension and thickening vary, and are presumably genetically regulated processes through as yet undescribed differences in matrix signaling between notochordal (more extension) and somitic (less extension) mesoderm [28]. Evidence supporting this balance of forces model comes from the mouse, where the somitic mesoderm normally converges and extends by MIB but also thickens as cells leave the surface (a negative radial intercalation). However, when MIB, and the convergence forces it produces, are lost in a mutant of PTK7 (a PCP gene), a previously unknown radial intercalation reveals itself, and because it is unopposed, it results in excessive thinning of the somitic tissue [29]. The idea that the proportions of tissue shape change are regulated by a balance of competing forces generated in several axial dimensions by mass cell intercalations, or other integrated cell behaviors, should be explored in more systems to determine its generality.
Matrix polarization of cells: lessons from the ascidian
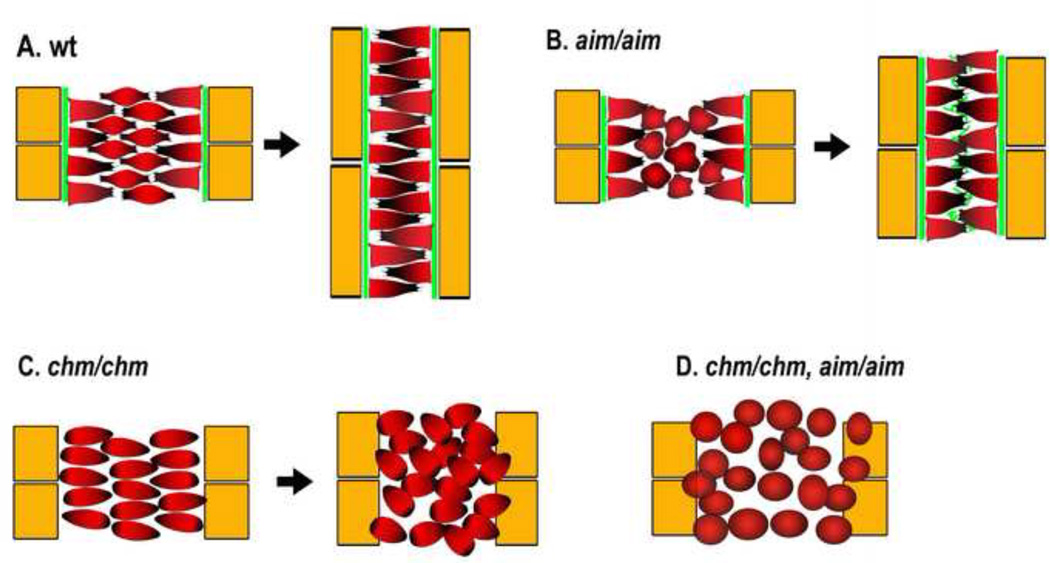
The ascidian notochord undergoes CE by mediolateral intercalation from an array of cells about 8 across to 1 tier across by a mechanism of polarized cell behavior and boundary capture. This intercalation produces about 2-fold extension in this period of development. Internal cells are bipolar and intercalate between one another, but any cell contacting the boundary ceases the invasive, intercalating protrusive activity and spreads on the boundary, never to leave again [22, 30, 31] (Fig. 3A), similar to the boundary capture in frog notochords [32]. Embryos mutant for prickle (aimless, aim) show decreased MIB and CE of the notochord, but next to its boundaries, where the matrix protein laminin (LN) is assembled, cells are nevertheless moderately polarized in the normal fashion; and when un-polarized internal cells happen to contact the boundaries, perhaps as the medially directed, monopolar protrusive activity of the boundary cells pulls them to the boundaries, they are likewise captured at the boundary, and become polarized, which is sufficient to produce significant CE [22] (Fig. 3B). In contrast, embryos mutant for laminin α3/4/5 (chongmague, chm), lack boundary-mediated polarization and captur and display a more severe loss of polarized behavior, a loss of notochord coherence, and less CE [22] (Fig. 3C). The double mutant results in complete failure of MIB and CE (Fig. 3D). These results suggest that matrix-mediated boundary capture/polarization is sufficient to produce substantial intercalation without PCP function, though the two normally complement one another. Also, in this ascidan, PCP function is not essential for initial surface assembly of notochord laminin matrix, as it is for FN in Xenopus, but plays some role, since laminin is assembled internally at later stages. Again, these results suggesting a complex, partially interdependent relationship between matrix deposition and core PCP signaling in regulating cell polarity during chordate embryo elongation [22].
Figure 3.
Diagrams show the patterns of intercalation of notochord cells, and the amount of CE, in wild type embryos (A), a mutant of the PCP gene prickle (aimless, aim, B), a mutant of the laminin 3/4/5 subunit (chongmague, chm, C)and the double mutant (D) in the ascidian. Notochord cells are indicated with red, somites with yellow, and laminin with green. See refs. 22, 30, 31. Adapted with permission from ref. 22 (issue 1, p. 39).
Signaling and mechanical functions of the notochordal-somitic boundary (NSB)
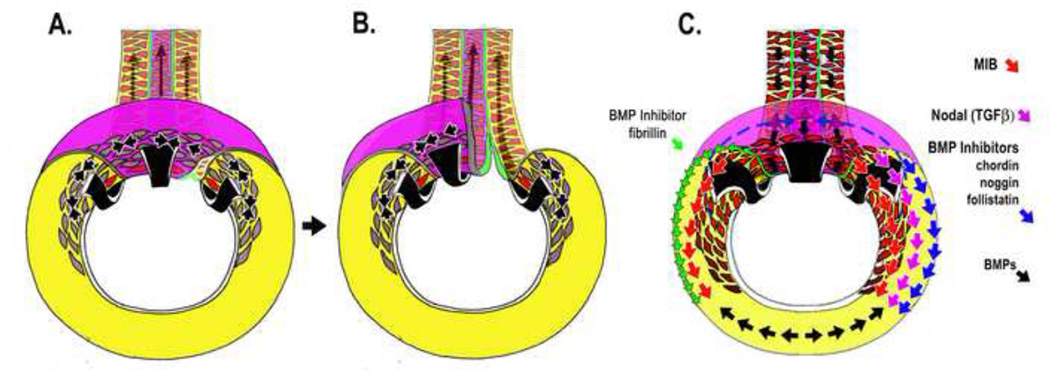
In Xenopus, the NSB forms progressively, from presumptive anterior-to-posterior [32], by sorting of two contiguous cell populations, one expressing β catenin at high levels (somitic) and the other at low levels (notochordal), indicating involvement of the canonical Wnt pathway, and surprisingly, not involving cadherin-mediated changes in cell adhesion [33]. Internal, bipolar cells vigorously intercalate between boundary cells, cease their invasive, intercalation behavior and spread in the plane of the boundary to occupy a defined area at these ends, leaving their internal/medial ends free to do a medially-directed, monopolar intercalation behavior [32]. Fibrillin2 (Fib2)-containing microfibrils appear in the boundary just after it becomes morphologically visible [34], and their assembly requires polarized microtubule growth, which in turn requires a PCPdependent polarization of those cells [35].
Fib2-containing microfibrils have dual structural/mechanical and signaling roles, the latter involving binding and inactivating BMP’s and interactions with other matrix molecules in signaling cascades. Mechanically, Fib2-mediated boundary capture stabilizes notochord CE. Perturbation of Fib-2 assembly or expression in Xenopus embryos or explants results in mechanically unstable, herniated and kinked notochords [36]. Kinked notochords also appear in zebrafish Fib2 mutants [37]. In Xenopus, these manipulations also result in separation of notochordal and somitic tissue and unclosed blastopores in embryos. The notochord slides posteriorly relative to the somitic mesoderm, parallel to its axis of extension, while being subjected to tensile convergence forces perpendicular to (across) its boundary, generated by the posteriorly progressing expression of MIB, which occurs shortly after the boundary forms just after involution (black arrows, Fig. 4A, B). In addition to CE of the notochordal and somitic mesoderm, convergence forces are generated by the posterior extension of the notochord. It forms a sliding spline, which is bound to somitic mesoderm on both sides by Fib2, and as the spline slides posteriorly, it zips these tissues together. Failure of this sliding, zipper joint on perturbation of Fib2 expession or function results loss of circumblastoporal tension, and failure of blastopore closure [36](also see [38, 39])(Fig. 4B).
Figure 4.
Diagrams of the vegetal aspect of the Xenopus embyo illustrates the mechanical function of the Fibrillin-2 (green) in reinforcement of notochordal (magenta)/somitic (yellow) boundary (NSB) in blastopore closure (A-B), concomitant with progressive dorsalization of posterior mesoderm (34, 36) (C). NSB formation proceeds progressively posteriorly in the mesoderm (green line, A-B) with the progress of MIB (transition of grey cells to red cells). Therefore large convergence forces (black arrows) develop across the newly formed NSB. Disruption of Fibrillin-2 results in fracture of this boundary and failure of blastopore closure (36) (B). Progression of MIB (red arrows) follows the earlier progress of nodal signaling (magenta arrows) and BMP inhibitors (blue arrows) into mesoderm of the ventral sector of the gastrula where these signals counter the effect of BMP signals (black arrows) and induce formation of posterior somitic mesoderm (C). The notochord and NSB, containing Fibrillin-2, potentially with BMP inhibiting activity, shears posteriorly along the lateral edge of the presumptive somitic mesoderm into ventral (presumptive posterior) regions (green arrows, C), where it may have dorsalizing activity. At the same time, it posterior movement has the mechanical effect of acting as a spline, a "zipper" that generates convergence forces as it pulls the pre-somitic mesoderm towards the midline (See 28, 34, 36, 40, 41; Dzamba, B. and DeSimone, D., personal communication).
In regard to signaling, expressing a fragment of Fib2 that inhibits Fib2 assembly blocks MIB and the strong boundary capture activity of the NSB, indicating localized microfibrils mediate this behavior [36]. Inactivating BMP signaling on the ventral side of the embryo induces a second axis in Xenopus and expressing putative BMP-binding fragments of Fibrillin also has this activity, providing another mechanism by which fibrillin can locally regulate signaling [40] (Dzamba B and DeSimone D, personal communication). This means that shearing of the notochord posteriorly with respect to the presumptive somitic mesoderm brings an additional BMP-inhibitor into the progressively more posterior, presumptive somitic mesoderm lying at the ventral side of the blastopore (green arrows, Fig.4C). Fibrillins also regulate TGFβ signaling [40, 41], and therefore the expression and dynamic movements of Fib2 during morphogenesis can have far-reaching consequences. In cultured fibroblasts, Fib1 assembly into microfibrils requires prior FN assembly [42], a dependence shared by other ECM molecules [43], and Fib-2 assembly around tissues is reduced in FN morphant embryos [44]. Therefore loss of Fibrillin may be responsible for some aspects of phenotypes associated with the upstream loss of FN function. These results highlight the importance of surface/boundary localized ECM that form stable, localized signaling centers, with the potential to regulate cell morphogenic behavior as well as their differentiation, but at the same time may move into new tissue contexts, and serve mechanical morphogenic roles as well.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- 3.Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- 5.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 6.Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 7.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 8.Davidson LA, Marsden M, Keller R, Desimone DW. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Davidson LA, Dzamba BD, Keller R, Desimone DW. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn. 2008;237:2684–2692. doi: 10.1002/dvdy.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latimer A, Jessen J. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 2010;29:89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Rozario T, Dzamba B, Weber GF, Davidson LA, DeSimone DW. The physical state of fibronectin matrix differentially regulates morphogenetic movements in vivo. Dev Biol. 2009;327:386–398. doi: 10.1016/j.ydbio.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George E, Georges-Labouesse E, Patel-King R, Rayburn H, Hynes R. Defects in mesoderm, neural tube and vascular develoment in mice lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 13.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 14.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibers controlled by cytoskeletal tension. Proc. Natl. Acad. Sci. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 16. Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16:421–432. doi: 10.1016/j.devcel.2009.01.008. This paper examines how cell-cell adhesion in a tissue, mediated by cadherins, relates to FN fibril assembly at the boundary of the tissue. Increasing cell - cell adhesion by over-expressing cadherin leads to both precocious assembly of FN, and an increase in cortical tension through a Rac-Pak-MRLC pathway. Cell-cell adhesion is normally regulated by non-canonical Wnt11 signals, generating a model in which PCP activates adhesion, which increases both cortical and tissue tension to support FN fibril assembly.
- 17.Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- 18.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 19.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 20.Khadka D, Liu W, Habas R. Non-redundant roles for Profilin2 and Profilin1 during vertebrate gastrulation. Developmental Biology. 2009;332:396–406. doi: 10.1016/j.ydbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 22. Veeman M, Nakatani Y, Hendrickson C, Ericson V, Lin C, Smith W. chongmague reveals an essential role for laminin-mediated boundary formation in chordate convergence and extension movements. Development. 2008;135:33–41. doi: 10.1242/dev.010892. This paper exploits a genetically tractable system to determine that both the PCP pathway and localized laminin assembly at the notochordal-somitic border each contribute, partially redundantly, to polarize notochordal cells for intercalation. The paper also describes a strong requirement for PCP signaling in maintaining and restricting laminin to the notochordal surface, but not for its initial deposition.
- 23. Julich D, Mould A, Koper E, Holley S. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. This paper provides evidence for a “reciprocal non-cell autonomous” inhibition of integrin function, which forms the basis for appealing model for why FN is not assembled between cells in a tissue but is restricted to the tissue surface. How this occurs mechanistically is an important unanswered question. The paper describes a mechanism for initially establishing tissue borders by reverse signaling through ephrins, but as the authors point out, border formation and relieving FN assembly inhibition may not be linked by direct signaling between an ephrin pathway and integrins. Thus this mechanism may function widely to limit FN assembly to tissue surfaces.
- 24.Wilson PA, Oster G, Keller R. Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants. Development. 1989;105:155–166. doi: 10.1242/dev.105.1.155. [DOI] [PubMed] [Google Scholar]
- 25.Wilson P, Keller R. Cell rearrangement during gastrulation of Xenopus: direct observation of cultured explants. Development. 1991;112:289–300. doi: 10.1242/dev.112.1.289. [DOI] [PubMed] [Google Scholar]
- 26.Ninomiya H, Winklbauer R. Epithelial coating controls mesenchymal shape change through tissue-positioning effects and reduction of surface-minimizing tension. Nat. Cell Biol. 2007:10. doi: 10.1038/ncb1669. [DOI] [PubMed] [Google Scholar]
- 27.Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 28.Keller R, Cooper MS, Danilchik M, Tibbetts P, Wilson PA. Cell intercalation during notochord development in Xenopus laevis. J Exp Zool. 1989;251:134–154. doi: 10.1002/jez.1402510204. [DOI] [PubMed] [Google Scholar]
- 29.Yen W, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto D, Crowther R. Formation of the notochord in living ascidian embryos. J Embryol Exp Morphol. 1985:1–17. [PubMed] [Google Scholar]
- 31.Munro E, Odell G. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002a;1:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development. 1992;116:915–930. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- 33.Reintsch W, Habring-Mueller A, Wang R, Schohl A, Fagotto F. beta-Catenin controls cell sorting at the notochord-somite boundary independently of cadherin-mediated adhesion. J Cell Biol. 2005;170:675–686. doi: 10.1083/jcb.200503009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skoglund P, Dzamba B, Coffman CR, Harris WA, Keller R. Xenopus fibrillin is expressed in the organizer and is the earliest component of matrix at the developing notochord-somite boundary. Dev Dyn. 2006;235:1974–1983. doi: 10.1002/dvdy.20818. [DOI] [PubMed] [Google Scholar]
- 35. Shindo A, Yamamoto T, Ueno N. Coodination of Cell Polarity during Xenopus Gastrulation. PLoS One. 2008;3:e1600. doi: 10.1371/journal.pone.0001600. These authors use EB-3GFP to localize the growing ends of microtubules in presumptive Xenopus notochrdal cells, and find that the orientation of microtubule growth occurs even before these cells adopt a bipolar morphology. Signals from the notochord-somite boundary are required for this orientation, and, in turn, microtubules are required for fibrillin assembly in the boundary. This boundary effect is mimicked in animal cap cells expressing distinct levels of nodal, which implicates a nodal gradient in boundary formation.
- 36.Skoglund P, Keller R. Xenopus fibrillin regulates directed convergence and extension. Dev Biol. 2007;301:404–416. doi: 10.1016/j.ydbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gansner J, Madsen E, Mecham R, Gitlin J. Essential Role of fibrillin-2 in Zebrafish notochord and Vascular Morphogenesis. Dev Dyn. 2008;237:2844–2861. doi: 10.1002/dvdy.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schectman The mechanics of amphibian gastrulationIGastrulationproducing interactions between various regions of an anuran egg (Ityla regila) Univ. Calif. Publ. Zool. 1942;51:1–39. [Google Scholar]
- 39.Keller R, Danilchik M. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development. 1988;103:193–209. doi: 10.1242/dev.103.1.193. [DOI] [PubMed] [Google Scholar]
- 40.Sengle G, Charbonneau N, Ono R, Sasaki T, Alvarez J, Keene D, Bachinger H, Sakai L. Targeting of Bone Morphogenetic Protein Growth Factor Complexes to Fibrillin. J Biol Chem. 2008:283. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez F, Rifkin DB. Extracellular microfibrils: contexual platforms for TGFbeta signaling. Curr Opin Cell Biol. 2009;21:616–621. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sabatier L, Chen D, Fagotto-Kaufmann C, Humbmacher D, McKee M, Annis D, Mosher D, Reinhardt D. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. This paper uncovers an absolute requirement for FN fibrils in the Fibrillin-1 assembly pathway, and provides data extending this requirement across the fibrillin family. These observations extend the role of FN in organizing other ECM components to the fibrillins, and suggests that some aspects of FN-targeted phenotypes may be indirect, i.e., due to defects in matrix components dependent on FN for their assembly or function rather than in FN itself.
- 43.Dallas S, Chen Q, Sivakumar P. Dynamics of Assembly and Reorganization of Extracellular Matrix Proteins. Curr Top Dev Biol. 2006;75:1–24. doi: 10.1016/S0070-2153(06)75001-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]