Abstract
Racial and sex disparities in chronic diseases and mortality are sources of health inequality and have been observed from infancy to adulthood. Disparities in health and mortality contribute to corresponding disparities in healthy life. I address two previously unanswered questions in the aging literature. First, does the racial and sex gap in healthy life narrow, persist, or expand over age and time, particularly considering severity of ill health, among the oldest old? Second, do some race-sex groups of birth cohorts live not just longer lives, but longer healthier lives, while others spend additional years in illness? To estimate the quantities, I employ a refined definition of physical disability and apply a new extension of Sullivan’s method to true birth cohorts. The results suggest among the oldest old, few racial or sex disparities exist over age and time in mild disability. Yet, racial and sex disparities persist over age and time in severe disability.
INTRODUCTION
Racial and sex disparities in chronic diseases and mortality are a source of health inequality and have been observed in infancy, adolescence, and adulthood. These disparities in health and mortality contribute to corresponding disparities in healthy life. They may be partially the result of a lifetime of biological, social, and psychological differences including education (Freedman and Martin, 1999), occupation (Darity, 2003), income and wealth (Williams and Jackson, 2005), access to health care and information (Kreps, 2006), health behaviors (Lantz et al., 1998), and social integration (Kawachi, 1999). An important research question, central to the ongoing exploration of aging in the United States, is whether racial and sex disparities in healthy life experienced at earlier ages and time periods narrow, persist, or expand over age and time among the oldest old. Assessment of changing patterns in sex and racial disparities in the oldest old population (85+) is important for several reasons. First, change over age and time in healthy life disparities is indicative of corresponding disparities in quality of life and racial inequality in health (Crimmins et al., 1989, 1997; Hayward and Heron, 1999). Second, assessment of disparities is critical because they are a major public health concern affecting future demand for medical care and services (Singer and Manton, 1998) and quality of life (Cutler, 2001). Third, disparities are also an important public policy concern. The long term fiscal viability of the Social Security and Medicare programs may be affected by differential changes in health and mortality. Additional years of productive disability-fee working life may contribute to favorable changes in the ratio of workers to Social Security and Medicare beneficiaries and extend the trust fund years into the future (Singer and Manton, 1998). Fewer years may contribute to greater health care expenditures and long term care needs and present a strain on the programs.
In this paper, I address two research questions important to the understanding of aging. First, does the racial and sex gap in healthy life narrow, persist, or expand over age and time, particularly considering severity of ill health, among the oldest old? Second, do some race-sex groups of birth cohorts live not just longer lives, but longer healthier lives (disability compression), while others spend additional years in illness (disability expansion)? To answer these questions, I estimate disparities in the expected remaining life in disability by sex and race in US cohorts born between 1904 and 1909. Estimation is based on a newly developed extension of Sullivan’s method to birth cohorts (Imai and Soneji, 2007) and a refined definition of physical disability (Verbrugge and Jette, 1994) using data from both community and institutionalized Medicare recipients. Estimation of the expected years of life in disability by level of severity along with total expected years of life allows assessment of these two related research questions. In particular, I examine age and temporal changes in the racial and sex gap in healthy life by level of severity and discuss implications in disability compression and expansion for all race-sex groups.
The existence of a sex and racial gap in healthy life has been widely observed over the last forty years. Yet changes over age and time in this gap has been the subject of much debate in the literature and explained by three competing theories of differential aging. First, age as leveler rests on selective survival and posits earlier gaps in healthy life narrow in advanced age. Convergence in the racial and sex gap has been found among the oldest old in chronic disease prevalence and physical disability (Johnson, 2000; Manton and Gu, 2001) and functional health (Gibson, 1991; Clark et al., 1993). Alternatively, the theory of persistent inequality asserts sex and racial gaps in earlier life continues through advanced ages. Such stable sex and racial gaps have been found in physical disability (Ferraro and Farmer, 1996a; Ferraro, 1987; Kelley-Moore and Ferraro, 2004). Finally, multiple jeopardy is rooted in cumulative disadvantage theory and argues the healthy life gap of minorities and women widens even in advanced age. Widening healthy life gaps, supporting the multiple jeopardy theory, has been noted in disability and institutionalization (Clark, 1997; Liao et al., 1999).
The vast majority of research into the theoretical understanding of sex and racial gaps in healthy life has three limitations. First, the use of cross-sectional data and hypothetical cohorts may ignore the influence of cohort effects and introduce bias, which makes assessment of changes in racial and sex gaps over age and time difficult (Bongaarts and Feeny, 2002). Second, assessment focusing exclusively on health measures among those alive masks the effect of differential mortality of race-sex groups at advanced ages. Finally, the use of binary indicators of physical disability masks levels of severity and limits ability to discern different patterns in disability. In contrast, this paper combines the health and mortality experience of birth cohorts and uses an expanded definition of physical disability.
To address the first limitation, I focus on the mortality and disability experience of true birth cohorts aging over time. In doing so, I avoid the influence of distorting cohort effects that may result from age-specific disability prevalence and mortality rates changing over time. Based on data from birth cohorts, I use an extension of Sullivan’s method developed by Imai and Soneji (2007) to estimate the expected remaining life with disability. The new methodology provides valid estimation and does not require the assumption of constant age-specific disability prevalence and mortality rates over time. The method also addresses the second limitation concerning differential mortality. By estimating the expected remaining life in disability among only those alive at a given age, I implicitly consider differential mortality.
To address the third limitation, I expand the definition of physical disability to indicate level of severity following the conceptual framework of Verbrugge and Jette (1994). I classify physical disability into mild and severe based on two set of criteria. First, the level of severity is based on the number of physical activities that are difficult or impossible to perform. Second, the level is based on whether the ability to perform a physical activity is possible with some difficulty, or if there is such great difficulty that personal or equipment assistance is required. Classification of physical disability into mild and severe may distinguish levels of limitation and impairment. The classification may be indicative of differences in medical care and rehabilitation. Both may be more complex and costly for the severely disabled who require greater services, increased dependency on family and caregivers, and experience greater morbidity.
The paper is organized as follows. First, I review largely period-based evidence of the existence of sex and racial disparities in health and mortality, leading to disparities in healthy life expectancy. I also discuss the need for similar research using birth cohorts. Second, I introduce a distinction in physical disability indicating level of severity. I also outline an application of Sullivan’s method to birth cohorts used to estimate disparities in the expected remaining life with disability. Third, I assess changing racial and sex disparities over age and time among the oldest old. I focus on the 1904 to 1909 US birth cohorts. Finally, I discuss conclusions and implications.
SEX, GENDER, AND RACIAL DISPARITIES
Examination of both sex and racial disparities is important for two reasons. The evidence for these disparities between racial and ethnic groups in health status and mortality is overwhelming and cause for public health and public policy concern (Schulz et al., 2000; Sudano and Baker, 2006). Additionally, a comparison by both race and sex is necessary because racial differences in health and mortality may differ in degree and possibly direction by sex (Manton and Stallard, 1997). While recognizing that both sex-linked biology and gender can independently or jointly affect health status and mortality, hereafter, I refer to both the biological construct of sex and sociological construct of gender as sex (Krieger, 2003).
Disparities in Mortality and Total Life Expectancy
In the US, men experience higher age-adjusted mortality rates for most, if not all, major causes of death (Nathanson, 1984; Arias, 2006). The greatest gender differences occur in diseases of the heart and malignant neoplasms, in which male mortality has exceeded that for women since at least 1900 and 1944, respectively (Ciocco, 1940; Linder and Grove, 1947). Consequently, life expectancy at all ages favors women. The gender gap is likely the result of a complex interaction of behavioral and biological factors (Newman and Brach, 2001). With respect to behavior, higher historical rates of smoking, obesity (Calle et al., 1999), and unintentional injury (NCHS, 2006) in addition to lower rates of preventive health care (Doyal, 2001) have contributed to greater male mortality rates. Biologically, differences in the level of various sex hormones between men and women may decrease the risk cardiovascular disease of the latter through favorable changes in blood pressure, serum lipids, insulin and glucose levels (Muller et al., 2003).
Important racial disparities in life expectancy also exist. Studies that assess disparities in cohort life expectancy consistently show blacks experience higher mortality than whites within sex (Manton and Stallard, 1997; Parnell and Owens, 1999; Harper et al., 2007). Comparisons of both cohort and period life expectancy by race are difficult because the accuracy of age reported on death certificate information and population counts are often questionable, especially for older blacks (Rosenwaike, 1968; Rosenwaike and Logue, 1983; Coale and Kisker, 1990). A significant source of unreliability for these older blacks is unregistered births (Elo and Preston, 1994). A second concern of cohort life expectancy is waiting time; meaningful estimation requires the cohort to be extinct, or at least nearly extinct (Wilmoth et al., 2005). As of 2006, for example, cohorts born later than 1916 still have a sizable proportion of their populations alive. Despite these data and waiting time considerations, the advantage of cohort life expectancy is that it represents the mortality experience of a real birth cohort aging over time and avoids the influence of distorting cohort effects.
Analyzing the mortality of birth cohorts, Manton and Stallard (1997) found racial disparities in the age and sex-specific mortality rates of the 1887 and 1892 birth cohorts using the method of extinct generations. In this method, the mortality rate at a given age is equal to the ratio of the number of deaths at this age to the number of deaths at this age and all later ages in all later years for this cohort (Vincent, 1951). The method reduces the impact of age of death misstatement on death certificates by allowing the error to appear in both the numerator and denominator of the mortality rate (Johnson, 2000). It allows estimation of mortality rates and life expectancy by race and sex based solely on death certificate information, avoiding biases in census estimates of blacks.
Careful consideration of data concerns, especially age misreporting, in period-based studies have also consistently shown disparities by sex and race. Using high quality Medicare data, Kestenbaum (1992) showed a racial crossover in the 1987 period at age 90. A racial crossover implies that white mortality was lower than black mortality until this age, at which point the relationship reversed. Considering both data quality and population heterogeneity, Lynch et al. (2003) found the variance in the distribution of ages of death decreased with blacks, but not whites, across time. Lynch et al. (2003) also found that a black-white period mortality crossover existed and the age of crossover increased over time.
Disparities in Chronic Diseases, Impairments, and Disability
Along with racial and sex differences in disease interaction and health care, disparities in chronic conditions contribute to corresponding disparities in functional loss and disability (Smith and Kington, 1997; Hayward et al., 2000). The most important chronic conditions that contribute to physical disability are osteoarthritis, stroke, heart disease, and diabetes (Guccione et al., 1994). In this section, I discuss disparities in each of these chronic conditions and their importance to physical disability.
First, rheumatoid diseases, especially osteoarthritis, are the most common chronic condition and leading cause of physical limitations among elderly females (Piacavet and Hazes, 2003; Verbrugge and Juarez, 2006). In 1993, the prevalence of self-reported arthritis was 1.43 times greater in 65–74 year old women and and 1.25 times greater in 75-plus year old women compared to men (Verbrugge, 1995). Among women, blacks were about twice as likely as whites to report knee osteoarthritis, controlling for age and weight. Yet among men, blacks were no more likely than whites (Anderson and Felson, 1988). Rheumatoid diseases impact the independence of older adults and are linked to higher activity limitation and incidence of physical disability (Katz, 1995; Song et al., 2006).
Second, disparities in circulatory diseases such as atherosclerosis, anemia, hypertension, heart disease, and stroke are equally important. Lipoprotein(a) is associated with heart disease and stroke and shown to be much higher in black women than white in the Kaiser Permanante Women Twins Study, conducted in 1989–1990 (Selby et al., 1994). Anemia is associated with physical disability and decreased physical performance and muscle strength (Penninx et al., 2004). Guralnik et al. (2004) found anemia to be approximately three times as prevalent in black men (27.5 percent) and black women (28.0 percent) as white men and women (9.2 percent and 8.7 percent, respectively). In a review of twenty-five community and population level studies, Gorey and Trevisan (1998) found hypertension approximately 2.59 times more prevalent among black women than white women in studies conducted between 1960 and 1975 and 1.77 times more prevalent in studies conducted between 1976 and 1990. The prevalence of hypertension among black men compared to white men was approximately 2.20 times greater in the first half and 1.38 times greater in the later second half. In the Duke MacArthur study, Gold et al. (1996) found that even among the healthiest elderly studied, racial differences in hypertension persisted.
Finally, adult onset diabetes mellitus is far more prevalent in blacks than whites (Brancati et al., 1996; Wray et al., 2006) and the excess prevalence among blacks is greater among women than men (Robbins et al., 2000). Diabetes is associated with physical disability. These disabilities are likely to substantially impair functionality and quality of life, especially during the later progression of the disease (Gregg et al, 2000).
Along with racial and sex disparities in disease interaction and health care, disparities in the chronic conditions discussed in this section likely contribute to corresponding disparities in functional loss and disability. Crimmins (2004) argues the most severe physical disability is an inability to provide self-care and is measured by activities of daily living (ADL) including: bathing or showering, dressing, eating, getting in or out of bed or chairs, walking, and using the toilet. Examining five waves of the National Long Term Care Survey from 1982 to 1999, Arbeev et al. (2004) found the highest ADL disability prevalence among black women age 70 and higher (22 to 27 percent) followed by white women (16 to 18 percent). ADL disability prevalence for black and white men was significantly lower, 16 to 23 percent and 10 to 13 percent, respectively. While disparities in these chronic conditions are a signifcant societal concern, they alone do not indicate disparities in quality of life. To estimate this, health and mortality infrmation can be combined into the single measure called disability life expectancy.
Disparities in Disability Life Expectancy
Disparities in disability and total life expectancy may ultimately culminate in disparities in the expected remaining life with disability (disability life expectancy). Defined analogously as total life expectancy, disability life expectancy (DLE) is the expected remaining life with disability (as opposed to without disability) of an individual of a given age. The measure, along with total life expectancy, is ideal for assessing the three competing theories of differential aging.
Assessment of the three competing theories of differential aging using DLE of true birth cohorts offers distinct advantages (Ferraro and Farmer, 1996a) and directly addresses important concerns of previous work. First, DLE and total life expectancy implicitly adjust for differential mortality. Second, estimation of disparities in DLE by cohort is important because it reflects the true health and mortality experience of cohorts aging over time. Cohort analysis based on a long history of consecutive cross-sectional surveys or many waves of panel data is also important. Analysis based on birth cohorts also avoids the infuence of cohort effects (Bongaarts and Feeny, 2002) and does not invoke tenuous stationarity assumptions in age-specifc disability prevalence and age-specifc mortality rates over time required in period-based estimation (Imai and Soneji, 2007).
Period-based evidence consistently shows disparities in DLE by sex, race, and socioeconomic status factors. The proportion of remaining life in disability is lower for men (Crimmins et al., 1989; Manton and Stallard, 1991), whites (Crimmins et al., 1989; Hayward and Heron, 1999), and the most educated (Land and Guralnik, 1994; Crimmins and Saito, 2001; Molla et al., 2004). Many period-based studies have also found widening racial and sex disparities (Dowd and Bengston, 1978; Chappell and Havens, 1980). Yet assessments of the competing theories of differential aging are tenuous given nonstationary mortality and disability over time.
Several cohort studies that account for differential mortality have examined racial and sex disparities in the prevalence of disability and ill health. Some cohort-based studies have found persistent racial and sex disparities in disability (Kelley-Moore and Ferraro, 2004) and subjective health (Ferraro and Farmer, 1996a,b). Other such studies have found widening racial disparities in self-assessed health (Ferraro et al., 1997). These mixed results from cohort studies may be due to differences in the defnition of ill-health. Instead, this work incorporates more nuanced definitions of physical function disability by level of severity.
MEASUREMENT OF DISABILITY AND DISABILITY LIFE EXPECTANCY
Mild and Severe Physical Disability
The biopsychosocial model of disability, developed by Nagi (1965), argues disablement results from the complex interaction of biological, personal, and social forces (Jette, 2006). Verbrugge and Jette (1994) expanded the disablement model by considering personal and environmental factors. Disability, in this framework, is a gap between personal capability and environmental demand (Verbrugge and Jette, 1994). The social and physical environment may become more hospitable for the disabled through the use of personal and equipment assistance, both of which serve as buffers that reduce dysfunction (Verbrugge and Sevak, 2002).
The number of ADL dysfunctions may indicate the level of personal capability and serves as a useful means to distinguish between mild and severe disability. One ADL dysfunction may be indicative of mildly degraded personal capability. Two or more ADL dysfunctions, on the other hand, may indicate severe degradation of both fine and gross motor skills and could involve both the upper and lower body.
Considering both personal capability and environment, Verbrugge and Jette (1994) distinguish intrinsic and actual disability. The former refers to difficulty performing an activity without personal or equipment assistance while the latter refers to difficulty even with the use of such assistance. For example, an individual with limited hand function because of osteoarthritis may face significant difficulty using eating utensils and suffer from an intrinsic disability. With the aid of special utensils with bendable shafts, the physical environment becomes less restrictive and disability lessens.
The use of assistance, either personal or equipment, is based on both need and access. In their study of elderly community residents, Verbrugge and Sevak (2002) found severity of disability and need were strongly associated with assistance use. Also, among the severely disabled, equipment assistance was preferred over personal assistance. In addition to need, both types of assistance require access. While access to personal assistance often comes in the form of co-residing family members, access to equipment assistance requires eligibility from Medicare, Medicaid, and supplemental private insurance or self-pays.
Medicare is the largest health insurance program in the U.S., which covered between 95.3 percent and 96.5 percent of the U.S. population age 65 and older between the years 1991 and 2003 (DeNavas-Walt et al., 2005). A person over age 65 is eligible for coverage if he or she has paid Medicare taxes for at least 40 credits. Typically, one credit is equal to one fiscal quarter. The spouse or widow of a worker who has earned enough credits is also eligible if they were married for at least ten years.
Equipment assistance is covered through Medicare’s durable medical equipment (DME) benefit, which, in 2003, totae 7.7 billion dollars (MedPAC, 2005). Assistive devices useful for ADL dysfunctions and covered under the Medicare DME benefit include: bed pans, canes, commodes, crutches, hospital beds, patient lifts, seat lifts, traction equipment, walkers, and wheelchairs (Iwashyna and Christie, 2007). Per approval of a beneficiary’s doctor or other health care provider, the Medicare DME benefit covers 80 percent of the equipment cost, after the deductible. The beneficiary is responsible for payment of the remaining 20 percent, which may come from state Medicaid for poor elderly, supplemental private insurance, or self-pay. Although chronically disabled elderly are eligible for the Medicare DME benefit, it may not provide sufficient access to equipment assistance because of prohibitive cost, dissatisfaction with quality and coordination of care, and supply barriers (Rosenbach, 1995; Wolff et al., 2005; Iwashyna and Christie, 2007; Hoffman et al., 2007).
I focus on the elderly Medicare population and use the the 1991 Medicare Current Beneficiary Survey (MCBS) (available through the Inter-university Consortium for Political and Social Research (ICPSR)), 1992 and 1993 MCBS Access to Care (available through the ICPSR), and 1994 to 2003 MCBS Cost and Use (available through the U.S. Department of Health and Human Services). The MCBS is a continuous, multi-purpose survey of a representative national sample of the Medicare population. The Medicare population includes both the noninstutitionalized and the institutionalized populations and is conducted by the Centers for Medicare and Medicaid Services. The main advantage of the MCBS over other national surveys such as the National Health Interview Survey is the inclusion of both noninstitutional-ized (i.e., community) and institutionalized (i.e., nursing home) residents.
Although the data includes both community and institutionalized Medicare recipients, results may not be generalizable for all elderly. As noted earlier, the overall proportion of elderly not eligible for Medicare is relatively small. Yet, among blacks the proportion uninsured is nearly is twice as high as that for whites (Mold et al, 2004). The uninsured are composed of the ineligible and unenrolled. Many elderly blacks may have worked in jobs in which they did not pay Medicare taxes or in companies that opted out of the program. Also, some uninsured elderly blacks are eligible but did not enroll because of poor access to program information.
Each respondent in the MCBS was asked the following question: “Because of a health or physical problem, do you have any difficulty ... ?” where “...” represent ADLs. Each respondent who responded affirmatively to an ADL dysfunction was also asked two questions regarding personal and equipment assistance. First, “Do you receive help from another person with ...?” and “Do you use special equipment or aide to help you with ...?” where ... represent a reported ADL dysfunction. Nearly all national health surveys ask a variation of these questions allowing estimation of physical function disability. Yet, no regular annual health survey, to the best of this author’s knowledge, directly addresses actual and intrinsic disability. To do so would require a survey question such as, “even with the use of special equipment or aide to help you with ..., do you still have any difficulty with ...” where ... again represents a reported ADL dysfunction. Despite the constraints of the survey questions and wording, assessing disparities by level of severity provides a more accurate description of the complex nature of disability.
Motivated by the disablement process, I assess level of disability using two set of criteria. Under the first set, based on the number of ADL dysfunctions, I define mild disability as zero to one dysfunction, severe between two and six. Under the second set, based on assistance, a respondent was considered to have mild disability if he/she self-reported at least one ADL dysfunction, but no use of personal assistance or special equipment to aide or help with this ADL. Severe disability was defined as a respondent who self-reported at least one ADL dysfunction and used either personal assistance or special equipment to aide or help with this ADL. In all the anlayses presented below, the survey weights are incorporated so that respondents are approximately weighted according to their population size.
Access would not be an important factor in a Medicare system with little or no copay on DME, high quality and efficient coordination of care, and few supply barriers. In such a system, usage would be based solely on need. The first criteria, based on number of ADL dysfunctions, would address intrinsic disability. The second criteria, based on use of personal assistance and assistive equipment, would address actual disability in this universal access environment. Yet, in the current Medicare system, limited access may prohibit use despite need. Those who report using personal assistance and especially special equipment to aide with an ADL dysfunction may only represent a select group with sufficient access out of the larger population with need.
Estimation of Disability Life Expectancy from a Cohort Life Table
DLE is estimated using the extension of Sullivan’s method to birth cohorts developed by Imai and Soneji (2007). The extension combines the disability prevalence and mortality rates of an birth cohort into a single summary measure of the cohort’s health status. The key idea of the new method is to combine the cohort life table with age-specific disability prevalence estimated from either consecutive cross-sectional surveys or a longitudinal survey. In particular, the new method simply partitions the total number of person-years lived, which is obtained from the cohort life table, into mild and severe DLE based on the proportion with mild and severe disability, which is in turn measured from the disability surveys.
Imai and Soneji (2007) prove that the cohort-based extension of Sullivan’s method provides a statistically unbiased and consistent estimator of mild and severe DLE and offers many advantages over the period-based Sullivan’s method and the multi-state life table method. First, the cohort extension is based on a cohort life table, which describes the mortality experience of a real cohort of individuals from birth of the first to death of the last member of the group (Chiang, 1984). Second, the cohort extension avoids tenuous stationarity assumptions, notably constant age-specific mortality rates and age-specific disability prevalence over time (e.g., Chiang, 1984; Preston et al., 2001). Third, compared to the multi-state life table method, the cohort extension of Sullivan’s method avoids theoretical and data driven assumptions that may affect estimation of DLE (Imai and Soneji, 2007).
A final advantage occurs when the disability surveys do not cover the entire age interval. In this case, Imai and Soneji (2007) apply the method of bounds and invoke assumptions about the nature of disability. For example, mortality rates for the 1909 birth cohort are observed for ages 61 to 94 because the mortality data is available from 1970 to 2003. Using the method of extinct generations, smoothed mortality rates are estimated for even higher ages until a specified maximum age (e.g., 100), at which point the cohort is considered extinct and the life table closed. Yet, disability prevalence for this birth cohort is only available for ages 82 to 94 because the disability surveys are available from 1991 to 2003. To estimate DLE at age 95, I can bound it by considering the minimum and maximum values of the contribution of disability person years within the last age intervals not covered by the disability survey. Disability prevalence is bounded between 0 and 1 for all ages. Then, the bounds for disabled person years for these intervals are bounded by 0 and the total person years in these intervals. These bounds may not be informative in practice.
In order to further narrow the bounds, Imai and Soneji (2007) entertain an additional monotonicity assumption regarding the nature of disability for older ages. In particular, disability prevalence is assumed to increase monotonically with age. In the example, disability prevalence for ages 95 and beyond is bounded between the observed disability prevalence for age 94 and 1. Then, the new bounds for disability person years at age 95 are between the disability person years at age 94 and the total person years at age 95. Similarly, if disability surveys do not cover earlier age intervals, I can obtain the bounds of DLE using the monotonicity assumption. In particular, disability prevalence for earlier ages is no greater than the first observed disability prevalence.
It is possible to obtain the simultaneous confidence interval of the lower and upper bound of DLE using the bootstrap method (Beran, 1988). This simultaneous confidence interval is asymptotically balanced and has correct overall coverage probability given sufficient number of bootstrap replications.
For this analysis, I estimate cohort life tables for birth cohorts born between 1904 and 1909 based on mortality data collected in US Vital Statistics and mortality rates calculated by the method of extinct generations by single year of age, sex, and race. These birth cohorts are chosen for study because they experience considerable disability and mortality during the window of available data. They are also old enough to be nearly extinct at the last year of available mortality information.
I then estimate mild and severe disability prevalence by single year of age, sex, and race for these cohorts using consecutive MCBS data from 1991 to 2003. Given the variability in observed disability prevalence, I use a model-based adjustment to estimate the disability prevalence as a smooth function of age and functions of year of survey, race, and sex. In particular, I model the disability prevalence using the generalized additive model (GAM) (Hastie and Tibshirani, 1990). Finally, I combine disability prevalence and mortality rates of birth cohorts to estimate mild and severe DLE using the methodology discussed in this section.
RESULTS
Mild and Severe Disability
Difficulty walking was the most prevalent ADL dysfunction for nearly all race-sex groups over age and time among the oldest old Medicare population. For example, Table 1 shows the age-adjusted prevalence of waking dysfunction for the 1909 birth cohort between ages 82 and 94. Black women reported the highest prevalence of this ADL dysfunction. Of the 57 percent who reported a walking dysfunction, 12 percent required only personal assistance, mostly from their husbands and children; 19 percent required only equipment assistance such as canes, walkers, and wheelchairs; and 18 percent required a combination of both types of assistance. White women and black men report the next highest prevalence of walking dysfunction (both 51 percent) followed by white men (41 percent). As previously discussed in the prevalence of disability with equipment usage among women and minorities may be a conservative estimate because of differentially constrained access.
TABLE 1.
Age-Adjusted Prevalence of Walking Dysfunction in the 1909 Birth Cohort Between Ages 82 and 94. This Table Shows the Age-Adjusted Prevalence of Self-Reported Walking Dysfunction Without Any Assistance, with Personal Assistance Only, with Assistive Equipment Usage Only, and with Both Personal Assistance and Assistive Equipment for all Race-Sex Groups of the 1909 Birth Cohort Between Ages 82 and 94
| Dysfunction |
|||||
|---|---|---|---|---|---|
| No Dysfunction | No Assistance | Personal Only | Equipment Only | Personal & Equipment | |
| White Men | 0.59 | 0.09 | 0.05 | 0.15 | 0.11 |
| White Women | 0.49 | 0.1 | 0.12 | 0.17 | 0.13 |
| Black Men | 0.49 | 0.1 | 0.05 | 0.23 | 0.13 |
| Black Women | 0.43 | 0.08 | 0.12 | 0.19 | 0.18 |
Considering the number of and use of assistance for ADL dysfunctions in this birth cohort, black women report the highest prevalence of both mild and severe physical disability among all race-sex groups. Table 2 presents the prevalence estimates for two ages, 82 and 93, by severity level. The prevalence of mild disability, measured by either criteria, is approximately equal for all race-sex groups at both ages considered. Severe disability, however, is higher among females than males. For example, among white women alive at age 82, between 18 percent and 19 percent reported two or more ADL dysfunctions compared to 14 percent to 15 percent for white men. Of those alive eleven years later, between 63 percent and 69 percent of white women reported severe disability compared to 56 percent to 63 percent of white men. Also of note is that the increase over age in severe disability, based on either criteria, was nearly equal for all race-sex groups. Assessing changes in race-sex disparities over age based solely on disability prevalence is problematic. As Table 2 indicates, severe disability prevalence was higher for black women than for white men and the change over age was approximately equal. Yet this could occur by many distinct processes. For example, mortality and severe disability incidence of white men could both have exceeded that for black women. Also possible is that the mortality of white men could have been higher than that of black women while severe disability rose much faster for the latter than for the former. Indeed, a major critique of the multiple jeopardy hypothesis is the failure to account for differential mortality (Ferraro and Farmer, 1996a).
TABLE 2.
Prevalence of Mild and Severe Disability for the 1909 Birth Cohort at Select Ages. This Table Shows the Prevalence of Mild and Severe Disability for the 1909 Birth Cohort at Ages 82 and 93, Severity Assessed by the Number of ADL Dysfunctions and Use of Personal or Equipment Assistance
| Age | Criterua | Severity
Level |
White |
Black |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men |
Women |
Men |
Women |
|||||||||||
| 95 Percent CI |
95 Percent CI |
95 Percent CI |
95 Percent CI |
|||||||||||
| Median | Lower | upper | Median | Lower | upper | Median | Lower | Upper | Median | Lower | Upper | |||
| 82 | Number ADL | Mild | 0.14 | 0.13 | 0.15 | 0.15 | 0.15 | 0.16 | 0.14 | 0.13 | 0.15 | 0.15 | 0.14 | 0.16 |
| Severe | 0.14 | 0.14 | 0.15 | 0.18 | 0.18 | 0.19 | 0.15 | 0.15 | 0.16 | 0.20 | 0.19 | 0.20 | ||
| Assistance | Mild | 0.14 | 0.13 | 0.16 | 0.16 | 0.14 | 0.17 | 0.15 | 0.14 | 0.16 | 0.16 | 0.15 | 0.17 | |
| Severe | 0.14 | 0.13 | 0.15 | 0.18 | 0.18 | 0.19 | 0.15 | 0.14 | 0.16 | 0.19 | 0.19 | 0.20 | ||
| 93 | Number ADL | Mild | 0.20 | 0.19 | 0.21 | 0.21 | 0.20 | 0.22 | 0.19 | 0.18 | 0.21 | 0.21 | 0.20 | 0.22 |
| Severe | 0.61 | 0.56 | 0.63 | 0.68 | 0.63 | 0.69 | 0.62 | 0.58 | 0.64 | 0.69 | 0.65 | 0.71 | ||
| Assistance | Mild | 0.09 | 0.08 | 0.10 | 0.09 | 0.08 | 0.10 | 0.09 | 0.08 | 0.10 | 0.09 | 0.09 | 0.10 | |
| Severe | 0.69 | 0.65 | 0.71 | 0.75 | 0.72 | 0.77 | 0.71 | 0.66 | 0.73 | 0.77 | 0.73 | 0.78 | ||
Changes Over Age and Time in Total and Disability Life Expectancy
Total life expectancy and DLE implicitly adjust for differential mortality. The latter considers the disability and mortality experience of only those alive at a given age. As previously discussed men experience higher mortality than women throughout infancy, adolescence, and adulthood. The same pattern holds true among the oldest old. As shown in the lower half of Figure 2, total life expectancy favors women for the 1909 birth cohort by 1.5 years at age 82, 0.70 years at age 88, and 0.25 years at age 93. These differences are consistent across race. A convergence of total life expectancy appears within sex in both birth cohorts considered in Figure 2. For example, by age 82, white and black men experienced nearly identical total life expectancy, as did white and black women. This near equality continued over the final ages of this cohort.
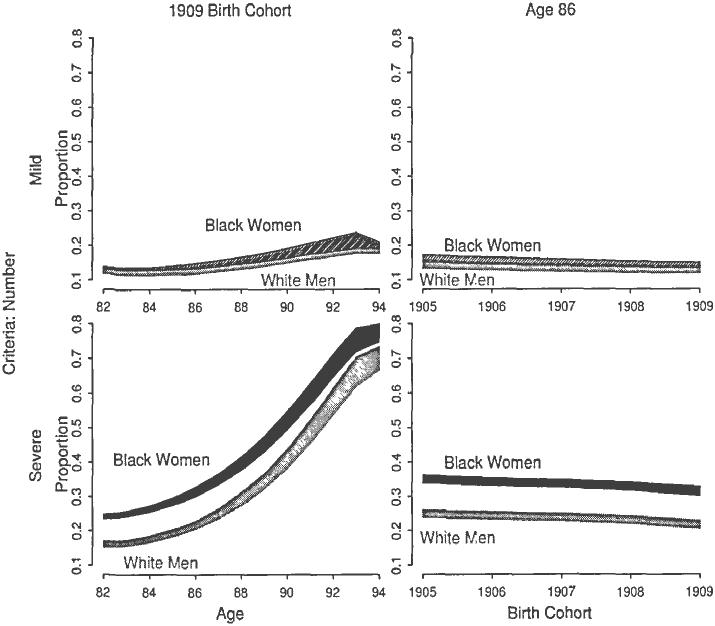
Fig. 2.
Proportion of Remaining Life in Mild and Severe Disability for White Men and Black Women. The shaded region represents the 95 percent confidence interval of the proportion of remaining life spent in mild and severe disability. Severity assessed by the number of ADL dysfunctions.
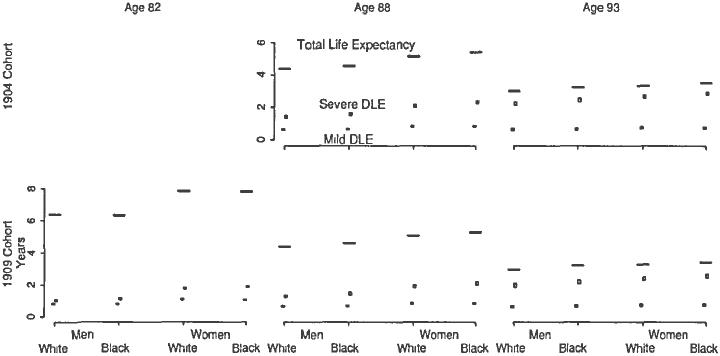
Examination of the older 1904 and younger 1909 birth cohorts across several ages allow direct assessment of the first research question. The upper half of Figure 2 shows mild and severe DLE (based on number of ADL dysfunctions) for ages 88 and 93 in the 1904 birth cohort. As with white and black men, the balanced 95 percent confidence interval of the bounds of mild DLE intersects at ages 88 and 93 for white and black women. The balanced 95 percent confidence intervals are estimated using the boostrap procedure described previously with 2,000 replications. The disparities in severe DLE persist across age for blacks within sex. For example, at age 88, severe DLE is at least 0.32 years higher for black men than white men. At age 93, the disparity only slightly lessens, with at least a 0.29 year difference.
A similar pattern over age appears in the younger 1909 birth cohort with mild DLE. Within sex, the balanced 95 percent confidence interval of the bounds of mild DLE intersects at all ages considered for whites and blacks. Yet, changes over age in severe disability in this younger cohort do not follow the same pattern observed in the older cohort. Within sex, severe DLE is significantly greater for blacks than whites at age 82. Unlike the 1909 birth cohort, the disparities narrow by age 88 and are nonexistent by age 93. A similar set of patterns appears with mild and severe DLE based on assistance.
In order to assess changing disparities across age and time by race and sex, I introduce an additional quantity of interest. The proportion of remaining life in disability adjusts for total life expectancy and allows relative comparison of race-sex groups. Two such comparisons are possible: white men versus black women and white women versus black men. The former is shown in Figure 2. The left half shows the proportion of remaining life spent in mild and severe disability over age for the 1909 birth cohort. Severity is assessed by the number of ADL dysfunctions, in this case. Although minorities and women experience disparities in health and mortality throughout life, by age 82, white men and black women experience nearly identical proportions in mild disability. For severe disability, however, black women experience significantly greater proportions than white men for all ages considered. At age 82, for example, the proportion of remaining life in severe disability is between 23 percent and 25 percent, while only between 15 percent and 17 percent for white men. Similar disparities existed in older birth cohorts.
Age-specific disparities persist across birth cohorts, as shown in the right half of Figure 2. The proportion of remaining life spent in mild disability at age 86, for example, decreased slightly across birth cohorts and was nearly identical for white men and black women. Yet disparities in the severe disability proportion persisted across time. A similar persistence of disparities in this proportion occurred for other advanced ages across birth cohorts.
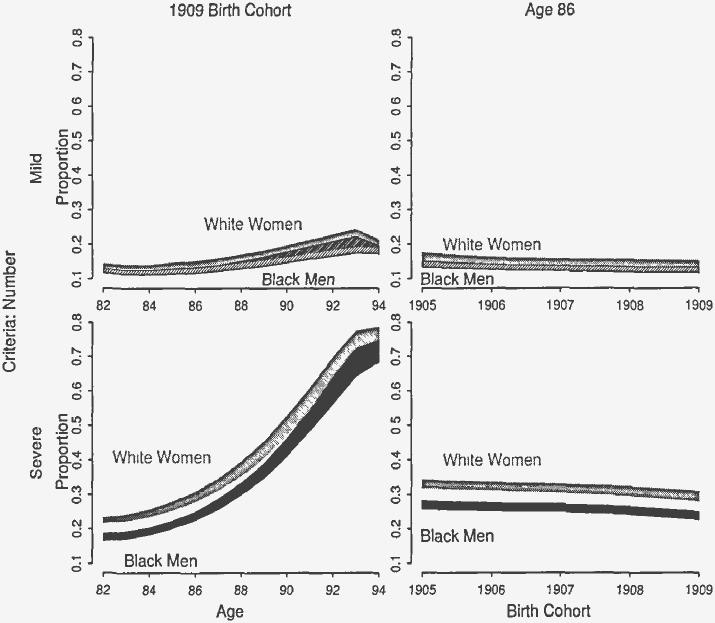
A similar comparison between white women and black men reveals surprising results as shown in Figure 3. White women and black spend nearly identical proportions of their remaining life in mild disability. Yet for severe disability, the proportion for white women is significantly higher between ages 82 and 90. At age 82, for example, the proportion of remaining life in severe disability is between 22 percent and 23 percent, while only between 15 percent and 17 percent for black men. Age-specific disparities in the proportion of remaining life spent in severe disability, though not in mild disability, persisted across birth cohorts. These results, along with those for white men and black women, provide direct evidence of persistent inequality by race and sex among the oldest old in severe disability. These results also show racial convergence and equality in mild disability among the surviving oldest old.
Fig. 3.
Proportion of Remaining Life in Mild and Sever Disability for White Women and Black Men. The shaded region represents the 95 percent confidence interal of the proportion of remaining life spent in mild and severe disability. Severity assessed by the number of ADL dysfunctions.
Disability Compression and Expansion
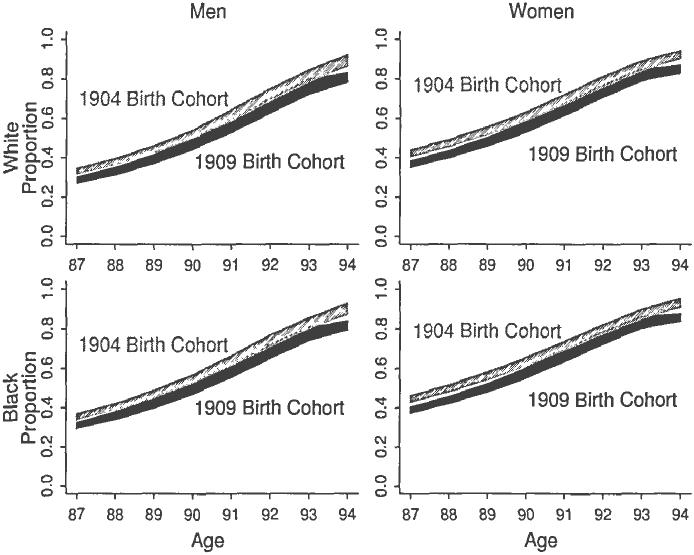
It is possible that age-specific racial and sex disparities in healthy life could narrow over time if black women experience greater disability compression compared to other race-sex groups. Such is the case with severe disability based on use of personal or equipment assistance as shown in Figure 4. For men, stationary age-specific mortality rates were associated with slightly decreasing age-specific severe disability prevalence over time. Consequently, the age-specific proportion of remaining life spent in this disability was nearly constant between the older 1904 and younger 1909 birth cohorts. As seen in the right half of Figure 4, a small, though significant, compression of severe disability occurred between these birth cohorts for women. Between the ages 87 and 90, the proportion of remaining life spent in severe disability decreased over the six years between cohorts.
Fig. 4.
Proportion of Remaining Life Spent in Severe Disability for the 1904 and 1909 Birth Cohorts by Sex and Race. Each panel shows the proportion of remaining life spent in severe disability for two birth cohorts, 1904 and 1909. The shaded region represents the 95 percent confidence interval of the proportion of remaining life spent in this disability. Severity assessed by the use of personal or equipment assistance.
Through Figure 4 presents local (both in age and time) evidence of greater disability compression by women, a conclusion of converging sex disparities would be premature. It remains unclear if such a pattern occurs in other ages and for other birth cohorts. For disparities to eventually converge, the disability compression of women would need to outpace that of men over many cohorts. The age window could be widened by invoking the monotonicity assumption discussed earlier. Yet doing so would increase the bounds because of the increased uncertainty in the unobserved disability prevalence of earlier and older ages. No evidence of disability compression or expansion was seen with mild disability based on assistance or with either mild or severe actual disability based on the number of ADL dysfunctions for the ages and cohorts considered.
CONCLUDING REMARKS
This paper produces several important results that help answer the question of whether the racial and sex gap in healthy life narrow, persist, or expand over age and time, particularly considering severity of ill health, among the oldest old. First, racial gaps within sex converge over age and time for both levels of severity. Second, sex gaps within race persist over age and time. Third, race-sex gaps were nonexistent in mild disability but persisted over age and time for severe disability among the oldest old. Finally, analysis over a relatively narow age and time window provided evidence of slight compression in severe disability for women.
This paper helps to explain the seemingly inconsistent results of previous studies assessing competing theories of differential aging among the oldest old. Changes in healthy life disparities likely differ by level of disability severity even in this advanced age group with nearly equivalent age and sex-specific mortality. Narrowing disparities in mild DLE are consistent with the findings of Johnson (2000); Manton and Gu (2001). The finding of persistent inequality in severe disability by race and sex is consistent with previous studies (Ferraro, 1987; Guralnik et al., 1993; Ferraro and Farmer, 1996a; Kelley-Moore and Ferraro, 2004).
Persistent race-sex inequality in severe DLE over age and time indicates the nature of disability may be far worse for women, especially black women. The disparities are likely to persist into the future as evidenced by only slightly greater compression in severe DLE among black women compared to other race-sex groups. They are indicative of greater medical care and services among these disadvantaged groups. Women, especially black women, may continue to experience sex inequality in health and quality of life even in the oldest old ages.
The present study provides a basis for future work in sex and racial disparities in healthy life. Additional years of disability survey would allow a wider age window for analysis and assessment of changes in racial and sex disparities over age and time. Also, a more detailed analysis of the differential use of special equipment and aides through the Medicare durable medical equipment benefit would allow greater insight into constrained access among women, especially black women.
Three major limitations to this study should be considered when interpreting these results. First, results may not be generalized for all oldest old in these birth cohorts because they are based on Medicare population. The proportion of uninsured blacks, either ineligible or unenrolled, is relatively higher than for whites. They may experience worse health and poorer access to assistance, especially costly equipment assistance.
Second, the study assesses changes in racial and sex disparities over a small range of birth cohorts, 1904 to 1909. The findings may not be generalizable to cohorts born many years earlier or later given the nonstationarity in advances in medicine, disability prevalence, and mortality rates. Finally, disability prevalence in this paper is based on self-reports. Cognitive impairment may be quite high among the oldest old and recollection of a doctor’s report of a chronic condition, disability, or equipment usage may become increasingly questionable with advanced age. Nevertheless, the evidence of equality in mild disability yet persistent inequality in severe disability in this paper provides motivation for further research into changing racial and sex health disparities over age and time.
Fig. 1.
Total Life Expectancy and Disability Life Expectancy (DLE) by Severity Level. This graph shows the total life expectancy and disability life expectancy by level of severity for select ages and birth cohorts. Mild disability is defined as less than three ADL dysfunctions. Severe disability is defined as three or more ADL dysfunctions. Total life expectancy is represented as a horizontal line. The balanced 95 percent confidence interval of the bounds of mild (severe) DLE is shown as a(n) closed (open) box. Estimates derived from US period of life tables and Medicare Current Beneficiary Survey.
Note: Data for the 1904 burn cohort at age 82 are not available because the Medicare Current Beneficiary Survey started in 1991.
REFERENCES
- Anderson J, Felson D. Factors associated with osteoarthritis of the knee in the first National Health and Nutrition Examination Survey (HANES I) American Journal of Epidemiology. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- Arbeev K, Butov A, Manton K, Sannikov I, Yashin A. Disability trends in gender and race groups of early retirement ages in the USA. Sozial-Und Praventivmedizen. 2004;49:142–151. doi: 10.1007/s00038-004-3041-y. [DOI] [PubMed] [Google Scholar]
- Arias E. United States life table, 2003. 2006;54:14. National Vital Statistics Reports. [PubMed] [Google Scholar]
- Beran R. Balanced simultaneous confidence sets. Journal of the American Statistical Association. 1988;83:679–686. [Google Scholar]
- Bongaarts J, Feeny G. How long do we live? Population and Development Review. 2002;28:13–29. [Google Scholar]
- Brancati F, Whelton P, Kuller L, Klag M. Diabetes mellitus, race, and socioeconomic status a population-based study. Annals of Epidemiology. 1996;6:67–73. doi: 10.1016/1047-2797(95)00095-x. [DOI] [PubMed] [Google Scholar]
- Calle E, Thun M, Petrelli J, Rodriguez C, Heath CJ. Body-mass index and mortality in a prospective cohort of U.S. adults. New England Journal of Medicine. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Chappell N, Havens B. Old and female: Testing the double jeopardy hypothesis. The Sociological Quarterly. 1980;21:157–171. [Google Scholar]
- Chiang CL. Robert E. Krieger Publisher, Co.; Malabar, Florida: 1984. The Life Table and Its Applications. [Google Scholar]
- Ciocco A. Sex differences in morbidity and mortality. The Quarterly Review of Biology. 1940;15:59–73. [Google Scholar]
- Clark D. US trends in disability and institutionalization among older blacks and whites. American Journal of Public Health. 1997;87:438–440. doi: 10.2105/ajph.87.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Maddox G, Steinhauser K. Race, aging, and functional health. Journal of Aging and Health. 1993;5:536–553. [Google Scholar]
- Coale A, Kisker E. Defects in data on old-age mortality in the United States: New procedures for calculating mortality schedules and life tables at the highest ages. Asian and Pacific Population Forum. 1990;4: 1–31. [Google Scholar]
- Crimmins E. Trends in the health of the elderly. Annual Review of Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Saito Y. Trends in health life expectancy in the United States, 1970-1990: gender, racial, and educational differences. Social Science and Medicine. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Saito Y, Ingeneri D. Changes in life epectancy and disability-free life expectancy in the United States. Population and Development Review. 1989;15:235–267. [Google Scholar]
- Crimmins E, Saito Y, Ingeneri D. Trends in disability-free life expectancy in the United States, 1970-90. Population and Development Review. 1997;23:555–572. [Google Scholar]
- Cutler D. The reduction in disability among the elderly. Proceedings of the National Academy of Science. 2001;98:6546–6547. doi: 10.1073/pnas.131201698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darity W. Employment discrimination, segregation, and health. American Journal of Public Health. 2003;93:226–231. doi: 10.2105/ajph.93.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor B, Hill Lee C. Current Population Reports, P60-229, U.S. Census Bureau. U.S. Government Printing Office; Washington, DC: 2005. Income, poverty, and health insurance coverage in the United States: 2004. Tech. Rep. [Google Scholar]
- Dowd J, Bengston V. Aging in minority populations: An examination of the double jeopardy hypothesis. Journal of Gerontology. 1978;33:427–36. doi: 10.1093/geronj/33.3.427. [DOI] [PubMed] [Google Scholar]
- Doyal L. Sex, gender, and health: the need for a new approach. British Medical Journal. 2001;323:1061–1063. doi: 10.1136/bmj.323.7320.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo I, Preston S. Estimating African-American mortality from inaccurate data. Demography. 1994;31:427–458. [PubMed] [Google Scholar]
- Ferraro K. Double jeopardy to health for Black older adults? Journal of Gerontology. 1987;42:528–533. doi: 10.1093/geronj/42.5.528. [DOI] [PubMed] [Google Scholar]
- Ferraro K, Farmer M. Double jeopardy, aging as leveler, or persistent health inequality? A longitudinal analysis of white and black Americans. Journal of Gerontology: Social Sciences. 1996a;51B:S319–S328. doi: 10.1093/geronb/51b.6.s319. [DOI] [PubMed] [Google Scholar]
- Ferraro K, Farmer M. Double jeopardy to health hypothesis for African Americans: Analysis and critique. Journal of Health and Social Behavior. 1996b;37:27–43. [PubMed] [Google Scholar]
- Ferraro K, Farmer M, Wybraniec J. Health trajectories: long-term dynamics among black and white adults. Journal of Health and Social Behavior. 1997;38:38–54. [PubMed] [Google Scholar]
- Freedman V, Martin L. The role of education in explaining and forecasting trends in functional limitations among older Americans. Demography. 1999;36:461–473. [PubMed] [Google Scholar]
- Gibson R. Age-by-race differences in the health functioning of elderly persons. Journal of Aging and Health. 1991;3:335–351. doi: 10.1177/089826439100300302. 28. [DOI] [PubMed] [Google Scholar]
- Gold D, Peiper C, Westlund R, Blazer D. Do racial differences in hypertension persist in successful agers? Findings from the MacArthur study of sucessful aging. Journal of Aging and Health. 1996;8:207–219. doi: 10.1177/089826439600800203. [DOI] [PubMed] [Google Scholar]
- Gorey K, Trevisan M. Secular trends in the United States black/white hypertension revalence ratio: Potential impact of diminishing response rates. Amerc Journal of Epidemiology. 1998;147:95–99. doi: 10.1093/oxfordjournals.aje.a009434. [DOI] [PubMed] [Google Scholar]
- Gregg E, Langlois J, Beckles G, Engelgau M, Williamson D, Narayan KV, Leveille S. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- Guccione A, Felson D, Anderson J. The effects of special medical conditions of the functional limitations of elders in the Framingham Study. American Journal of Public Health. 1994 doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik J, Eisenstaedt R, Ferrucci L, Klein H, Woodman R. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- Guralnik J, Land K, Blazer D, Fillenbaum G, Branch L. Educational status and active life expectancy among older blacks and whites. New England Journal of Medicine. 1993;329: 110–116. doi: 10.1056/NEJM199307083290208. [DOI] [PubMed] [Google Scholar]
- Harper S, Lynch J, Burris S, Smith GD. Trends in the black-white life expectancy gap in the United States, 1983–2003. Journal of the American Medical Association. 2007;297:1224–1232. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani R. Chapman Hall, London: 1990. Generalized Additive Models. [Google Scholar]
- Hayward M, Heron M. Racial inequality in active life among adult Americans. Demogrphy. 1999;36:77–91. [PubMed] [Google Scholar]
- Hayward M, Miles T, Crimmins E, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review. 2000;65:910–930. [Google Scholar]
- Hoffman J, Shumway-Cook A, Yorkston K, Ciol M, Dudgeon B, Chan L. Associaton of mobility limitations with health care satisfaction and use of preventive care: A survey of Medicare beneficiaries. Archives of Physical Medicine and Rehabilitation. 2007;88: 583–588. doi: 10.1016/j.apmr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Imai K, Soneji S. On the estimation of disability free life expectancy: Sullivan’s method ad its extension. Journal of the American Statistcal Association Forthcoming. 2007 doi: 10.1198/016214507000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna T, Christie J. Low use of durable medical equipment by chronically disabled elderly. Journal of Pain and Symptom Management. 2007;33:324–330. doi: 10.1016/j.jpainsymman.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Jette A. Toward a common language for function, disability, and health. Physical Therapy. 2006;86:726–734. [PubMed] [Google Scholar]
- Johnson N. The racial crossover in comorbidity, disability, and mortality. Demography. 2000;37:267–283. [PubMed] [Google Scholar]
- Katz P. The impact of rheumatoid aithritis on life activities. Arthritis Care and Research. 1995;8:272–278. doi: 10.1002/art.1790080411. [DOI] [PubMed] [Google Scholar]
- Kawachi I. Social capital and community effects on population and individual health. Annals of the New York Academy of Sciences. 1999;896:120–130. doi: 10.1111/j.1749-6632.1999.tb08110.x. [DOI] [PubMed] [Google Scholar]
- Kelley-Moore J, Ferraro K. The black/white disability gap: Persistent inequality in later life? The Journal of Gerontology. 2004;59B:S34–S43. doi: 10.1093/geronb/59.1.s34. 30. [DOI] [PubMed] [Google Scholar]
- Kestenbaum B. A description of the extreme aged population based on improved Medicare enrollment data. Demography. 1992;29:565–580. [PubMed] [Google Scholar]
- Kreps G. Communication and racial inequalities in health care. American Behavioral Scientist. 2006;49:760–774. [Google Scholar]
- Krieger N. Genders, sexes, and health: what are the connections–and why does it matter? International Journal of Epidemiology. 2003;32: 652–657. doi: 10.1093/ije/dyg156. [DOI] [PubMed] [Google Scholar]
- Land K, Guralnik J. Estimating increment-decrement life tables with multiple covariates from panel data: The case of active life expectancy. Demogrpahy. 1994;31:297–319. [PubMed] [Google Scholar]
- Lantz P, House J, Lepkowski J, Williams D, Mero R, Chen J. Socioeconomic factors, health behaviors, and mortality. Journal of the American Medical Association. 1998;279:1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- Liao Y, Mcgee D, Cao G, Cooper R. Black-white differences in disability and morbidity in the last years of life. American Journal of Epidemiology. 1999;149:1097–1103. doi: 10.1093/oxfordjournals.aje.a009763. [DOI] [PubMed] [Google Scholar]
- Linder F, Grove R. United States Public Health Service, National Office of Vital Statistics; Washington, D.C.: 1947. Vital statistics in the United States 1900–1940. Tech. rep. [Google Scholar]
- Lynch S, Brown S, Harmsen K. Black-White differences in mortality compression and deceleration and the mortality crossover reconsidered. Research on Aging. 2003;25:456–483. [Google Scholar]
- Manton K, Gu X. Changes in the prevalence of chronic disability in the United States black and nonblack population above age 65 from 1982 to 1999. Proceedings of the National Academy of Sciences. 2001;98:6354–6359. doi: 10.1073/pnas.111152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton K, Stallard E. Cross-sectional estimates of active life expectancy for the U.S. elderly and oldest-old populations. Journal of Gerontology. 1991;46:S170–S182. doi: 10.1093/geronj/46.3.s170. 31. [DOI] [PubMed] [Google Scholar]
- Manton K, Stallard E. National Academy Press; Washington, D.C.: 1997. Racial and ethnic differences in the health of older Americans, chap. Health and Disability Differences Among Racial and Ethnic Groups. [Google Scholar]
- MedPAC . MedPAC; Washington, DC: 2005. A data book: Healthcare spending and the medicare program. Tech. rep. [Google Scholar]
- Mold J, Fryer G, Thomas C. Who are the uninsured elderly in the United States. Journal of the American Geriatric Society. 2004;52:601–606. doi: 10.1111/j.1532-5415.2004.52169.x. [DOI] [PubMed] [Google Scholar]
- Molla M, Madans J, Wagener D. Differentials in adult mortality and activity limitation by years of education in the united states at the end of the 1990s. Population and Development Review. 2004;30:625–646. [Google Scholar]
- Muller M, Van Der Schouw Y, Thussen J, Grobbee D. Endogenous sex homones and cardiovascular disease in men. Journal of Clinical Endocrinology and Metabolism. 2003;88:5076–5086. doi: 10.1210/jc.2003-030611. [DOI] [PubMed] [Google Scholar]
- Nagi S. Sociology and Rehabilitation. American Sociological Association; Washington, DC: 1965. Some conceptual issues in disability and rehabilitation; pp. 100–113. [Google Scholar]
- Nathanson C. Sex differences in mortality. Annual Review of Sociology. 1984;10:191–213. doi: 10.1146/annurev.so.10.080184.001203. [DOI] [PubMed] [Google Scholar]
- NCHS . National Center for Health Statistics; Hyattsville, MD.: 2006. Health, United States, 2006 with chartbook on trends in the health of Americans. Tech. rep. [PubMed] [Google Scholar]
- Newman A, Brach J. Gender gap in longevity and disability in older persons. Epidemiological Reviews. 2001;23:343–350. doi: 10.1093/oxfordjournals.epirev.a000810. [DOI] [PubMed] [Google Scholar]
- Parnell A, Owens C. Evaluation of U.S. mortality patterns at old ages using the Medicare Enrollment Database. Demographic Research. 1999;1:1–30. doi: 10.4054/demres.1999.1.2. 32. [DOI] [PubMed] [Google Scholar]
- Penninx B, Pahor M, Cesari M, Corsi AM, Woodman R, Bandinelli S, Guralnik J, Ferrucci L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. Journal of the American Geriatric Society. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- Piacavet H, Hazes J. Prevalence of self reported musculoskeletal diseases is high. Annals of the Rheumatic Diseases. 2003;62:644–650. doi: 10.1136/ard.62.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S, Heuveline P, Guillot M. Blackwell Publishing; Malden, MA: 2001. Demography: measuring and modeling population processes. [Google Scholar]
- Robbins J, Vaccario V, Zhang H, Kasl S. Excess type 2 diabetes in African-American women and mean aged 40-74 and socioeconomic status: evidence from the Third National Health and Nutrition Examination Survey. Journal of Epidemiology and Community Health. 2000;54:839–845. doi: 10.1136/jech.54.11.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbach M. Access and satisfaction within the disabled medicare population. Health Care Financing Review. 1995;17:147–167. [PMC free article] [PubMed] [Google Scholar]
- Rosenwaike I. On measuring the extreme aged in the population. Journal of the American Statistical Association. 1968;63:29–40. [Google Scholar]
- Rosenwaike I, Logue B. Accuracy of death certificate ages for the extreme aged. Demography. 1983;20:569–585. [Google Scholar]
- Schulz A, Israel B, Willams D, Parker E, Becker A, James S. Social inequalities, stressors ansd self reported health status among African American and white women in Detroit metropolitan area. Social Science and Medicine. 2000;51:1639–1653. doi: 10.1016/s0277-9536(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Selby J, Austin M, Sandholzer C, Quesenberry C, Zhang D, Mayer E, Utermann G. Environmental and behavioral influences of plasma lipoprotein(a) concentration in women twins. Preventive Medicine. 1994;23:345–353. doi: 10.1006/pmed.1994.1048. [DOI] [PubMed] [Google Scholar]
- Singer B, Manton K. The effects of health changes on projections of health service needs for the elderly population of the United States. Proceedings of the National Academy of Science. 1998;95:15618–15622. doi: 10.1073/pnas.95.26.15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Kington R. Demographic and economic correlates of health and old age. Demography. 1997;34:159–170. [PubMed] [Google Scholar]
- Song J, Chang R, Dunlop D. Population impacts of arthritis on disability in older adults. Arthritis Care and Research. 2006;55:248–255. doi: 10.1002/art.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudano J, Baker D. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: The roles of socioeconomic status, health behaviors, and healht insurance. Social Science and Medicine. 2006;62:909–922. doi: 10.1016/j.socscimed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Verbrugge L. Women, men, and osteoarthritis. Arthritis Care and Research. 1995;8:212–220. doi: 10.1002/art.1790080404. [DOI] [PubMed] [Google Scholar]
- Verbrugge L, Jette A. The disablement process. Social Science and Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Verbrugge L, Sevak P. Use, type, and efficacy of assistance for disability. journal of Gerntology: Social Science. 2002;57B:S366–S379. doi: 10.1093/geronb/57.6.s366. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Juarez L. Profiles of arthritis disability: II. Arthritis and Rheumatism (Arthritis Care and Research) 2006;55:102–113. doi: 10.1002/art.21694. [DOI] [PubMed] [Google Scholar]
- Vincent P. La mortalit’e des viellards. Population. 1951;VI:l81–204. [Google Scholar]
- Williams D, Jackson PB. Social sources of racial disparities in health. Health Affairs. 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. 34. [DOI] [PubMed] [Google Scholar]
- Wilmoth J, Andreev K, Jdanov D, Glei D. Methods protocol for the human mortality database. University of California at Berkeley and Max Planck Institute for Demographic Research; 2005. Tech. rep. [Google Scholar]
- Wolff J, Agree E, Kasper J. Wheelchairs, walkers, and canes: What does Medicare pay for, And who benefits? Health Affairs. 2005;24:1140–1149. doi: 10.1377/hlthaff.24.4.1140. [DOI] [PubMed] [Google Scholar]
- Wray L, Alwin D, Manning T, Best L. Social status, risky health behavior and diabetes in middle-aged and older adults. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2006;61:S290–S298. doi: 10.1093/geronb/61.6.s290. [DOI] [PubMed] [Google Scholar]