Abstract
Injuries to the musculoskeletal system are common, debilitating and expensive. In many cases, healing is imperfect, which leads to chronic impairment. Gene transfer might improve repair and regeneration at sites of injury by enabling the local, sustained and potentially regulated expression of therapeutic gene products; such products include morphogens, growth factors and anti-inflammatory proteins. Proteins produced endogenously as a result of gene transfer are nascent molecules that have undergone post-translational modification. In addition, gene transfer offers particular advantages for the delivery of products with an intracellular site of action, such as transcription factors and noncoding RNAs, and proteins that need to be inserted into a cell compartment, such as a membrane. Transgenes can be delivered by viral or nonviral vectors via in vivo or ex vivo protocols using progenitor or differentiated cells. The first gene transfer clinical trials for osteoarthritis and cartilage repair have already been completed. Various bone-healing protocols are at an advanced stage of development, including studies with large animals, and human trials are envisaged. Other applications in the repair and regeneration of skeletal muscle, intervertebral disc, meniscus, ligament and tendon are in preclinical development. In addition to scientific, medical and safety considerations, clinical translation is constrained by social, financial and logistical issues.
Introduction
More than 20 million injuries are inflicted on the musculoskeletal system each year in the USA; sprains, fractures and contusions are the most common. Collectively, they cost the US healthcare system $150 billion per annum1 Musculoskeletal tissues vary considerably in their ability to repair spontaneously after injury2 Most fractures of long bones, for example, heal by themselves, whereas large segmental defects do not. Articular cartilage has almost no intrinsic reparative activity, irrespective of the size of the lesion, and tendons often heal but form a regenerate of inferior quality. Minor muscle injuries, such as strains, heal without intervention, but severe injuries result in the formation of a dense scar. In many cases, the ability of a tissue to regenerate is affected by inflammation and the degree of damage to surrounding tissues.
Much research is devoted to developing technologies that enhance the repair or regeneration of damaged musculoskeletal tissues. Many such strategies depend upon the delivery of morphogens, often proteinaceous growth factors, for example insulin-like growth factor 1 (IGF-1), to orchestrate this process. The importance of this function is reflected by the volume of research devoted to developing scaffolds with the ability to deliver the appropriate factors in a controlled and sustained manner (reviewed elsewhere3–5); this endeavour is proving difficult.
Gene transfer is an alternative technology for delivering gene products to sites of tissue injury.6,7 It offers the prospect of sustained and, ultimately, regulated local synthesis of one or more morphogens in situ. Unlike many recombinant growth factors produced in bioreactors and subjected to packaging and storage, the gene products are nascent proteins synthesized locally with post-translational modification. Gene transfer is also superior to traditional methods for delivering products with an intracellular site of action, such as transcription factors, signalling molecules and noncoding species of RNA, as well as proteins (such as receptors) that need to be inserted into a specific cellular compartment. Extensive preclinical literature supports the concept of using gene therapy to repair and regenerate various components of the musculoskeletal system, and the first human clinical trials have taken place. Other protocols are being advanced towards clinical translation. This Review focuses on the translational aspects of using gene therapy to aid the restoration of the musculoskeletal system.
A gene therapy primer
Vectors
Vectors are used to transfer genes (usually cDNAs) of interest into host cells in a manner that facilitates translocation to the nucleus with subsequent high levels of transgene expression. Viruses are widely used as vectors, because of their inherent ability to translocate their own genetic material efficiently. To create a vector for gene therapy, sequences of the viral genome that contribute to virulence and disease are normally removed and replaced with genes of interest and their regulatory sequences, while retaining infectivity. A number of viruses have been engineered in this way and >1,200 human gene-therapy trials have been performed using viral vectors.8
Different viruses confer different properties on their derivative vectors. The major considerations for human medicine are their biology, safety, ease of manufacture and cost-effectiveness. Biological considerations include the carrying capacity of the vector, the length of time its genome will persist in the body to sustain transgene expression, and the degree to which it generates a neutralizing immune response. Safety concerns have centred on whether or not the vector inserts (integrates) its genetic material into that of the host cell, a process that can lead to insertional mutagenesis and cancer.9 Immune responses to the vector can limit the duration of transgene expression and prevent repeated dosing. In one example, a robust immune reaction to an adenoviral vector led to the death of a participant in a gene therapy trial for ornithine transcarbamylase deficiency.10
Production of recombinant viral vectors is not trivial and influences the ability to perform preclinical studies in the laboratory, the ease of testing in large-animal models, the cost of clinical trials and, ultimately, the price of a gene therapy products in the medical marketplace. The last of these factors is increasingly important and could severely restrict the use of an otherwise successful gene therapy. For example, Glybera® (alipogene tiparvovec; uniQure, Amsterdam, Netherlands), a gene therapy treatment for lipoprotein lipase deficiency, approved by the European Medicines Agency, might cost as much as €1 million for a one-time treatment. Intellectual property issues are also important for research translation. The properties of commonly used viral vectors of relevance to tissue repair and regeneration have been reviewed elsewhere.11
Because of the complexities of viral gene transfer, nonviral vectors are of particular interest. These vectors can be as simple as DNA plasmids, but are usually associated with liposomes or various types of polymer to enhance uptake. Physical stimuli, such as electroporation and sonication, can also enhance transfection efficiency. In general, nonviral vectors are less expensive and easier to construct than viral vectors, but they are much less efficient. As a result, fewer human trials have taken place with nonviral vectors.8 Nonviral gene delivery has been reviewed elsewhere.12
Gene delivery to sites of tissue damage
Vectors can be introduced directly into the body (in vivo delivery) or extracorporeally into cells that are subsequently implanted into the site of injury (ex vivo delivery). The former strategy is simpler and less expensive, but raises greater safety concerns because, after the vectors are introduced into the body, direct control over their activities is not possible. Successful in vivo delivery also requires the existence of a sufficient population of healthy cells, within the damaged tissue, to take up and express the transgene endogenously at appropriate levels. As injuries to the musculoskeletal system are often associated with considerable cell death, this requirement is not always met.
Ex vivo gene delivery obviates these problems and has the advantage of introducing cells, in addition to gene products, to the injury site. Ex vivo gene transfer also meshes well with traditional tissue-engineering approaches in which cell populations are removed from the body, expanded, modified, seeded on a scaffold, incubated in a bioreactor and reimplanted. Although potentially successful, such technologies are likely to be expensive, as using autologous cells in this manner requires two surgeries and a GMP (good manufacturing practice) facility for growing and extensive testing of the genetically modified cells.13 Moreover, primary cell populations do not always expand or transduce well. In view of these contraints, there is interest in using expedited ex vivo gene transfer in which tissue is removed from the patient, genetically modified and reimplanted into the injury site during the course of one operation.13 An alternative strategy is to use allografted cells that can be universal donors.
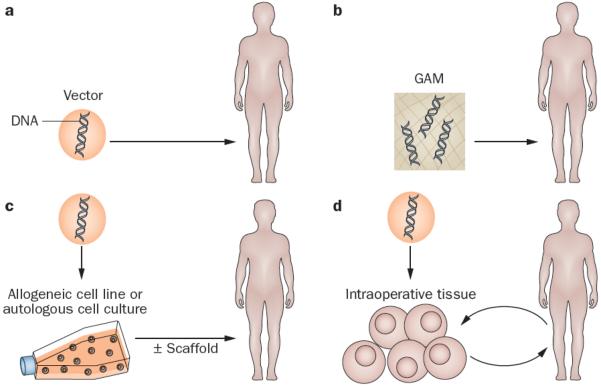
Four different strategies for gene therapy have emerged in the context of treating musculoskeletal injuries, two of them ex vivo and two of them in vivo (Figure 1). The traditional ex vivo approach recovers cells (often progenitor cells) from the patient, expands the population and genetically modifies them prior to reimplantation during a second surgical procedure. This process can be streamlined with the use of allogeneic cell lines. Alternative, expedited ex vivo approaches use tissue biopsy samples (such as bone marrow, fat or muscle) that are subjected to genetic modification intraoperatively, and then immediately reimplanted into the defect during the same operation.13 In the most common in vivo approach, the vector is introduced by direct injection into the defect site. An alternative technology uses a gene-activated matrix (GAM) in which the vector is associated with an implanted scaffold.14
Figure 1.
Strategies for gene transfer to defects into sites of musculoskelet al injury. For in vivo gene delivery (right), the vector is introduced directly into the site of the lesion, either as a free suspension (top right) or incorporated into a GAM (bottom right). For ex vivo delivery (left), vectors are not introduced directly into the defect. Instead, they are used for the genetic modification of cells, which are subsequently implanted. Traditional ex vivo methods (top left) usually involve the establishment of autologous cell cultures, which are genetically modified in vitro. Alternatively, an established allograft cell line can be used. Modified cells are then introduced into the lesion, often after seeding onto an appropriate scaffold. Expedited ex vivo methods (bottom left) avoid the need for cell culture and scaffolds by genetically modifying tissues (such as bone marrow, muscle and fat) intraoperatively and inserting them into the defect during a single operation. Abbreviation: GAM, gene-activated matrix.
Gene therapy progress
Articular cartilage
Damage to the articular cartilage can lead to pain and subsequent osteoarthritis (OA). Because articular cartilage has a limited capacity to regenerate spontaneously, a number of surgical procedures have been developed for its repair.15 In terms of gene therapy, the most pertinent procedures are microfracture and autologous chondrocyte implantation (ACI).
Microfracture is one of several related techniques that enable communication between a full-thickness chondral lesion and the underlying bone marrow. Progenitor cells from the subchondral region enter the lesion and become trapped in the ensuing fibrin clot, where some of them differentiate along a chondrogenic lineage to form repair tissue. Microfracture is thought to have some efficacy for the treatment of small focal lesions, but not larger lesions, for which ACI is usually indicated. The newly formed tissue resulting from microfracture is fibrocartilage, which is less durable than articular cartilage, and is sometimes compromised by the presence of intralesional osteophytes. Nevertheless, this inexpensive and simple technique is reasonably effective and the FDA requires that new cartilage repair methods are superior to microfracture, a ruling with serious implications for clinical trial design.
Two gene-based approaches attempt to improve the effectiveness of microfracture; both are simple intraoperative methods. Using one method, recombinant adeno-associated virus (AAV) is applied directly to the exudate that enters the osteochondral lesion.15–17 In a rabbit osteochondral defect model, fibroblast growth factor 2 (FGF-2),15 IGF-1,16 and the transcription factor SOX9,17 have been delivered by transgene, with promising results.
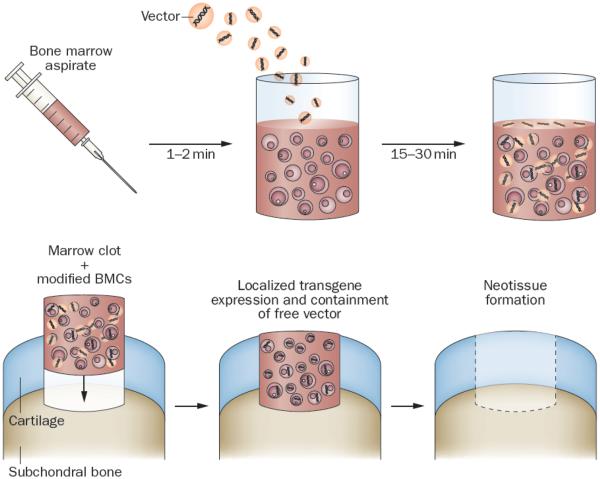
The alternative approach of Pascher et al.18 accelerates the process by removing bone marrow and mixing it with adenovirus vectors during clotting. The clotted bone marrow, containing transduced cells and vector, known as a `gene plug', is then press-fit into the cartilage lesion (Figure 2). Adenovirus is available to transduce additional cells as they migrate into the defect, a process aided by the superior transducing properties of adenovirus when bound to a matrix.19 Promising results have been reported with this method in rabbits using cDNA that encodes bone morphogenetic protein (BMP)-2, although delayed, progressive endochondral ossification was noted.20 In analogous experiments, cDNA encoding Indian hedgehog protein was superior to that encoding BMP-2, repairing cartilage without endochondral ossification.20
Figure 2.
Gene transfer to osteochondral defects using genetically modified, clotted, autologous bone marrow (gene plugs). Freshly aspirated marrow is mixed with a vector that carries appropriate reparative cDNAs, and is allowed to clot in a suitably shaped container. The clot , which now contains genetically modified bone marrow cells and bound vector, is press-fit into the defect. Expression of the transgenes promotes differentiation of progenit or cells within the lesion along the appropriate lineages to regenerate cartilage and subchondral bone. Modified from Pascher, A. et al.,18 with permission.
Ivkovic et al.21 used gene plug technology in a full-thickness chondral defect model in sheep, with TGFB1 cDNA as the transgene. This method improved the outcome, but did not lead to complete repair of the lesion, possibly because the gene plugs did not contain sufficient numbers of chondroprogenitors and were not in communication with the bone marrow as a source of additional cells. The number of progenitors in a gene plug can be augmented by the addition of cells during clotting of the aspirate.18
Another technology involves transplantation of genetically modified autologous skeletal muscle or fat into the defect. These tissues can be harvested, genetically modified, and then press-fit into osteochondral lesions during a single surgery. Results from pilot experiments with rabbits, using adenovirus vectors carrying BMP2 cDNA, are encouraging. Of interest, the implanted tissues formed bone in the subchondral region and cartilage above, indicating the importance of local cues in cell fate.23
Large chondral lesions are sometimes treated by ACI, which requires articular cartilage to be harvested from a lesser-weight-bearing part of the joint. This cartilage is a source of autologous chondrocytes that are expanded in culture and implanted into the defect. Good clinical results have been reported, equal or superior to microfracture.14 The nature of this process lends itself to ex vivo gene therapy.
The application of ACI has been constrained by the high cost of autologous therapy, in which the cell population needs to be expanded before reimplantation, and by the need for two surgeries. The cost and complexity would be greatly reduced if allografted cells could be used. The basis for cartilage repair using genetically modified allografts was provided by Kang et al.,23 who first showed, in rabbits, that genetically modified allografted chondrocytes could persist and express transgenes in osteochondral defects.
A large body of data from small-animal models (using rabbits and rats) confirms that genetically modified allogeneic or autogenous chondrocytes are effective agents of cartilage repair.24,25 Confirmation of efficacy in larger animals has been provided by Nixon and colleagues, who used horse models.26–29 Implantation of allograft chondrocytes following adenoviral transduction with BMP-7 accelerated the early repair process, but by 8 months there was little difference compared with controls.26 Transduction with IGF-1, by contrast, provided a sustained improvement in repair.27–28 In their most recent work, this group used AAV to transfer IGF-1 to autologous chondrocytes, noting improved repair of full-thickness chondral defects.29
Genetically modified allograft chondrocytes have been used in human clinical trials by the South Korean company Kolon Industries.30 A line of human chondrocytes was established from a newborn with polydactyly, and one cohort of cells was transduced with a retrovirus carrying TGFB1 cDNA.31 For cartilage repair, the transduced cells are surgically introduced into cartilage lesions using a fibrin scaffold. Because retrovirus vectors integrate into the host genome and are, thereby, potentially carcinogenic,9 the transduced cells are irradiated prior to implantation and mixed with nontransduced, nonirradiated cells to amplify the effect. This method is undergoing further clinical trials in South Korea.31
Patients with OA often require restoration of articular cartilage, but the process is complicated by a concomitant disease process that produces an environment hostile to cartilage repair. In particular, NFκB-activating proinflammatory cytokines (such as IL-1) inhibit chondrogenesis from mesenchymal stem cells (MSCs) in the joints of patients with OA.32 This circumstance provides additional opportunities for gene therapy as a means of controlling the activities of inflammatory mediators. A GAM approach used tethered lentivirus vectors expressing IL-1 receptor antagonist (IL-1Ra) to address this issue.33 Of note, an AAV–IL-1Ra construct is presently undergoing regulatory approval for human clinical use to treat OA.11 IL-4 is another cytokine of interest as an anti-inflammatory cytokine.35 Co-delivery of cDNAs that encode an anti-inflammatory product, such as IL-1Ra or IL-10, and a cartilage growth factor, such as IGF-1, have also been studied.35,36 Strategies for treating OA usually involve the injection of vectors or genetically modified cells into the joint.11 Under these conditions, the primary site of transgene expression is the synovium and all intra-articular tissues, including the cartilage, are exposed to the gene product via diffusion through the synovial fluid.
In four clinical trials in the USA and Korea, suspensions of allogeneic chondrocytes that express TGF-β1 were injected into knee joints of patients with OA.11 These studies have completed phase I38 and phase II,39 and phase III trials40 are in preparation. Gene therapy for OA has been reviewed elsewhere.11,43,44
Future refinements of this approach include the use of progenitor cells, rather than chondrocytes, as agents of ex vivo gene transfer for cartilage repair.26,43,44 Interest in the use of GAMs is also high,45,46 and research into improving the efficiency and targeting of nonviral vectors continues. Pi et al.,47 for example, identified peptides that traffic specifically to chondrocytes and enhance transfection when attached to polyethylenimine. Gene delivery for cartilage repair has been reviewed elsewhere.48
Bone
Bone is often misconstrued as the `low-hanging fruit' of tissue regeneration because it is one of the few organs in the body that normally heals well without scarring. However, the purposeful therapeutic regeneration of bone is difficult. Most research has been performed with animal models of cranial defects and large segmental defects in long bones. Spine fusion, fracture healing, nonunions, restoration of bone after avascular necrosis, and the repair of alveolar, mandibular and periodontal defects have also been studied.2
Research into gene therapy has mostly focused on the delivery of morphogens (particularly BMPs,49,50 Wnt proteins,51 angiogenic factors such as vascular endothelial growth factor (VEGF),52 osteogenic transcription factors,53 LIM-domain proteins (LMPs)54 and cyclooxygenase-2.55 The potential use of microRNAs is also of interest.56 Selection of transgenes is complicated by the choice between the endochondral route to bone formation, which requires the initial formation of cartilage,57 or the intramembranous route. This dichotomy is most apparent in the need for a blood supply; chondrogenesis occurs in an avascular environment, whereas osteogenesis has an absolute requirement for vasculogenesis. The latter is convincingly demonstrated by the synergy between cDNAs that encode VEGF and BMPs in healing osseous defects.58
Most studies have used traditional ex vivo approaches with adenoviral, retroviral, lentiviral or nonviral vectors in combination with muscle-derived stem cells (MDSCs) or MSCs derived from bone marrow or fat.59 Considerable success has been reported using rodent and rabbit models in which a critical-sized defect is created in a long bone or the cranium (reviewed elsewhere60). Relatively few of these studies have progressed to using large-animal models that are needed in advance of human clinical trials. However, success has been reported in goat,61 pig62 and horse63 models using adenovirus to transfer cDNA of BMPs into long-bone defects, cranial defects and sites of osteonecrosis of the hip, with bone-marrow-derived MSCs or dermal fibroblasts as carriers.
The direct injection of adenovirus carrying BMP-encoding cDNA64 has shown promise in the treatment of segmental defects in small animals (rabbits65,66 and rats67), but was ineffective in treating sheep,68 possibly owing to an immune reaction to the vector and, in that case, to human BMP-2.69 Nevertheless, success was reported in some, but not all, studies of adenoviral delivery of BMP-2 or BMP-6 to horses.70,71
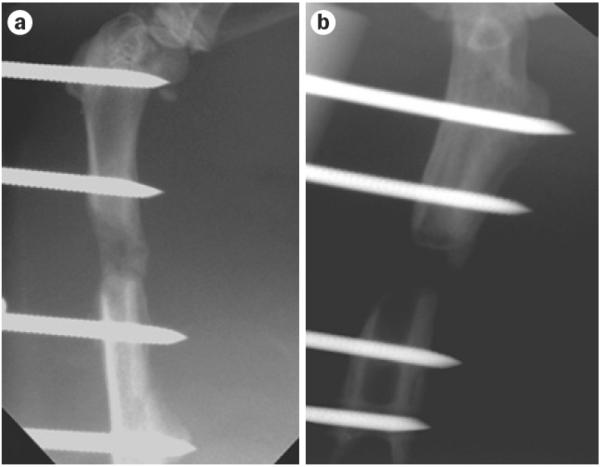
Expedited ex vivo procedures that use autologous fat and muscle do not provoke neutralizing antibody responses to an adenovirus vector,23 and more reliably heal segmental defects in rats than the direct injection of the same vector (Figure 3). This technology is effective in a rat xenotransplantation model using genetically modified sheep muscle (C. H. Evans, unpublished work), which is encouraging for ongoing studies in sheep.
Figure 3.
Healing of critical-size femoral defect in the rat using genetically modified muscle graft. Skelet al muscle was transduced with adenovirus expressing a marker gene (right) or BMP2 (left). X-rays were taken after 4 weeks and show bridging in the defect receiving the BMP2 cDNA, whereas expression of a marker gene did not form new bone. Modified from Evans, C.H. et al.,22 with permission.
An alternative approach to expediting ex vivo delivery is a method in which lentivirus vectors are used to transduce and reimplant autologous bone marrow cells intraoperatively.72 Previous experiments from the same group, using an expanded bone-marrow-derived MSC population in a rat segmental defect model, showed that BMP-2 was expressed for a longer period of time and produced better quality bone when delivered by a lentivirus vector than when delivered by adenovirus.73 In the expedited approach, the bone marrow is fractionated to isolate the buffy-coat layer prior to transduction with lentivirus. Because lentivirus vectors integrate into the host genome, studies are underway to include a `suicide gene' to be activated in case of malignant transformation.74
A safe, genetically modified, allograft osteogenic cell line would both expedite and simplify the process of ex vivo gene delivery. However, unlike cartilage repair, bone is not repaired by genetically modified allogeneic cells unless an immunosuppressive drug is used.75 Sonnet et al.76 addressed this problem by encapsulating allogeneic cells, transduced with adenovirus expressing BMP-2, in resorbable hydrogel particles. Remarkably, efficient bone-healing was noted in a rat segmental defect model, even though BMP-2 expression was low.
Much excitement was caused by the first publication of GAM technology,14,77 because an impressive level of osteogenesis was stimulated in large segmental defects in rats and dogs using plasmid DNA. Plasmids encoding the first 34 amino acids of parathyroid hormone (the basis of teriparatide; Forteo®, Eli Lilly, USA) and BMP-4 were delivered in association with a collagen sponge. This material is stable and could form the basis of an `off-the-shelf' product. Subsequent development was slowed by the weak transfection capability of DNA plasmids in this setting, so emphasis has shifted to improving the transfection ability of the matrices78 and using viral vectors in association with scaffolds.79 Nonviral gene therapy for bone regeneration is reviewed elsewhere.80
Allograft revitalization is an innovative modification of GAM technology. In this application, allograft bone is used as the scaffold and is coated with AAV. This method reflects the clinical use of allograft bone, the efficacy of which is limited by its inability to integrate and undergo turnover. When coated with AAV, infiltrating cells become transduced and, with the appropriate transgenes, promote osteogenesis at the same time as stimulating osteoclastic resorption of the allograft. Proof-of-principle was first established in mice using transgenes encoding VEGF and RANKL (receptor activator of NFκB ligand).81 Effectiveness was subsequently shown with AAV carrying BMP2 cDNA82 and constitutively active activin receptor type 1.83 No clinical trial has used gene transfer to promote bone healing.
Skeletal muscle
Muscle injuries account for a large number of injuries sustained by participants in professional and recreational sports. In fact, muscle injuries constitute 10–55% of all injuries sustained by athletes, depending on the type of sport.84 Whereas relatively minor muscle injuries (such as strains) can heal completely without intervention, severe muscle injuries typically result in the formation of dense scar tissue that impairs muscle function and can lead to muscle contracture and chronic pain. Regenerative medicine strategies for such severe muscle injuries have not been optimized. Injured muscle undergoes a sequential cycle of healing phases, including muscle degeneration, inflammation, angiogenesis, regeneration and fibrosis.84 Although biological approaches developed to improve skeletal muscle healing have targeted these different phases of the healing process, the most promising work has been in the areas of muscle regeneration and fibrosis. The challenge for muscle repair is to stimulate the regeneration of native tissue while preventing fibrosis. IGF-1 has myogenic properties and has been delivered as a transgene using adenovirally transduced myoblasts.85 An alternative approach is transfection-based delivery of IGF-1 to improve muscle healing.86
Because healing after enhancement of muscle regeneration is often associated with fibrosis, clinical application of this approach needs to be coupled with antifibrotic therapy. One such strategy exploits the antifibrotic properties of molecules (such as decorin, IFN-γ, losartan, relaxin and suramin) that antagonize the actions of TGF-β, a major promoter of fibrosis. These agents can block fibrosis and improve muscle healing after injury.87–95 For example, delivery of the gene encoding decorin, DCN, to laceration-injured skeletal muscle via an AAV is capable of inhibiting the formation of fibrosis and promoting skeletal muscle regeneration.87
Healing of skeletal muscle is also dependent on angiogenesis, and muscle-derived progenitor cells that express VEGF stimulate angiogenesis and reduce fibrosis in mice.96 Similar effects were achieved in rats using transfected myoblasts; synergy was noted between VEGF and stromal-cell-derived factor 1.97 Methods using muscle progenitor cells might be improved by transduction with myoblast determination protein 1, which can promote their differentiation into myoblasts.98
Tendon and ligament
As reviewed by Docheva et al.,99 several gene therapy strategies have been used to heal tendon and ligament. Morphogens, including BMP-12 (also known as growth/differentiation factor [GDF]-7), BMP-13 (also known as GDF-6) and BMP-14 (also known as GDF-5), are able to induce ligament and tendon formation from progenitors. Promising results have been reported in animal models of tendon healing, using transfer of cDNAs encoding BMP-12100,101 and BMP-14,102–103 but not BMP-13,104 even though all three induce tenogenesis in other systems105 and GDF6 (encoding BMP-13) transfer into MSCs induces ligamentogenesis in vitro;106 mechanical factors might account for this discrepancy.107 Transfer of the transcription factor scleraxis promotes the differentiation of MSCs into tenocytes in vitro108 and, when used ex vivo with MSCs, enhances healing of the rotator cuff in a rat model.109 Similar results were reported using a combination of cDNAs encoding BMP-2 and SMAD8.110 Less-specific strategies use transgenes that encode proteins (FGF-2, IGF-1, PDGF, TGF-β or VEGF) that are general stimulators of matrix formation, angiogenesis and cell proliferation.111–114
Gene transfer has also been used to promote osteogenesis at the bone insertion site after surgical reconstruction, thereby enhancing fixation of tendons into bone,115–117 and to promote healing by reducing inflammation.118 The use of small interfering RNAs (siRNAs) has also been explored as a method of preventing ectopic ossification within the healing tissue.119,120
Intervertebral disc
The approaches described in this Review can be applied to the intervertebral disc.121–122 Proof-of-principle has been established in rabbit models of disc degeneration using adenovirus and AAV vectors to deliver growth factors, cytokine antagonists and tissue inhibitors of metalloproteinases.123 Because the intervertebral disc is immunologically isolated, long-term transgene expression can be achieved, even when using highly antigenic vectors such as adenovirus, although this protection could be diminished in degenerate discs.
Meniscus
Gene transfer to the meniscus has been achieved with several different viral124–126 and nonviral127,128 vectors with in vivo and ex vivo strategies. Transgenes encoding IGF-1,128 FGF-2125 and TGF-β126 have been used. Most research has used cell culture, tissue explants and small-animal models, but one encouraging study used goats.128 Mesenchymal stromal cells were transfected with an IGF1 construct and transplanted within a calcium alginate gel into full-thickness defects in the white zone of the meniscus. 16 weeks after surgery, the mensical lesion was repaired with what seemed to be authentic meniscal tissue.128
Unresolved issues
Despite the abundance of preclinical success with animal models, a number of important matters are unresolved. For example, little information exists on how much, and at which stage of the healing process, a given growth factor or morphogen is needed. Most investigators introduce genes soon after injury using strong constitutive promoters and assume that more is better. Given the dynamic biology of tissue repair and regeneration, this approach might be inappropriate. For example, delayed administration of BMP-2 in the healing of segmental defects of bone produces a superior result.129 Also, although BMP-2 can promote osteogenesis, high concentrations are inflammatory and promote bone resorption. One advantage of gene delivery is the potential to regulate expression quantitatively and temporally. However, until we know how much transgene to express, and when to express it, this capability is redundant and has not, therefore, been extensively explored.
Another matter requiring clarification is the appropriate cell type for ex vivo gene therapy. Progenitor cells are frequently used for this purpose, but whether the origin of these cells matters is unclear. Detailed studies comparing, for example, stem cells derived from fat, bone marrow and muscle in the same model system have not been performed. Progenitor cells are used on the basis that they not only deliver transgene products, but also differentiate into the cells of the regenerated tissue. However, unequivocal demonstration of the presence of large numbers of donor cells in the repair tissue has been difficult. This difficulty raises the question of whether it is necessary that the cells used for gene delivery are capable of differentiation in this manner. Genetically modified skin cells, for example, have been used successfully to heal osseous lesions in horses.63
Depending upon the application, inflammatory and immune responses to viral vectors can be problematic. Adenovirus is particularly antigenic, activating both the innate and adaptive components of the immune system. In an extreme case, unrelated to regenerative medicine, this antigenicity led to the death of a participant in a gene therapy trial.10 For the applications discussed in this Review, the major concern is that the immune system will interfere with the efficiency of gene transfer, and excessive inflammation will inhibit regeneration. Most humans, unlike experimental animals, have pre-existing immunity to adenovirus serotype 5, which will inhibit transduction after in vivo delivery. This limitation can be obviated, initially, by the use of alternative serotypes, but these will generate immune responses of their own that prohibit repeat dosing. AAV is generally considered the least antigenic of the common viral vectors, generating humoral, but not cell-mediated, immune responses in experimental animals. However, a human clinical trial using AAV to deliver factor IX in patients with haemophilia revealed a strong, unforeseen, cell-mediated response that limited transgene expression.130 More-extensive studies report different immunogenicities of different AAV serotypes in different species.131 One advantage of nonviral vectors is that many have low immunogenicity.
Barriers to translation
Although the scientific literature describes numerous examples of the successful use of gene therapy to restore injured musculoskeletal tissues in small laboratory animals, we are aware of only two protocols that are in clinical trials. These protocols are used in the OA and cartilage repair trials32,38–40 using genetically modified allogeneic chondrocytes. Several factors contribute to the lack of translation.132,133
Any attempt to bring gene therapy into the clinic for a nonlethal nongenetic indication will undergo intense scrutiny by regulatory bodies, whose main concern is safety and the risk-to-benefit ratio. The latter is skewed by the fact that most musculoskeletal injuries are not life-threatening. The pharmacology, toxicology and biodistribution studies that will undoubtedly be necessary require GLP (good laboratory practice) facilities and are expensive and time-consuming. Moreover, demonstration of efficacy in a large-animal model is also likely to be required. Such activities are difficult to accomplish in academia and the large pharmaceutical companies are reluctant to participate in this type of gene therapy enterprise, especially in the early phases, which they view as medically and commercially risky (discussed in detail elsewhere42,132).
Conclusions
Despite few clinical trials, there are grounds for cautious optimism. Gene therapy as a whole is undergoing a resurgence, and several protocols are entering phase III trials. The first gene therapy has been approved for clinical use by the European Medicines Agency, joining the only other approved gene therapy drug, gendicine, which is used in China to treat cancer of the head and neck.
Although the attention of the gene therapy world is focused on Mendelian diseases and cancer, applications of the type described in this Review should benefit collaterally as the field of gene therapy develops and expands. As noted, one protocol for the restoration of articular cartilage has entered phase III trials in Korea32 and four related OA trials in the USA and Korea32,38–40 have been completed. At some point, gene therapy should attract serious attention from the pharmaceutical industry, especially as there is a huge unmet market requirement for ways to restore the injured musculoskeletal system.
Key points.
Gene transfer offers a solution to the problem of being unable to deliver morphogens and other regenerative products sustainably to sites of injury
Nascent proteins synthesized locally after gene transfer are likely to have undergone authentic post-translational modification and have higher activity than recombinant counterparts
Gene transfer can regulate transgene expression and deliver products with an intracellular action (for example, transcription factors and noncoding RNAs) or proteins that need to be inserted into a membrane (for example, receptors)
Several strategies exist for transferring genes to sites of injury using different viral or nonviral vectors in vivo or by ex vivo delivery in conjunction with progenitor or differentiated cells
Preclinical progress has been made in cartilage repair, bone healing and the regeneration of muscle, intervertebral disc, meniscus, tendon and ligament
A small number of osteoarthritis and cartilage repair clinical trials have taken place
Acknowledgements
The authors' work in this area has been supported by the AO Foundation and by NIH grants R01 AR050243, R01 AR052809, R01 AR43623, R21 AR049606, R01 AR048566, R01 AR057422 and R01 AR051085 from National Institute for Arthrit is and Musculoskelet al Skin Diseases, 1P01AG043376-01A1 from National Institute on Aging, X01 NS066865 from National Center for Advancing Translation Sciences, and W81XWH-13-2-0052 from the Department of Defense (the Armed Forces Institute of Regenerative Medicine II).
Footnotes
Competing interests
C.H.E. declares that he is a co-inventor on patents pertaining to the subject matter of this Review, and that he is a scientific advisory board member for TissueGene, Inc. J.H. declares that he receives remuneration as a consultant and royalties from Cook Myosite, Inc., a company he cofounded.
Author contributions
Both authors contributed to researching data for the article, discussing the content, writing and review/editing of the manuscript before submission.
References
- 1.Jacobs JJ. The Burden of Musculoskeletal Diseases in the United States. 2nd edn Ch. 6. American Academy of Orthopaedic Surgeons; 2011. pp. 129–179. [Google Scholar]
- 2.Evans CH. Advances in regenerative orthopedics. Mayo Clin. Proc. 2013;88:1323–1339. doi: 10.1016/j.mayocp.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs. 2012;26:163–175. doi: 10.2165/11631850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2012;29:1–63. doi: 10.1615/critrevtherdrugcarriersyst.v29.i1.10. [DOI] [PubMed] [Google Scholar]
- 5.Lam J, Lu S, Kasper FK, Mikos AG. Strategies for controlled delivery of biologics for cartilage repair. Adv. Drug Deliv. Rev. doi: 10.1016/j.addr.2014.06.006. http://dx.doi.org/10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed]
- 6.Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clin. Orthop. Relat. Res. 1999;(367 Suppl):S410–S418. doi: 10.1097/00003086-199910001-00040. [DOI] [PubMed] [Google Scholar]
- 7.Evans C. Using genes to facilitate the endogenous repair and regeneration of orthopaedic tissues. Int. Orthop. 2014;38:1761–1769. doi: 10.1007/s00264-014-2423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012—an update. J. Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 9.Hacein-Bey-Abina S, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 10.Raper SE, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat. Rev. Rheumatol. 2011;7:244–249. doi: 10.1038/nrrheum.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Li W, Ma N, Steinhoff G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013;14:46–60. [PubMed] [Google Scholar]
- 13.Evans CH, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 14.Minas T. A primer in cartilage repair. J. Bone Joint Surg. 2012;94:141–146. doi: 10.1302/0301-620X.94B11.30679. [DOI] [PubMed] [Google Scholar]
- 15.Cucchiarini M, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV- mediated overexpression of human fibroblast growth factor 2. Mol. Ther. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Cucchiarini M, Madry H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 2014;21:811–819. doi: 10.1038/gt.2014.58. [DOI] [PubMed] [Google Scholar]
- 17.Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J. Mol. Med. (Berl.) 2013;91:625–636. doi: 10.1007/s00109-012-0978-9. [DOI] [PubMed] [Google Scholar]
- 18.Pascher A, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. GeneTher. 2004;11:133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 19.Neumann AJ, Schroeder J, Alini M, Archer CW, Stoddart MJ. Enhanced adenovirus transduction of hMSCs using 3D hydrogel cell carriers. Mol. Biotechnol. 2013;53:207–216. doi: 10.1007/s12033-012-9522-y. [DOI] [PubMed] [Google Scholar]
- 20.Sieker JT, et al. Direct bone morphogenetic protein 2 and indian hedgehog gene transfer for articular cartilage repair using bone marrow coagulates. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2014.11.008. http://dx.doi.org/10.1016/j.joca.2014.11.008. [DOI] [PubMed]
- 21.Ivkovic A, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010;17:779–789. doi: 10.1038/gt.2010.16. [DOI] [PubMed] [Google Scholar]
- 22.Evans CH, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur. Cell. Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang R, et al. Ex vivo gene transfer to chondrocytes in full-thickness articular cartilage defects: a feasibility study. Osteoarthritis Cartilage. 1997;5:139–143. doi: 10.1016/s1063-4584(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 24.Orth P, et al. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:2119–2130. doi: 10.1007/s00167-011-1448-6. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hidaka C, et al. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J. Orthop. Res. 2003;21:573–583. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 27.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J. Bone Joint Surg. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 28.Brower-Toland BD, et al. Direct adenovirus- mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum. Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 29.Ortved KF, Begum L, Mohammed HO, Nixon AJ. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol. Ther. doi: 10.1038/mt.2014.198. http://dx.doi/org/10.1038/mt.2014.198. [DOI] [PMC free article] [PubMed]
- 30.Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-β-1 in degenerative arthritis patients. Cytotherapy. 2012;14:247–256. doi: 10.3109/14653249.2011.629645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US National Library of Medicine ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01825811.
- 32.Wehling N, et al. Interleukin-1β and tumor necrosis factor α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass KA, et al. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials. 2014;35:5921–5931. doi: 10.1016/j.biomaterials.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachakonda PS, Rai MF, Schmidt MF. Application of inflammation-responsive promoter for an in vitro arthritis model. Arthritis Rheum. 2008;58:2088–2097. doi: 10.1002/art.23598. [DOI] [PubMed] [Google Scholar]
- 35.Haupt JL, et al. Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J. Orthop. Res. 2005;23:118–126. doi: 10.1016/j.orthres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Neumann E, et al. Inhibition of cartilage destruction by double gene transfer of IL-1Ra and IL-10 involves the activin pathway. Gene Ther. 2002;9:1508–1519. doi: 10.1038/sj.gt.3301811. [DOI] [PubMed] [Google Scholar]
- 37.Watson RS, et al. scAAV-mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints. Gene Ther. 2013;20:670–677. doi: 10.1038/gt.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US National Library of Medicine ClinicalTrials.gov [online] 2010 https://clinicaltrials.gov/ct2/show/NCT00599248.
- 39.US National Library of Medicine ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01671072.
- 40.US National Library of Medicine ClinicalTrials.gov [online] 2015 https://www.clinicaltrials.gov/ct2/show/NCT02072070.
- 41.Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11:379–389. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- 42.Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy and its tortuous path into the clinic. Transl. Res. 2013;161:205–216. doi: 10.1016/j.trsl.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagnotto MR, et al. Adeno-associated viral gene transfer of transforming growth factor-β1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–813. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 44.Gelse K, et al. Cell-based resurfacing of large cartilage defects: long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheum. 2008;58:475–488. doi: 10.1002/art.23124. [DOI] [PubMed] [Google Scholar]
- 45.Needham CJ, et al. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014;10:4103–4112. doi: 10.1016/j.actbio.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunger JM, et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl Acad. Sci. USA. 2014;111:E798–E806. doi: 10.1073/pnas.1321744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pi Y, et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324–6332. doi: 10.1016/j.biomaterials.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Saraf A, Mikos AG. Gene delivery strategies for cartilage tissue engineering. Adv. Drug Deliv. Rev. 2006;58:592–603. doi: 10.1016/j.addr.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Betz OB, et al. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J. Bone Joint Surg. Am. 2006;88:355–365. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 50.Wright V, et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol. Ther. 2002;6:169–178. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 51.Gao F, Zhang CQ, Chai YM, Li XL. Lentivirus-mediated Wnt10b overexpression enhances fracture healing in a rat atrophic non- union model. Biotechnol. Lett. doi: 10.1007/s10529-014-1703-2. http://dx.doi.org/10.1007/s10529-014-1703-2. [DOI] [PubMed]
- 52.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J. Orthop. Res. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 53.Han D, Li J. Repair of bone defect by using vascular bundle implantation combined with Runx II gene-transfected adipose-derived stem cells and a biodegradable matrix. Cell Tissue Res. 2013;352:561–571. doi: 10.1007/s00441-013-1595-9. [DOI] [PubMed] [Google Scholar]
- 54.Lattanzi W, et al. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–1343. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rundle CH, et al. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J. Gene Med. 2008;10:229–241. doi: 10.1002/jgm.1148. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, et al. The promotion of bone regeneration through positive regulation of angiogenic- osteogenic coupling using microRNA-26a. Biomaterials. 2013;34:5048–5058. doi: 10.1016/j.biomaterials.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 57.Scotti C, et al. Engineering of a functional bone organ through endochondral ossification. Proc. Natl Acad. Sci. USA. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng H, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gates CB, Karthikeyan T, Fu F, Huard J. Regenerative medicine for the musculoskeletal system based on muscle-derived stem cells. J. Am. Acad. Orthop. Surg. 2008;16:68–76. doi: 10.5435/00124635-200802000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Evans C. Gene therapy for the regeneration of bone. Injury. 2011;42:599–604. doi: 10.1016/j.injury.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu L, Chuanchang D, Wei L, Yilin C, Jiasheng D. Enhanced healing of goat femur- defect using BMP7 gene-modified BMSCs and load-bearing tissue-engineered bone. J. Orthop. Res. 2010;28:412–418. doi: 10.1002/jor.20973. [DOI] [PubMed] [Google Scholar]
- 62.Chang SC, et al. Large-scale bicortical skull bone regeneration using ex vivo replication- defective adenoviral-mediated bone morphogenetic protein-2 gene-transferred bone marrow stromal cells and composite biomaterials. Neurosurgery. 2009;65:75–81. doi: 10.1227/01.NEU.0000345947.33730.91. [DOI] [PubMed] [Google Scholar]
- 63.Ishihara A, Zekas LJ, Litsky AS, Weisbrode SE, Bertone AL. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J. Orthop. Res. 2010;28:403–411. doi: 10.1002/jor.20978. [DOI] [PubMed] [Google Scholar]
- 64.Baltzer AWA, et al. Potential role of direct adenoviral gene transfer in enhancing facture repair. Clin. Orthop. Relat. Res. 2000;379(Suppl):S120–S125. doi: 10.1097/00003086-200010001-00016. [DOI] [PubMed] [Google Scholar]
- 65.Bertone AL, et al. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. J. Orthop. Res. 2004;22:1261–1270. doi: 10.1016/j.orthres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Baltzer AW, et al. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 1999;7:197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- 67.Betz VM, et al. Healing of segmental bone defects by direct percutaneous gene delivery: effect of vector dose. Hum. Gene Ther. 2007;18:907–915. doi: 10.1089/hum.2007.077. [DOI] [PubMed] [Google Scholar]
- 68.Egermann M, et al. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006;13:1290–1299. doi: 10.1038/sj.gt.3302785. [DOI] [PubMed] [Google Scholar]
- 69.Egermann M, et al. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum. Gene Ther. 2006;17:507–517. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 70.Southwood LL, et al. Evaluation of direct in vivo gene transfer in an equine metacarpal IV ostectomy model using an adenoviral vector encoding the bone morphogenetic protein-2 and protein-7 gene. Vet. Surg. 2012;41:345–354. doi: 10.1111/j.1532-950X.2011.00947.x. [DOI] [PubMed] [Google Scholar]
- 71.Menendez MI, et al. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthritis Cartilage. 2011;19:1066–1075. doi: 10.1016/j.joca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Virk MS, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol. Ther. 2011;19:960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Virk MS, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–931. doi: 10.1016/j.bone.2007.12.216. [DOI] [PubMed] [Google Scholar]
- 74.Alaee F, et al. Suicide gene approach using a dual-expression lentiviral vector to enhance the safety of ex vivo gene therapy for bone repair. Gene Ther. 2014;21:139–147. doi: 10.1038/gt.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsuchida H, Hashimoto J, Crawford E, Manske P, Lou J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J. Orthop. Res. 2003;21:44–53. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 76.Sonnet C, et al. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J. Orthop. Res. 2013;31:1597–1604. doi: 10.1002/jor.22407. [DOI] [PubMed] [Google Scholar]
- 77.Fang J, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc. Natl Acad. Sci. USA. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tierney EG, Duffy GP, Hibbitts AJ, Cryan SA, O'Brien FJ. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release. 2012;158:304–311. doi: 10.1016/j.jconrel.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 79.Dupont KM, et al. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res. 2012;347:575–588. doi: 10.1007/s00441-011-1197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wegman F, Oner FC, Dhert WJ, Alblas J. Non-viral gene therapy for bone tissue engineering. Biotechnol. Genet. Eng. Rev. 2013;29:206–220. doi: 10.1080/02648725.2013.801227. [DOI] [PubMed] [Google Scholar]
- 81.Ito H, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat. Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yazici C, et al. Self-complementary AAV2.5- BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol. Ther. 2011;19:1416–1425. doi: 10.1038/mt.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koefoed M, et al. Biological effects of rAAV- caAlk2 coating on structural allograft healing. Mol. Ther. 2005;12:212–218. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 84.Gharaibeh B, et al. Biological approaches to improve skeletal muscle healing after injury and disease. Birth Defects Res. C Embryo. Today. 2012;96:82–94. doi: 10.1002/bdrc.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasemkijwattana C, et al. Development of approaches to improve the healing following muscle contusion. Cell Transplant. 1998;7:585–598. doi: 10.1177/096368979800700609. [DOI] [PubMed] [Google Scholar]
- 86.Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther. 2006;13:1657–1664. doi: 10.1038/sj.gt.3302817. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, et al. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol. Ther. 2007;15:1616–1622. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- 88.Zhu J, et al. Relationships between transforming growth factor-β1, myostatin, and decorin: implications for skeletal muscle fibrosis. J. Biol. Chem. 2007;282:25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- 89.Fukushima K, et al. The use of an antifibrosis agent to improve muscle recovery after laceration. Am. J. Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 90.Nozaki M, et al. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am. J. Sports Med. 2008;36:2354–2362. doi: 10.1177/0363546508322886. [DOI] [PubMed] [Google Scholar]
- 91.Foster W, Li Y, Usas A, Somogyi G, Huard J. Gamma interferon as an antifibrosis agent in skeletal muscle. J. Orthop. Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Negishi S, Sakamoto M, Usas A, Huard J. The use of relaxin improves healing in injured muscle. Ann. NY Acad. Sci. 2005;1041:395–397. doi: 10.1196/annals.1282.060. [DOI] [PubMed] [Google Scholar]
- 93.Terada S, et al. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J. Bone Joint Surg. Am. 2013;95:980–988. doi: 10.2106/JBJS.L.00266. [DOI] [PubMed] [Google Scholar]
- 94.Kobayashi T, et al. The timing of administration of a clinically relevant dose of losartan influences the healing process after contusion induced muscle injury. J. Appl. Physiol. 2013;114:262–273. doi: 10.1152/japplphysiol.00140.2011. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am. J. Sports Med. 2008;36:1548–1554. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- 96.Deasy BM, et al. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol. Ther. 2009;17:1788–1798. doi: 10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou W, et al. Angiogenic gene-modified myoblasts promote vascularization during repair of skeletal muscle defects. J. Tissue Eng. Regen. Med. doi: 10.1002/term.1692. http://dx.doi.org/10.1002/term.1692. [DOI] [PubMed]
- 98.Goudenege S, et al. Enhancement of myogenic and muscle repair capacities of human adipose- derived stem cells with forced expression of MyoD. Mol. Ther. 2009;17:1064–1072. doi: 10.1038/mt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv. Drug Deliv. doi: 10.1016/j.addr.2014.11.015. http://dx.doi.org/10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed]
- 100.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J. Orthop. Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 101.Majewski M, et al. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139–1146. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- 102.Basile P, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol. Ther. 2008;16:466–473. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rickert M. BMP-14 gene therapy increases tendon tensile strength in a rat model of achilles tendon injury. J. Bone Joint Surg. Am. 2008;90:445. author reply 445–446. [PubMed] [Google Scholar]
- 104.Gulotta LV, Kovacevic D, Packer JD, Ehteshami JR, Rodeo SA. Adenoviral- mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am. J. Sports Med. 2011;39:180–187. doi: 10.1177/0363546510379339. [DOI] [PubMed] [Google Scholar]
- 105.Wolfman NM, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J. Clin. Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haddad-Weber M, et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2010;12:505–513. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eliasson P, Fahlgren A, Aspenberg P. Mechanical load and BMP signaling during tendon repair: a role for follistatin? Clin. Orthop. Relat. Res. 2008;466:1592–1597. doi: 10.1007/s11999-008-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alberton P, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21:846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am. J. Sports Med. 2011;39:1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 110.Hoffmann A, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J. Clin. Invest. 2006;116:940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang JB, et al. Adeno-associated virus-2- mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. J. Bone Joint Surg. Am. 2008;90:1078–1089. doi: 10.2106/JBJS.F.01188. [DOI] [PubMed] [Google Scholar]
- 112.Hou Y, et al. Effects of transforming growth factor-β1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28:324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 113.Nakamura N, et al. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998;5:1165–1170. doi: 10.1038/sj.gt.3300712. [DOI] [PubMed] [Google Scholar]
- 114.Schnabel LV, et al. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 115.Coen MJ, Chen ST, Rundle CH, Wergedal JE, Lau KH. Lentiviral-based BMP4 in vivo gene transfer strategy increases pull-out tensile strength without an improvement in the osteointegration of the tendon graft in a rat model of biceps tenodesis. J. Gene Med. 2011;13:511–521. doi: 10.1002/jgm.1604. [DOI] [PubMed] [Google Scholar]
- 116.Martinek V, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J. Bone Joint Surg. Am. 2002;84:1123–1131. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 117.Lattermann C, et al. Gene transfer to the tendon-bone insertion site. Knee Surg. Sports Traumatol. Arthrosc. 2004;12:510–515. doi: 10.1007/s00167-003-0482-4. [DOI] [PubMed] [Google Scholar]
- 118.Ricchetti ET, et al. Effect of interleukin-10 overexpression on the properties of healing tendon in a murine patellar tendon model. J. Hand Surg. Am. 2008;33:1843–1852. doi: 10.1016/j.jhsa.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin L, et al. Adenovirus-mediated transfer of siRNA against Runx2/Cbfa1 inhibits the formation of heterotopic ossification in animal model. Biochem Biophys. Res. Commun. 2006;349:564–572. doi: 10.1016/j.bbrc.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 120.Xue T, et al. Non-virus-mediated transfer of siRNAs against Runx2 and Smad4 inhibit heterotopic ossification in rats. Gene Ther. 2010;17:370–379. doi: 10.1038/gt.2009.154. [DOI] [PubMed] [Google Scholar]
- 121.Evans C. Potential biologic therapies for the intervertebral disc. J. Bone Joint Surg. Am. 2006;88(Suppl. 2):95–98. doi: 10.2106/JBJS.E.01328. [DOI] [PubMed] [Google Scholar]
- 122.Nishida K, Gilbertson LG, Robbins PD, Evans CH, Kang JD. Potential applications of gene therapy to the treatment of intervertebral disc disorders. Clin. Orthop. Relat. Res. 2000;379(Suppl):S234–S241. doi: 10.1097/00003086-200010001-00031. [DOI] [PubMed] [Google Scholar]
- 123.Woods BI, Vo N, Sowa G, Kang JD. Gene therapy for intervertebral disk degeneration. Orthop. Clin. North Am. 2011;42:563–574. doi: 10.1016/j.ocl.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Goto H, et al. Transfer of lacZ marker gene to the meniscus. J. Bone Joint Surg. Am. 1999;81:918–925. doi: 10.2106/00004623-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and α-SMA expression in human meniscal lesions. Gene Ther. 2009;16:1363–1372. doi: 10.1038/gt.2009.91. [DOI] [PubMed] [Google Scholar]
- 126.Steinert AF, et al. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-β 1 complementary deoxyribonucleic acid. Tissue Eng. 2007;13:2227–2237. doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 127.Lee HP, Kaul G, Cucchiarini M, Madry H. Nonviral gene transfer to human meniscal cells. Part I: transfection analyses and cell transplantation to meniscus explants. Int. Orthop. 2014;38:1923–1930. doi: 10.1007/s00264-014-2410-2. [DOI] [PubMed] [Google Scholar]
- 128.Zhang H, Leng P, Zhang J. Enhanced meniscal repair by overexpression of hIGF-1 in a full-thickness model. Clin. Orthop. Relat. Res. 2009;467:3165–3174. doi: 10.1007/s11999-009-0921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Betz OB, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14:1039–1044. doi: 10.1038/sj.gt.3302956. [DOI] [PubMed] [Google Scholar]
- 130.Manno CS, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 131.Calcedo R, Wilson JM. Humoral immune response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy—lost in translation? J. Cell. Physiol. 2012;227:416–420. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Madry H, et al. Barriers and strategies for the clinical translation of advanced orthopaedic tissue engineering protocols. Eur. Cell. Mater. 2014;27:17–21. doi: 10.22203/ecm.v027sa04. [DOI] [PubMed] [Google Scholar]