Abstract
The strict human pathogen Neisseria gonorrhoeae has caused gonorrhea for thousands of years, and currently gonorrhea is the second most prevalent bacterial sexually transmitted infection worldwide. Given the ancient nature of N. gonorrhoeae and its unique obligate relationship with humankind over the millennia, its remarkable ability to adapt to the host immune system and cause repeated infections, and its propensity to develop resistance to all clinically useful antibiotics, the gonococcus is an ideal pathogen on which to study the evolution of bacterial pathogenesis, including antimicrobial resistance, over the long term and within the host during infection. Recently, the first gonococcus displaying high-level resistance to ceftriaxone, identified in Japan, was characterized in detail. Ceftriaxone is the last remaining option for empirical first-line treatment, and N. gonorrhoeae now seems to be evolving into a true “superbug.” In the near future, gonorrhea may become untreatable in certain circumstances. Herein, the history of antibiotics used for treatment of gonorrhea, the evolution of resistance emergence in N. gonorrhoeae, the linkage between resistance and biological fitness of N. gonorrhoeae, lessons learned, and future perspectives are reviewed and discussed.
Keywords: Neisseria gonorrhoeae, gonorrhea, antimicrobial resistance, genetics, evolution
Introduction
Gonorrhea is currently the second most prevalent bacterial sexually transmitted infection (STI) and disease worldwide and, accordingly, gonorrhea remains a major public health concern.1 Historically, the strict human pathogen Neisseria gonorrhoeae has caused gonorrhea for thousands of years. In this respect a passage in the Old Testament (Leviticus 15:1–3), which warns: “When any man has a bodily discharge, the discharge is unclean,” gives reason to believe that gonorrhea is an ancient disease and has successfully adapted itself to existing with Homo sapiens. The term gonorrhea can be attributed to the second century Greek physician Galen who coined the disease “as flow of seed.”
Given the apparent ancient nature of gonorrhea, and its unique relationship with humankind over the millennia, its remarkable ability to cause repeated infections in the same host (the interested reader is encouraged to read Ober’s writings,2 of the 18th century Englishman James Boswell who documented to the biographer Samuel Jackson his 17 cases of gonorrhea), and its propensity to develop resistance to all clinically useful antibiotics, the gonococcus is an ideal bacterial STI pathogen on which to study the evolution of bacterial pathogenesis, including antimicrobial resistance, over the long term and within the host during infection. As a strict human pathogen, it is not surprising that the gonococcus has developed multiple mechanisms to cope with the innate and adaptive immunity systems that are typically associated with the host defense. For example, to escape the immunological defense system of the host, the gonococcus uses mechanisms such as antigenic and phase variation of outer membrane structures, blocking antibodies, molecular mimicry, and inhibition and/or induction of apoptosis.3 This ability of gonococci to escape host defensive systems emphasizes its adaptive ability and provides a framework for understanding the evolutionary processes used by gonococci to resist the myriad of antibiotics used to treat infections for the past 70 years.
Its comparative ease of cultivation in the laboratory, the ability to genetically manipulate strains, and recent advances in genomics make the gonococcus an ideal STI pathogen to study in terms of the evolution of antibiotic resistance, including its mechanisms, expressed by such pathogens. It is important to stress that in the absence of effective vaccines, appropriate diagnostics and subsequent effective antibiotic therapy remains the principal method to stop the spread of STIs in the community. Emergence of resistant strains causing an STI can endanger the reproductive health (and in some instances, overall health) of the afflicted when such resistance is not initially evident. Continued sexual encounters by those harboring a resistant strain can result in spread of the pathogen within social networks. Once resistance is determined, alternative and sometimes more expensive or unavailable antibiotics (e.g., in poor countries) are required to cure the infection. During the last 70–80 years, N. gonorrhoeae has developed resistance to mainly all antimicrobials introduced for treatment of gonorrhea. Worryingly, the antibiotic treatment options now seem to be running out, and in the near future, gonorrhea may become untreatable in certain circumstances (see later).1
History of antibiotics recommended for treatment and resistance emergence in N. gonorrhoeae
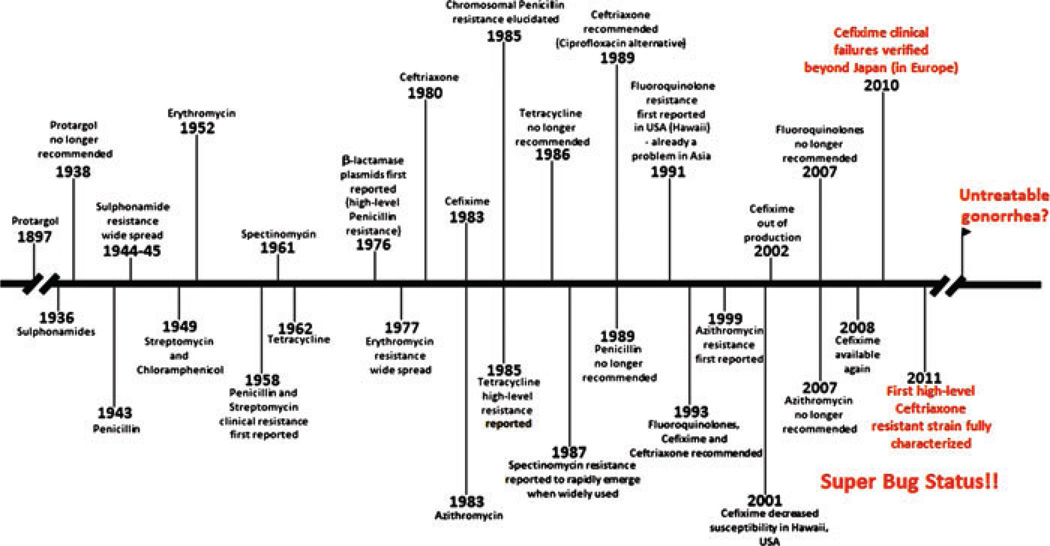
Apart from the use of the silver-based compound termed protargol in the late 1890s,4 the age of effective chemotherapy for gonorrhea can be traced to the use of sulfonamides introduced in the mid-1930s.5 While initially effective, gonococcal resistance to sulfa drugs became widespread by the mid-1940s.6 Fortunately, at the time, the “new miracle drug” penicillin was highly effective against gonococci and was reported and advertised as a curative agent as early as 1943.7 Indeed, at this time, gonococci were highly sensitive to penicillin (minimum inhibitory concentration [MIC] values typically ≤0.05 µg/mL). Penicillin became the first-line drug for treatment. Within 10–15 years (see Fig. 1), however, the relatively low doses of penicillin required for cure had to be increased due to enhanced MICs in circulating gonococcal strains resulting in treatment failures.8 This gradual decrease in penicillin susceptibility was due to the sequential accumulation of chromosomal mutations by gonococci.4 Although two types of β-lactamase encoding plasmids, originating in Asia and Africa, causing high-level penicillin resistance were reported in certain gonococci in 1976,9,10 these strains remained relatively rare in the United States and several other geographical regions worldwide. By the early to mid-1980s, it was feared that penicillin’s usefulness had run its course. Indeed, this fear was realized with the isolation of a strain in Durham, North Carolina that caused penicillin-resistant gonorrhea due only to chromosomally based mutations.11 By the late 1940s and 1950–1960s other antibiotics were used in the treatment of gonorrhea for those patients where penicillin was contraindicated; these alternative antibiotics included certain aminoglycosides, macrolides, and tetracycline. Not surprisingly, strains resistant to these alternative antibiotics rapidly emerged due to chromosomal mutations or gene acquisition events. In this respect, the appearance of strains with the tetM gene (“tetracycline resistance protein” mediating high-level tetracycline resistance) carried on the conjugal plasmid, which was first reported in 1985,12 resulted in the removal of tetracycline as a treatment option in the mid- to late-1980s in most countries. Fortunately, the fluoroquinolones, such as ciprofloxacin, were highly active against gonococci, allowing options for treatment. However, the work of Belland et al.13 on laboratory derived mutants of gonococci expressing resistance to ciprofloxacin was especially prescient as they showed that mutations in gyrA and parC (encoding DNA gyrase and DNA topoisomerase IV, respectively) developed during in vitro selection using subinhibitory concentrations of ciprofloxacin and together these mutations provided a level of resistance that was clinically important. Such resistant clinical strains also emerged and were transmitted in South East Asia already in the mid-1990s,14,15 and soon they were spreading worldwide. In the United States, they first appeared in Hawaii,16–18 likely due to importation from Asia; the prevalence of these strains rapidly increased and later they appeared in the west coast states, initially spreading especially among men who have sex with men (MSM).19–21 By 2007, fluoroquinolone-resistant strains were of sufficient prevalence throughout the United States that all fluoroquinolones were removed from the recommended treatment regimen.22 This was an important moment because N. gonorrhoeae was now recognized by the Centers for Disease Control and Prevention (CDC) as a “superbug.”4 Due to the high prevalence of ciprofloxacin resistance, many Asian and European countries had excluded ciprofloxacin as first-line antibiotic for treatment already in the early or mid-2000s.
Figure 1.
History of antibiotic treatment of gonorrhea and evolution of resistance in N. gonorrhoeae in the United States that is highly influenced from other geographic regions, especially through the import of resistant strains from Asia. Modified from a figure prepared by Paul Johnson (Emory University School of Medicine).
Fortunately, the third-generation cephalosporins were still effective at low concentrations. For all intents and purposes, third-generation cephalosporins (cefixime and ceftriaxone) were the main antibiotics, and in most countries recommended first-line drugs, that could be used with confidence. However, in recent years the susceptibility to these third-generation cephalosporins has rapidly decreased globally.1,21,23–25 Cefixime treatment failures have been identified in Japan within the last several years,1,26,27 and recently the first clinical failures beyond Japan were confirmed in Europe.28 Unfortunately, given history, it is likely that cefixime resistance will continue to spread globally and also ceftriaxone will have the same fate. This fear is justified with the recent emergence and detailed characterization of the first gonococcus displaying high-level clinical resistance to ceftriaxone (MIC of 2–4 µg/mL) identified in Kyoto, Japan.29,30 This is particularly worrisome as ceftriaxone is the last remaining option for empirical first-line treatment of gonorrhea. N. gonorrhoeae now seems to be evolving into a true superbug and, in the near future, gonorrhea may become untreatable in certain circumstances.
The history of antibiotic treatment of gonorrhea and evolution of resistance in N. gonorrhoeae in the United States, highly influenced by other geographic regions, can be seen in Figure 1.
Genetics of antibiotic resistance in Neisseria gonorrhoeae
As with other heritable changes, resistance to antibiotics in gonococci develops due to spontaneous mutation and/or gene (whole or parts) acquisition, which are effectively selected due to antibiotic pressure in patients and, in general, in society. Once established, resistance determinants can be donated to other gonococci largely by transformation, which substantially facilitates spread of resistance alleles. An excellent example of conjugal transfer of DNA into and among gonococci appears to be the transfer of the tetM gene carried on the conjugal plasmid in certain strains. This conjugal plasmid, with or without carriage of the tetM gene, can also display relatively high efficiency in intercellular conjugal transfer of β-lactamase-encoding plasmids between different N. gonorrhoeae strains, as well as to N. meningitidis, Haemophilus influenzae, and Escherichia coli.31–35 However, this conjugal plasmid and its conjugation system is substantially less efficient, compared to the transformation system (see later), and fails to mobilize chromosomal genes. In general, resistance determinants seem to be stably maintained in gonococci even though the antibiotic has been removed from treatment regimen decades earlier. This maintenance of the resistance determinants may be due to the antibiotics used to treat other bacterial infections, inappropriate use of the antibiotic, or antigonococcal agents used topically to prevent STIs and HIV transmission or pregnancy (e.g., the spermicide nonoxynol-9), which could inadvertently maintain selective pressure in the community for resistant strains. However, the persistence may also be because the resistance determinants (i) do not affect the biological fitness (no benefits for the bacteria to get rid of them); (ii) do lower the biological fitness; however, this fitness cost is compensated by second-site mutation (not influencing the resistance); or (iii) the resistance determinants may even cause a higher biological fitness and, in fact, make these clones more successful with regard to transmission and virulence (see later).
Transformation has played a key role in the evolution of antibiotic resistance in the gonococcus. Gonococci are highly competent for transformation (natural competence during the entire life cycle) by their own DNA and to a lesser extent, although still quite significant, that of other closely related bacteria, that is especially commensal Neisseria species and N. meningitidis. Accordingly, for example, pharyngeal gonorrhea, where gonococci frequently coincide with other neisserial species, may act as an asymptomatic reservoir for infection but also for initial emergence of antibiotic resistance, by transformation, in the gonococci.1 Donor DNA from these other species can create mosaic genes in recipient gonococci, such as penA mosaic alleles, that encode variants of penicillin-binding protein 2 (PBP2) having reduced affinity for β-lactam antibiotics. These emerged mosaic genes (commonly resistance determinants) can subsequently effectively spread among gonococcal strains.1 Nevertheless, also other closely related bacterial species can act as DNA donors, which is exemplified by the gonococcal β-lactamase plasmids (see above) that seem to have been initially donated by a Haemophilus species.36
The development of penicillin resistance in gonococci is illustrative of how antibiotic pressure and selection can drive resistance; this topic has been extensively reviewed by Shafer et al.4 As noted above, the development of chromosomally determined resistance took nearly 40 years and was the result of changes (mutations and gene acquisition) in at least five single locus (or at least in some cases “cooperative loci”). Early work by Sparling and coworkers showed that sequential accumulation of polymorphisms in loci termed penA, mtr, and penB resulted in graded increases in penicillin resistance. The latter two resistance determinants also affect the susceptibility to several other antimicrobials, such as tetracycline, macrolides, and cephalosporins, which are also affected by polymorphisms in penA (see below). penA encodes PBP2, which is the main lethal target for penicillin (and other β-lactam antibiotics) and responsible for peptidoglycan cross-linking at the septum during cell division.37 The Spratt laboratory has provided insightful data revealing that a single amino acid insertion (Asp345A) in PBP2 downstream from the penicillin acylation site substantially decreased the affinity for penicillin.38 This Asp345A insertion was most probably generated by transformation from donor DNA originating from commensal Neisseria residing in the nasopharynx. The mtr (multiple transferable resistance) determinant was first identified by Maness and Sparling in 197339 as a phenotype that resulted in increased, nonspecific resistance of gonococci to a panel of hydrophobic antimicrobials and the less hydrophobic penicillin. Mtr was first thought to decrease the outer membrane permeability of gonococci to these antimicrobials, but is now known to be mainly due to mutations in a gene encoding a transcriptional repressor (MtrR) or its promoter.4,40,41 MtrR binds to and represses an adjacent, but divergent, promoter used for transcription of an efflux pump operon (mtrCDE), which encodes a tripartite export system that expels antimicrobials from the bacterial periplasmic space.4 Veal et al.42 showed that overexpression of the mtrCDE-encoded efflux pump is important in strains expressing high-level penicillin resistance and determined that mutations that abrogate pump function can result in a mutant strain expressing hypersusceptibility to penicillin; note that this observation supports the concept that inhibitors of drug efflux pumps could allow for a return of previously used and now discarded antibiotics. The penB resistance determinant is due to specific mutations in the gene encoding the major outer membrane porin protein termed PorB1b.43 This porin, PorB, exists in two allelic forms termed PorB1a and PorB1b. PorB1b–producing gonococci are often slightly less susceptible than PorB1a strains to penicillin and penB mutations can further decrease such susceptibility. Specific amino acid replacements in loop 3 (G120K and A121D) of PorB1b have been linked to the penB resistance determinant.44 These mutations were thought to decrease entry of penicillin through the PorB1b porin,43,44 but a conflicting view has been presented.4,45,46 Interestingly, penB mutations are only phenotypically evident when the strain has a coresident mtrR mutation, suggesting some interaction between PorB1b and the MtrCDE efflux pump.4,45,46 At least two additional mutations are needed for penicillin resistance (MIC of ≥2 µg/mL), but these are less well understood. Specific mutation in ponA (ponA1; results in the amino acid replacement L421P) causes a decreased affinity for penicillin to the encoded PBP1, and further decreased susceptibility to penicillin.47 Finally, the penC (currently more commonly named pilQ2) mutation occurs in the pilQ gene, which encodes the secretin PilQ of the type IV pilin.47,48 pilQ2 (encoding the amino acid replacement E666K) can decrease the stability of the PilQ doughnut-like multimeric structure in the outer membrane, which seems to decrease entry of penicillin.47,48 However, since pilQ2 mutations influence proper piliation, which is important for gonococcal disease, it is hard to envision how pilQ mutations would afford a selective advantage in the community and accordingly be of importance for wide spread of clinical penicillin resistance.
Against this background, it is important to ask: is it possible that history is repeating itself with respect to the emergence of strains displaying decreased susceptibility and resistance to cefixime and ceftriaxone? As mentioned earlier, this now seems to be an approaching reality. Accordingly, in recent years, the susceptibility to the recommended first-line cefixime and ceftriaxone has decreased worldwide (and in response, higher doses of ceftriaxone have been administered), 1,21,23–25 cefixime treatment failures are identified,1,26–28 and recently the first gonococcus displaying high-level clinical resistance to ceftriaxone, which is the last remaining option for empirical first-line treatment, was reported.29,30 Furthermore, the evolution of the resistance to these third-generation cephalosporins also seems to be highly similar to the evolution of penicillin resistance, that is, the most common mechanism for decreased susceptibility is alteration of penA, that is, acquisition of a penA mosaic allele (many different kinds with divergent effects on the MICs exist) or alterations of amino acid A501 in PBP2 1,23,24,28–30,49–60 The same mutations, as seen in penicillin resistance, in especially the promoter of mtrR further decrease the susceptibility.1,23,24,30,50,53,57,60 Moreover, alterations of amino acid G101 and A102 in PorB1b (penB resistance determinant) result in further decreased susceptibility.1,23,24,30,50,53,57,60 However, on the relatively few studies and the currently circulating gonococcal strains ponA1 or mutations in pilQ do not seem to substantially enhance the MICs.30,50,60,61 As in chromosomally mediated penicillin resistance, at least one unknown (“factor X”), nontransformable resistance determinant seems to exist.30,50,53,57,60 Worryingly, the detailed characterization (including also transformation experiments verifying the resistance mechanisms) of the first gonococcus displaying high-level clinical resistance to ceftriaxone showed that only a few additional amino acid replacements in a “traditional” mosaic PBP2 (PBP2 mosaic X allele, which has been correlated with cefixime treatment failures in Japan) were needed, that is, together with the resistance determinants mtrR, penB, and “factor X,” to develop the ceftriaxone MIC of 2–4 µg/mL (cefixime MIC of 8 µg/mL).30 This novel PBP2 allele contained only 12 polymorphic PBP2 amino acids compared to the PBP2 mosaic X allele, and four of these alterations were unique compared with any neisserial PBP2 sequence previously described. These four unique amino acid alterations consisted of A311V, T316P, A328T, and T484S. Although additional studies are needed, A311V and T316S are likely the alterations causing the high resistance to ceftriaxone (and all other extended-spectrum cephalosporins), that is, due to the proximity to the β-lactam active site in PBP2.30 Any secondary spread of this strain has not yet been verified. Despite the suboptimal Japanese surveillance systems (very limited gonococcal antimicrobial resistance surveillance), this fact may indicate that the strain has a lower biological fitness that results in limited further spread. Accordingly, the biological fitness of this first strain with high-level ceftriaxone resistance is crucial to investigate in an appropriate model and well-designed study.
Based on all historical precedents, strains with decreased susceptibility and resistance to third-generation cephalosporins will become more common and analysis of their genetic profiles are crucial, in order to understand the mechanisms for emergence and spread (national and international) of this resistance. Accordingly, it also seems inevitable that strains with clinical resistance to ceftriaxone will emerge and spread internationally, and the only question is when, and not if, we will identify these strains spreading worldwide.
An additional question of interest and high importance, discussed in more detail below, is why penicillin-resistant gonococci have persisted in the community, even though the antibiotic, in most countries worldwide, was removed from the treatment regimen decades ago? Is this because of a selection due to the general antibiotic pressure in the society, or is it because the biological fitness is unaltered (with or without compensatory mutations), OR do such strains in fact have a selective advantage, due to higher biological fitness, in the community? Answering these questions will likely give us important clues about the mechanisms for future spread and persistence also of cephalosporin resistant strains in the society.
Linkage between antibiotic resistance and biological fitness of gonococci
For several years, studies on antibiotic resistance and bacterial pathogenesis have been treated as unlinked areas of interest. In part, this is because mechanisms of resistance to classical antibiotics do not normally influence virulence. If anything, in the absence of the selective antibiotic, resistant strains have been considered less fit in the laboratory setting. However, this view is now changing, in part, due to recent work by Jerse’s laboratory on the gonococcal MtrCDE efflux pump and control of gene expression by transcriptional regulators and cis-acting regulatory mutations. This multidrug efflux pump recognizes both classical and nonclassical antibiotics. The latter group includes host-derived antimicrobials that are at the first line of innate host defense and include such structurally diverse compounds as antimicrobial peptides,62 bile salts,63 and progesterone.64 In 2003, Jerse et al.65 reported that gonococci lacking a functional MtrCDE efflux pump were unable to cause a sustained vaginal infection in female Balb/C mice. In subsequent experiments,64,66 that measured and compared in vivo fitness of gonococci bearing defined null mutations in genes encoding MtrR or the transcriptional activator (MtrA) of mtrCDE, the group showed that levels of efflux pump gene expression could significantly modulate fitness during infection. Moreover, cis-acting regulatory sequences that regulate mtrR and/or mtrCDE expression also influenced in vivo fitness. Most importantly, they found evidence for in-host evolution of gonococci that resulted in the generation of gonococcal variants with enhanced fitness in vivo. These in vivo-generated variants contained nonsense or missense mutations in the mtrR gene that derepressed expression of mtrCDE. Collectively, these studies strongly indicate that the capacity of gonococci to resist mediators of innate host defense through the efflux action of MtrCDE contributes to their survival during infection. Since this pump also recognizes penicillin, mutations that enhance pump expression, which are known to be critical for chromosomally mediated resistance to penicillin, may have been maintained despite the removal of penicillin from the treatment regimen. Accordingly, this is an excellent example of how a mechanism of antibiotic resistance can increase bacterial survival and fitness during infection. Furthermore, it has recently been shown that the MtrR repressor can regulate expression of nearly 70 genes scattered throughout the gonococcal chromosome, and accordingly this repressor may be of high importance, not only for repressing transcription of the mtrCDE-encoded efflux pump, but also perhaps for many processes involved in the basic metabolism, adaptation, biological fitness, and pathogenicity.67 Finally, recent indications of horizontal gene transfer from human to N. gonorrhoeae were published.68 Despite that the function of this 685 bp sequence (98–100% identical to the human long interspersed nuclear element L1) in gonococci remains unknown, this finding impacts our consideration of the coevolution of host and bacterial pathogens (and commensal bacteria),69 which may also involve evolution of antibiotic resistance, effects on biological fitness, bacterial adaptation, survival and virulence?
What might be the solutions to the problem of resistance, and what does the future hold?
N. gonorrhoeae now has shown its ability to also develop high-level resistance to ceftriaxone,29,30 which is the last remaining option for empirical first-line treatment of gonorrhea; this STI pathogen, therefore, seems to be evolving into a true superbug and, in the near future, may become untreatable in certain circumstances.
For future treatment of ceftriaxone resistant gonococcal strains, exploration of using increased doses and/or multiple doses of ceftriaxone is important.1,70 However, based on the history of evolution of penicillin resistance, these approaches would provide only a short-term solution. A more attractive approach would be to use antibiotic combination therapy. Combination therapy may, due to synergistic or additive effects, be effective in the treatment although the gonococcal strain is even resistant to both antibiotics when they are administered as single drugs. Combination therapy may also reduce future development of antimicrobial resistance, or at least the pace of this development, in N. gonorrhoeae.1 Unfortunately, combination therapy (as well as using multiple doses) would be problematic in less-resourced settings worldwide, which suffer from the highest gonorrhea burden, because of cost and compliance issues. Spectinomycin remains an option for treatment, with the exception of pharyngeal gonorrhea, but unfortunately rapid emergence of resistance to spectinomycin was observed when it was widely used in the past,71 and this drug is not available in many countries worldwide.1,23 Regarding new or “rediscovery” of old antimicrobials or other substances (e.g., plant extract) for treatment, there are no promising alternatives in sight.1,72 Nevertheless, gentamicin has been used as first-line treatment in Malawi during the past 15 years without any observed emergence of resistance, and the gonococcal population is susceptible in Europe as well.73 However, more in vitro and in vivo evaluations, including appropriately designed clinical treatment studies, are crucial, and based on its pharmacokinetics/pharmacodynamics, use of gentamicin solely for pharyngeal gonorrhea especially might be questionable. More promising seems to be to use gentamicin as one arm (together with, e.g., the new extended-release micro-sphere formulation of azithromycin that has improved gastrointestinal tolerability) for combination treatment. Carbapenems (such as ertapenem) may be a treatment option;30,74 however, this depends on the ceftriaxone resistance mechanisms and especially the penA alterations, of which most of them substantially also affect the carbapenem MICs (Unemo and Tapsall, unpublished data). However, all these new treatment regimens need up to date and comprehensive in vitro studies and randomized controlled treatment efficacy trials (evaluating effectiveness, side-effects and other safety issues, resistance selection (also among other bacterial species), pharmacokinetics and pharmacodynamics, and cost). Unfortunately, none seems ideal as a long-term solution, especially not in all settings including also less-resourced ones.
Accordingly, it seems crucial to start to “think out of the box” and investigate new targets and strategies for treating gonorrhea (as well as several other infectious diseases). After years of virtual neglect, which can be attributed to many reasons, the overall problem of antibiotic-resistant pathogens has rekindled interest in developing new anti-infectives. For instance, efflux pump inhibitors (EPIs) have been shown to increase the susceptibility of Gram-negative bacteria to certain antibiotics; this topic has been discussed by others.75 In light of the work by Jerse’s group,64–66 EPIs might also enhance bacterial susceptibility to mechanisms of innate host defense. The biosynthesis of gonococcal lipid A, the section of lipooligosaccharide imbedded in the outer membrane, has also been targeted by Nicholas and his group at the University of North Carolina-Chapel Hill (personal communication).76,77 Chemical inhibitors of LpxC have been found by them to have potent antigonococcal activity in vitro and further studies will test their ability to eradicate gonococci during an experimental gonococcal infection in the murine lower genital tract infection model. Finally, host defense peptides such as LL-37 (multifunctional cathelicidin peptide) that have both direct and indirect antibacterial actions are also being considered as a new class of anti-infectives.78
What does the future hold? This is not an easy question to answer. Clearly, the massive global public health problem of antibiotic resistance in general has caught the attention of many. Any solution will require the joint efforts of governments, industry, academia and the healthcare establishments. With respect to gonorrhea and other agents of STIs, we must first convince those in the decision-making process that the reproductive health of the world’s population will be severely compromised if we do not act soon. The recent emergence of the first gonococcal strain expressing high-level resistance to ceftriaxone,29,30 the last remaining option for empirical first-line treatment of gonorrhea, should be the “wake-up call.” In the meantime, in order to at least delay widespread dissemination of ceftriaxone-resistant gonococcal strains we need to maximize our active surveillance efforts (perform susceptibility testing of gonococcal isolates and appropriate identification of treatment failures), globally, nationally, and especially at the local level, and use effective antibiotics (of appropriate quality and in optimized dose).1 In certain regions, discontinued antibiotics might be used, but this strategy would require a return to the practice of culturing and antibiotic resistance testing in the clinical laboratory, which probably will not be feasible and/or affordable. Advances in molecular diagnostics and rapid genome sequencing efforts can also help to predict antibiotic resistance and ideally also future evolution of resistance. However, in regard to third-generation cephalosporins, such as cefixime and ceftriaxone, sufficient data to accurately correlate the presently known resistance determinants to the MICs of the gonococcal isolates and, further, to the treatment outcome remain lacking. None of this, however, will be effective unless there is a willingness to discuss STIs in the context of the overall healthcare initiatives that currently exist or lie in the future.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae . Expert Rev. Anti. Infect. Ther. 2009;7:821–834. doi: 10.1586/eri.09.63. [DOI] [PubMed] [Google Scholar]
- 2.Ober WB. Boswell’s clap. JAMA. 1970;212:91–95. [PubMed] [Google Scholar]
- 3.Sparling PF. Biology of Neisseria gonorrhoeae . In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH, editors. Sexually Transmitted Diseasess. 4th ed. New York, USA: McGraw-Hill Professional; 2007. pp. 607–626. [Google Scholar]
- 4.Shafer WM, Folster JP, Nicholas RA. Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseriae. In: Genco C, Wetzler L, editors. Neisseria: Molecular Mechanisms of Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 245–267. [Google Scholar]
- 5.Kampmeier RH. Introduction of sulfonamide therapy for gonorrhea. Sex. Transm. Dis. 1983;10:81–84. doi: 10.1097/00007435-198304000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop EMC. Gonorrhoea and the sulphonamides. Br. J. Vener. Dis. 1949;25:81–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Mahoney JF, Ferguson C, Buchholtz M, van Slyke CJ. The use of penicillin sodium in the treatment of sulfonamide-resistant gonorrhea in men. A preliminary report. Am. J. Gonorr. Vener. Dis. 1943;27:525–528. [Google Scholar]
- 8.Willcox RR. A survey of problems in the antibiotic treatment of gonorrhoea. With special reference to South-East Asia. Br. J. Vener. Dis. 1970;46:217–242. doi: 10.1136/sti.46.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashford WA, Golash RG, Hemming VG. Penicillinase-producing Neisseria gonorrhoeae . Lancet. 1976;2:657–658. doi: 10.1016/s0140-6736(76)92467-3. [DOI] [PubMed] [Google Scholar]
- 10.Phillips I. Beta-lactamase producing, penicillin-resistant gonococcus. Lancet. 1976;2:656–657. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- 11.Faruki H, Kohmescher RN, McKinney WP, Sparling PF. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally-mediated resistance) N. Engl. J. Med. 1985;313:607–611. doi: 10.1056/NEJM198509053131004. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control (CDC) Tetracycline-resistant Neisseria gonorrhoeae—Georgia, Pennsylvania, New Hampshire. MMWR. Morb. Mortal. Wkly. Rep. 1985;34:563–570. [PubMed] [Google Scholar]
- 13.Belland RJ, Morrison SG, Ison C, Huang WM. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol. Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Kumazawa J, Matsumoto T, Kobayashi I. High prevalence of Neisseria gonorrhoeae strains with reduced susceptibility to fluoroquinolones in Japan. Genitourin. Med. 1994;70:90–93. doi: 10.1136/sti.70.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapsall JW. Antibiotic resistance in Neisseria gonor-rhoeae . Clin. Infect. Dis. 2005;41(Suppl. 4):S263–S268. doi: 10.1086/430787. [DOI] [PubMed] [Google Scholar]
- 16.Knapp JS, Ohye R, Neal SW, et al. Emerging in vitro resistance to quinolones in penicillinase-producing Neisseria gonorrhoeae strains in Hawaii. Antimicrob. Agents Chemother. 1994;38:2200–2203. doi: 10.1128/aac.38.9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knapp JS, Fox KK, Trees DL, Whittington WL. Fluoroquinolone resistance in Neisseria gonorrhoeae . Emerg. Infect. Dis. 1997;3:33–39. doi: 10.3201/eid0301.970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Fluoroquinolone-resistance in Neisseria gonorrhoeae, Hawaii, 1999, and decreased susceptibility to azithromycin in N. gonorrhoeae, Missouri, 1999. MMWR. Morb. Mortal. Wkly. Rep. 2000;49:833–837. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Increases in fluoroquinolone-resistant Neisseria gonor-rhoeae—Hawaii and California, 2001. MMWR. Morb. Mortal. Wkly. Rep. 2002;51:1041–1044. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR. Morb. Mortal. Wkly. Rep. 2004;53:335–338. [PubMed] [Google Scholar]
- 21.Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: are cephalosporins next? Curr. Infect. Dis. Rep. 2011;13:196–204. doi: 10.1007/s11908-011-0169-9. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control Prevention (CDC) Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR. Morb. Mortal. Wkly. Rep. 2007;56:332–336. [PubMed] [Google Scholar]
- 23.Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert Opin. Pharmacother. 2009;10:555–577. doi: 10.1517/14656560902731993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis DA. The gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 2010;86:415–421. doi: 10.1136/sti.2010.042648. [DOI] [PubMed] [Google Scholar]
- 25.Tapsall JW. Antimicrobial resistance in Neisseria gonorrhoeae WHO/CDS/CSR/DRS/2001.3. Geneva: World Health Organization; 2001. [Accessed: August 16, 2011]. 2001. Available at: http:/www.who.int/entity/drugresistance/Antimicrobial_resistanc_in_Neisseria_gonorrhoeae.pdf. [Google Scholar]
- 26.Deguchi T, Yasuda M, Yokoi S, et al. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J. Infect. Chemother. 2003;9:35–39. doi: 10.1007/s10156-002-0204-8. [DOI] [PubMed] [Google Scholar]
- 27.Yokoi S, Deguchi T, Ozawa T, et al. Threat to cefixime treatment of gonorrhea. Emerg. Infect. Dis. 2007;13:1275–1277. doi: 10.3201/eid1308.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unemo M, Golparian D, Syversen G, et al. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro. Surveill. 2010;15:i19721. doi: 10.2807/ese.15.47.19721-en. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg. Infect. Dis. 2011;17:148–149. doi: 10.3201/eid1701.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohnishi M, Golparian D, Shimuta K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 2011;55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backman A, Orvelid P, Vazquez JA, et al. Complete sequence of abeta-lactamase-encoding plasmid in Neisseria meningitidis . Antimicrob. Agents Chemother. 2000;44:210–212. doi: 10.1128/aac.44.1.210-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flett F, Humphreys GO, Saunders JR. Intraspecific and intergeneric mobilization of non-conjugative resistance plasmids by a 24.5 megadalton conjugative plasmid of Neisseria gonorrhoeae . J. Gen. Microbiol. 1981;125:123–129. doi: 10.1099/00221287-125-1-123. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda F, Tsuji A, Kaneko Y, et al. Conjugal transfer of beta-lactamase-producing plasmids of Neisseria gonorrhoeae to Neisseria meningitidis . Microbiol. Immunol. 1986;30:737–742. doi: 10.1111/j.1348-0421.1986.tb03000.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberts MC. Plasmids of Neisseria gonorrhoeae and other Neisseria species. Clin. Microbiol. Rev. 1989;2(Suppl.):S18–S23. doi: 10.1128/cmr.2.suppl.s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts MC, Knapp JS. Transfer of β-lactamase plasmids from Neisseria gonorrhoeae to Neisseria meningitidis and commensal Neisseria species by the 25.2-megadalton conjugative plasmid. Antimicrob. Agents Chemother. 1988;32:1430–1432. doi: 10.1128/aac.32.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elwell LP, Roberts M, Mayer LW, Falkow S. Plasmid-mediated beta-lactamase production in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 1977;11:528–533. doi: 10.1128/aac.11.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougherty TJ. Genetic analysis and penicillin-binding protein alterations in Neisseria gonorrhoeae with chromosomally mediated resistance. Antimicrob. Agents Chemother. 1986;30:649–652. doi: 10.1128/aac.30.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowson CG, Jephcott AE, Gough KR, Spratt BG. Penicillinbinding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae . Mol. Microbiol. 1989;3:35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 39.Maness MJ, Sparling PF. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae . J. Infect. Dis. 1973;128:321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Hagman KE, Pan W, Spratt BG, et al. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 41.Zarantonelli L, Borthagaray G, Lee EH, Shafer WM. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob. Agents Chemother. 1999;43:2468–2472. doi: 10.1128/aac.43.10.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veal WL, Nicholas RA, Shafer WM. Overex-pression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae . J. Bacteriol. 2002;184:5619–5624. doi: 10.1128/JB.184.20.5619-5624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill MJ, Simjee S, Al-Hattawi K, et al. Gonococcal resistance to β-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 1998;42:2799–2803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olesky M, Hobbs M, Nicholas RA. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2002;46:2811–2820. doi: 10.1128/AAC.46.9.2811-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olesky M, Zhao S, Rosenberg RL, Nicholas RA. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 2006;188:2300–2308. doi: 10.1128/JB.188.7.2300-2308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shafer WM, Folster JP. Towards an understanding of chromosomally mediated penicillin resistance in Neisseria gonorrhoeae: evidence for a porin-efflux pump collaboration. J. Bacteriol. 2006;188:2297–2299. doi: 10.1128/JB.188.7.2297-2299.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ropp PA, Hu M, Olesky M, Nicholas RA. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2002;46:769–777. doi: 10.1128/AAC.46.3.769-777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao S, Tobiason DM, Hu M, et al. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol. Microbiol. 2005;57:1238–1251. doi: 10.1111/j.1365-2958.2005.04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ameyama S, Onodera S, Takahata M, et al. Mosaiclike structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 2002;46:3744–3749. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golparian D, Hellmark B, Fredlund H, Unemo M. Emergence, spread and characteristics of Neisseriagon-orrhoeae isolates with in vitro decreased susceptibility and resistance to extended-spectrum cephalosporins in Sweden. Sex. Transm. Infect. 2010;86:454–460. doi: 10.1136/sti.2010.045377. [DOI] [PubMed] [Google Scholar]
- 51.Ito M, Deguchi T, Mizutani KS, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob. Agents Chemother. 2005;49:137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SG, Lee H, Jeong SH, et al. Various penA mutations together with mtrR porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J. Antimicrob. Chemother. 2010;65:669–675. doi: 10.1093/jac/dkp505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindberg R, Fredlund H, Nicholas R, Unemo M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: Association with genetic polymorphisms in penA mtrR porB1b, and ponA . Antimicrob. Agents Chemother. 2007;51:2117–2122. doi: 10.1128/AAC.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandori M, Barry PM, Wu A, et al. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob. Agents Chemother. 2009;53:4032–4034. doi: 10.1128/AAC.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahata S, Senju N, Osaki Y, et al. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2006;50:3638–3645. doi: 10.1128/AAC.00626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka M, Nakayama H, Huruya K, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int. J. Antimicrob. Agents. 2006;27:20–26. doi: 10.1016/j.ijantimicag.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Tomberg J, Unemo M, Davies C, Nicholas RA. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry. 2010;49:8062–8070. doi: 10.1021/bi101167x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unemo M, Fasth O, Fredlund H, et al. Phenotypic and genetic characterization of the 2008 WHO Neisseria gon-orrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimi-crob. Chemother. 2009;63:1142–1151. doi: 10.1093/jac/dkp098. [DOI] [PubMed] [Google Scholar]
- 59.Whiley DM, Limnios A, Ray S, et al. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 2007;51:3111–3116. doi: 10.1128/AAC.00306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao S, Duncan M, Tomberg J, et al. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2009;53:3744–3751. doi: 10.1128/AAC.00304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whiley DM, Jacobsson S, Tapsall JW, et al. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J. Antimicrob. Chemother. 2010;65:2543–2547. doi: 10.1093/jac/dkq377. [DOI] [PubMed] [Google Scholar]
- 62.Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagman KE, Lucas CE, Balthazar JT, et al. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology. 1997;143(Pt 7):2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 64.Warner DM, Shafer WM, Jerse AE. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 2008;70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jerse AE, Sharma ND, Simms AN, et al. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 2003;71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warner DM, Folster JP, Shafer WM, Jerse AE. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae . J. Infect. Dis. 2007;196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- 67.Johnson PJ, Stringer VA, Shafer WM. Off-target gene regulation mediated by transcriptional repressors of antimicrobial efflux pump genes in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2011;55:2559–2565. doi: 10.1128/AAC.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson MT, Seifert HS. Opportunity and means: horizontal gene transfer from the human host to a bacterial pathogen. MBio. 2011;2:e00005–e00011. doi: 10.1128/mBio.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shafer WM, Ohneck EA. Taking the gonococcus-human relationship to a whole new level: implications for the coevolution of microbes and humans. MBio. 2011;2:e00067–e00011. doi: 10.1128/mBio.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chisholm SA, Mouton JW, Lewis DA, et al. Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J. Antimicrob. Chemother. 2010;65:2141–2148. doi: 10.1093/jac/dkq289. [DOI] [PubMed] [Google Scholar]
- 71.Boslego JW, Tramont EC, Takafuji ET, et al. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and penicillinase-producing Neisseria gonorrhoeae . N. Engl. J. Med. 1987;317:272–278. doi: 10.1056/NEJM198707303170504. [DOI] [PubMed] [Google Scholar]
- 72.Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 2007;44(Suppl 3):S84–S101. doi: 10.1086/511422. [DOI] [PubMed] [Google Scholar]
- 73.Chisholm SA, Quaye N, Cole MJ, et al. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J. Antimicrob. Chemother. 2010;66:592–595. doi: 10.1093/jac/dkq476. [DOI] [PubMed] [Google Scholar]
- 74.Livermore DM, Alexander S, Marsden B, et al. Activity of ertapenem against Neisseria gonorrhoeae . J. Antimicrob. Chemother. 2004;54:280–281. doi: 10.1093/jac/dkh272. [DOI] [PubMed] [Google Scholar]
- 75.Lomovskaya O, Watkins WJ. Efflux pumps: their role in antibacterial drug discovery. Curr. Med. Chem. 2001;8:1699–1711. doi: 10.2174/0929867013371743. [DOI] [PubMed] [Google Scholar]
- 76.Lee CJ, Liang X, Chen X, et al. Species-specific and inhibitor-dependent conformations of LpxC: implications for antibiotic design. Chem. Biol. 2011;18:38–47. doi: 10.1016/j.chembiol.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mao H, Zhou P, Nicholas RA. LpxC inhibitors as a novel class of antibiotics against Neisseria gonorrhoeae; Banff, Alberta, Canada. Abstr.. Proceedings of the 17th International Pathogenic Neisseria Conference; 2010. p. P011. [Google Scholar]
- 78.Bucki R, Leszczyńska K, Namiot A, Sokołowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch. Immunol. Ther. Exp. (Warsz.) 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]