Abstract
Young children engage cognitive control reactively in response to events, rather than proactively preparing for events. Such limitations in executive control have been explained in terms of fundamental constraints on children’s cognitive capacities. Alternatively, young children might be capable of proactive control but differ from older children in their meta-cognitive decisions regarding when to engage proactive control. We examined these possibilities in three conditions of a task-switching paradigm, varying in whether task cues were available before or after target onset. Reaction times, ERPs, and pupil dilation showed that 5-year-olds did engage in advance preparation, a critical aspect of proactive control, but only when reactive control was made more difficult, whereas 10-year-olds engaged proactive control whenever possible. These findings highlight meta-cognitive processes in children’s cognitive control, an understudied aspect of executive control development.
Keywords: executive control, meta-cognition, proactive control, reactive control, cognitive development, children
Many everyday activities require efficient control over one’s own thoughts and actions to ensure goal attainment (e.g., to keep working rather than check the news or daydream). Such executive control can be temporally engaged in different ways. For instance, when walking to a new place, one may engage control proactively, searching for directions beforehand, or reactively, figuring out directions while actually walking. Proactive and reactive modes of control, as first highlighted by the Dual Mechanisms of Control framework (Braver, 2012; Braver et al., 2007), present complementary advantages and limitations. Proactive control allows individuals to anticipate and prepare for upcoming events, hence engaging mental effort early to bias the cognitive system to prevent or minimize the effects of interference before it occurs. Proactive control is generally very efficient, but highly demanding on working memory, due to active maintenance of goal-relevant information through sustained activity in lateral prefrontal cortex (PFC) over relatively long periods. In contrast, reactive control is mobilized later in response to unforeseen events to resolve interference after it occurs. As such, it is less demanding because of transient activation of goal-relevant information and transient recruitment of lateral PFC (Braver, 2012; Marklund & Persson, 2012).
Although adults vary in the recruitment of proactive and reactive control as a function of trait factors (e.g., working memory capacity), overall they flexibly engage the most adaptive control mode depending on contextual demands (e.g., whether or not upcoming events can be reliably predicted), as evidenced by changes in lateral PFC activity and pupil dilation in response to experimental manipulations that encourage reactive or proactive control (Braver, Paxton, Locke, & Barch, 2009; Chiew & Braver, 2013). Such flexibility in control mode engagement is observed from age 8 on, but not in younger children, who appear to rely on reactive control even in situations where proactive control would seem to be more efficient (Blackwell & Munakata, 2013; Chatham, Frank, & Munakata, 2009; Chevalier et al., 2014; Vallesi & Shallice, 2007). For instance, Chatham et al. (2009) used pupil dilation, a well-established index of cognitive effort (e.g., Beatty, 1982), to track early and late mental effort engagement associated with proactive and reactive control, respectively. They observed that, when asked to respond to specific cue-target associations, 3-year-olds show greater late mental effort (as shown by greater pupil dilation) after target onset, showing no anticipation of the target, whereas 8-year-olds show greater early mental effort before target onset; suggesting a shift from reactive to proactive control during childhood. The shift to proactive control seems to occur around 6 years of age, although proactive control continues to develop through late adolescence (Andrews-Hanna et al., 2011; Chatham, Provan, & Munakata, in revision; Chevalier & al., 2014; Lucenet & Blaye, 2014). Understanding the reasons why young children do not engage the most mature and efficient forms of control is critical to uncover the mechanisms underpinning executive control development and to design effective interventions early in childhood.
Young children may rely on reactive control because limited cognitive resources prevent the use of proactive control. For example, because of lower working memory capacity (e.g., Gathercole, Pickering, Ambridge, & Wearing, 2004), they may not be able to actively maintain task-relevant information active long enough to engage proactive control. From this perspective, quantitative increase in cognitive resources may support diversification of children’s control modes with age. Alternatively, changes in the meta-cognitive coordination of control modes may drive executive control development. Specifically, young children may be able to exert control proactively, but may differ from older children and adults in the conditions under which they determine proactive control should be engaged. For example, proactive control is likely to be more effortful and less accurate for young children, due to lower cognitive resources or lack of practice. Therefore, young children may have a higher threshold for engaging it and may not realize the advantages of this more demanding control mode in some situations.
The meta-cognitive coordination hypothesis builds on evidence that, across a variety of domains, multiple strategies can co-exist from early on, and the best strategy in a given situation (i.e., the strategy that allows the best performance with minimal cognitive effort) can be selected based on the automatic calculation of the respective costs and benefits of the available strategies (e.g., Adolph, 1997; Crowley & Siegler, 1993). Importantly, with age, information accumulates about the costs and benefits of each strategy in various situations, leading to more frequent selection and better execution of the most efficient strategies (e.g., Chen & Siegler, 2000; Lemaire & Brun, 2014; Siegler, 2007). According to the Expected Value of Control (EVC) theory (Shenhav, Botvinick, & Cohen, 2013), such evaluation of the costs and benefits of control and adequacy with task demands is critical to adulthood executive control. Dorsal anterior cingulate cortex (dACC) may integrate information on task demands and how well currently engaged control serves these demands in order to determine how control should be exerted (e.g. reactively or proactively). Increasing meta-cognitive coordination of control may drive control development in children (see Zelazo, 2004), and account for its relation with dACC activity in children (Fjell et al., 2012; Kelly et al., 2009; Kharitonova, Martin, Gabrieli, & Sheridan, 2013).
If young children rely on reactive control because of how they coordinate control, then making reactive control more difficult should weaken its dominance, and encourage them to select an alternative approach from their repertoire (e.g., Siegler, 2007), increasing the likelihood of proactive control. In contrast, young children should not engage proactive control if they have insufficient cognitive resources for proactive control to be part of their repertoire, even when reactive control is more difficult. The cued task-switching paradigm (Meiran, 1996) is well suited to test these hypotheses because it allows manipulating the possibility to engage reactive and/or proactive control (e.g., Czernochowski, 2014). It requires switching back and forth between multiple tasks (e.g., color- and shape-matching tasks) as a function of a task cue signaling the upcoming task (e.g., a palette of colors for color-matching, a palette of geometric shapes for shape-matching). Proactive control in this paradigm is multi-faceted, including processes related to task set maintenance and monitoring of upcoming task difficulty (e.g., Paxton et al., 2008; Czernochowski, 2014; Waxer & Morton, 2011), but perhaps its most prominent aspect is advance preparation based on task cues. In adults, early cue presentation yields better performance (e.g., Altmann, 2004; Monsell, 2003) because it allows them to proactively determine the relevant task goal and task rules, hence preparing to process the upcoming target effectively (DeBaene & Brass, in press).
Such advance preparation is also evidenced through event-related potentials (ERPs), whose excellent temporal resolution of brain activity is especially valuable for capturing the temporal dynamic of control. Specifically, early cue presentation is associated with a cue-locked late posterior positivity over parietal channels that reflects task selection (e.g., Jamadar, Michie, & Karayanidis, 2010; Karayanidis, Coltheart, Michie, & Murphy, 2003; Karayanidis, Jamadar, Ruge, Phillips, & Heathcote, 2010; Jamadar, Hughes et al., 2010; Karayanidis, Mansfield et al., 2009). During childhood, similar behavioral and ERP effects are observed from 7 years of age onward (e.g., Cepeda, Kramer, & Gonzalez de Sather, 2001; Manzi, Nessler, Czernochowski, & Friedman, 2011), suggesting that school-age children also adopt a proactive approach, when they are offered the possibility to prepare in advance. In contrast, young children may not benefit as much from early cue presentation due to their bias toward reactive control (Chatham et al., 2009) and suboptimal processing of task cues (Chevalier & Blaye, 2009; Chevalier, Huber, Wiebe, & Espy, 2013).
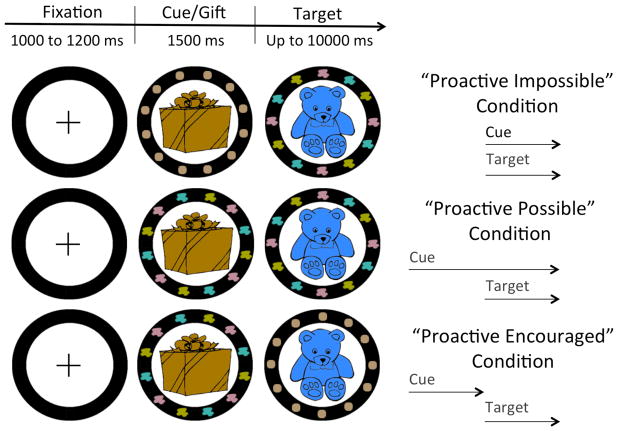
To address whether proactive, advance preparation can be encouraged during childhood, the present study targeted two age groups: 5-year-old children, who start switching ably between tasks but still engage reactive control preferentially (Blackwell & Munakata, 2013; Chatham, Provan, & Munakata, in revision; Chevalier et al., 2014; Vallesi & Shallice, 2007), and 10-year-olds, who likely engage proactive control whenever this control mode is possible (Chatham et al., 2009). The cue-target interval was manipulated in three conditions of an age-appropriate, cued task-switching paradigm (Figure 1). Specifically, in the “Proactive Impossible” condition, the task cue (e.g., patches of color) was presented simultaneously with the target (e.g., blue bear), hence preventing proactive preparation and encouraging reactive control. In the “Proactive Possible” condition, early cue presentation was continued after target onset, giving children the opportunity to prepare proactively for the upcoming target, but children could still reactively process the task cue after target onset. Finally, early cue presentation was terminated on target onset in the “Proactive Encouraged” condition, encouraging children to process the cue proactively by increasing the difficulty of reactive control, since the cue would had to be retrieved from memory if not processed before target onset. Besides behavioral indices, the temporal dynamic of control was directly examined using cue-locked ERPs, through the late posterior positivity associated with cue-based task selection, and pupil dilation, whose temporal fluctuations reflect changes in mental effort—both indices have been previously used to capture reactive and proactive control (Chatham et al., 2009; Chiew & Braver, 2013; Karayanidis et al., 2011).
Figure 1.
Illustration of the cued task-switching paradigm used in each condition. Children had to switch between sorting the target by shape or by color. In this example, the multiple patches of color signal that color is the relevant sorting dimension. In the “Proactive Impossible” condition, target onset was preceded by neutral information (brown circles) regarding the upcoming task, hence making proactive preparation impossible. In the “Proactive Possible” condition the task cue was presented earlier but remained visible after target onset, making proactive preparation possible but not necessary. In the “Proactive Encouraged” condition, the early presentation of the task cue was discontinued after target onset, hence providing a strong incentive to proactively process the cue before target onset.
We expected 10-year-olds to engage proactive control whenever it was possible, that is, in the “Proactive Possible” and “Proactive Encouraged” conditions. Specifically, these children should show faster reaction times, more pronounced cue-locked posterior positivity and greater cue-related pupil dilation in both conditions, relative to the “Proactive Impossible” condition. In contrast, we expected that the “Proactive Possible” condition would not provide a strong enough incentive for 5-year-olds to prepare proactively, hence yielding no difference from the “Proactive Impossible” condition. Of prime interest was performance in the “Proactive Encouraged” condition. If 5-year-olds’ limited working-memory capacity prevents the utilization of proactive control, they should not show ERP or pupil dilation markers of proactive control in this condition either. Because this condition makes reactive control particularly challenging, RTs should increase and accuracy decrease. In contrast, if 5-year-olds are capable of proactive control, they should engage it in this condition because reactive control is much more challenging. They should show faster reaction times, more pronounced cue-locked posterior positivity and pupil dilation than in the other two conditions. ERPs and pupil dilation may provide the clearest indication of proactive control engagement, because behavior does not always initially change when children transition to new approaches (Siegler & Jenkins, 1989).
Method
Participants
Study participants included 36 five-year-old children (mean age = 68.5 months; SD = 2.7 months; 20 girls and 14 boys) and 28 ten-year-old children (mean age = 118.6 months; SD = 1.7 months; 17 girls and 13 boys). Four additional 5-year-olds were excluded because of fussing. Initial recruitment included more 5-year-olds than 10-years-olds to compensate for greater exclusion rate due to poorer noise/signal ratio in younger children’s data. Most children were Caucasian (48 White, one Black, two Asian; race was not reported for the remaining 13 participants) and from middle to high socio-economic background (55 had at least one parent with a four-year college degree or higher, and three had at least one parent with some college education; parental education was not reported for the remaining six participants), which is reflective of the local community. All children were right-handed. Prior to participation, parental informed consent was obtained for all children. Ten-year-olds also gave informed assent.
Materials and Procedure
A trained experimenter tested all participants individually in a single 90-minute session at the laboratory. Each session started with sensor net application, followed by eye-tracking calibration. Children sat 60 cm away from the computer monitor and completed three successive conditions of an age-appropriate, cued task-switching paradigm, and a selective sustained attention task (not presented here). After task completion, children received a developmentally appropriate toy and their accompanying parents received monetary compensation.
Each participant completed three conditions of the “Santa Claus Game”, run with E-Prime 1.2 (Psychology Software Tools, Pittsburgh, PA). In these adaptations of the cued task-switching paradigm, children helped Santa to prepare for next Christmas by sorting toys (i.e., the targets) by either their shape or their color. Three combinations of shapes and colors were used (one for each condition), bear-car-bluered, airplane-doll-green-orange, and train-horse-purple-pink. On each trial, one 8 × 8-cm bidimensional target (e.g., a blue bear) was presented within a black circle at the center of the screen and four 2 × 2-cm unidimensional response pictures (e.g., a bear, a red patch, a car and a blue patch) were constantly presented below the black circle. Each response picture corresponded to one of the four horizontally aligned buttons on a response pad. Children were asked to keep their four fingers (index and middle fingers of each hand) on the response pad and respond by pressing the response button that matched either the color or the shape of the target as a function of the task cues. Task cues were displayed on the black circle (12 gray geometrical shapes signaled that shape was relevant, whereas 12 patches of different colors signaled that color was relevant).
On each trial, children saw a fixation cross within the black circle for 1000 to 1200 ms (jittered inter-trial interval) followed by a Christmas gift (i.e., brown wrapped box), which contained the toy that they had to sort next. After 1500 ms, the target replaced the box and remained on screen until a response was entered or for up to 10 sec. Critically, the timing of cue presentation was manipulated across conditions (Figure 1). In the “Proactive Impossible” condition, the task cue was presented at the same time as the target, so that proactive cue processing was impossible (non-informative brown circles were presented instead of the cue while the gift was displayed), hence forcing participants to select the relevant task after target onset. In contrast, the cue was presented along with the gift and remained visible after target onset in the “Proactive Possible” condition, so that cue-based proactive preparation was possible but not necessary. Finally, early cue presentation (i.e., along with the gift) was terminated before target onset in the “Proactive Encouraged” condition, hence making reactive control more challenging and providing a strong incentive to process the cue proactively (select the relevant task, ahead of target presentation). Cue conditions were blocked and their order was counterbalanced across participants. Participants were explicitly informed of the change in cue presentation as they started a new condition. For the sake of pupillometric data, objects presented before and after target onset were roughly equivalent in complexity (similar cartoon design with similar levels of detail) and luminance (similar color brightness calculated based on the RGB color model). In addition, the spatial location of the patches of colors/geometric shapes/non-informative brown circles was slightly rotated within the black circle on target onset so that a perceptual change occurred at the level of the cue in all three conditions.
Each condition started with two demonstration trials in which the experimenter demonstrated how to sort toys according to one of the dimensions (e.g., color), followed by four practice trials completed by the child. Demonstration and practice trials were repeated for the other dimension. Children were then instructed that they would play the Color and Shape games at the same time and completed 15 practice trials after six demonstration trials. Guidance and feedback were provided during these trials that could be repeated if necessary. Although response pictures were displayed on the buttons and on the screen to help children match each button with its corresponding response picture, they were subsequently removed from the button box (but remained visible on the screen) during the test phase to reduce head movements (and related artifacts in the EEG data). In the test phase, children completed three series of 21 trials separated by short breaks (generally below two minutes). Test trials contained 30 no-switch trials, where the relevant task repeated, and 30 switch trials where the relevant task changed (plus three start trials)1. Switch and no-switch trials alternated unpredictably.
Data Recording and Processing
Behavioral data
Accuracy was calculated as the percent of correct responses on switch and no-switch trials. Reaction times (RTs) were examined for correct responses after discarding outliers, that is, values greater than M + 3 SD, and values lower than 200 ms or M – 3 SD (1.6%). Only one 5-year-old did not have at least five values per experimental cell and was excluded from RT analysis. For the remaining children, on average 21 and 26 trials per experimental cell (out of 30) were included in RT analysis at ages 5 and 10, respectively. Reaction times were log-transformed prior to statistical analyses to control for age-related baseline differences (Meiran, 1996). For the sake of clarity, we reported both log-transformed and raw values.
ERP data
EEG data were recorded at a 250 Hz sampling rate using 128-channel Hydrocel Electrical Geodesic Sensor Net and Net Station 4.3.1 (Electrical Geodesics Inc, EGI, Eugene, OR). Impedances were kept below 50 kO. Recording in every channel was vertex-referenced. The EEG data were processed offline using Net Station 4.3.1 and EP Toolkit 2.23 (Dien, 2010) for filtering, segmentation, artifact detection, eye blink correction, bad channel replacement, and averaging. The continuous data were first digitally filtered using a 30-Hz low-pass filter. Next, correct switch trials and no-switch trials were segmented into a 1200 ms time window around cue/gift onset, including a 200 ms baseline period. Artifact removal was performed twice, first within Net Station and then using EP Toolkit 2.23. In Net Station, a channel was considered bad if its amplitude for a specific trial varied by over 160μV during the entire segment. Eye blinks were rejected if the amplitude varied by over 200μV. Channels were rejected if they were marked bad on more than 20% of the segments. A segment was rejected if it contained more than 16 bad channels. Then, using EP Toolkit 2.23, an automated independent components analysis (ICA) corrected any eye blinks that were not identified by Net Station. A channel was considered bad for a specific trial if its amplitude varied by over 100μV within that trial or rejected for the entire task if they were marked as bad on at least 20% of the trials. Bad channels were corrected using spline interpolation based on neighboring channels (M = 3.3, SD = 1.0 at age 5; M = 2.5; SD = 1.0 at age 10). Trials were rejected if 10% or more of the channels had poor signal. Following artifact correction, data were re-referenced to the average reference and baseline corrected using the 200 ms pre-cue period. Correct switch and no-switch trials in each condition were analyzed. Children with at least 10 good segments in all of the experimental cells were included in subsequent analyses, which falls within the range recommended and used with young children (DeBoer, Scott, & Nelson, 2005; Rueda, Posner, Rothbart, & Davis-Stober, 2004). On average, there were 18 good segments per experimental cell (out of 30) at age 5 (N = 28) and 26 at age 10 (N = 28).
Pupillometric data
Pupil dilation was recorded at a 50-Hz sampling rate using a Tobii x50 eye-tracker and ClearView software (Tobii, Stockholm, Sweden). Calibration was achieved after net application and before completing the task-switching paradigm, using a 5-point procedure. Analysis of pupil dilation was limited to the first 2500 ms of each trial, that is, the 1500 ms gift/cue presentation and the first 1000 ms following target onset. The 200 ms immediately preceding cue/gift onset (during which a fixation cross was presented) were used as baseline for each trial and pupil dilation was calculated as percent change from this baseline, in order to control for age-related differences in baseline pupil diameter (Chatham et al., 2009). Measurements for correct trials were averaged into consecutive 60-ms bins. Trials that contained valid data points for less than half of the segment were discarded. Children with at least 5 good trials in each experimental cell were included in the statistical analyses (N = 26 at age 5 and N = 24 at age 10). On average, there were 19 good segments per experimental cell (out of 30) at age 5 and 26 at age 10.
Unless otherwise noted, all measures were analyzed using 3 (Condition: Proactive Impossible, Proactive Possible, Proactive Encouraged) × 2 (Trial Type: No-switch, Switch) × 2 (Age: 5, 10) mixed design ANOVAs2.
Results
As expected, 10-year-olds showed proactive preparation whenever it was possible (in both the “Proactive Possible” and “Proactive Encouraged” conditions), across reaction times, ERPs, and pupil dilation. Five-year-olds showed the capacity for proactive preparation across these measures, but only when reactive control was made more difficult (in the “Proactive Encouraged” condition).
Behavioral Analyses
Reaction times
RTs are shown in Figure 2A. Age, condition, and trial type significantly affected RTs, F(1, 61) = 149.912, p < .001, η2p = .711, F(2, 122) = 50.968, p < .001, η2p = .455, and F(1, 61) = 70.376, p < .001, η2p = .536, respectively. These effects were qualified by significant two-way interactions between age and condition, F(2, 122) = 5.592, p = .005, η2p = .084, and age and trial type, F(1, 61) = 7.838, p = .007, η2p = .114, and a three-way interaction between age, condition and trial type, F(2, 122) = 5.261, p = .006, η2p = .079. As expected, 5-year-olds (7.79 ln ms; 2791 ms) responded slower than 10-year-olds (7.04 ln ms; 1317 ms), and children were faster on no-switch trials (7.38 ln ms; 1989 ms) than switch trials (7.46 ln ms; 2120 ms), hence showing significant switch costs. Most importantly, 5-year-olds performed faster in the “Proactive Encouraged” condition (7.63 ln ms; 2324 ms) than the other two conditions, ps < .001, whereas RTs in the “Proactive Possible” condition (7.83 ln ms; 2937 ms) were only marginally faster than in the “Proactive Impossible” condition (7.94 ln ms; 3200 ms), p = .066. In contrast, 10-year-olds responded faster in both the “Proactive Possible” and “Proactive Encouraged” conditions (6.86 ln ms; 1224 ms, and 6.94 ln ms; 1105 ms, respectively) than the “Impossible” condition (7.32 ln ms; 1624 ms), ps < .001, whereas RTs in the first two conditions did not significantly differ, p = .115. In addition, 10-year-olds showed significantly smaller switch costs in the “Proactive Impossible” condition (0.05 ln ms, 76 ms) than the “Proactive Possible” (0.13 ln ms; 130 ms) and “Proactive Encouraged” conditions (0.17 ln ms; 160 ms), ps < .040, whereas switch costs did not differ across conditions at age 5, all ps > .154.
Figure 2.
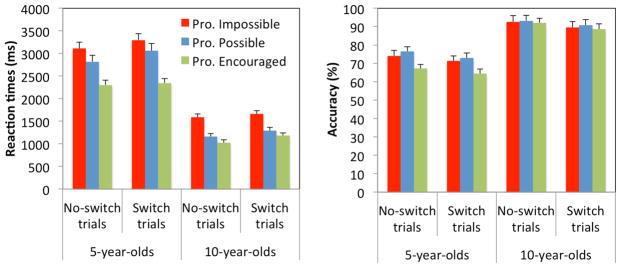
Mean reaction times (left) and accuracy rates (right) for 5- and 10-year-olds in each condition. Error bars indicate stander errors. Five-year-olds responded faster only when they were strongly encouraged to prepare proactively, whereas 10-year-olds showed faster RTs whenever proactive preparation was possible. Accuracy was lower in the “Proactive Encouraged” condition than in the other two conditions at age 5.
Accuracy
The ANOVA on accuracy revealed significant main effects of age, F(1, 62) = 38.424, p < .001, η2p = .383, trial type, F(1, 62) = 26.356, p < .001, η2p = .298, and condition, F(2, 124) = 4.671, p = .011, η2p = .070. As expected, 5-year-olds achieved lower accuracy rates (71.1%) than 10-year-olds (91.1%), and children showed lower accuracy on switch trials (79.6%) than no-switch trials (82.6%). Accuracy was significantly lower in the “Proactive Encouraged” condition (78.0%) relative to the “Proactive Possible” condition (83.4%), p < .001, and marginally so relative to the “Proactive Impossible” condition (81.8%), p = .051; the latter two conditions did not differ significantly, p = .445 (Figure 2B). Although the Condition × Age interaction did not reach significance, F(2, 124) = 2.359, p = .099, η2p = .037, follow-up analyses showed the effect of condition was significant at age 5, F(2, 70) = 5.089, p = .009, η2p = .127, but not at age 10, p = .934. The other interactions were not significant, ps > .842.
Five-year-olds’ faster RTs, accompanied by lower accuracy, in the “Proactive Encouraged” condition did not seem to reflect a speed/accuracy tradeoff (e.g., more impulsive responding) rather than the use of proactive control, given that the effect of condition on RTs held even when controlling for accuracy in a mixed model, F(2, 169) = 45.87, p < .001, η2 = .66. RTs were slower in both the “Proactive Impossible” and “Proactive Possible” conditions than in the “Proactive Encouraged” condition, estimated coefficient: .247, t(169) = 5.14, p < .001, and estimated coefficient: .105, t(169) = 2.16, p = .031, respectively.
ERP Analyses
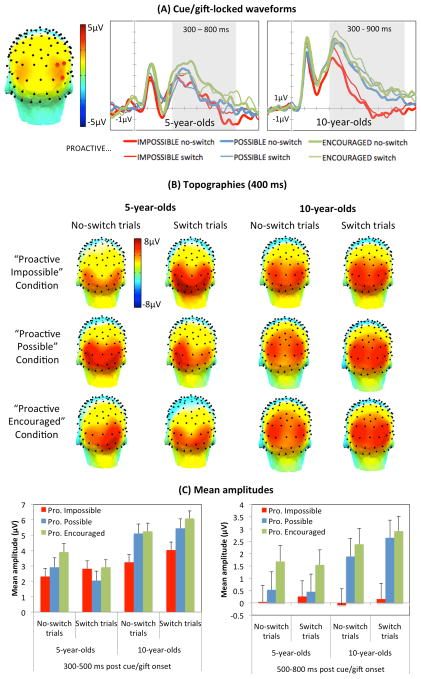
The cue/gift-locked waveforms showed on all trial types an initial posterior peak around 400 ms that extended into a positive-going slow-wave over posterior channels between 500 and 900 ms post cue onset, corresponding to the posterior positivity related to proactive preparation (Figure 3A and 3B). The timing and topography is consistent with a previous report with children (Manzi et al., 2011). The peak channels were circumscribed with a spatial principal component analysis (PCA), using the covariance matrix and infomax rotation. Based on the scree plot (Cattell, 1966), 13 spatial factors were retained that accounted for approximately 83% of the spatial variance in the ERPs data. The topography of the first spatial factor, which accounted for 11% of the variance, matched the late posterior positivity. Waveforms were averaged across the channels whose factor loadings on the first spatial factor were greater than .6 (Dien, 2010): 84, 85 and 91 (Figure 3A).
Figure 3.
Cue/gift-locked posterior positivity. (A) The topography of the spatial factor corresponding to the late posterior positive slow-wave is shown on the left, with peak channels (factor loading > 0.6) marked in red. Waveforms, averaged across the peak channels, are shown in the middle and right panels, with grayed areas signaling the windows during which amplitude significantly differed across conditions. (B) Topographies of the late posterior positivity. (C). Mean amplitudes. Vertical bars indicate standard errors. At age 5, the posterior positivity was more pronounced in the “Proactive Encouraged” condition than the other conditions. At age 10, the posterior positivity was more pronounced after cue onset in both the “Proactive Encouraged” and “Proactive Possible” conditions, relative to the “Proactive Impossible” condition.
The initial peak was indexed by the mean amplitude between 300 and 500 ms after cue/gift onset within each condition separately (Figure 3C, left). Visual inspection of the data showed that this window included the peaks from all participants while not overlapping with other components. There were a significant effect of condition, F(2, 108) = 8.342, p < .001, η2p = .134, and a significant Condition × Age interaction, F(2, 108) = 3.123, p = .048, η2p = .0553. On both trial types, amplitude tended to be more pronounced in the “Proactive Encouraged” condition than in the “Proactive Possible” and “Proactive Impossible” conditions at age 5, although pairwise comparisons fell short of significance, p = .093 and p = .076, respectively. The latter conditions did not differ from each other, p = .885. In contrast, 10-year-olds showed a greater amplitude in both the “Proactive Encouraged” and “Proactive Possible” conditions, relative to the “Proactive Impossible” condition, ps < .003; with no difference between the former two conditions, p = .362. Finally, there was a significant interaction between trial type and age, F(2, 108) = 6.929, p = .011, η2p = .114, due to greater amplitude on switch trials than no-switch trials at age 10, p = .015, whereas switch and no-switch trials did not differ at age 5, p = .188.
As the peak extended into a positive slow-wave, it was also explored by averaging magnitude across 100-ms intervals, between 500 and 900 ms, following Manzi et al. (2011). This was done separately for each age group because of age-related differences in the time course of the posterior positivity. Preliminary analyses showed that condition had a significant effect on mean amplitude between 500 and 800 ms at age 5, and between 500 and 900 ms at age 10. Therefore, the two age groups were compared on the interval during which condition had a significant effect for both age groups, that is, 500 to 800ms after cue/gift onset (Figure 3C, right). The effect of condition, F(2, 108) = 20.086, p < .001, η2p = .271, interacted with age, F(2, 108) = 4.259, p = .017, η2p = .073. On both trial types, the amplitude was greater at age 5 in the “Proactive Encouraged” condition than the other two conditions, ps < .012, which did not differ from each other, p = .478. In contrast, 10-year-olds showed greater amplitudes in both the “Proactive Encouraged” and the “Proactive Possible” conditions relative to the “Proactive Impossible” condition, ps < .001, with no significant difference between the former two, p = .402.
Pupillometric Analyses
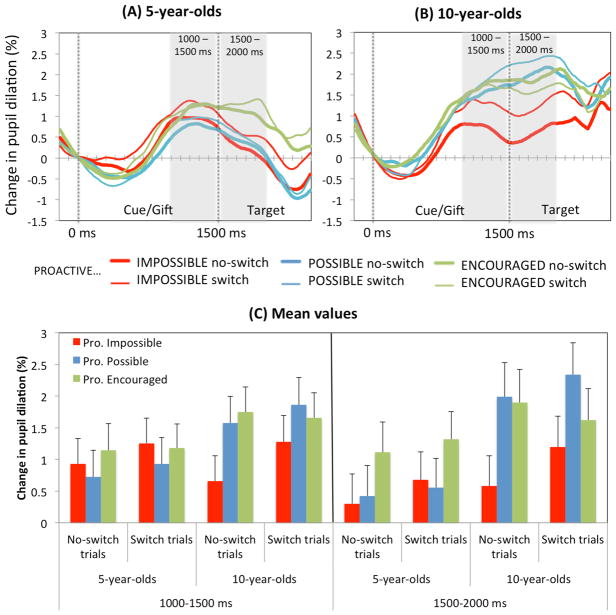
Pupil size increases progressively and peaks about one second after the relevant event onset in adults (e.g., Wierda, Rijn, Taatgen, & Martens, 2012). Consistently, we observed a peak in pupil dilation between 1000 and 1500 ms after cue/gift onset for both age groups (Figure 4A). Interestingly, these peaks extended during the following 500 ms in some of the conditions. Therefore, we examined mean pupil dilation during these two windows.
Figure 4.
Change in pupil dilation over the course of a trial at ages 5 (A) and 10 (B) in each condition. Grayed areas show the windows used for statistical analysis. The first gray dotted line signals cue/gift onset (0ms) and the second line signals target onset (1500ms). Mean values are shown in the lower panel (C). Vertical bars indicate standard errors. The initial peak observed between 1000 and 1500ms after cue onset did not differ across conditions. However, the peak extended over the following 500ms only in the “Proactive Encouraged” condition at age 5, and in both the “Proactive Possible” and “Proactive Encouraged” conditions at age 10.
Between 1000 and 1500 ms after cue/gift onset, pupil dilation tended to change more on switch (1.36%) than no-switch trials (1.13%), F(1, 48) = 3.724, p = .060, η2p = .072 (Figure 4B). During the following 500 ms, change in pupil dilation was affected by condition4, F(2, 96) = 4.888, p = .010, η2p = .092. There was also a significant Condition × Age interaction, F(2, 96) = 3.34, p = .040, η2p = .065. Five-year-olds showed greater change in pupil dilation in the “Proactive Encouraged” condition (1.22%) than in the “Proactive Impossible” condition (0.49%), p = .038, and in the “Possible” condition (0.48%), p = .037, whereas the latter two conditions did not differ, p = .999. In contrast, 10-year-olds showed greater change in pupil dilation in both the “Proactive Encouraged” and “Proactive Possible” conditions (1.76% and 2.17%, respectively), relative to the “Proactive Impossible” condition (0.89%), ps < .019; the former two did not differ from each other, p = .370. Finally, although pupil dilation tended to show greater change on switch trials (1.29%) than no-switch trials (1.05%), the effect of trial type failed to reach significance, F(1, 48) = 2.936, p = .093.
Discussion
The present study addressed the nature of young children’s bias towards reactive control by examining whether 5- and 10-year-old children engage proactive control when the advantage of proactive control over reactive control is manipulated in a task-switching paradigm. Ten-year-olds engaged in proactive, advance preparation whenever this control mode was possible, as shown by faster RTs, more pronounced cue-locked posterior positivity, and greater cue-related pupil dilation in the “Proactive Possible” (and “Proactive Encouraged”) condition than the “Proactive Impossible” condition. In contrast, 5-year-olds displayed a bias towards reactive control even when proactive control was possible, hence extending to a paradigm involving set shifting previous evidence for a shift from reactive to proactive control observed during childhood in measures of inhibition and working memory (Chatham et al., 2009; Chevalier et al., 2014; Lucenet & Blaye, 2014; see also Vallesi & Shallice, 2007). Critically, despite being biased towards reactive control, 5-years-olds showed a proactive profile when the advantage of proactive control over reactive control was increased. As discussed below, these findings suggest that 5-year-olds reliance on reactive control does not result from fundamental limitations on working-memory capacity preventing proactive control, but from age-related differences in meta-cognitive coordination of control modes.
At 5 years of age, children responded faster and showed a more pronounced posterior positivity and greater cue-related pupil dilation when proactive control was encouraged and reactive control was more difficult. These findings contrast with the pattern expected if children had engaged reactive control in the “Proactive Encouraged” condition, which included slower reaction times. Although faster responding was accompanied by lower accuracy in this condition, greater impulsivity is unlikely to account for our results because RTs were significantly faster in the “Proactive Encouraged” condition even after controlling for accuracy. Moreover, 5-year-olds’ engagement of proactive control is clearly shown by the ERP and pupillometric markers of advance preparation observed in the “Proactive Encouraged” condition. These findings rule out the hypothesis that too little working memory capacity puts proactive control totally out of these children’s reach. Indeed, 5-year-olds showed a proactive pattern in the “Proactive Encouraged” condition although the discontinuation of cue presentation after target onset increased working memory demands by requiring greater active maintenance of the task goal.
Since proactive control is already part of young children’s repertoire of control modes, 5-year-olds likely differ from 10-year-olds in the conditions under which they engage each control mode. Lower accuracy when using proactive control suggests that young children may have a threshold for engaging proactive control that is higher than in adults. Given the relatively underdeveloped nature of their proactive control, young children may engage proactive control only when reactive control is much more difficult to implement. Greater effort or lower accuracy associated with proactive control may bias young children against this control mode, such that their higher threshold for engaging proactive control may be adaptive. With age and greater practice, proactive control becomes less demanding, leading children to progressively associate proactive control with a more advantageous cost/benefit ratio resulting in its more frequent engagement. Consistently, children continue to engage proactive control more systematically through late adolescence (Andrews-Hanna et al., 2011; Killikelly & Szűcs, 2013; Waxer & Morton, 2011).
Although lower accuracy when 5-year-olds engaged proactive control suggests that the bias towards reactive control may be adaptive at that age, lower accuracy may have also resulted from additional working memory demands due to early discontinuation of cue presentation in the “Proactive Encouraged” condition. Had 5-year-olds engaged proactive control in the “Proactive Possible” condition, they may have shown faster RTs without lower accuracy. Asking children to label the relevant task before target onset (with cue information still visible afterwards) is one way to encourage proactive control without such additional working-memory demands. Labeling has repeatedly been shown to benefit older children (Karbach & Kray, 2007; Kray, Eber, & Karbach, 2008), and preliminary findings suggest that it may also speed up responding at age 5 without decreasing accuracy (Lucenet et al., 2012).
Therefore, when both reactive and proactive controls are possible, reactive control may not always be the most adaptive option for young children, which implies that coordination of control modes may refine and become more adaptive during childhood. Specifically, over childhood, children may build more explicit or complete representations of available control modes and potential alternatives, hence facilitating the evaluation of the adequacy between task demands and available control means (e.g., proactive control is more efficient than reactive control when the upcoming task can be reliably predicted by early cue presentation). An open question is the extent to which this coordination is achieved intentionally or not. With age, children may increasingly reflect on their own functioning (Zelazo, 2004), which is consistent with increasing meta-cognitive processes in tasks tapping executive control at school age (e.g., Roderer & Roebers, 2014). Alternatively, the evaluation of control mode adequacy and control coordination may be largely unintentional and refined through practice and dACC maturation (see Shenhav et al., 2013).
Since switching to a new task is more demanding than repeating the same task, one may have expected proactive preparation to benefit switch trials mostly, unlike the similar effect of condition on switch and no-switch trials observed here. Such an expectation relies on the incorrect assumption that implementing switches is the greatest executive challenge for children in the task-switching paradigm. Previous research has shown that both no-switch and switch trials within mixed-task blocks are highly demanding for children. Indeed, change in performance across the lifespan is driven by performance increase on both switch and no-switch trials within mixed-task blocks (relative to single-task blocks) to a much greater extent than difference between switch and no-switch trials within mixed-task blocks (e.g., Dibbets & Jolles, 2006; Karbach & Kray, 2007; Reimers & Maylor, 2005). Hence, the most critical challenge for young children is to identify the relevant task under conditions of task uncertainty, even on trials where no task switch is expected, and this ability is one of the most critical elements driving age-related change in performance. Proactive control can be just as helpful for children to determine the relevant task on no-switch trials as on switch trials, since early identification of the relevant task should facilitate target processing, even on trials where the relevant task happens to repeat. This is probably even more so for younger children who tend to approach switch and no-switch trials in a more similar fashion than do adults (Chevalier et al., 2013), and for whom simpler tasks tap executive control to a larger degree than later in development (Cepeda, Blackwell, & Munakata, 2013). Relatedly, overall faster performance when advance preparation was possible was accompanied by greater switch costs at age 10. These children may have sustained the task rules more robustly in working memory when the task was prepared for in advance, making it harder to switch from that rule on the next trial, in a phenomenon akin to asymmetrical switch costs (e.g., Ellefson et al., 2006). Younger children may not have shown this pattern because of less robust working memory; so that variations based on whether the task was prepared for in advanced had less effect on subsequent trials.
The present study focused on advance preparation, the most prominent aspect of proactive control in the task-switching paradigm. How other aspects of proactive control (e.g. task set maintenance, monitoring upcoming task difficulty) develop and are coordinated during childhood remain to be clarified in future studies. Of particular interest is the interplay between advance preparation and these other aspects in yielding increasingly proactive profiles with advancing age.
In addition, reactive control was inferred here from the lack of advance preparation, given that children of this age are capable of reactive control (e.g., Chatham et al., 2009) and task-switching taps control processes even in young children. For example, performance on task-switching paradigms in early childhood correlates with other cognitive control tasks, such as Go/No-Go and working memory span tasks (e.g., Chevalier et al., 2012), and loads onto the same latent factor as other cognitive control tasks (e.g., Clark et al., 2014). These findings make it likely that children in our study who did not show proactive profiles of performance instead relied on reactive control, rather than on non-controlled processes. Nonetheless, reactive control should be directly assessed in future studies, for instance by probing how task conflict affects performance and by examining reactive control-related ERPs, such as the pre-response negativity in adults (Czernochowski et al., 2010; Czernochowski, 2014).
In conclusion, the present study showed that children as young as 5 years can successfully engage proactive control. Their bias toward reactive control does not result from fundamental limitations in working memory capacity, but from meta-cognitive decisions regarding when to engage proactive control. Executive control development should not be conceived exclusively in terms of quantity of control or growing repertoire of control modes with advancing age, but also in terms of changes on the meta-cognitive coordination of these control modes. Such a proposal shifts our perspective on executive control development, and is consistent with the prominent role that is often attributed to meta-cognition in cognitive development more broadly (e.g., Kuhn, 2000; Chen & Siegler, 2000; Roderer & Roebers, 2014; Siegler, 2007; Schneider, 2008). These findings also have important implications for improving cognitive control early in development. Extant intervention programs try to improve children’s control abilities per se with long training regimens that have met limited success, especially in terms of transfer effects (Diamond, 2012; Wass, Scerif, & Johnson, 2012). Our results suggest that modifying the environment to guide children in the utilization of the most adaptive form of control is a promising alternative that could be easily implemented, inexpensive, and immediately effective.
Acknowledgments
This research was supported by grants from the National Institutes of Health (RO1 HD37163 and P50-MH079485). The authors thank Chris Bird, Krystin Corby, Julia Campbell, and Ronny Wiegand who helped set up the EEG system and collect the data.
Contributor Information
Nicolas Chevalier, University of Edinburgh.
Shaina Bailey Martis, University of Colorado Boulder.
Tim Curran, University of Colorado Boulder.
Yuko Munakata, University of Colorado Boulder.
References
- Adolph KE. Learning in the development of infant locomotion. Monographs of the Society for Research in Child Development. 1997;62(3, Serial No. 251) [PubMed] [Google Scholar]
- Altmann EM. Advance preparation in task switching: what work is being done? Psychological Science. 2004;15(9):616–22. doi: 10.1111/j.0956-7976.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PloS one. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;9(1):276–292. [PubMed] [Google Scholar]
- Blackwell KA, Munakata Y. Costs and benefits linked to developments in cognitive control. Developmental Science. 2013:1–9. doi: 10.1111/desc.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–13. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford, England: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7351–6. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB. The Scree test for the number of factors. Multivariate Behavioral Research. 1966;1(2):245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JCM. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37(5):715–729. doi: 10.1037//0012-1649.37.5.715. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Blackwell KA, Munakata Y. Speed isn’t everything: complex processing speed measures mask individual differences and developmental changes in executive control. Developmental Science. 2013;16(2):269–86. doi: 10.1111/desc.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5529–33. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Provan LS, Munakata Y. An intermediate phase in the transition from reactive to proactive control in revision. [Google Scholar]
- Chen Z, Siegler RS. VII. Components of Strategic Change. Monographs of the Society for Research in Child Development. 2000;65(2):43–58. doi: 10.1111/1540-5834.00080. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Blaye A. Setting goals to switch between tasks: effect of cue transparency on children’s cognitive flexibility. Developmental Psychology. 2009;45(3):782–97. doi: 10.1037/a0015409. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Blaye A, Dufau S, Lucenet J. What visual information do children and adults consider while switching between tasks? Eye-tracking investigation of cognitive flexibility development. Developmental Psychology. 2010;46(4):955–72. doi: 10.1037/a0019674. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Jame TD, Wiebe SA, Nelson JM, Espy KA. Contribution of reactive and proactive control to children’s working memory performance: Insight from item recall durations. Developmental Psychology. 2014 doi: 10.1037/a0036644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Huber KL, Wiebe SA, Espy KA. Qualitative change in executive control during childhood and adulthood. Cognition. 2013;128(1):1–12. doi: 10.1016/j.cognition.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Sheffield TD, Nelson JM, Clark CAC, Wiebe SA, Espy KA. Underpinnings of the costs of flexibility in preschool children: The roles of inhibition and working memory. Developmental Neuropsychology. 2012;37(2):99–118. doi: 10.1080/87565641.2011.632458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology. 2013;4(January):15. doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Nelson JM, Garza J, Sheffield TD, Wiebe SA, Espy KA. Gaining control: changing relations between executive control and processing speed and their relevance for mathematics achievement over course of the preschool period. Frontiers in Psychology. 2014;5:107. doi: 10.3389/fpsyg.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K, Siegler RS. Flexible strategy use in young children’s Tic-Tat-Toe. Cognitive Science. 1993;17:531–561. [Google Scholar]
- Czernochowski D. ERPs dissociate proactive and reactive control: Evidence from a task-switching paradigm with informative and uninformative cues. Cognitive, Affective & Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0302-y. [DOI] [PubMed] [Google Scholar]
- Czernochowski D, Nessler D, Friedman D. On why not to rush older adults - relying on reactive cognitive control can effectively reduce errors at the expense of slowed responses. Psychophysiology. 2010;47(4):637–646. doi: 10.1111/j.1469-8986.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaene W, Brass M. Dissociating strategy-dependent and independent components in task preparation. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2014.04.015. in press. http://dx.doi.org/10.1016/j.neuropsychologia.2014.04.015i. [DOI] [PubMed]
- DeBoer T, Scott LS, Nelson CA. ERPs in developmental populations. In: Handy TC, editor. Event-related potentials, a methods handbook. Cambridge, MA: The MIT Press; 2005. pp. 263–298. [Google Scholar]
- Diamond A. Activities and programs that improve children’s executive functions. Current Directions in Psychological Science. 2012;21(5):335–341. doi: 10.1177/0963721412453722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: an open source program for advanced statistical analysis of event related potential data. Journal of Neuroscience Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dibbets P, Jolles J. The Switch Task for Children: Measuring mental flexibility in young children. Cognitive Development. 2006;21(1):60–71. doi: 10.1016/j.cogdev.2005.09.004. [DOI] [Google Scholar]
- Ellefson MR, Shapiro LR, Chater N. Asymmetrical switch costs in children. Cognitive Development. 2006;21:108–130. doi: 10.1016/j.cogdev.2006.01.002. [DOI] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Dale AM. Multimodal imaging of the self-regulating developing brain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(48):19620–5. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40(2):177–90. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F. The spatial and temporal dynamics of anticipatory preparation and response inhibition in task-switching. Neuroimage. 2010;51(1):432–449. doi: 10.1016/J.Neuroimage.2010.01.090. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Michie PT, Karayanidis F. Sequence effects in cued task switching modulate response preparedness and repetition priming processes. Psychophysiology. 2010;47(2):365–86. doi: 10.1111/j.1469-8986.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Coltheart M, Michie PT, Murphy K. Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology. 2003;40(3):329–48. doi: 10.1111/1469-8986.00037. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Jamadar S, Ruge H, Phillips N, Heathcote A. Advance preparation in task-switching: Converging evidence from behavioural, brain activation and model-based approaches. Frontiers in Psychology. 2010 doi: 10.3389/fpsyg.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Mansfield EL, Galloway KL, Smith JL, Provost A, Heathcote A. Anticipatory reconfiguration elicited by fully and partially informative cues that validly predict a switch in task. Cognitive Affective and Behavioral Neuroscience. 2009;9(2):202–215. doi: 10.3758/CABN.9.2.202. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Whitson LR, Heathcote A, Michie PT. Variability in proactive and reactive cognitive control processes across the adult lifespan. Frontiers in psychology. 2011;2(November):318. doi: 10.3389/fpsyg.2011.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach J, Kray J. Developmental changes in switching between mental task sets: The influence of verbal labeling in childhood. Journal of Cognition and Development. 2007;8(2):205–236. [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19(3):640–57. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Developmental Cognitive Neuroscience. 2013;6:61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killikelly C, Szűcs D. Delayed development of proactive response preparation in adolescents: ERP and EMG evidence. Developmental Cognitive Neuroscience. 2013;3:33–43. doi: 10.1016/j.dcn.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J, Eber J, Karbach J. Verbal self-instructions in task switching: a compensatory tool for action-control deficits in childhood and old age? Developmental Science. 2008;11(2):223–36. doi: 10.1111/j.1467-7687.2008.00673.x. [DOI] [PubMed] [Google Scholar]
- Kuhn D. Metacognitive development. Current Directions in Psychological Science. 2000;9(5):178–181. [Google Scholar]
- Lemaire P, Brun F. Effects of strategy sequences and response-stimulus intervals on children’s strategy selection and strategy execution: A study in computational estimation. Psychological Research. 2014 doi: 10.1007/s00426-013-0501-0. [DOI] [PubMed] [Google Scholar]
- Lucenet J, Blaye A. Age-related changes in the temporal dynamics of executive control: a study in 5- and 6-year-old children. Frontiers in Psychology. 2014;5:831. doi: 10.3389/fpsyg.2014.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucenet J, Kray J, Chevalier N, Blaye A. Asking preschoolers to label in task-switching: a benefit beyond the reorientation of attention. Poster presented to the Development of Executive Functions Workshop; Utrecht, Netherlands. 2012. Apr, [Google Scholar]
- Manzi A, Nessler D, Czernochowski D, Friedman D. The development of anticipatory cognitive control processes in task-switching: an ERP study in children, adolescents, and young adults. Psychophysiology. 2011;48(9):1258–75. doi: 10.1111/j.1469-8986.2011.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund P, Persson J. Context-dependent switching between proactive and reactive working memory control mechanisms in the right inferior frontal gyrus. NeuroImage. 2012;63(3):1552–60. doi: 10.1016/j.neuroimage.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22(6):1423–1442. doi: 10.1037/0278-7393.22.6.1423. [DOI] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7(3):134–140. doi: 10.1016/S1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18(5):1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: effects of age on general and specific switch costs. Developmental Psychology. 2005;41(4):661. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Roderer T, Roebers CM. Can you see me thinking (about my answers)? Using eye-tracking to illuminate developmental differences in monitoring and control skills and their relation to performance. Metacognition and Learning. 2014;9(1):1–23. [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK, Davis-Stober CP. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC neuroscience. 2004;5:39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. The Development of Metacognitive Knowledge in Children and Adolescents: Major Trends and Implications for Education. Mind, Brain, and Education. 2008;2(3):114–121. doi: 10.1111/j.1751-228X.2008.00041.x. [DOI] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–40. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler RS. Cognitive variability. Developmental Science. 2007;10(1):104–9. doi: 10.1111/j.1467-7687.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- Siegler RS, Jenkins E. How Children Discover New Strategies. L. Erlbaum; New York, NY: 1989. [Google Scholar]
- Vallesi A, Shallice T. Developmental dissociations of preparation over time: deconstructing the variable foreperiod phenomena. Journal of Experimental Psychology. Human Perception and Performance. 2007;33(6):1377–88. doi: 10.1037/0096-1523.33.6.1377. [DOI] [PubMed] [Google Scholar]
- Wass SV, Scerif G, Johnson MH. Training attentional control and working memory – Is younger, better? Developmental Review. 2012;32(4):360–387. [Google Scholar]
- Waxer M, Morton JB. The development of future-oriented control: an electrophysiological investigation. NeuroImage. 2011;56(3):1648–54. doi: 10.1016/j.neuroimage.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Wierda SM, Rijn H, Van Taatgen NA, Martens S. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proceedings of the National Academy of Sciences of the Unites States of America. 2012 doi: 10.1073/pnas.1201858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD. The development of conscious control in childhood. Trends in Cognitive Science. 2004;8(1):12–17. doi: 10.1016/j.tics.2003.11.001. [DOI] [PubMed] [Google Scholar]