Abstract
Quadrato Motor Training (QMT) is a whole-body movement contemplative practice aimed at increasing health and well-being. Previous research studying the effect of one QMT session suggested that one of its means for promoting health is by enhancing cognitive flexibility, an important dimension of creativity. Yet, little is known about the effect of a longer QMT practice on creativity, or the relative contribution of the cognitive and motor aspects of the training. Here, we continue this line of research in two inter-related studies, examining the effects of prolonged QMT. In the first, we investigated the effect of 4-weeks of daily QMT on creativity using the Alternate Uses (AUs) Task. In order to determine whether changes in creativity were driven by the cognitive or the motor aspects of the training, we used two control groups: Verbal Training (VT, identical cognitive training with verbal response) and Simple Motor Training (SMT, similar motor training with reduced choice requirements). Twenty-seven participants were randomly assigned to one of the groups. Following training, cognitive flexibility significantly increased in the QMT group, which was not the case for either the SMT or VT groups. In contrast to one QMT session, ideational fluency was also significantly increased. In the second study, we conducted a pilot longitudinal structural magnetic resonance imaging and diffusion tensor imaging (4-weeks QMT). We report gray matter volume and fractional anisotropy changes, in several regions, including the cerebellum, previously related to interoceptive accuracy. The anatomical changes were positively correlated with cognitive flexibility scores. Albeit the small sample size and preliminary nature of the findings, these results provide support for the hypothesized creativity-motor connection. The results are compared to other contemplative studies, and discussed in light of theoretical models integrating cognitive flexibility, embodiment and the motor system.
Keywords: Quadrato Motor Training, creativity, cerebellum, MRI, embodiment
Introduction
Creativity, Training, and Health
The lexeme in the English word creativity comes from the Latin term creō, meaning “to create, make.” Thus, creativity means bringing into being, as it involves generation of novelty and transformation of existent information (Chávez-Eakle et al., 2007). Creativity requires, as well as generates, new information that transcends informational boundaries, yet is integrated with existing information in a manner exhibiting value (Horan, 2007). Behaviorally, the first component, namely the generation of new information, can be measured by divergent thinking tests; whereas the second component, concerning existing information constraints, can be measured by convergent thinking tests. Here we focus on divergent thinking, studying ideational fluency and cognitive flexibility, two important measures of creativity. Specifically, cognitive flexibility is diminished in several neurodegenerative conditions, such as Parkinson’s (Tomer et al., 2002). Thus, sustaining and improving cognitive flexibility may serve a significant role in maintaining cognitive and emotional well-being and health (Schmid, 2005). In the current study, we aimed at investigating the link between flexibility of behavior and cognitive flexibility. To this end, we employ Quadrato Motor Training (QMT by Patrizio Paoletti – see below), which requires constant flexibility in the movement and behavior, and assess its impact on cognitive flexibility and ideational fluency using the AUs Task (Chermahini and Hommel, 2010).
The QMT is a whole-body movement meditation, aimed at improving well-being, by enhancing attention, coordination and cognitive flexibility (Ben-Soussan et al., 2013, 2015). The QMT requires a state of enhanced attention, as it combines dividing attention to the motor response and cognitive processing for producing the correct direction of movement to the next point in the Quadrato space (Ben-Soussan et al., 2013; see Figure 1). QMT can further be viewed as ‘Mindful movement,’ due to the increased awareness it requires to the body and its location in space. Mindful movement is a general term for practices that involve bringing awareness to the detailed experience of movement (Kabat-Zinn, 2009), such as walking meditation, Yoga and Tai Chi. Similar to other Mindful movement practices, QMT requires balance control, which is known to integrate inputs from the motor cortex, cerebellum, and basal ganglia, as well as feedback from vestibular and proprioceptive systems required to maintain an upright posture (Chang et al., 2010, 2014; Wayne et al., 2014). In line with that, a previous study demonstrated that a month of daily QMT practice significantly enhanced cerebellar oscillatory function (Ben-Soussan et al., 2014a). Yet, in comparison to other Mindful movement practices, QMT has the advantage of being a relatively short training (possibly several minutes), and can be practiced in limited spaces. These unique aspects render the QMT a technique warranting scientific exploration, with the future aim of implementing this technique in various health-promoting and educational setups.
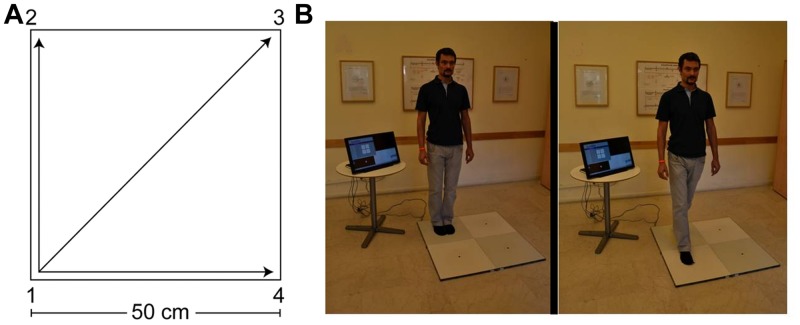
FIGURE 1.
The Quadrato Motor Training (QMT). (A) A graphical illustration of the QMT. (B) A participant during the QMT while waiting for the next instruction (left) and following the instruction (right).
We have previously reported that a single session of QMT increased cognitive flexibility, but not fluency, as well as improved spatial cognition and reflectivity (Ben-Soussan et al., 2013, 2014b) in contrast to two different control groups controlling for cognitive and motor load.
Long-term effects of a month of daily QMT on divergent thinking were previously studied in comparison to simple walking training (WT; Venditti et al., 2014). Both fluency and flexibility increased only in the QMT group (Venditti et al., 2014). These significant differences might have stemmed from the cognitive aspect of the contemplative movement (QMT), relatively missing from the non-contemplative simple walking (WT).
This has led us in the present study (Study A) to compare 4-weeks of daily QMT to two control groups, tapping on the cognitive and motor aspects of the QMT: Verbal Training (VT, identical cognitive training with verbal response) and Simple Motor Training (SMT, similar motor training with reduced choice requirements). At the same time, the present study replicates the 1-month QMT in a different language. In this study, we predicted that: (1) cognitive flexibility would increase following QMT compared to the other control groups, while (2) fluency is predicted to increase in both the QMT and the VT groups.
A direct continuation of Study A is to start uncovering the possible underlying mechanism mediating QMT-induced enhancement in divergent thinking. Indeed, a fascinating and under-explored aspect of creativity is its possible connection to the motor system, suggested by several authors (Cotterill, 2001; Dietrich, 2004; Chávez-Eakle et al., 2007; Vandervert et al., 2007; Carruthers, 2011; Koziol et al., 2012). Building on the previously hypothesized creativity-motor connection, we set out in the pilot Study B to examine a possible correlation between change in divergent thinking measures and structural changes in brain regions related to motor activation. To this end, we employed structural Magnetic Resonance Imaging (sMRI), and investigated structural changes and AU scores following 4-weeks of daily QMT. Here, we predicted that: (1) QMT would induce anatomical changes in motor regions; and (2) anatomical changes in motor regions would be correlated with increased cognitive flexibility.
Study A
Methods
Participants and Design
Twenty-seven female students (mean ± SD age: 24 ± 3 years) participated in the study, none of whom practiced QMT before. All were right-handed with no medical history that might affect their performance. Since gender-dependent differences have been frequently observed in both the motor and the cognitive realms (Baron-Cohen and Hammer, 1997) we chose to focus here on females. The study was conducted in the EEG/MEG unit at the Gonda Brain Research Center, and was approved by the ethics committee of Bar-Ilan University.
Upon entering the lab, the participant signed a written informed consent. Subsequently, participants were seated in a quiet room, in front of a computer screen and completed the AU Task. All data were collected both before and after 4 weeks of daily practice. Participants were randomly allocated to one of three groups: (1) Quadrato Motor Training (QMT- three choices and whole-body response); (2) SMT (one choice and whole-body response); and (3) VT (three choices and verbal response). Although the initial group sizes were identical (n = 9 each), the final number of participant finishing the 4-weeks training varied between the groups (QMT, n = 6; SMT, n = 7; and VT, n = 5).
Training Groups
Quadrato Motor Training
The QMT, by Patrizio Paoletti, requires standing at one corner of a 0.5 m×0.5 m square and making movements in response to verbal instructions given by an audio tape recording. There were three optional directions of movement. The instructions direct participants to keep the eyes focused straight ahead, hands loose at the side of the body. They are also told to immediately continue with the next instruction and not to stop due to mistakes. At each corner, there are three possible directions to move (for example, from corner 1 the participant can move to corner 2, to corner 3 or to corner 4). The training thus consists of 12 possible movements (3 directions × 4 corners): 2 forward, 2 backward, 2 left, 2 right and 4 diagonals. The participant is required to move from one corner to another according to the number on the recording. For example, if the sequence required is 1, 2, 1, 2, 1, 2, 3, 2, 4, 3, 1…. this means moving to the first corner, then to the second, then back to the first, and so on (see Figure 1). The daily training consisted of a sequence of 69 commands, lasting 7 min. For additional data regarding the training groups, see Ben-Soussan et al. (2013).
Simple Motor Training
The SMT group moved from corner to corner on the square in exactly the same manner as the QMT group (pace, duration, auditory cue), but their movement was consistently 1-2-3-4-1 etc. This group also practiced with the same recordings as the QMT group. However, while the QMT group was told that each number represented a different corner of the square, the SMT group was told to simply begin at a certain corner and to continue to the next corner clockwise in response to the instructions. That is, regardless of the number specified on the tape, they always moved in the same sequence. This reduced the uncertainty regarding the direction of the movement, compared to the QMT group. The SMT group thus provided a control of similar motor performance but with reduced cognitive demands.
Verbal Training
The VT group was designed to control for the motor load while keeping the same cognitive load and uncertainty. The participants, who were instructed to only make verbal responses, stood 1 m in front of the square, but did not move on the corners at all. Instead, they responded to the taped commands verbally by stating what direction of movement would be required in order to reach the corner specified by the command. For example, for a movement from corner 1 to corner 2, they were required to say “straight.” All other training parameters were kept identical to the QMT (pace, duration, auditory cue).
The Alternate Uses (AUs) Task
The AU Task is an established measure assessing creativity (Guilford et al., 1978). Two main features of creativity are fluency, defined as the total number of ideas generated, and flexibility, defined as the tendency to generate a heterogeneous pool of responses, or to use a variety of categories and themes when producing ideas (Guilford, 1950; Guilford et al., 1978; Runco, 1986). Flexibility conveys information that is not conveyed by fluency (Guilford, 1968; Runco, 1986). This task was chosen here as it was previously used to assess change in cognitive flexibility following motor training (Netz et al., 2007; Venditti et al., 2014).
In a previous study with the aim of examining changes in creativity following training (reported in Ben-Soussan et al., 2013), we clustered a 908-word database developed by Levy-Drori and Henik (2006), using hierarchical cluster analysis, and grouped those words having similar ratings of concreteness, availability of context and familiarity assigned by Levy-Drori and Henik. Concreteness was rated by them on a scale from one to seven, where “1” indicated very low concreteness (very abstract word) to “7” which indicated a very concrete word. Availability of context was defined as ranging from 1 to 7, with “1” indicating that it is very difficult to think of a context and “7” indicating that it is very easy. Familiarity was defined as ranging from “1” indicating that it is not very familiar and “7” indicating that it is very familiar. We then chose 18 words from the three largest clusters for which the level of concreteness, similarity on familiarity and availability features were highest (see Supplementary Table S1). These 18 words were divided into two groups (nine words in each group). A total of 60 participants received one of the two lists, and were asked to produce as many AUs as possible, 1 min being allocated for each item. Each word was shown on a single page on which the participant had to write down the various uses. The scores for each word were analyzed by counting the number of AUs produced. The words were then divided into six sets of three words so that each set had a similar rating for familiarity, concreteness and availability. One of the sets (set d) resulted in higher fluency scores (see Supplementary data, where corrections maintain all reported results).
The presentation order of each set of words was counterbalanced using a Latin square (see Supplementary Table S2). Three names of objects were shown on a computer screen before the training and after the training (at the beginning and at the end of the month). Six pairs of sets (e.g., a–f) were used with internal order counterbalanced across six participants. The internal order of the three items per set (Table S1 in supplementary material) was presented in a random manner. Importantly, sets a, b, c, d, e, and f each appear once in each ordinal position.
In this task, the participant is required to name as many different ways in which a given item might be used, within a certain time frame (1 min). The fluency score was defined as the mean number of uses given by the participant for the three items. On the basis of all the uses made by the participants, 10 independent categories were defined across all the items. These included broad categories of usage such as “a weapon” or “a costume.” The flexibility score was defined as the mean number of different categories employed by the participant across all three words presented (Russ, 1998). Hence, in order to calculate the flexibility score, all responses for a given item were first divided into the different independent categories. Two independent judges who were naïve to the identity of the participants and their training groups scored the test independently for flexibility, and consistency between judges was tested. We examined the correlation between the scores of the two judges using a two-tailed Pearson correlation coefficient test. A high correlation was found between scores given by the two judges both in the scores before the training and after the training: r = 0.88 and 0.86, n = 18, respectively.
Statistical Analysis
We ran a Group (QMT, SMT, VT) × Training (pre, post) analysis of variance (ANOVA) for creativity (separately on fluency and flexibility scores). Then, post hoc paired t-tests were conducted.
Results
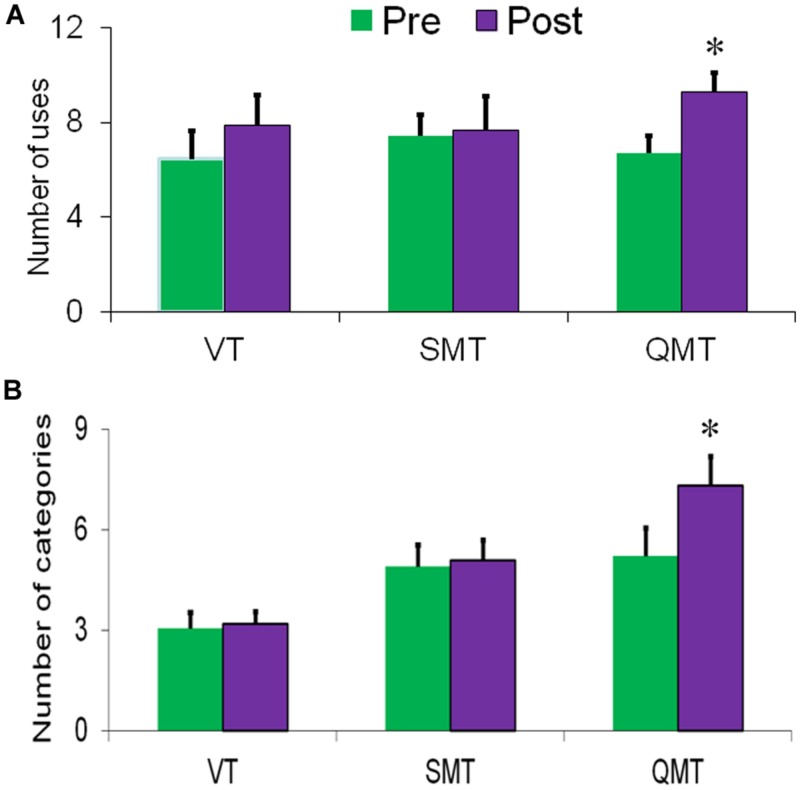
The first ANOVA, conducted for fluency, revealed a main effect for Training [F(1,15) = 8.80, MSE = 17.25, p = 0.01], with post-training being generally higher compared to pre-training (Figure 2A). Albeit the Group × Training interaction was not significant [F(2,15) < 1], we tested directly each group’s effect on fluency, to better evaluate the results compared to our previous studies. Post hoc t-tests showed that fluency significantly increased only in the QMT group [t(5) = -4.21, p < 0.01], and not in the SMT or VT groups [t(6) = -0.31, t(4) = -1.33, ns, respectively]. The second ANOVA conducted for flexibility similarly yielded a main effect for Training [F(1,15) = 8.01, MSE = 5.81, p < 0.05]. In addition, a significant Group × Training interaction was found [F(2,15) = 5.20, MSE = 0.727, p < 0.05]. For the QMT group, flexibility significantly increased [t(5) = -3.20, p < 0.05] in contrast to the SMT and VT groups who showed no change following training (see Figure 2B).
FIGURE 2.
Change in creativity as a function of Group and Training. (A) Fluency and (B) Flexibility. Data are expressed as mean ± SEM, ∗p < 0.05.
Importantly, correcting for AU set d did not change these results (see supplementary materials).
Study B
Methods
Participants and Design
Three healthy women participated in this pilot study (mean ± SD age: 41.5 ± 11.4 years), none of whom practiced QMT before, with no previous head injury which might have affected their brain structure. In this study, the QMT sequence consisted of 258 commands, and lasted 12 min. The reason for the change in procedure was due to the fact that this study is a part of a larger study conducted with the longer QMT practice paradigm aimed at examining longer sequences for neurodegenerative disease. The study took place at the St. Andrea Hospital, Rome. Upon entering the lab, the participant signed a written informed consent. sMRI was acquired immediately after the AU Task. The study was approved by the ethical committee of Università Campus Bio-Medico di Roma. The AUs task was employed similarly to the one reported in Study A, with different sets given to the participants before and following a month of QMT training. The AU task was completed prior to entering the magnet.
MRI Data
MRI Scans
For each subject, high-resolution 3D T1-weighted sMRI and diffusion tensor imaging (DTI) data were acquired at the beginning (pre-QMT) and after 4-weeks of daily QMT practice (post-QMT) using a Siemens MAGNETOM Sonata (Erlangen, Germany) 1.5 T scanner (sMRI: 3D T1-weighted MP-RAGE sequence, TR = 3000 ms, TE = 4.38 ms, flip angle = 15°, matrix = 192 × 192, FOV = 240 mm2, 160 sagittal slices, voxel size = 1.25 mm × 1.25 mm × 1.20 mm; DTI: 12 non-collinear direction sequence, TR = 7100 ms, TE = 94 ms, flip angle = 90°, matrix = 256 × 192, FOV = 240 mm × 320 mm, b = 0 and 900 s/mm2, 48 axial slices, voxel size = 1.25 mm × 1.25 mm × 3.0 mm). We computed two anatomical measures: gray matter (GM) volume, and fractional anisotropy (FA). GM volume is a measure of the amount of cortical GM corresponding to each region and is computed using both surface area and cortical thickness (Frye et al., 2010). FA is a marker of white matter integrity, which is thought to reflect anatomical features of white matter, such as axon caliber, fiber density and myelination (Scholz et al., 2009).
Image Analysis
The sMRI data were analyzed using the voxel-based morphometry technique (VBM, Good et al., 2001). DTI data were analyzed using the Tract-Based Spatial Statistics (TBSS, Smith et al., 2006); SMRI were longitudinally processed with VBM2 toolbox1 of SPM22. In short, pre-QMT images were aligned to the T1 template and, then, post-QMT images were co-registered to the pre-QMT T1-aligned scans. All scans were bias corrected and segmented into gray and white, and CSF compartments. GM maps were normalized to MNI atlas space (1 mm × 1 mm × 1 mm voxels) and smoothed using an 8 mm FWHM Gaussian kernel. Preprocessing of DTI data was conducted with FSL3. DTIs were corrected for motion and eddy currents distortions and then skull-stripped using the Brain Extraction Tool (BET; Smith, 2002). Maps of FA were computed by fitting a tensor model to the raw diffusion data using the FMRIB’s Diffusion Toolbox (FDT). These maps were projected onto a mean FA tract skeleton, before applying voxelwise within-subject statistics to compare them between time points, as described elsewhere (Scholz et al., 2009).
Statistical Analysis
Voxel-based morphometry technique data were analyzed using a general linear model (GLM). Anatomical localization was carried out with the MSU–MNI Space Utility toolbox of SPM using the AtlasQuery FSL tool. Statistical analysis of TBSS data was performed by using a permutation-based inference tool for non-parametric statistical thresholding. Pre- and post-QMT FA and VBM data were compared by using a paired t-test, with subject’s age as a covariate, controlling for the potential effect of this variable (Luders et al., 2009). Significance level for the t-tests was set with a minimum cluster size of 700 voxels for the GM volume (Brubaker et al., 2010) and one of 100 voxels or more for the FA as the cluster-size threshold (Ocklenburg et al., 2013).
Results
Alternate Uses Task
Similar to the results of Study A, flexibility significantly improved following 4-weeks of daily QMT [t(2) = -6.05, p < 0.05; Figure 3]. Although fluency also increased, it did not reach significance [t(2) = -0.39, ns].
FIGURE 3.
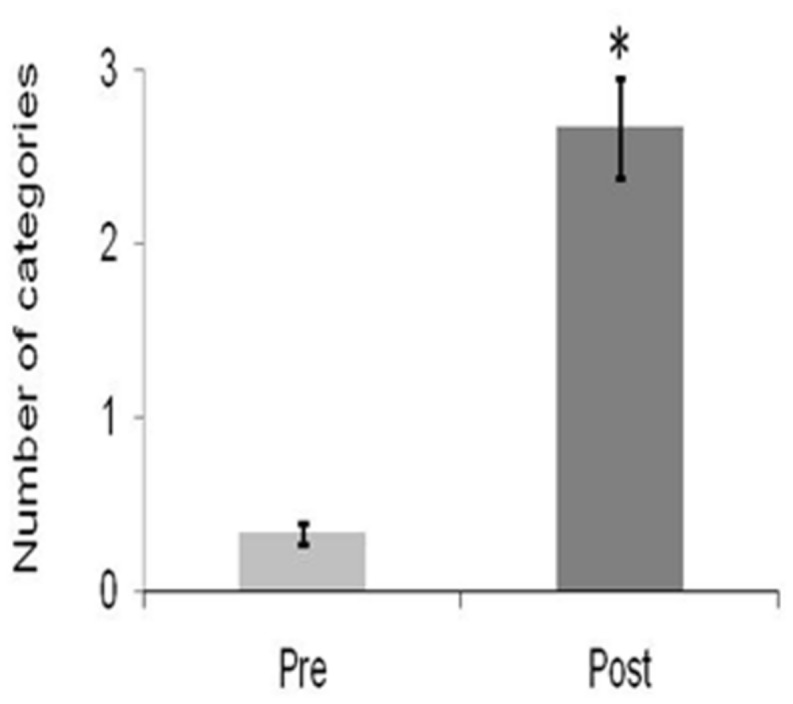
Change in creativity as a function of Training for Flexibility. Data are expressed as mean ± SEM, ∗p < 0.05.
Brain Anatomy Results
Voxel-based morphometry technique analysis results showed significant (p < 0.001, uncorrected) GM volume increases, localized in left and right cerebellum, and frontal areas, mainly in the inferior frontal and middle frontal gyri (Table 1; Figure 4A). FA analysis results showed significant (p < 0.01, uncorrected) increases localized mainly in the middle cerebellar peduncles (Table 2; Figure 4B).
Table 1.
Significant increases in GM volume after 4-weeks of daily QMT.
| Coordinates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| k | p-value | Z-value | x | y | z | Hemisphere | Lobe | Region | BA |
| 1584 | 0.000 | 3.63 | 24 | -52 | -22 | Right cerebellum | Anterior lobe | Culmen | |
| Posterior lobe | Cerebellar tonsil | ||||||||
| Declive | |||||||||
| Tuber | |||||||||
| Uvula | |||||||||
| 1128 | 0.000 | 3.79 | -6 | 13 | 65 | Left cerebrum | Frontal lobe | Medial frontal gyrus | 6 |
| Middle frontal gyrus | 6 | ||||||||
| Superior frontal gyrus | 8 | ||||||||
| 851 | 0.000 | 4.07 | -15 | 21 | -23 | Left cerebrum | Frontal lobe | Inferior frontal gyrus | 47 |
| Middle frontal gyrus | 11 | ||||||||
| Orbital gyrus | 47 | ||||||||
| Subcallosal gyrus | 47 | ||||||||
| Superior frontal gyrus | 11 | ||||||||
| 706 | 0.000 | 4.34 | -17 | -37 | -29 | Left cerebellum | Anterior lobe | Culmen | |
| 580 | 0.000 | 4.32 | -32 | -62 | -33 | Left cerebellum | Posterior lobe | Cerebellar tonsil | |
| Culmen | |||||||||
| Declive | |||||||||
| Pyramis | |||||||||
| Tuber | |||||||||
| Uvula | |||||||||
| Anterior lobe | Culmen | ||||||||
| 471 | 0.000 | 3.88 | -37 | -5 | -7 | Left cerebrum | Sub-lobar | Claustrum | |
| Extra-nuclear | 13 | ||||||||
| Temporal lobe | Superior temporal gyrus | 38 | |||||||
| 297 | 0.000 | 4.10 | -54 | -37 | -3 | Left cerebrum | Temporal lobe | Middle temporal gyrus | 21 |
| Superior temporal gyrus | 22 | ||||||||
| 262 | 0.000 | 4.18 | 11 | 28 | -33 | Right cerebrum | Frontal lobe | Orbital gyrus | 11 |
| Rectal gyrus | 11 | ||||||||
| 102 | 0.001 | 3.19 | 21 | -6 | -38 | Right cerebrum | Limbic lobe | Parahippocampal gyrus | 35 |
| Uncus | 36 | ||||||||
Cluster extension (k), which represents the number of contiguous voxels passing the threshold of ≥100. All clusters meet the significance level set at p < 0.001 uncorrected. Neuroanatomical labels are referred to the Talairach coordinates x, y, z, by means of the MSU SPM tool.
FIGURE 4.
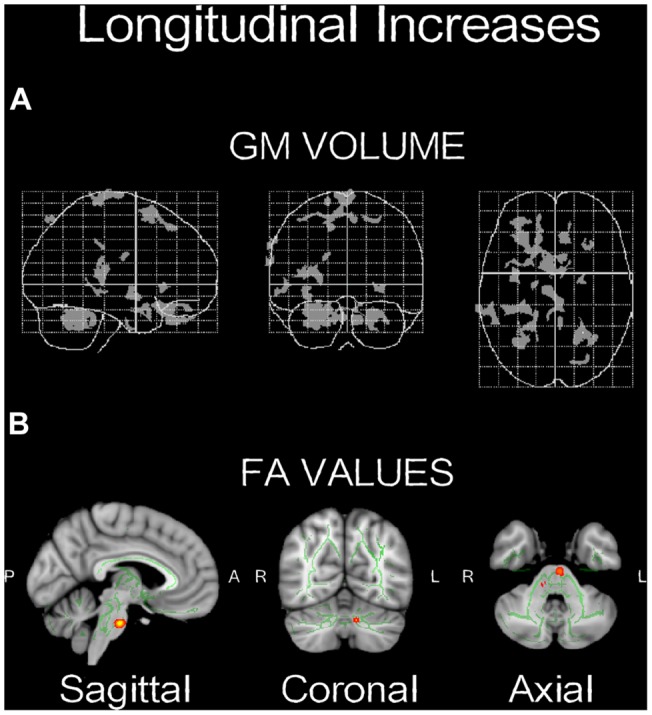
Neuroplasticity following longitudinal (4-weeks) daily QMT. (A) Significant increases in GM volume (p < 0.001, uncorrected) projected onto the glass brain; (B) Significant increases in FA values (p < 0.01, uncorrected) onto brain template. Red-yellow color scale is related to the p-values (in which yellow is highest).
Table 2.
Significant increases in FA values after 4-weeks of daily QMT.
| Coordinates |
|||||
|---|---|---|---|---|---|
| k | p-value | x | y | z | WM structures |
| 283 | 0.010 | 102 | 102 | 41 | Middle cerebellar peduncle |
| 59 | 0.004 | 94 | 107 | 38 | Corticospinal tract L |
| 31 | 0.008 | 100 | 62 | 40 | Anterior thalamic radiation L |
| 27 | 0.008 | 54 | 71 | 74 | Posterior thalamic radiation R |
The number of voxels (k) represents the number of voxels belonging to certain WM structures. Significance level set at p < 0.01 uncorrected. Neuroanatomical localization was obtained using AtlasQuery FSL tool with JHU White-Matter Tractography and the JHU ICBM-DTI-81 White-Matter Labels as reference atlases.
We then examined the correlation between the anatomical changes and changes in flexibility, both of which significantly increased post-training. Multiple regression analyses were performed to investigate whether GM and FA map changes were correlated with the change in flexibility, as previously reported for a similar group size (Ben-Soussan et al., 2015). We used the results of VBM and TBSS analyses as explicit masks to ensure that only the appropriate GM and fiber ROI which changed following the training were included in the analysis (Denier et al., 2013). These masks were subsequently inserted as explicit masks in two correlation analyses with flexibility, one for the GM and one for the FA.
As seen in Figure 5A, a positive correlation (p < 0.005, n = 3) was found between change in flexibility and the GM increment in the right cerebellum and the superior frontal gyrus (Table 3). In addition, a positive correlation (p < 0.05) was found between increased flexibility and FA changes, mostly located in the left corticospinal tract and the middle cerebellar peduncles (Table 4; Figure 5B).
FIGURE 5.
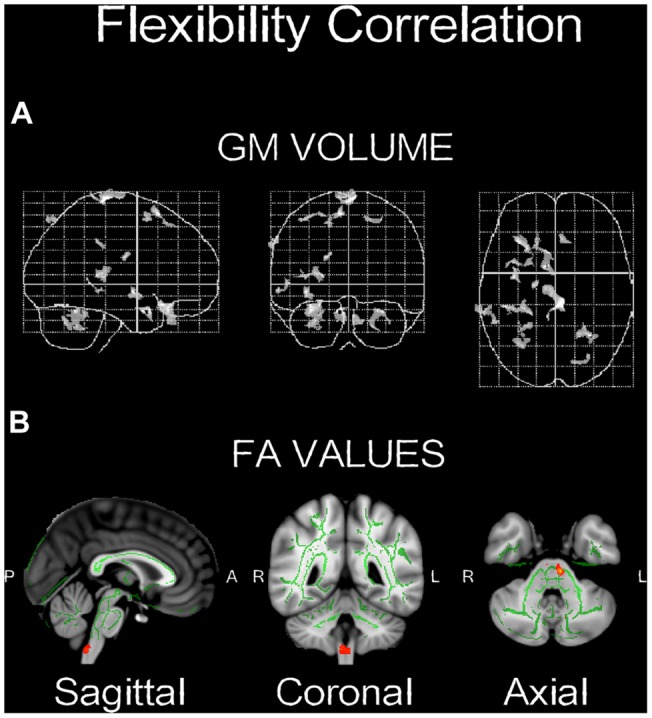
Significant correlations between change and GM volume (A,B) FA values. Red-yellow color bar range is based on min-max statistical threshold. Refer to corresponding supplementary tables for more neuroanatomical and statistical details (n = 3).
Table 3.
Positive correlation between flexibility values and GM maps showing volume increases after 4-weeks of daily QMT.
| Coordinates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| k | p-value | Z-value | x | y | z | Hemisphere | Lobe | Region | BA |
| 612 | 0.000 | 3.73 | 21 | -56 | -37 | Right cerebellum | Anterior lobe | Culmen | |
| Posterior lobe | Cerebellar tonsil | ||||||||
| Declive | |||||||||
| 301 | 0.001 | 4.41 | -6 | 18 | 62 | Left cerebrum | Frontal lobe | Superior frontal gyrus | 6, 8 |
| 292 | 0.001 | 3.47 | -29 | -64 | -32 | Left cerebellum | Posterior lobe | Cerebellar tonsil | |
| Pyramis | |||||||||
| Tuber | |||||||||
| Uvula | |||||||||
| Culmen | |||||||||
| Declive | |||||||||
| Anterior lobe | Culmen | ||||||||
| 248 | 0.002 | 3.83 | -37 | -2 | -7 | Left cerebrum | Sub-lobar | Extra-nuclear | 13 |
| Claustrum | |||||||||
| 235 | 0.000 | 3.76 | -25 | 22 | -18 | Left cerebrum | Frontal lobe | Inferior frontal gyrus | 47 |
| Middle frontal gyrus | 11 | ||||||||
| 180 | 0.005 | 3.55 | -37 | 9 | 58 | Left cerebrum | Frontal lobe | Middle frontal gyrus | 6 |
| Superior frontal gyrus | 6 | ||||||||
| 133 | 0.002 | 3.83 | -37 | -4 | -5 | Left cerebrum | Temporal lobe | Middle temporal gyrus | 21 |
| Superior temporal gyrus | 22 | ||||||||
| 65 | 0.001 | 3.35 | 5 | 31 | -28 | Right cerebrum | Frontal lobe | Orbital gyrus | 11 |
| Rectal gyrus | 11 | ||||||||
k ≥ 100; p < 0.005 uncorrected. Refer to Table 1 for a detailed explanation of the table layout.
Table 4.
Positive correlation between flexibility values and FA maps showing increases after 4-weeks of daily QMT.
| Coordinates |
|||||
|---|---|---|---|---|---|
| k | p-value | x | y | z | WM structures |
| 21 | 0.018 | 96 | 101 | 37 | Corticospinal tract L |
| 5 | 0.047 | 102 | 101 | 41 | Middle cerebellar peduncle |
Significance level set at p < 0.05 uncorrected. Refer to Table 2 for a detailed explanation of the table layout.
Discussion
QMT Improves Divergent Thinking Creativity
The results of Study A show that 4-weeks of daily QMT practice induce increased cognitive flexibility and ideational fluency, which was not the case in either the SMT group or in the VT group, representing the motor and cognitive aspects of the training, respectively (Figure 2). This is in line with our first hypothesis, and with two previous studies. In the first relevant study, we reported findings regarding the effects of a single session of QMT (Ben-Soussan et al., 2013), where a similar increase in flexibility was observed in contrast to SMT or VT. However, no significant change was reported for fluency. Our results show that the increase in flexibility was not significantly different between one session and 4-weeks of training, possibly suggesting that participants might have reached a ceiling effect in the AU task after one session, underscoring the need to utilize in the future different tasks to assess QMT-induced cognitive flexibility. Yet, as opposed to one session of training (Ben-Soussan et al., 2013), long-term QMT also increased ideational fluency, which is probably mediated by an accumulated effect of daily QMT sessions. Yet, the fluency results should be treated with caution as the Group × Training interaction was not significant. We further acknowledge the reduction in statistical power given participant attrition, in part because participants who successfully complete all follow-up measurements may have differed from those respondents lost to attrition. Nevertheless, no baseline differences were found in the AU scores between those who continued and those who dropped out (see Supplementary data and Table S4).
In another recent study, we examined the effects of a month of daily QMT as opposed to simple WT, tapping the motor aspect of QMT (Venditti et al., 2014). This study reported increased flexibility and fluency following QMT but not WT. Our results replicate the previous results in the QMT group, as expected. This demonstrates that there is no language bias affecting the results, as the current study was conducted with Hebrew speakers, whereas the previous study was conducted with Italian speakers. Importantly, while we hypothesized an increase in fluency following VT, tapping the cognitive aspect of the QMT, no change in fluency occurred in both the VT and SMT groups. This suggests that the combination of the cognitive and motor aspects of the QMT, and not the cognitive aspect per se, renders the fluency enhancement. Put differently, we suggest that this stems from the complexity of the movement, indicating that it is the combination of the motor and cognitive aspects embedded in QMT, which is important for increasing fluency.
In Study B, flexibility again significantly increased, as expected (Figure 3). Yet, although a trend was observed for fluency, it did not reach significance, possibly due to the small sample size. However, the possibility that the discrepancy between the results of Study A and Study B in terms of fluency is due to the altered QMT sequence in the second study (12 min in Study B vs. 7 min in Study A) cannot be ruled out. Therefore, the effect of QMT sequence length on different aspects of creativity should be further examined, underlying the importance of examining additional sequences of the QMT.
QMT as a Mindful Practice
Our results are in line with the proposal that contemplative practices increase creativity (Horan, 2007), albeit early evidence is inconsistent with that, possibly due to the wide variety of meditation techniques and creativity measures employed (Horan, 2007; Lippelt et al., 2014). Although there are many meditative techniques, we adopt here the conceptualization of Lutz et al. (2008), grossly dividing meditation into two forms: focused attention – learned control over the focus of one’s attention by using a stable object with the goal of quieting the mind, and open monitoring (OM) – maximizing the breadth and clarity of maintained attention in order to bring higher momentary awareness to internal processes (the latter includes for example Mindfulness and Zen). Importantly, when focusing only on studies investigating the effect of OM techniques on creativity, the overall picture is of OM practices increasing divergent thinking (Cowger and Torrance, 1982; Zabelina et al., 2011; Colzato et al., 2012; Greenberg et al., 2012; Ding et al., 2014).
Here, we wish to draw attention to the mindful nature of QMT, supported by the similarity of the training effects in enhancing divergent thinking. Mindful practices have been noted in the literature using varied terminology depending on the discipline called upon, including “the act of becoming more aware,” “a reflective act,” or “mindfulness” (Depraz et al., 2000). It has been claimed that the mindful act has three interdependent phases: a first phase of suspension from the habitual act of allowing the mind and body to go where they want; a second phase of redirection of attention inwardly; and a third phase of receptivity toward the experience (Depraz et al., 2000). Mindfulness meditation, as conceptualized in the Western tradition, incorporates all three phases (Kabat-Zinn, 2009). Similarly, QMT can be thought of as training in all the above three phases. First, one suspends the automatic movement. Then, one redirects attention toward the external cue and the internally generated movement. Finally, one quietly stands in a receptive manner in between instructions, without correcting motor or decision errors. QMT is also dependent on the moment by-moment re-investment of attention. In support of that, we reported increased ability to respond in a non-habitual fashion following 5–12 min QMT (Moore and Malinowski, 2009; Ben-Soussan et al., 2014b). To sum, QMT can be considered to be a Mindful Movement practice, and similarly to other OM practices, leads to increased divergent thinking.
Structural Changes Following QMT
Structural changes in GM volume were found mainly in the left and right cerebellum, as well as in the left frontal lobe, whereas the main FA increase was found in the middle cerebellar peduncle (Figure 4). The changes in the cerebellum and the frontal cortex could be explained by their role in the acquisition of motor sequences (Exner et al., 2002). The cerebellum is important for the integration of somatosensory and motor information relevant to coordinate context-dependent planning and execution of coordinate motion (Ivry, 2000; Tesche and Karhu, 2000). Although interoception was not directly measured here, it is important to note in the context of this special issue, that the cerebellum is closely linked to interoceptive awareness reflecting explicit awareness of bodily processes (Critchley et al., 2004; Mercader et al., 2010). The left frontal regions, mainly in the inferior frontal and middle frontal gyri, are related to motor learning, action observation and intention understanding (Exner et al., 2002; Dapretto et al., 2005). In addition, both the inferior frontal and the middle frontal gyri (see Table 1) have been consistently related to working memory and response inhibition (Leung et al., 2002; Morin and Michaud, 2007; Swick et al., 2008) and selection among competing alternatives (Paulesu et al., 1993) which may increase following QMT. Notably, the spatial proximity of the GM and FA increases in the cerebellum following the training suggests that the QMT-induced increase in GM volume is related to an altered organization of underlying white matter pathways. Importantly, we have previously reported that following 3-months QMT cerebellar GM volume and FA increased, in positive correlation with increased brain-derived neurotrophic factor (BDNF) level (Ben-Soussan et al., 2015), a neurotrophin closely linked to interoceptive awareness (Mercader et al., 2010). In addition, QMT was previously reported to increase cerebellar low-rhythm activity (Ben-Soussan et al., 2014a). This has led to a theoretical account emphasizing the cerebellum as an important candidate which possibly mediates the QMT effects on well-being (Ben-Soussan et al., 2015). The current anatomical results provide further support to such a proposition, and show that 1-month QMT is sufficient to induce measurable cerebellar anatomical alterations.
The current results are in line with previous meditation studies which have found activation in areas closely linked to motor learning, such as the frontal gyrus and the cerebellum. For example, Pagnoni and Cekic (2007) reported increased GM volume in Zen meditation practitioners compared to matched controls, especially in the putamen which is closely involved in the control of voluntary movement (Nambu et al., 2002). This result was interpreted as being related to the cognitive processes engaged by meditation, and especially to the conscious regulation of attention and posture (Pagnoni and Cekic (2007). Vestergaard-Poulsen et al. (2009) examined the effects of Tibetan Buddhist meditation, and found that in addition to increased GM density in the medulla oblongata, left superior and inferior frontal gyri, and left fusiform gyrus, an increment was found in the anterior lobe of the cerebellum in the meditator group compared to the control group. In addition, Hölzel et al. (2011) studied the effect of mindfulness-based stress reduction (MBSR) in meditation-naïve participants, before and after 8-weeks of training. In addition to other regions, they found increased GM density in the cerebellum in the MBSR group compared to controls. Hölzel et al. (2011) address their cerebellar-related results in relation to its role in the regulation of emotion and cognition. They further mention the claim made by Schmahmann (2004), that in the same way that the cerebellum regulates the rate, force, rhythm, and accuracy of movements, it also regulates the speed, capacity, consistency, and appropriateness of cognitive and emotional processes.
Compared to sitting meditation, the anatomical examination of whole-body movement-based contemplative practices is surprisingly rare. One exception is a recent study examining the effects of Tai Chi Chuan (TCC), a whole-body contemplative practice involving movement, such as weight-shifting between the right and left legs and asymmetrical diagonal leg movements (Wei et al., 2013). Wei et al. (2013) demonstrated that TCC practitioners showed greater cortical thickness in prefrontal cortex and temporal cortex relative to the matched control group. Interestingly, neuroimaging studies have consistently shown similar findings following aerobic exercise, as this was shown to lead to increased gray and white matter volume in the prefrontal cortex of older adults (Colcombe et al., 2006). In addition, greater amounts of physical activity, such as walking, are associated with sparing of prefrontal and temporal brain regions of late adulthood (Erickson et al., 2010). Taken together, the above studies show that both sitting and movement contemplative practices induce neuroplasticity in motor regions. This strengthens the claim that the results shown here might not be due to the movement per se, but involve higher cognitive modulation.
Creativity and the Motor System
Albeit the small sample size and the preliminary nature of the findings, a positive correlation was found between increased flexibility and cerebellar changes, both in the GM volume and the FA values (Figure 5). Due to the low power of the correlation analysis, this finding should be treated as being suggestive, but can still guide the hypotheses of future larger studies. This correlation supports the previously suggested link between creativity and the motor system (Dietrich, 2004; Carruthers, 2011; Koziol et al., 2012). For example, Cotterill (2001) suggested that: “If cognition is linked to overt or covert movement, intelligence becomes the ability to consolidate individual motor elements into more complex patterns, and creativity is the outcome of a race-to-threshold process which centers on the motor areas.” (p. 1). Similarly, Carruthers (2011) wrote: “creativity lies in the assembly and activation of action-schemata, with creative thoughts arising subsequently from the mental rehearsal of those actions” (p. 437). The creativity-motor connection has been advanced to the point that it has been provocatively claimed that: “we were not born to think. We were born to move. Human creative ideas are nothing in the absence of the manual dexterity that allows tools to be made, complex architecture to be constructed, art to be created, and instruments to be played” (Koziol et al., 2012, p. 515).
The motor-creativity hypothesized connection was previously supported by several reports. For example, a positive correlation was found between figural and verbal creativity and cerebral blood flow in the right cerebellum (Chávez-Eakle et al., 2007). Takeuchi et al. (2010) measured GM volume using voxel-based morphometry and found a positive correlation between divergent thinking and the right dorsolateral prefrontal cortex and the bilateral striata. In accord with our anatomical results, other reports have also emphasized the involvement of the cerebellum and the precentral gyrus in creative processes (Cotterill, 2001; Chávez-Eakle et al., 2007; Vandervert et al., 2007). The current findings also provide preliminary insight regarding the possible relationship between anatomical changes in motor-related areas and creativity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpsyg.2015.01021
References
- Baron-Cohen S., Hammer J. (1997). Is autism an extreme form of the “male brain”? Adv. Infancy Res. 11 193–218. [Google Scholar]
- Ben-Soussan T. D., Avirame K., Glicksohn J., Goldstein A., Harpaz Y., Ben-Shachar M. (2014a). Changes in cerebellar activity and inter-hemispheric coherence accompany improved reading performance following Quadrato Motor Training. Front. Syst. Neurosci. 8:81 10.3389/fnsys.2014.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Soussan T. D., Berkovich-Ohana A., Glicksohn J., Goldstein A. (2014b). A suspended act: increased reflectivity and gender-dependent electrophysiological change following Quadrato Motor Training. Front. Psychol. 5:55 10.3389/fpsyg.2014.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Soussan T. D., Berkovich-Ohana A., Glicksohn J. (2015). From cerebellar activation and connectivity to cognition: a review of the Quadrato Motor Training. Biomed. Res. Int. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Soussan T. D., Glicksohn J., Goldstein A., Berkovich-Ohana A., Donchin O. (2013). Into the square and out of the box: the effects of Quadrato Motor Training on creativity and alpha coherence. PLoS ONE 8:e55023 10.1371/journal.pone.0055023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Soussan T. D., Piervincenzi C., Venditti S., Verdone L., Caserta M., Carducci F. (2015). Increased cerebellar volume and BDNF level following Quadrato Motor Training. Synapse 69 1–6. 10.1002/syn.21787 [DOI] [PubMed] [Google Scholar]
- Brubaker C. J., Dietrich K. N., Lanphear B. P., Cecil K. M. (2010). The influence of age of lead exposure on adult gray matter volume. Neurotoxicology 31 259–266. 10.1016/j.neuro.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers P. (2011). Creative action in mind. Philos. Psychol. 24 437–461. 10.1080/09515089.2011.556609 [DOI] [Google Scholar]
- Chang Y. K., Nien Y. H., Chen A. G., Yan J. (2014). Tai Ji Quan, the brain, and cognition in older adults. J. Sport Health Sci. 3 36–42. 10.1016/j.jshs.2013.09.003 [DOI] [Google Scholar]
- Chang Y. K., Nien Y. H., Tsai C. L., Etnier J. L. (2010). Physical activity and cognition in older adults: the potential of Tai Chi Chuan. J. Aging Phys. Act. 18 451–472. [DOI] [PubMed] [Google Scholar]
- Chávez-Eakle R. A., Graff-Guerrero A., García-Reyna J. C., Vaugier V., Cruz-Fuentes C. (2007). Cerebral blood flow associated with creative performance: a comparative study. Neuroimage 38 519–528. 10.1016/j.neuroimage.2007.07.059 [DOI] [PubMed] [Google Scholar]
- Chermahini S. A., Hommel B. (2010). The (b)link between creativity and dopamine: spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition 115 458–465. 10.1016/j.cognition.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Colcombe S. J., Erickson K. I., Scalf P. E., Kim J. S., Prakash R., McAuley E., et al. (2006). Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 61 1166–1170. 10.1093/gerona/61.11.1166 [DOI] [PubMed] [Google Scholar]
- Colzato L. S., Ozturk A., Hommel B. (2012). Meditate to create: the impact of focused-attention and open-monitoring training on convergent and divergent thinking. Front. Psychol. 3:116 10.3389/fpsyg.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterill R. M. (2001). Cooperation of the basal ganglia, cerebellum, sensory cerebrum and hippocampus: possible implications for cognition, consciousness, intelligence and creativity. Prog. Neurobiol. 64 1–33. 10.1016/S0301-0082(00)00058-7 [DOI] [PubMed] [Google Scholar]
- Cowger E. L., Torrance E. P. (1982). Further examination of the quality of changes in creative functioning resulting from meditation (Zazen) trraining. Creat. Child Adult Q. 7 211–217. [Google Scholar]
- Critchley H. D., Wiens S., Rotshtein P., Öhman A., Dolan R. J. (2004). Neural systems supporting interoceptive awareness. Nat. Neurosci. 7 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Dapretto M., Davies M. S., Pfeifer J. H., Scott A. A., Sigman M., Bookheimer S. Y., et al. (2005). Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 9 28–30. 10.1038/nn1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier N., Schmidt A., Gerber H., Schmid O., Riecher-Rössler A., Wiesbeck G. A., et al. (2013). Association of frontal gray matter volume and cerebral perfusion in heroin addiction: a multimodal neuroimaging study. Front. Psychiatry 4:135 10.3389/fpsyt.2013.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depraz N., Varela F. J., Vermersch P. (2000). “The gesture of awareness: an account of its structural dynamics,” in Investigating Phenomenal Consciousness ed. Velmans M. (Philadelphia, PA: John Benjamins; ) 121–136. 10.1075/aicr.13.10dep [DOI] [Google Scholar]
- Dietrich A. (2004). The cognitive neuroscience of creativity. Psychon. Bull. Rev. 11 1011–1026. 10.3758/BF03196731 [DOI] [PubMed] [Google Scholar]
- Ding X., Tang Y.-Y., Tang R., Posner M. I. (2014). Improving creativity performance by short-term meditation. Behav. Brain Funct. 10 1–8. 10.1186/1744-9081-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Raji C. A., Lopez O. L., Becker J. T., Rosano C., Newman A. B., et al. (2010). Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 75 1415–1422. 10.1212/WNL.0b013e3181f88359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner C., Koschack J., Irle E. (2002). The differential role of premotor frontal cortex and basal ganglia in motor sequence learning: evidence from focal basal ganglia lesions. Learn. Mem. 9 376–386. 10.1101/lm.48402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. E., Liederman J., Malmberg B., McLean J., Strickland D., Beauchamp M. S. (2010). Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb. Cortex 20 2625–2635. 10.1093/cercor/bhq010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C. D., Johnsrude I., Ashburner J., Henson R. N., Friston K. J., Frackowiak R. S. (2001). Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 14 685–700. 10.1006/nimg.2001.0857 [DOI] [PubMed] [Google Scholar]
- Greenberg J., Reiner K., Meiran N. (2012). “Mind the trap”: mindfulness practice reduces cognitive rigidity. PLoS ONE 7:e36206 10.1371/journal.pone.0036206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford J. P. (1950). Creativity. Am. Psychol. 5 444–454. 10.1037/h0063487 [DOI] [PubMed] [Google Scholar]
- Guilford J. P. (1968). Creativity, Intelligence, and their Educational Implications. San Diego, CA: Robert R. Knapp. [Google Scholar]
- Guilford J. P., Christensen P. R., Merrifield P. R., Wilson R. C. (1978). Alternate Uses: Manual of Instructions and Interpretation. Orange, CA: Sheridan Psychological Services. [Google Scholar]
- Hölzel B. K., Carmody J., Vangel M., Congleton C., Yerramsetti S. M., Gard T., et al. (2011). Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 191 36–43. 10.1016/j.pscychresns.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan R. (2007). The relationship between creativity and intelligence: a combined yogic-scientific approach. Creat. Res. J. 19 179–202. 10.1080/10400410701397230 [DOI] [Google Scholar]
- Ivry R. (2000). Exploring the role of the cerebellum in sensory anticipation and timing: commentary on Tesche and Karhu. Hum. Brain Mapp. 9 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. (2009). Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Delacour. [Google Scholar]
- Koziol L. F., Budding D. E., Chidekel D. (2012). From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum 11 505–525. 10.1007/s12311-011-0321-y [DOI] [PubMed] [Google Scholar]
- Leung H. C., Gore J. C., Goldman-Rakic P. S. (2002). Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J. Cogn. Neurosci. 14 659–671. 10.1162/08989290260045882 [DOI] [PubMed] [Google Scholar]
- Levy-Drori S., Henik A. (2006). Concreteness and context availability in lexical decision tasks. Am. J. Psychol. 119 45–65. 10.2307/20445318 [DOI] [PubMed] [Google Scholar]
- Lippelt D. P., Hommel B., Colzato L. S. (2014). Focused attention, open monitoring and loving kindness meditation: effects on attention, conflict monitoring, and creativity – a review. Front. Psychol. 5:1083 10.3389/fpsyg.2014.01083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Gaser C., Narr K. L., Toga A. W. (2009). Why sex matters: brain size independent differences in gray matter distributions between men and women. J. Neurosci. 29 14265–14270. 10.1523/JNEUROSCI.2261-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., Slagter H. A., Dunne J. D., Davidson R. J. (2008). Attention regulation and monitoring in meditation. Trends Cogn. Sci. 12 163–169. 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader J. M., Fernandez-Aranda F., Gratacòs M., Aguera Z., Forcano L., Ribasés M., et al. (2010). Correlation of BDNF blood levels with interoceptive awareness and maturity fears in anorexia and bulimia nervosa patients. J. Neural Transm. 117 505–512. 10.1007/s00702-010-0377-8 [DOI] [PubMed] [Google Scholar]
- Moore A., Malinowski P. (2009). Meditation, mindfulness and cognitive flexibility. Conscious. Cogn. 18 176–186. 10.1016/j.concog.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Morin A., Michaud J. (2007). Self-awareness and the left inferior frontal gyrus: inner speech use during self-related processing. Brain Res. Bull. 74 387–396. 10.1016/j.brainresbull.2007.06.013 [DOI] [PubMed] [Google Scholar]
- Nambu A., Kaneda K., Tokuno H., Takada M. (2002). Organization of corticostriatal motor inputs in monkey putamen. J. Neurophysiol. 88 1830–1842. [DOI] [PubMed] [Google Scholar]
- Netz Y., Tomer R., Axelrad S., Argov E., Inbar O. (2007). The effect of a single aerobic training session on cognitive flexibility in late middle-aged adults. Int. J. Sports Med. 28 82–87. 10.1055/s-2006-924027 [DOI] [PubMed] [Google Scholar]
- Ocklenburg S., Hugdahl K., Westerhausen R. (2013). Structural white matter asymmetries in relation to functional asymmetries during speech perception and production. Neuroimage 83 1088–1097. 10.1016/j.neuroimage.2013.07.076 [DOI] [PubMed] [Google Scholar]
- Pagnoni G., Cekic M. (2007). Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol. Aging 28 1623–1627. 10.1016/j.neurobiolaging.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Paulesu E., Frith C. D., Frackowiak R. S. (1993). The neural correlates of the verbal component of working memory. Nature 362 342–345. 10.1038/362342a0 [DOI] [PubMed] [Google Scholar]
- Runco M. A. (1986). Flexibility and originality in children’s divergent thinking. J. Psychol. 120 345–352. 10.1080/00223980.1986.9712632 [DOI] [Google Scholar]
- Russ S. W. (1998). Play, creativity, and adaptive functioning: implications for play interventions. J. Clin. Child Psychol. 27 469–480. 10.1207/s15374424jccp2704_11 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D. (2004). Disorders of the cerebellum: ataxia, dysmetria of thought and the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 16 367–378. 10.1176/appi.neuropsych.16.3.36 [DOI] [PubMed] [Google Scholar]
- Schmid T. (ed.) (2005). “Promoting health through creativity: an introduction,” in Promoting Health through Creativity: for Professionals in Health, Arts and Education ed. Schmid T. (London: Whurr Publishers; ). [Google Scholar]
- Scholz J., Klein M. C., Behrens T. E., Johansen-Berg H. (2009). Training induces changes in white-matter architecture. Nat. Neurosci. 12 1370–1371. 10.1038/nn.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T. E., Mackay C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 9:102 10.1186/1471-2202-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Sassa Y., Hashizume H., Sekiguchi A., Fukushima A., et al. (2010). Regional gray matter volume of dopaminergic system associate with creativity: evidence from voxel-based morphometry. Neuroimage 51 578–585. 10.1016/j.neuroimage.2010.02.078 [DOI] [PubMed] [Google Scholar]
- Tesche C. D., Karhu J. J. (2000). Anticipatory cerebellar responses during somatosensory omission in man. Hum. Brain Mapp. 9 119–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R., Fisher T., Giladi N., Aharon-Perez J. (2002). Dissociation between spontaneous and reactive flexibility in early Parkinson’s disease. Neuropsychiatry Neuropsychol. Behav. Neurol. 15 106–112. [PubMed] [Google Scholar]
- Vandervert L. R., Schimpf P. H., Liu H. (2007). How working memory and the cerebellum collaborate to produce creativity and innovation. Creat. Res. J. 19 1–18. 10.1080/10400410709336873 [DOI] [Google Scholar]
- Venditti S., Verdone L., Pesce C., Tocci N., Caserta M., Ben-Soussan T. D. (2014). Creating well-being: increased creativity and proNGF decrease following Quadrato Motor Training. Biomed. Res. Int. 2015:275062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P., van Beek M., Skewes J., Bjarkam C. R., Stubberup M., Bertelsen J., et al. (2009). Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport 20 170–174. 10.1097/WNR.0b013e328320012a [DOI] [PubMed] [Google Scholar]
- Wayne P. M., Gow B. J., Costa M. D., Peng C. K., Lipsitz L. A., Hausdorff J. M., et al. (2014). Complexity-based measures inform effects of Tai Chi training on standing postural control: cross-sectional and randomized trial studies. PLoS ONE 9:e114731 10.1371/journal.pone.0114731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G. X., Xu T., Fan F. M., Dong H. M., Jiang L. L., Li H. J., et al. (2013). Can tai chi reshape the brain? A brain morphometry study. PLoS ONE 8:e61038 10.1371/journal.pone.0061038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabelina D. L., Robinson M. D., Ostafin B. D., Council J. R. (2011). Manipulating mindfulness benefits creative elaboration at high levels of neuroticism. Empir. Stud. Arts 29 243–255. 10.2190/EM.29.2.g [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.