Abstract
Background
Neoadjuvant chemotherapy (NAC) may allow breast-conserving therapy (BCT) in patients who require mastectomy at presentation. Breast MRI is more accurate than mammography in assessing treatment response, but combined test reliability in identifying BCT candidates after NAC is not well described. We evaluated whether post-NAC breast MRI alone and with mammography accurately identifies BCT candidates.
Methods
In this retrospective study of 111 consecutive breast cancer patients receiving NAC, all had pre- and postchemotherapy MRI, followed by surgery. Posttreatment MRI and mammography results were correlated with surgical outcomes and pathologic response.
Results
Fifty-one of 111 (46 %) patients presented with multicentric or inflammatory breast cancer and were not BCT candidates. The remaining 60 (54 %) were considered BCT candidates after downstaging (mean age: 47 years). All 60 had at least a partial response to NAC and were suitable for BCT on MRI after NAC. Forty-five of 60 (75 %) underwent lumpectomy; 15 of 60 (25 %) chose mastectomy. Forty-one of 45 (91 %) of lumpectomies were successful; 4 of 45 (9 %) required mastectomy. Twelve of 15 (80 %) patients choosing mastectomy could have undergone BCT based on pathology; 3 of 15 (20 %) did require mastectomy. Two of these three patients had extensive microcalcifications on mammogram, indicating the need for mastectomy despite MRI suitability for BCS. MRI alone correctly predicted BCS in 53 of 60 (88 %) patients. MRI plus mammography was correct in 55 of 60 (92 %), although only 9 of 45 (20 %) BCT patients and 4 of 15 (27 %) potentially conservable mastectomy patients had complete pathologic responses.
Conclusions
Posttreatment MRI plus mammography is an accurate method to determine whether BCT is possible after NAC is given to downstage disease.
Despite the availability of screening mammography, some patients present with cancers whose size, relative to the size of the breast, precludes breast-conserving therapy (BCT) if surgery is the initial step in treatment. Randomized trials have demonstrated that neoadjuvant chemotherapy (NAC) allowed BCT in 23 and 33 % of patients who were believed to require mastectomy at presentation.1,2 Although rates of pathologic complete response (pCR) have increased with improvements in drug therapy, BCT rates after NAC remain low, in part because evaluation of response to NAC is problematic.3,4
Multiple studies have demonstrated that MRI is the most accurate imaging examination for assessment of response to NAC, with a strong correlation between tumor size on MRI and residual tumor size at pathologic examination.5,6 MRI also defines the pattern of residual tumor as contiguous or scattered, an important factor in selecting surgical therapy. However, the reliability of MRI in identifying candidates for BCT after NAC alone, or in combination with mammography, is not well described. We evaluated the accuracy of MRI and mammography in determining suitability for BCT after NAC.
METHODS
Patients
This retrospective study was Institutional Review Board approved with waiver of consent and was HIPAA compliant. A total of 111 consecutive patients who underwent pre- and post-NAC MRI followed by definitive surgery between 2009 and 2012 were included in this study population. To minimize selection bias, all patients receiving NAC who had both pre- and posttreatment MRIs were included in the initial patient population even though some were not candidates for downstaging to BCT based on presenting tumor characteristics. Initial diagnosis of breast cancer was made through core biopsy. Patients received NAC either as part of two prospective protocols, Cancer and Leukemia Group B (CALGB) 40601 and 40603 for patients with clinical stage II–III HER2-positive and HER2-negative, hormone receptor-poor breast cancer, respectively, or as part of standard clinical care.7,8 In CALGB 40601, HER2 positivity was defined as IHC 3+ or FISH-amplified with ratio of ≥2.0. In CALGB 40603, hormone receptor-poor disease was defined as estrogen and progesterone receptor staining of ≤10 %. Time between pre- and posttreatment MRIs ranged from 9 to 28 weeks (mean 16.5, median 16).
For patients with HER2-positive tumors, on study, they received paclitaxel with trastuzumab, lapatinib, or both. Off-study patients received doxorubicin (A) or epirubicin (E) and cyclophosphamide (C) followed by paclitaxel with trastuzumab. In patients with HER2-negative tumors, on study, they received paclitaxel alone or with bevacizumab or carboplatin, or bevacizumab plus carboplatin followed by AC ± bevacizumab. Off study, they received a standard anthracycline-based therapy followed by paclitaxel.
MRI
Some pretreatment MRI examinations were performed at other institutions using a variety of techniques. Those considered technically adequate were used for initial tumor evaluation. All posttreatment MRI exams were performed at Memorial Sloan Kettering Cancer Center with the patient prone in a dedicated surface breast coil on a 1.5-T or 3.0-T commercially available system (General Electric Medical Systems, Milwaukee, WI). Both breasts were imaged simultaneously using VIBRANT. The standard exam included a localizing sequence followed by a sagittal fat-suppressed T2-weighted and a sagittal T1-weighted sequence. A T1-weighted three-dimensional, fat-suppressed, fast-spoiled, gradient-echo sequence was performed before and three times after an intravenous rapid bolus injection of 0.1 mmol/L of gadopentetate dimeglumine (Magnevist, Berlex, and Wayne, NJ) per kilogram of body weight. Images were acquired in a sagittal projection following contrast injection followed by a saline bolus. Axial T1 postcontrast sequence and diffusion-weighted imaging also were performed. Section thickness was 0.3 cm with no gap and a minimum matrix of 256 × 256. Unenhanced images were subtracted from the contrast-enhanced images on a pixel-by-pixel basis producing three subtracted postcontrast subtraction sequences. Maximum intensity projection images were created using the first postcontrast sequence and the first postcontrast subtracted data.
The breast MRI examinations, including those performed at outside institutions, were interpreted by 1 of 19 experienced breast imagers. Changes in the enhancing tumor size from pre-NAC to post-NAC MRI examinations were documented. Complete resolution of enhancement was deemed a complete response.
Diagnostic mammograms of the index breast were performed after NAC in 39 of the 46 patients for whom conservation was planned. The statistical comparison of MRI alone versus MRI plus mammography was performed using McNemar’s test.
RESULTS
The 111 patients receiving NAC had stage IIA–IIIA (T2-3N0 or T1-3N1-2a) disease, multicentric breast cancer, or inflammatory breast cancer. Mean patient age was 46.8 (range 25–74) years. A total of 104 (93.7 %) patients had invasive ductal cancer (IDC). Patients with estrogen receptor (ER) and progesterone receptor (PR)-negative tumors were overrepresented, comprising 77 % of the group (44 HER2-positive, 42 HER2-negative). Of the 25 ER-positive patients, 10 were HER2-positive.
Fifty-one of 111 (46 %) patients presented with multicentric or inflammatory breast cancer and were not candidates for downstaging to BCT. MRI, mammographic, and pathologic findings of the remaining 60 (54 %) patients are the subject of this study. Patient characteristics are summarized in Table 1. A single patient (2 %) had T1 disease with bulky adenopathy, 39 (65 %) had T2 cancers, and 20 (33 %) had T3 tumors.
TABLE 1.
Patient characteristics
| Number of patients | All patients: 111 | BCT: 60 |
| Age, years (range 25–74) | Mean 46.8 | Mean 47.6 |
| Cancer type | ||
| IDC | 104 | 56 |
| IMC | 4 | 2 |
| ILC | 3 | 2 |
| Markers | ||
| ER/PR-positive HER2-negative | 15 | 10 |
| ER/PR-positive HER2-positive | 10 | 5 |
| ER/PR-negative HER2-positive | 44 | 20 |
| ER/PR-negative HER2-negative | 42 | 25 |
BCT breast-conserving therapy, IDC invasive ductal carcinoma, IMC invasive mammary carcinoma not otherwise specified, ILC invasive lobular carcinoma, ER estrogen receptor, PR progesterone receptor
All 60 patients potentially eligible for BCT had at least a partial response to NAC. No residual enhancement was seen on MRI in 20 of 60 (33 %; Fig. 1). In 40 of 60 (67 %), there was a decrease in both the size and degree of enhancement of the known cancer. In 34 patients (85 %), this consisted of a decrease in the size of a unifocal mass, whereas in the remaining 6, there was a decrease in multifocal tumor.
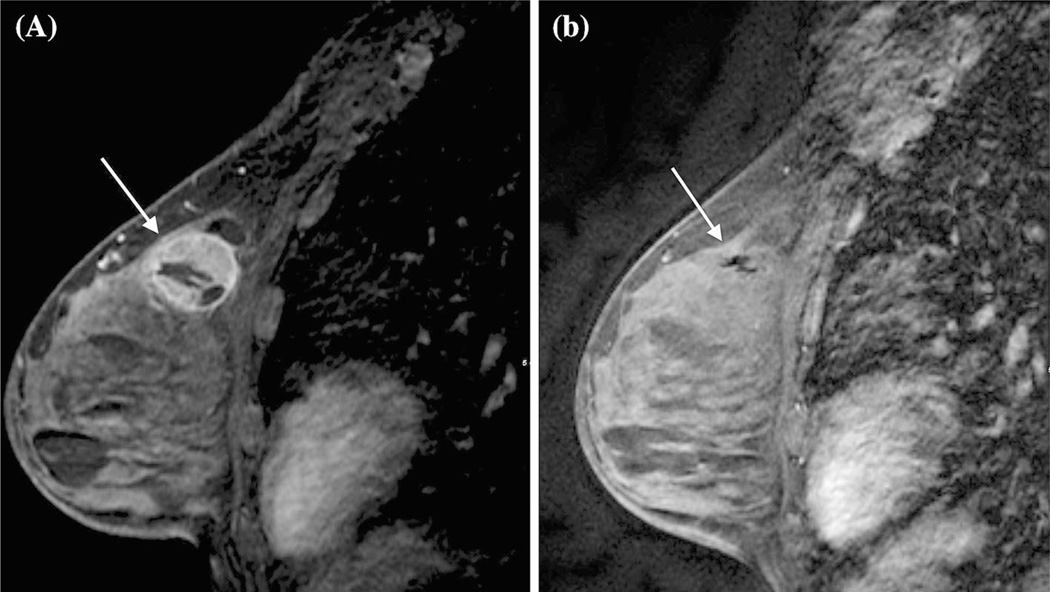
FIG. 1.
A 38-year-old with unifocal triple-negative disease with complete response by MRI. a Pretreatment MRI demonstrating large enhancing mass in the upper left breast. b Posttreatment MRI demonstrating complete resolution of mass and abnormal enhancement
Lumpectomy was attempted in 45 of 60 (75 %) women, and 15 (25 %) patients who were potential lumpectomy candidates chose mastectomy. Negative margins were obtained in 41 of 45 (91 %) lumpectomy patients. Forty of these women had posttreatment mammograms. In 37 of 41 patients who had successful BCT, mammography concurred with MRI that conservation was feasible. One patient who was believed to be a good candidate for BCT based on physical examination and MRI response had extensive residual malignant-appearing calcifications on mammography, necessitating needle localization and a larger lumpectomy to obtain negative margins. Nine of 45 (20 %) lumpectomy patients had pCR. Four of 45 (9 %) patients attempting BCT required mastectomy due to residual disease. Three of these patients had post-NAC mammograms, which also incorrectly predicted suitability for BCT. All four had IDC. Two cancers were T2 at presentation, two were T3, and three were ER-positive (1 HER2-positive); one was ER-negative and HER2-positive.
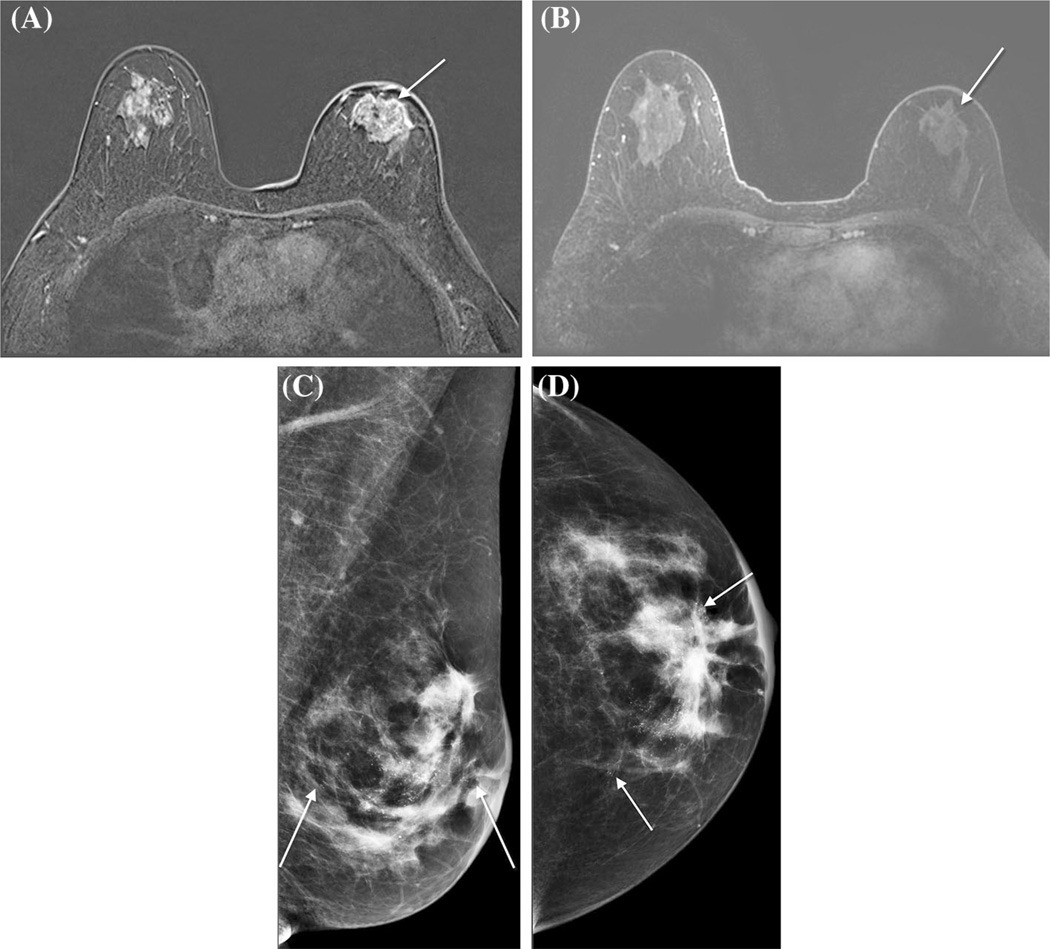
Twelve of 15 patients opting for mastectomy would have been candidates for BCT based on pathology of the mastectomy specimen, which showed limited or no residual carcinoma (pCR 4/15, 27 %), whereas in 3, the extent of residual disease suggested that mastectomy would have been medically necessary. Two of these three patients had mammograms with extensive microcalcifications, indicating the need for mastectomy despite the MRI suggesting suitability for BCT (Fig. 2).
FIG. 2.
A 44-year-old with unifocal invasive ductal carcinoma (ER 10 %/ PR 40 % HER2 negative). a Pretreatment MRI demonstrating large enhancing mass in the central left breast. b Posttreatment MRI showing resolution of abnormal enhancement and decrease in the size of the mass, suggesting suitability for breast-conserving therapy. c, d MLO and CC views of the posttreatment mammogram demonstrating extensive pleomorphic calcifications in the central breast measuring 6 cm by 5 cm, not apparent on the post-treatment MRI and felt to preclude breast-conserving therapy. ER estrogen receptor, PR progesterone receptor, MLO mediolateral oblique, CC craniocaudal
MRI alone was correct in predicting the ability to conserve the breast in 53 of 60 (88 %) patients. MRI combined with mammography was correct in 55 of 60 (92 %; p = 0.479).
DISCUSSION
NAC results in equivalent disease-free and overall survival compared with adjuvant chemotherapy while increasing the number of women who are eligible for BCT.7–9 The ability to perform BCT after neoadjuvant therapy is dependent upon the response of the tumor and the ability to evaluate the extent of residual disease preoperatively, and should be increased in patients with pCR. Improvements in systemic therapy and the availability of targeted therapy have increased the rates of pCR, but, paradoxically, a corresponding increase in the rates of BCT has not been observed. For example, in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B27 trial, the addition of docetaxel to AC increased rates of pCR to 26.1 % compared with 13.7 % in patients treated with AC alone (p < 0.001). Despite this, BCT rates did not differ between groups and were 61.6 and 63.7 %, respectively.4 In the Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization trial (NeoALTTO) in HER2-overexpressing patients, chemotherapy plus dual blockade with trastuzumab and lapatinib produced pCR in 51.3 % of patients compared with 24.7 % who received chemotherapy plus lapatinib and 29.5 % in those receiving chemotherapy and trastuzumab. Despite this large increase in the pCR rate, BCT rates in patients who were not candidates for the procedure before NAC were 26.4 % in the dual blockade group, 26.4 % in the lapatinib group, and 27.7 % in the trastuzumab group.3 These findings suggest the need for a more accurate assessment of the degree of response prior to the performance of surgery. There is little consensus about what should be the appropriate imaging evaluation for patients having NAC. Current National Comprehensive Cancer Network (NCCN) guidelines state that the multidisciplinary team should reach consensus on what studies to do pretreatment and that abnormal studies should be repeated posttreatment.10
In this study, we demonstrate that the combination of MRI and mammography post-NAC correctly predicted the ability to perform BCT in 92 % of patients. In the post- NAC setting, MRI has clearly been shown to be superior to physical examination, mammography, and ultrasound in determining the presence and extent of viable tumor within the breast. Rosen et al. showed that MRI had a correlation coefficient with histology of 0.75, whereas that of physical examination was 0.61.5 In a multi-institutional study of 41 women with stage IIB or III palpable breast cancers, Yeh and colleagues showed that MRI had the best correlation with pathology compared with physical examination, mammography, and ultrasound, whereas data from 216 women undergoing NAC in the I-SPY trial also demonstrated that MRI imaging was a stronger predictor of pathologic response than clinical assessment.6,11 Despite its superior performance, the accuracy of MRI in prediction of pCR is limited. In a meta-analysis of 25 studies of MRI’s ability to predict complete response, pooled estimates of sensitivity and specificity were 0.63 and 0.91, respectively, and were related to the pCR rate.12 Clinically, pCR is not a requirement for the performance of BCT after NAC. Patients whose tumors decrease in size and who do not have scattered viable tumor foci throughout the breast are appropriate candidates for BCT. The pCR rate in our study was only 20 % in patients who had successful lumpectomy, indicating that MRI provides valuable information even in the absence of pCR.
In addition to our study, two other studies also support the utility of MRI in identifying suitable candidates for BCT after NAC. Straver et al. examined pre- and post-NAC MRI exams in 208 patients and defined differences of tumor size on MRI and in the pathologic specimen of >2 cm as potentially leading to inappropriate attempts at BCT. In 35 cases (17 %), MRI underestimated tumor size by >2 cm, which would have led to inappropriate attempts at BCT in 27 patients (13 %). Conversely, MRI overestimated the extent of disease resulting in unnecessary mastectomy in nine patients. The overall accuracy of MRI for the selection of surgical treatment was 83 %.13 However, cancers >3 cm in size after NAC were considered a contraindication to BCT, limiting the generalizability of the study findings. Julius et al. reported that BCT was successful in 18 of 20 patients who underwent the procedure based on MRI findings after NAC.14 Charehbili et al. reported the relationship between MRI estimation of tumor size and surgical margins in 182 patients receiving NAC.15 In 86 patients (47 %), MRI underestimated tumor size by a median of 1.2 cm. In 10 % of cases, tumor size was underestimated by >2 cm, and these patients were significantly more likely to have positive margins (33.3 vs. 11.5 %, p = 0.005) than those in whom extent of tumor was underestimated to a lesser degree or overestimated.
Studies suggest that the accuracy of MRI for assessing response varies based on ER, PR, and HER2 status. Chen et al. demonstrated that the diagnostic accuracy of MRI for assessing response is better in HER2-positive tumors than HER2-negative cancers.16 De Los Santos et al. evaluated the ability of MRI to predict pCR in 179 of 764 (23.4 %) patients who achieved pCR. Overall accuracy was 74 %. The greatest positive predictive value was in patients with triple-negative and HER2-positive disease, likely due to their superior pCR rate.17 These observations may help to explain the excellent results of MRI in our study, because the patient population was heavily weighted toward patients with HER2-overexpressing and triple-negative breast cancers, groups known to have higher rates of response to NAC than patients with ER-positive cancers. In a recent multicenter study of 770 patients receiving NAC at 8 institutions, the mastectomy rate was 58 % in patients who had a partial response by MRI compared with 43 % in those who had a complete response (p = 0.0003), and lack of a complete response remained a significant predictor of mastectomy on multivariate analysis. However, receptor status, T stage at diagnosis, young age, and treating institution were found to be more significant determinants of surgical choice than MRI response data, indicating that factors other than the degree of response play a role in surgical decisions post NAC.18
Although MRI alone predicted the ability to perform BCT in 88 % of patients, this was improved to 92 % with the addition of mammography due to the visualization of microcalcifications, which increased the extent of disease compared to that seen on MRI. In two patients, this precluded conservation, and in one, resulted in a larger lumpectomy. These three patients draw attention to the importance of obtaining a posttreatment mammogram in addition to an MRI. Although Weiss et al. reported that in 53 patients with cancers containing calcifications who received NAC, MRI had a higher correlation with pathologic tumor size than mammography, malignant-appearing calcifications associated with viable tumor and those associated with necrotic tumor cannot be reliably distinguished at this time, and mammography and MRI are complementary studies in determining the appropriate extent of surgical resection after NAC.19
There are several limitations to our study. First, this was a retrospective, single-institution study with patients on several different treatment protocols based on the tumor subtype. The sample size was relatively small and precluded subgroup analysis. Additionally, hormone receptor-positive patients were underrepresented, potentially limiting the generalizability of the results. A number of the pretherapy MRI examinations were obtained at outside institutions utilizing different MRI protocols. However, the patient selection criteria and performance of MRI at multiple sites are reflections of what currently occurs in clinical practice. Additionally, we were unable to evaluate the relative contribution of MRI versus mammography in this retrospective study, because all patients had both tests at presentation and the majority had both after NAC. Mammography clearly provided important information regarding extent of resection in patients with cancer containing calcifications. The precise impact of loss of enhancement on MRI or identification of contiguous versus scattered tumor growth on the decision to perform BCT could not be quantitated in this study. However, the primary point of our study is that MRI and mammography in combination identify patients who can undergo BCT with a high degree of reliability.
In conclusion, our study demonstrates that MRI is a useful imaging modality to predict the feasibility of breast conservation in patients after NAC. However, posttreatment mammography also is necessary to identify the presence and extent of any residual malignant-appearing calcifications for accurate localization before breast conservation. More studies are needed to validate these findings.
ACKNOWLEDGMENT
This study was funded in part through NIH/NCI Cancer Center Support Grant No. P30 CA008748.
Footnotes
CONFLICT OF INTEREST None.
REFERENCES
- 1.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181(5):1275–1282. doi: 10.2214/ajr.181.5.1811275. [DOI] [PubMed] [Google Scholar]
- 6.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184(3):868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 7.Carey LA, Berry DA, Cirrincione C, et al. CALGB 40601: Phase III Trial of Lapatinib Added to Neoadjuvant Therapy of HER-2+ Breast Cancer. J Clin Oncol. 2013;31(Suppl) abstr 500. [Google Scholar]
- 8.Sikov WM, Berry DA, Perou CM, et al. Addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer. J Clin Oncol. 2015;1:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Breast Cancer Version 3.2014. Preoperative Systemic Therapy Guideline. J Natl Compr Canc Netw. 2014;12(4):544–546. [Google Scholar]
- 11.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263(3):663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y, Chen XS, Liu SY, Shen KW. Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a meta-analysis. AJR Am J Roentgenol. 2010;195(1):260–268. doi: 10.2214/AJR.09.3908. [DOI] [PubMed] [Google Scholar]
- 13.Straver ME, Loo CE, Rutgers EJ, et al. MRI-model to guide the surgical treatment in breast cancer patients after neoadjuvant chemotherapy. Ann Surg. 2010;251(4):701–707. doi: 10.1097/SLA.0b013e3181c5dda3. [DOI] [PubMed] [Google Scholar]
- 14.Julius T, Kemp SE, Kneeshaw PJ, Chaturvedi A, Drew PJ, Turnbull LW. MRI and conservative treatment of locally advanced breast cancer. Eur J Surg Oncol. 2005;31(10):1129–1134. doi: 10.1016/j.ejso.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Charehbili A, Wasser MN, Smit VT, et al. Accuracy of MRI for treatment response assessment after taxane- and anthracycline-based neoadjuvant chemotherapy in HER2-negative breast cancer. Eur J Surg Oncol. 2014;40(10):1216–1221. doi: 10.1016/j.ejso.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Chen JH, Bahri S, Mehta RS, et al. Breast cancer: evaluation of response to neoadjuvant chemotherapy with 3.0-T MR imaging. Radiology. 2011;261(3):735–743. doi: 10.1148/radiol.11110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119(10):1776–1783. doi: 10.1002/cncr.27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire KP, Hwang ES, Cantor A, et al. Surgical Patterns of Care in Patients with Invasive Breast Cancer Treated with Neoadjuvant Systemic Therapy and Breast Magnetic Resonance Imaging: Results of a Secondary Analysis of TBCRC 017. Ann SurgOncol. 2015;22:75–81. doi: 10.1245/s10434-014-3948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss A, Lee KC, Romero Y, et al. Calcifications on mammogram do not correlate with tumor size after neoadjuvant chemotherapy. Ann Surg Oncol. 2014;21(10):3310–3316. doi: 10.1245/s10434-014-3914-0. [DOI] [PubMed] [Google Scholar]