Abstract
Objective
Skin and musculoskeletal involvement are frequently present early in diffuse cutaneous systemic sclerosis (dcSSc). The current study examined the correlates for skin and musculoskeletal measures in a 1-year longitudinal observational study.
Methods
Patients with dcSSc were recruited at 4 US centers and enrolled in a 1-year study. Prespecified and standardized measures included physician and patient assessments of skin involvement, modified Rodnan skin score (MRSS), durometer score, Health Assessment Questionnaire disability index, serum creatine phosphokinase, tender joint counts, and presence/absence of tendon friction rubs, small joint contractures, and large joint contractures. Additionally, physician and patient global health assessments and health-related quality of life assessments were recorded. Correlations were computed among the baseline global assessments, skin variables, and musculoskeletal variables. Using the followup physician and patient anchors, effect sizes were calculated.
Results
A total of 200 patients were studied: 75% were women, mean ± SD age was 50.0 ± 11.9 years, and mean ± SD disease duration from first non–Raynaud’s phenomenon symptom was 1.6 ± 1.4 years. Physician global health assessment had large correlations with MRSS (r = 0.60) and physician-reported skin involvement visual analog scale in the last month (r = 0.74), whereas patient global assessment had large correlations with MRSS, the Short Form 36 health survey physical component scale, skin interference, and skin involvement in the last month (r = 0.37–0.72). Four of 9 skin variables had moderate to large effect sizes (0.51–1.09).
Conclusion
Physician and patient global assessments have larger correlations with skin measures compared to musculoskeletal measures. From a clinical trial perspective, skin variables were more responsive to change than musculoskeletal variables over a 1-year period, although both provide complementary information.
INTRODUCTION
Systemic sclerosis (SSc; scleroderma) is a connective tissue disease, hallmarks of which include thickening of the skin, vascular obliteration, and involvement of internal organ systems, including the cardiopulmonary, renal, and gastrointestinal systems (1). Diffuse cutaneous SSc (dcSSc) is the form of the disease that includes proximal skin thickening, earlier occurrence of more severe organ involvement, and association with high mortality and a significant impairment in health-related quality of life (HRQOL) (2–5).
Symptomatic musculoskeletal and skin disease are frequent manifestations of dcSSc and have detrimental impact on patients’ disease burden (6–10). For example, using a patient-reported symptom burden index, Kallen et al found that hand involvement and skin problems were reported as the second and third most burdensome symptoms (6). Bassel et al surveyed 464 patients with SSc and found that 5 of the 8 most frequently experienced symptoms were related to musculoskeletal or skin involvement (8). Other groups have found skin features to be among the most commonly mentioned SSc-related problems (9,10).
The Combined Response Index for Systemic Sclerosis (CRISS) study is a 200-patient, observational 1-year longitudinal cohort of patients with dcSSc and a disease duration of <5 years. CRISS seeks to develop a composite index for SSc by including various measurements of organ system involvement and function (11). Our goal was to develop a data-based approach to disease measurement, particularly in the context of future interventional trials. We used data from the CRISS cohort to 1) assess the correlates of baseline measures for skin and musculoskeletal involvement, and 2) evaluate the responsiveness to change of skin and musculoskeletal measures over 1 year.
PATIENTS AND METHODS
Patients
dcSSc was defined as skin thickening proximal, as well as distal, to the elbows or knees with or without involvement of the face and neck, and early disease was considered ≤5 years since the onset of the first sign or symptom of SSc, other than Raynaud’s phenomenon. The study was approved by the institutional review boards of the participating centers.
Outcome measures
The CRISS study included the core set outcome measures proposed through a consensus methodology as previously described (12). These measures cover 11 domains: skin, musculoskeletal, cardiac, pulmonary, gastrointestinal, renal, Raynaud’s phenomenon, digital ulcers, HRQOL and function, global health, and biomarkers.
Skin measures
Physician- and patient-reported measures
There were 3 physician and 4 patient assessments of skin involvement employed in the study. Both physicians and patients were asked to indicate “activity” of skin involvement in the last month and in the last year, respectively, on a scale of 0–10, where 0 indicated “not active” and 10 denoted “extremely active.” Physicians also provided an assessment of the skin severity on a scale of 1 (very mild) to 5 (very severe). These scales were created for CRISS as they were considered to be important for assessing activity and severity of skin involvement in early disease. Additionally, patients provided assessments of skin condition interference with daily activities in the last month and in the last year, respectively, recorded on a scale from 0 (indicating that the skin involvement “does not limit activity”) to 10 (“very severe limitation”).
Objective measures
The modified Rodnan skin score (MRSS) is a clinical measure of the extent and severity of skin thickening (13–15). Skin thickening is assessed in 17 body areas: fingers, hands, forearms, arms, feet, legs, and thighs (bilaterally), and face, chest, and abdomen (singularly) (16). Each area is scored from 0 to 3, with 0 representing normal skin and 3 being severe thickening. Cumulatively, MRSS ranges from 0 (no thickening) to 51 (severe thickening in all 17 areas) (15).
The durometer is a handheld device that measures the hardness of a surface. It has been used to measure skin hardness in patients with SSc and was found to be feasible, reliable, and responsive to change in a recent clinical trial (17). Durometer measurements in patients with SSc typically range from approximately 4 durometer units (DUs) for uninvolved skin to around 70 DUs for maximally involved skin (18). Durometry has been shown to have high correlation (r = 0.69) with MRSS in a pilot study and was included as an objective measure (17).
Musculoskeletal measures
Patient-reported measure
The Health Assessment Questionnaire (HAQ) disability index (DI) is a disease-specific, arthritis-targeted measure intended for assessing functional ability in arthritis (19). It is a self-administered 20-question instrument that assesses a patient’s level of functional ability and includes questions about both upper and lower extremities. The score is determined by summing the highest item score in each of the 8 domains and dividing the sum by 8, resulting in a score ranging from 0 (no disability) to 3 (severe disability) (20). Several studies have reported the reliability, validity, and prognostic value of the HAQ DI as a measure of musculoskeletal involvement in SSc (20–22).
Laboratory-reported measures
Serum creatine phosphokinase (CPK) was assessed using the local laboratory. In a subset of patients, antinuclear antibody, anticentromere antibody, and anti-Scl 70 antibody were recorded based on measurements by local laboratories.
Physical examination measures
The presence or absence of palpable tendon friction rubs (TFRs) was assessed at baseline and at 1-year and were coded as present/absent at each site (23). The sites included bilateral wrists, knees, ankles, as well as other sites where TFRs were noted during clinical examination (e.g., fingers). Small and large joint contractures were assessed bilaterally. Small joint contractures were evaluated in the fingers and wrists, and large joint contractures were assessed in the knees, elbows, and shoulders. Tender joint counts were evaluated bilaterally at the following joints: shoulders, elbows, wrists, metacarpophalangeals (as a group), proximal interphalangeals (as a group), hips, knees, ankles, and metatarsophalangeals.
Global health and HRQOL measures
We determined baseline global assessment of overall SSc using physician and patient assessments of health in the week prior to the study visit. In both cases, the patient or physician was asked to rate the patient’s overall health in the past week on a scale from 0 (excellent) to 10 (extremely poor). The generic HRQOL was evaluated using the Short Form 36 (SF-36) health survey physical component score (PCS) and the mental component score (MCS). The SF-36 has been previously validated for use in SSc (20). A modified Likert scale (transition health question) was employed for physicians and patients at the 1-year followup to determine the change in overall condition in the past year on a scale from 1 (“much better”) to 5 (“much worse”). Responses of 1 or 2 were considered an overall improvement, ratings of 4 or 5 were considered a decline in health, and a rating of 3 meant that there was no appreciable change in overall health.
Statistical analysis
We calculated summary statistics for all clinical and demographic variables collected on the subjects enrolled in the CRISS study. For the continuous variables we computed the mean, SD, and interquartile range (difference between the 75th and the 25th percentile). For the binary or discrete variables, we computed the percentage of patients satisfying a given condition.
To determine whether there was an association between the different skin and musculoskeletal variables, we computed Pearson’s (and when appropriate Spearman’s) correlations among the skin and the musculoskeletal variables. Pearson’s correlation coefficients were interpreted as proposed by Cohen: 0.0–0.10 indicates negligible correlation, 0.10–0.23 indicates a small correlation coefficient, 0.24–0.36 indicates a moderate correlation, and ≥0.37 indicates a large correlation coefficient (24).
For each skin and musculoskeletal variable we also evaluated responsiveness to change through the effect size (ES) using the transition health question (see Global health and HRQOL measures above). ES was calculated by deriving the mean change from baseline to followup for the group of patients whose SSc condition improved based on physician/ patient assessment and dividing it by the baseline SD. Cohen’s “rule-of-thumb” for interpreting ES is that a value of 0.20–0.49 represents a small change, 0.50–0.79 a medium change, and ≥0.80 a large change (25).
Finally, we considered logistic regression models for the log-odds of being improved according to physician and patient assessment, respectively. In each logistic regression, the log-odds were regressed on the change in each variable.
RESULTS
Outcomes
The CRISS study enrolled 200 participants with early dcSSc; 150 were women (75%), with a mean ± SD age of 50.0 ± 11.9 years, and a mean ± SD body mass index of 25.5 ± 5.5 kg/m2. The majority of the participants were white (79%) and reported non-Hispanic ethnicity (90%). The mean ± SD disease duration assessed from first non–Raynaud’s phenomenon sign or symptom was 1.6 ± 1.4 years. See Table 1 for additional details of the cohort.
Table 1.
Baseline characteristics of 200 patients with early diffuse systemic sclerosis*
| Baseline characteristic | No. | Value | IQR |
|---|---|---|---|
| Demographics | |||
| Age, years | 200 | 50.0 ± 11.9 | 42.9–37.6 |
| BMI, kg/m2 | 177 | 25.5 ± 5.5 | 21.6–28.5 |
| Disease duration, years | 193 | 1.6 ± 1.4 | 0.5–2.7 |
| Women, % | 150 | 75 | NA |
| Race, % | |||
| White | 157 | 79 | NA |
| African American | 18 | 9 | NA |
| Asian | 16 | 8 | NA |
| Other | 9 | 4 | NA |
| Hispanic | 19 | 10 | NA |
| Physician global assessment | 175 | 4.3 ± 2.2 | 3.0–6.0 |
| Patient global assessment | 177 | 3.9 ± 2.7 | 2.0–6.0 |
| SF-36 PCS | 174 | 37.9 ± 12.8 | 28.3–46.4 |
| SF-36 MCS | 174 | 44.2 ± 6.1 | 39.9–48.9 |
| Skin involvement | |||
| Physician reported | |||
| In the last month (0–10) | 183 | 4.0 ± 2.9 | 1.5–6.0 |
| In the last year (0–10) | 178 | 4.7 ± 3.0 | 2.0–7.0 |
| Skin severity (1–5) | 200 | 4.1 ± 1.5 | 3.0–5.0 |
| Patient reported | |||
| Skin condition interference with daily activities in last month (0–10) | 157 | 3.9 ± 3.2 | 1.0–7.0 |
| Skin condition interference with daily activities in last year (0–10) | 157 | 4.0 ± 3.2 | 1.0–7.0 |
| Skin involvement in the last month (0–10) | 171 | 3.3 ± 3.2 | 0.0–5.0 |
| Skin involvement in the last year (0–10) | 172 | 4.6 ± 3.3 | 2.0–7.3 |
| Physical examination | |||
| Modified Rodnan skin score | 200 | 20.6 ± 10.1 | 13.0–28.0 |
| Durometer, DU | 135 | 266.3 ± 66.6 | 219.2–307.9 |
| Skin progression rate | 193 | 63.8 ± 184.7 | 7.1–48.9 |
| Musculoskeletal | |||
| Patient reported | |||
| HAQ DI | 200 | 1.0 ± 0.8 | 0.1–1.5 |
| Laboratory/serology | |||
| Serum creatine phosphokinase, IU/liter | 161 | 167.1 ± 403.6 | 49.0–160.0 |
| ANA positive | 151 | 83 | – |
| Anticentromere positive | 103 | 12 | – |
| Anti-Scl 70 positive | 148 | 29 | – |
| Physical examination | |||
| Tendon friction rubs, % | 189 | 24 | NA |
| Small joint contractures, % | 182 | 52 | NA |
| Large joint contractures, % | 182 | 26 | NA |
| Tender joint count | 198 | 1.3 ± 2.7 | 0.0–1.8 |
Values are the mean ± SD unless indicated otherwise. IQR = interquartile range; BMI = body mass index; NA = not applicable; SF-36 = Short Form 36 health survey; PCS = physical component score; MCS = mental component score; DU = durometer units; HAQ = Health Assessment Questionnaire; DI = disability index; ANA = antinuclear antibody.
Patient and physician global assessments and HRQOL
The mean ± SD physician-reported global assessment of health (on a 0–10 scale) was 4.3 ± 2.2, while the mean ± SD patient-reported global assessment was 3.9 ± 2.7. The mean ± SD for the SF-36 PCS and MCS scores were 37.9 ± 12.8 and 44.2 ± 6.1, respectively, indicating a moderate to severe level of physical and mental well-being.
Skin involvement
Physician- and patient-reported assessments of skin involvement on a 0–10 visual analog scale (VAS) revealed that, on average, participants had moderate skin activity in the last year (Table 1). Mean ± SD baseline MRSS was 20.6 ± 10.1, while mean ± SD baseline durometer was 266.3 ± 66.6 DUs.
Musculoskeletal involvement
Mean ± SD baseline HAQ DI was 1.0 ± 0.8, mean ± SD baseline serum CPK was 167.1 ± 403.6 IU/liter, and mean ± SD number of tender joints was 1.3 ± 2.7. Twenty-four percent of the participants had tendon friction rubs, 26% had large joint contractures, and 52% had small joint contractures.
Correlation coefficients for skin and musculoskeletal variables
There was a large correlation (r = 0.43) between physician and patient global assessments at baseline. In addition, physician global assessment and patient global assessment had large (r = −0.53 and r = −0.72) negative correlations with SF-36 PCS, respectively. Both global assessments had negligible correlation with SF-36 MCS.
Skin measures
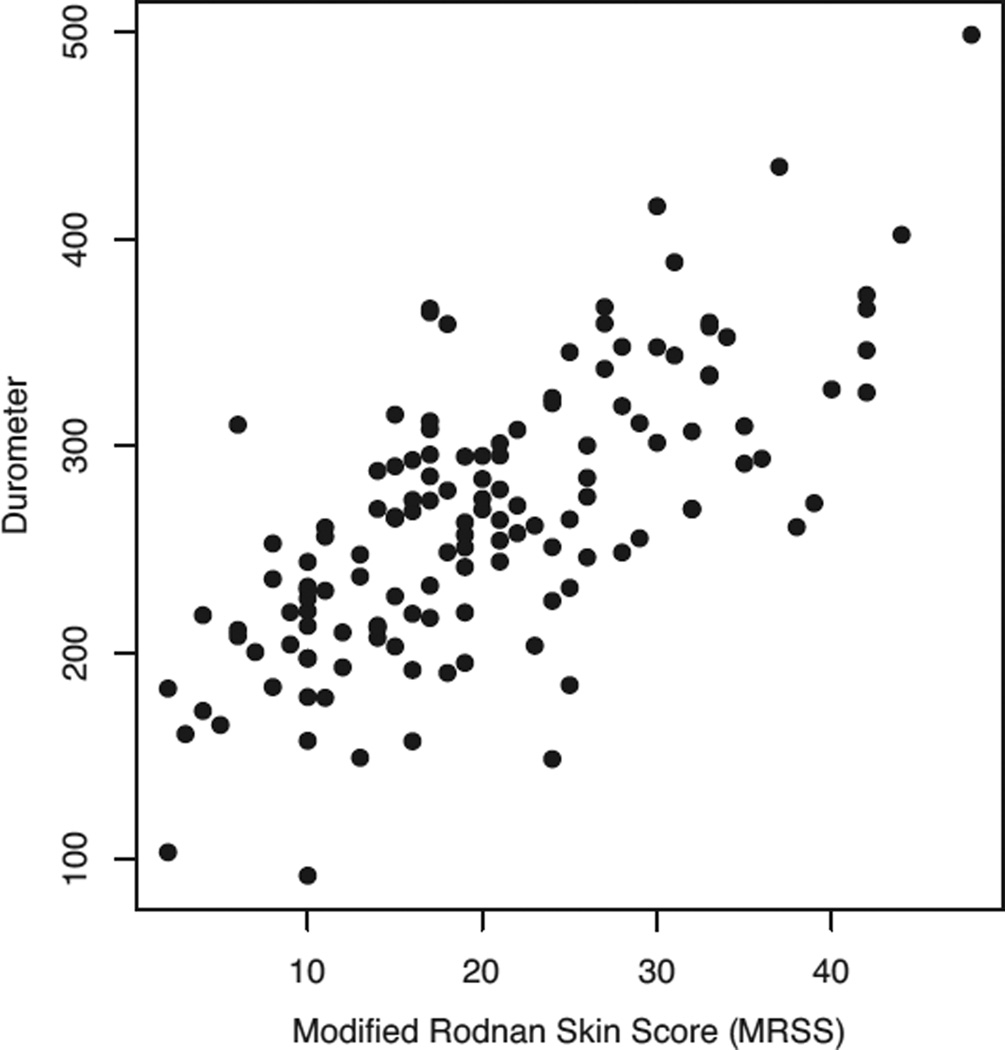
The physician global health assessment VAS had large correlations with the physician assessment of skin involvement in the last month VAS (r = 0.74) and with the patient reported skin involvement in the last month VAS (r = 0.44) (Table 2). MRSS had a large correlation with the physician-reported global health (r = 0.60), SF-36 PCS (r = −0.43), and many of the skin-related physician- and patient-reported variables, as well durometer readings (r = 0.69) (Figure 1). Other correlations are listed in Table 2.
Table 2.
Baseline correlates of skin variables*
| Skin variables | MD global health |
Pt. global health |
SF-36 PCS |
SF-36 MCS |
MD skin last month |
MD skin last year |
Skin severity |
Skin interference last month |
Skin interference last year |
Pt. skin last month |
Pt. skin last year |
MRSS | Durometer | Skin progression rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global assessment | ||||||||||||||
| Physician | – | 0.43 | −0.53 | 0.07 | 0.74 | 0.58 | 0.21 | 0.50 | 0.38 | 0.44 | 0.30 | 0.60 | 0.33 | 0.58 |
| Patient | 0.43 | – | −0.72 | 0.05 | 0.32 | 0.23 | 0.17 | 0.54 | 0.37 | 0.51 | 0.34 | 0.32 | 0.15 | 0.33 |
| SF-36 PCS | −0.53 | −0.72 | – | −0.35 | 0.38 | −0.29 | −0.22 | −0.62 | −0.53 | −0.57 | −0.44 | −0.43 | −0.21 | −0.35 |
| SF-36 MCS | 0.07 | 0.05 | −0.35 | – | 0.05 | 0.02 | 0.01 | 0.08 | 0.13 | 0.14 | 0.04 | 0.15 | 0.21 | 0.05 |
| Physician skin | ||||||||||||||
| Last month | 0.74 | 0.32 | −0.38 | 0.05 | – | 0.69 | 0.24 | 0.45 | 0.32 | 0.44 | 0.31 | 0.58 | 0.44 | 0.60 |
| Last year | 0.58 | 0.23 | −0.29 | 0.02 | 0.69 | – | 0.24 | 0.31 | 0.23 | 0.40 | 0.40 | 0.54 | 0.35 | 0.59 |
| Skin severity | 0.21 | 0.17 | −0.22 | 0.01 | 0.24 | 0.24 | – | 0.33 | 0.23 | 0.22 | 0.18 | 0.44 | 0.33 | 0.22 |
| Patient | ||||||||||||||
| Skin interference with ADLs | ||||||||||||||
| Last month | 0.50 | 0.54 | −0.62 | 0.08 | 0.45 | 0.31 | 0.33 | – | 0.79 | 0.63 | 0.46 | 0.52 | 0.36 | 0.29 |
| Last year | 0.38 | 0.37 | −0.53 | 0.13 | 0.32 | 0.23 | 0.23 | 0.79 | – | 0.44 | 0.57 | 0.45 | 0.33 | 0.13 |
| Skin involvement | ||||||||||||||
| Last month | 0.44 | 0.51 | −0.57 | 0.14 | 0.44 | 0.40 | 0.22 | 0.63 | 0.44 | – | 0.59 | 0.47 | 0.28 | 0.48 |
| Last year | 0.30 | 0.34 | −0.44 | 0.04 | 0.31 | 0.40 | 0.18 | 0.46 | 0.57 | 0.59 | – | 0.33 | 0.13 | 0.45 |
| Physical examination | ||||||||||||||
| MRSS | 0.60 | 0.32 | −0.43 | 0.15 | 0.58 | 0.54 | 0.44 | 0.52 | 0.45 | 0.47 | 0.33 | – | 0.69 | 0.53 |
| Durometer | 0.33 | 0.15 | −0.21 | 0.21 | 0.44 | 0.35 | 0.33 | 0.36 | 0.33 | 0.28 | 0.13 | 0.69 | – | 0.25 |
| Skin progression rate | 0.58 | 0.33 | −0.35 | 0.05 | 0.60 | 0.59 | 0.22 | 0.29 | 0.13 | 0.48 | 0.45 | 0.53 | 0.25 | – |
MD = physician; Pt. = patient; SF-36 = Short Form 36 health survey; PCS = physical component score; MCS = mental component score; MRSS = modified Rodnan skin score; ADLs = activities of daily living.
Figure 1.
Pairwise scatterplot of modified Rodnan skin scores and durometer scores (correlation: r = 0.69).
Musculoskeletal measures
There were large correlations between HAQ DI and SF-36 PCS (r = −0.79), physician global health assessment and baseline large joint contractures (r = 0.39), and between both physician and patient global health assessments and HAQ DI (r = 0.43 and r = 0.57, respectively). There were small to moderate correlations between the remaining baseline musculoskeletal variables and the global health assessments (Table 3).
Table 3.
Baseline correlates of musculoskeletal variables*
| Musculoskeletal variables | Physician global health |
Patient global health |
SF-36 PCS |
SF-36 MCS |
HAQ DI | CPK | Tendon friction rubs |
Small joint contractures |
Large joint contractures |
Tender joint count |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient reported | ||||||||||
| HAQ DI | 0.43 | 0.57 | −0.79 | 0.29 | – | 0.06 | 0.25 | 0.22 | 0.36 | 0.23 |
| Laboratory | ||||||||||
| Serum CPK | 0.19 | 0.13 | −0.15 | 0.06 | 0.04 | – | 0.29 | 0.00 | 0.14 | 0.01 |
| Physical examination | ||||||||||
| Tendon friction rubs | 0.36 | 0.21 | −0.24 | 0.09 | −0.06 | 0.29 | – | 0.18 | 0.21 | 0.10 |
| Small joint contractures | 0.36 | 0.13 | −0.19 | 0.09 | 0.07 | 0.00 | 0.18 | – | 0.50 | 0.20 |
| Large joint contractures | 0.39 | 0.28 | −0.29 | −0.01 | 0.07 | 0.14 | 0.21 | 0.50 | – | 0.14 |
| Tender joint count | 0.31 | 0.21 | −0.32 | 0.00 | 0.09 | 0.01 | 0.10 | 0.20 | 0.14 | – |
SF-36 = Short Form 36 health survey; PCS = physical component score; MCS = mental component score; HAQ = Health Assessment Questionnaire; DI = disability index; CPK = creatine phosphokinase.
Responsiveness to change
One-year data were available for 150 of the 200 study participants. Based on the physician assessment for change in overall SSc condition in the previous year, 58.6% of patients were categorized as improved, 26.9% as worsened, and 14.4% as unchanged. The patients’ assessments of change in health over 1 year revealed that 56.7% believed that the overall condition of their SSc improved, 26.8% reported that their condition declined, and 16.5% responded that their condition stayed the same.
Physician assessments of skin involvement in the past month and year, respectively, and MRSS had medium ES (0.51– 0.66) (Table 4). Physician assessment of overall skin severity had a large ES (0.83–1.09). For musculoskeletal variables, the ES were negligible (HAQ DI [0.07– 0.10]) to small (0.23– 0.33) (Table 4).
Table 4.
Responsiveness to change of skin and musculoskeletal variables over 1 year (effect size)*
| Variable | Physician anchor |
Patient anchor |
|---|---|---|
| Skin | ||
| Physician reported | ||
| Skin involvement in last month | −0.66 | −0.51 |
| Skin involvement in last year | −0.56 | −0.54 |
| Skin severity | −1.09 | −0.83 |
| Patient reported | ||
| Skin condition interference with daily activities in last month | −0.53 | −0.34 |
| Skin condition interference with daily activities in last year | −0.38 | −0.26 |
| Skin involvement in last month | −0.12 | −0.22 |
| Skin involvement in last year | 0.03 | −0.03 |
| Physical examination | ||
| Modified Rodnan skin score | −0.58 | −0.65 |
| Durometer | −0.02 | −0.25 |
| Musculoskeletal | ||
| Patient reported | ||
| HAQ DI | −0.10 | −0.07 |
| Laboratory | ||
| Serum creatine phosphokinase | −0.23 | −0.26 |
| Physical examination Tender joint count | −0.33 | −0.31 |
Small joint contractures, large joint contractures, and tendon friction rubs are not included as they are binary variables. HAQ = Health Assessment Questionnaire; DI = disability index.
Of the objective outcome measures, 3 items are measures of disease activity, defined as items that are reversible (either with treatment or spontaneously), i.e., serum CPK, tendon friction rubs, and tender joint count (26). Other objective measures, such as MRSS and durometer, assess severity (combination of activity and damage). Measures of activity were not more responsive than measures of severity (Table 4).
Logistic regression based on physician and patient assessments of improvement
In the univariate models, improvements in physician global assessment, MRSS, physician- reported skin severity, and physician evaluation of skin involvement in the last month are significantly associated with the odds of being improved as rated by physician. As an example, for a 1-unit increase in MRSS from baseline to the 1-year followup, there is a 6% decrease in the odds that the patient is rated improved by a physician (Table 5).
Table 5.
Logistic regression for the log-odds of being improved according to physician or patient assessment*
| Change in characteristic | Physician assessment, OR (95% CI) |
Patient assessment, OR (95% CI) |
|---|---|---|
| Physician global assessment | 0.65 (0.51–0.82)† | 0.68 (0.53–0.88)† |
| Physician skin involvement last month | 0.76 (0.63–0.92)† | 0.81 (0.66–0.99)† |
| Physician skin involvement last year | 0.86 (0.71–1.03) | 0.87 (0.71–1.07) |
| Skin severity (physician reported) | 0.65 (0.48–0.88)† | 0.86 (0.65–1.14) |
| SF-36 PCS | 1.07 (1.01–1.14)† | 1.03 (0.98–1.07) |
| SF-36 MCS | 0.98 (0.92–1.05) | 1.02 (0.96–1.09) |
| Durometer | 1.00 (0.99–1.00) | 0.99 (0.98–1.00) |
| MRSS | 0.94 (0.89–0.99)† | 0.88 (0.81–0.95)† |
| HAQ DI | 0.56 (0.27–1.17) | 0.69 (0.34–1.36) |
| CPK | 1.00 (0.99–1.00) | 0.99 (0.98–1.00)† |
| Total joint count | 0.97 (0.83–1.12) | 1.00 (0.86–1.17) |
| Patient-reported skin involvement last month | 0.92 (0.79–1.06) | 1.02 (0.89–1.17) |
| Patient-reported skin involvement last year | 0.96 (0.85–1.09) | 1.04 (0.92–1.18) |
| Skin interference with daily activities last month | 0.97 (0.79–1.19) | 0.87 (0.71–1.07) |
| Skin interference with daily activities last year | 1.01 (0.87–1.18) | 0.97 (0.83–1.13) |
OR = odds ratio; 95% CI = 95% confidence interval; SF-36 = Short Form 36 health survey; PCS = physical component score; MCS = mental component score; MRSS = modified Rodnan skin score; HAQ = Health Assessment Questionnaire; DI = disability index; CPK = creatine phosphokinase.
P < 0.05.
When considering patient self-assessment of disease at 1-year followup, our analysis revealed a significant association between the odds that the patient rated himself/herself as improved and improvements in physician global assessment, physician assessment of skin involvement last month, MRSS, and CPK. In particular, for a 1-unit increase in MRSS from baseline to 1-year followup there is a 12% decrease in the odds that the patient considered himself or herself as improved.
DISCUSSION
Diffuse cutaneous SSc is associated with poor HRQOL and high mortality, with skin and musculoskeletal symptoms being of particular importance to patients with this disease (4–6,9,27). There is a need to carefully evaluate the outcome measures used in clinical trials of dcSSc (11). This 1-year observational study found that physician global assessment of health correlates with objective measurements of skin involvement in addition to many other physician- and patient-reported assessments, while patient global health assessment has large correlations with patient- reported skin interference in daily activities and the PCS of the SF-36 questionnaire. In addition, MRSS and physician- and patient-reported skin variables were responsive to change. For musculoskeletal variables, only serum CPK and tender joint count showed responsiveness to change while contractures did not change. However, the musculoskeletal measures were less responsive than skin measures.
Hudson et al evaluated 803 patients with SSc and also reported that physician assessments of the overall disease condition in SSc patients are associated with objective skin measures, while patient assessments of overall disease are influenced by more subjective factors such as pain, fatigue, gastrointestinal symptoms, and other manifestations that affect HRQOL (28). The influence of objective skin symptoms on physician assessments of overall disease is likely due to evidence in the literature showing that, for dcSSc, skin involvement is predictive of mortality and is associated with internal organ involvement (22). Also, the use of MRSS is common as the primary/secondary outcome measure in multiple clinical trials (14,29,30). These findings, along with our findings on the responsiveness to change, support the conclusion that MRSS is a good indicator of improvement or progression in SSc and is a suitable measure for use in clinical trials. In addition, durometer measurement was found to be feasible, as 68% of participants had a baseline evaluation in this multicenter cohort, which is consistent with a previous clinical trial (17). In the current study, there was a large correlation (r = 0.69) between durometer and MRSS at baseline.
In general, skin measures had higher correlations with patient and physician measures of global health and were more responsive to change compared to musculoskeletal measures. In addition, physician assessment of global health correlated more highly with physician-reported skin involvement in the last month and MRSS. However, patient global assessment had large correlation with patient-reported skin condition interference and moderate correlation with MRSS, suggesting that, while objective skin involvement and severity has a greater effect on physician assessment of disease, skin interference with daily life and MRSS are both important for the patient.
Previous studies have suggested that musculoskeletal involvement is concerning to patients with dcSSc (8,31). For example, Clements et al found a significant correlation between HAQ DI and various musculoskeletal symptoms, including hand problems, small joint contractures, and tendon friction rubs (22). Change in tendon friction rubs has also been shown to predict change in HAQ DI (23). However, our data suggest that both physicians and patients consider skin involvement and impact of skin on day-to-day activity as contributing more to overall disease assessment than musculoskeletal involvement. The HAQ DI was the only variable with moderate correlations with physician and patient global assessments. MRSS and HAQ DI have a large correlation of 0.39, a finding consistent with a prior report (22).
The ability of HRQOL instruments to detect clinically important changes is crucial to their usefulness in determining the effectiveness of different therapies (20,32). The magnitude of responsiveness as measured by these instruments is useful in assessing treatment efficiency and assessing sample size for future trials. Responsiveness to change was larger for skin variables compared to musculoskeletal variables suggesting that skin variables (both objective and subjective) are better outcome measures for clinical trials.
The CRISS cohort is generally representative of patients enrolled in large multicenter random controlled trials (RCTs) of dcSSc when compared to the combined data from 3 large RCTs in dcSSc (33). The combined trial population had a similar mean age (48 years versus 50 years in CRISS), disease duration (27 months versus 19 months in CRISS), MRSS (25.3 versus 20.6 units in CRISS), tender joint count (1.3 versus 1.3 in CRISS), HAQ DI (1.2. versus 1.0 in CRISS), and physician global assessment (4.7 versus 4.3).
Our study has several strengths. First, it provides data on 200 patients with early dcSSc collected at 4 expert scleroderma centers. Second, it carefully evaluated outcome measures endorsed by experts in SSc via an international Delphi and nominal group technique (12). Third, we employed anchors to assess responsiveness to change for the outcome measures.
The current study also has some limitations. First, information about treatment was not collected at baseline or at followup. Treatment is a possible confounder for the current analyses since patients with more severe symptoms at baseline might have been treated more aggressively, resulting in a greater improvement over the 1-year study. However, the effect of treatment is beyond the scope of this analysis. The purpose of the current study was to assess performance of skin and musculoskeletal variables independent of treatment. Second, evaluations were performed at baseline and 1 year with no intervening evaluations, which did not allow time-series analysis; however, the correlations and anchors allowed us to ascertain responsiveness despite this. Third, the present data apply only to relatively early dcSSc and do not address the utility of these variables and their relation to other outcomes in patients with later, atrophic dcSSc nor to those with limited SSc.
In conclusion, in a multicenter early dcSSc cohort we found that physician global assessment and patient global assessment are associated with both objective (MRSS) and subjective assessments of skin severity and interference with skin involvement, although the strength of associations was different. Both assessments accounted for physical disability as assessed by the SF-36 PCS and HAQ DI. Our data offer strong support for the use of MRSS as an outcome measure in dcSSc. Other measures will likely apply in other clinical circumstances.
Significance & Innovations.
In an early diffuse cutaneous systemic sclerosis (dcSSc) population, patient and physician global assessments of disease have greater correlations with skin variables than with musculoskeletal variables.
The current study supports the use of different patient-reported and objective measures (such as modified Rodnan skin score, skin involvement in the last month, and the Health Assessment Questionnaire disability index) as clinical outcome measures in early dcSSc.
ACKNOWLEDGMENTS
The authors wish to acknowledge the participation of the following investigators at each site: Philip J. Clements (University of California, Los Angeles); Kristine Phillips, Elena Schiopu (University of Michigan); Robert Simms (Boston University); and Shervin Assassi (University of Texas at Houston), and would also like to thank the coordinators at each site who helped with successful completion of the study.
Supported by the NIH/National Institute for Arthritis and Musculoskeletal and Skin Disease (grant UO1-AR-055057). Ms Wiese’s work was supported by the Rheumatology Research Foundation Medical Student Research Preceptorship. Drs. Berrocal and Khanna’s work was supported by the NIH/National Institute for Arthritis and Musculoskeletal and Skin Disease (grant K24-AR-063120-02).
Dr. Seibold has received consultant fees, speaking fees, and/or honoraria (less than $10,000 each) from Intermune, EMD Serono, Biogene Idec, Roche, and (more than $10,000 each) from DART Therapeutics and Sigma Tau. Dr. Mayes has received consultant fees, speaking fees, and/or honoraria (less than $10,000) from Actelion. Dr. Khanna has received consultant fees, speaking fees, and/or honoraria (less than $10,000 each) from Actelion, Bayer, Biogene Idec, BMS, Digna, Genentech/Roche, Intermune, Merck, and Sanofi-Aventis/Genzyme.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Khanna had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Wiese, Berrocal, Furst, Seibold, Merkel, Mayes, Khanna.
Acquisition of data. Seibold, Merkel, Mayes, Khanna.
Analysis and interpretation of data. Wiese, Berrocal, Furst, Seibold, Merkel, Mayes, Khanna.
REFERENCES
- 1.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 3.Mura G, Bhat KM, Pisano A, Licci G, Carta M. Psychiatric symptoms and quality of life in systemic sclerosis. Clin Pract Epidemiol Ment Health. 2012;8:30–35. doi: 10.2174/1745017901208010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danieli E, Airo P, Bettoni L, Cinquini M, Antonioli CM, Cavazzana I, et al. Health-related quality of life measured by the Short-Form 36 (SF-36) in systemic sclerosis: correlations with indexes of disease activity and severity, disability, and depressive symptoms. Clin Rheumatol. 2005;24:48–54. doi: 10.1007/s10067-004-0970-z. [DOI] [PubMed] [Google Scholar]
- 5.Del Rosso A, Boldrini M, D’Agostino D, Placidi GP, Scarpato A, Pignone A, et al. Health-related quality of life in systemic sclerosis as measured by the Short Form 36: relationship with clinical and biologic markers. Arthritis Rheum. 2004;51:475–481. doi: 10.1002/art.20389. [DOI] [PubMed] [Google Scholar]
- 6.Kallen MA, Mayes MD, Kriseman YL, de Achaval SB, Cox VL, Suarez-Almazor ME. The symptom burden index: development and initial findings from use with patients with systemic sclerosis. J Rheumatol. 2010;37:1692–1698. doi: 10.3899/jrheum.090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Achaval S, Kallen MA, Mayes MD, Lopez-Olivo MA, Suarez-Almazor ME. Use of the patient-generated index in systemic sclerosis to assess patient-centered outcomes. J Rheumatol. 2013;40:1337–1343. doi: 10.3899/jrheum.120978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology (Oxford) 2011;50:762–767. doi: 10.1093/rheumatology/keq310. [DOI] [PubMed] [Google Scholar]
- 9.Van Lankveld WG, Vonk MC, Teunissen H, van der Hoogen FH. Appearance self-esteem in systemic sclerosis: subjective experience of skin deformity and its relationship with physician- assessed skin involvement, disease status and psychological variables. Rheumatology (Oxford) 2007;46:872–876. doi: 10.1093/rheumatology/kem008. [DOI] [PubMed] [Google Scholar]
- 10.Suarez-Almazor ME, Kallen MA, Roundtree AK, Mayes M. Disease and symptom burden in systemic sclerosis: a patient perspective. J Rheumatol. 2007;34:1718–1726. [PubMed] [Google Scholar]
- 11.Khanna D, Distler O, Avouac J, Behrens F, Clements PJ, Denton C, et al. Measures of response in clinical trials of systemic sclerosis: the Combined Response Index for Systemic Sclerosis (CRISS) and Outcome Measures in Pulmonary Arterial Hypertension Related to Systemic Sclerosis (EPOSS) J Rheumatol. 2009;36:2356–2361. doi: 10.3899/jrheum.090372. [DOI] [PubMed] [Google Scholar]
- 12.Khanna D, Lovell DJ, Giannini E, Clements PJ, Merkel PA, Seibold JR, et al. Development of a provisional core set of response measures for clinical trials of systemic sclerosis. Ann Rheum Dis. 2008;67:703–709. doi: 10.1136/ard.2007.078923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements PJ, Lachenbruch P, Siebold JR, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 14.Khanna D, Merkel PA. Outcome measures in systemic sclerosis: an update on instruments and current research. Curr Rheumatol Rep. 2007;9:151–157. doi: 10.1007/s11926-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 15.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus lowdose penicillamine trial. Arthritis Rheum. 2000;43:2445–2454. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Amjadi S, Maranian P, Furst DE, Clements PJ, Wong WK, Postlethwaite AE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60:2490–2498. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkel PA, Silliman NP, Denton CP, Furst DE, Khanna D, Emery P, et al. for the CAT-192 Research Group and the Scleroderma Clinical Trials Consortium. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 2008;59:699–705. doi: 10.1002/art.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kissin EY, Schiller AM, Gelbard RB, Anderson JJ, Falanga V, Simms RW, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006;55:603–609. doi: 10.1002/art.22093. [DOI] [PubMed] [Google Scholar]
- 19.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 20.Cole JC, Khanna D, Clements PJ, Seibold JR, Tashkin DP, Paulus HE, et al. Single-factor scoring validation for the Health Assessment Questionnaire-disability index (HAQ-DI) in patients with systemic sclerosis and comparison with early rheumatoid arthritis patients. Qual Life Res. 2006;15:1383–1394. doi: 10.1007/s11136-006-0018-8. [DOI] [PubMed] [Google Scholar]
- 21.Khanna D, Furst DE, Clements PJ, Park GS, Hays RD, Yoon J, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire disability index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32:832–840. [PubMed] [Google Scholar]
- 22.Clements PJ, Wong WK, Hurwitz EL, Furst DE, Mayes M, White B, et al. The disability index of the Health Assessment Questionnaire is a predictor and correlate of outcome in the high-dose versus low-dose penicillamine in systemic sclerosis trial. Arthritis Rheum. 2001;44:653–661. doi: 10.1002/1529-0131(200103)44:3<653::AID-ANR114>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Khanna PP, Furst DE, Clements PJ, Maranian P, Indulkar L, Khanna D. Tendon friction rubs in early diffuse systemic sclerosis: prevalence, characteristics and longitudinal changes in a randomized controlled trial. Rheumatology (Oxford) 2010;49:955–959. doi: 10.1093/rheumatology/kep464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Erlbaum; 1988. [Google Scholar]
- 25.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Medsger TA, Jr, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis [review] Clin Exp Rheumatol. 2003;(Suppl 29):S42–S46. [PubMed] [Google Scholar]
- 27.Hudson M, Steele R, Lu Y, Thombs BD, Panopalis P, Baron M. Clinical correlates of self-reported physical health status in systemic sclerosis. J Rheumatol. 2009;36:1226–1229. doi: 10.3899/jrheum.081057. [DOI] [PubMed] [Google Scholar]
- 28.Hudson M, Impens A, Baron M, Seibold JR, Thombs BD, Walker JG, et al. Discordance between patient and physician assessments of disease severity in systemic sclerosis. J Rheumatol. 2010;37:2307–2312. doi: 10.3899/jrheum.100354. [DOI] [PubMed] [Google Scholar]
- 29.Au K, Mayes MD, Maranian P, Clements PJ, Khanna D, Steen VD, et al. Course of dermal ulcers and musculoskeletal involvement in systemic sclerosis patients in the scleroderma lung study. Arthritis Care Res (Hoboken) 2010;62:1772–1778. doi: 10.1002/acr.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna D, Clements PJ, Furst DE, Korn JH, Ellman M, Rothfield N, et al. for the relaxin investigators and the Scleroderma Clinical Trials Consortium. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:1102–1111. doi: 10.1002/art.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steen VD. Clinical manifestations of systemic sclerosis. Semin Cutan Med Surg. 1998;17:48–54. doi: 10.1016/s1085-5629(98)80062-x. [DOI] [PubMed] [Google Scholar]
- 32.Khanna D, Tsevat J. Health-related quality of life: an introduction [review] Am J Manag Care. 2007;9:S218–S223. [PubMed] [Google Scholar]
- 33.Gladue H, Furst DE, Berrocal V, Seibold JR, Merkel PA, Mayes MD, et al. Comparison of baseline characteristics of the Combined Response Index for Systemic Sclerosis (CRISS) cohort to patients enrolled in clinical trials of diffuse systemic sclerosis [abstract] Arthritis Rheum. 2012;64:1464. [Google Scholar]