Abstract
Objective
To investigate the safety and efficacy of oral bovine type I collagen (CI) treatment in patients who have had diffuse cutaneous systemic sclerosis (dc-SSc; scleroderma) for ≤10 years.
Methods
One hundred sixty-eight patients with dcSSc were enrolled in a double-blind, placebo-controlled trial of oral CI (500 µg/day) or placebo administered over 12 months, with a followup visit at month 15. The primary outcome was the modified Rodnan skin thickness score (MRSS). Other clinical and immune system parameters were also assessed.
Results
Intent-to-treat and modified intent-to-treat analyses showed that for the total population of patients with dcSSc, there were no significant differences in the mean change in MRSS or other key clinical parameters between the CI and placebo treatment groups at 12 months or at 15 months. However, in a subanalysis of the available data at month 15, the CI-treated group of patients with late-phase dcSSc experienced a significant reduction in the MRSS compared with that in the placebo-treated patients with late-phase dcSSc (change in MRSS at month 15 –7.9 versus −2.9; P = 0.0063).
Conclusion
Although the results from this trial did not meet the primary outcome goals, the findings from exploratory analyses indicated that CI treatment may benefit patients with late-phase dcSSc. This new treatment strategy and preliminary clinical observations in patients with dcSSc need to be corroborated.
Systemic sclerosis (SSc; scleroderma) is an autoimmune disease that is associated with altered immune responses as well as widespread fibrosis (1,2). In the skin, lungs, and other organs, the infiltrating cells consist of activated T cells, plasma cells, mast cells, and monocytes/macrophages, and studies have indicated that T cells undergo clonal expansion in response to an antigen that is persistently present and widely distributed (1–11). A variety of autoantigens have been identified in patients with SSc, including type I collagen (CI), the most abundant protein in the body and a component of all tissues, organs, and blood vessels involved in the disease processes of SSc (1,12–17).
Studies in animal models of autoimmune diseases have resulted in successful treatment with orally administered autoantigens. Moreover, bovine myelin basic protein, type II collagen, and bovine retinal antigen have been safely administered orally in clinical trials involving treatment of patients with multiple sclerosis, those with rheumatoid arthritis, and those with uveoretinitis, respectively (18–24). This prompted us to use a similar strategy with oral bovine CI in patients with diffuse cutaneous SSc (dcSSc).
In this trial, we sought to induce oral immune tolerance to CI and to assess the ability of oral CI to improve the modified Rodnan skin thickness score (MRSS) in patients with dcSSc. The effects of oral CI on other clinical outcome variables were also assessed. A number of immunologic parameters were measured in all patients at baseline and at 12 months. In the present report, we describe the effects of this treatment on the MRSS and other clinical outcomes; data related to immune responses will be presented in a separate report.
PATIENTS AND METHODS
Study design
The study was a 12-center, randomized, double-blind, placebo-controlled clinical trial conducted under the oversight of a Data Safety and Monitoring Board and with approval from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the US Food and Drug Administration (FDA), and the Institutional Review Boards of all participating centers.
One hundred sixty-eight eligible male and female patients with dcSSc whose disease duration was ≤3 years (early-phase dcSSc) or >3–10 years (late-phase dcSSc) were enrolled in the study. All patients were age ≥18 years, had a clinical diagnosis of dcSSc according to the American College of Rheumatology (formerly, the American Rheumatism Association) 1980 criteria (25), and had an MRSS of ≥16 at screening that had remained stable during the 6 months preceding enrollment, as determined by history review or physical examination. Use of immunomodulatory therapies, herbal therapies, megavitamins, or nonsteroidal antiinflammatory drugs was not allowed.
Eligible patients were assigned to a treatment group using computer-generated random numbers. The patients were randomized to receive either oral CI (500 µg in 0.1N acetic acid [HAc]) or an identical-appearing placebo (HAc alone) daily. The dose and duration of treatment for this Phase II trial were based on data obtained from an earlier Phase I study in which it was demonstrated that maximal immune tolerance was induced after 6 months of oral administration of 500 µg/day bovine CI in patients with SSc (13). The study medication (CI or placebo) was continued for 12 months. All patients, whether they completed the trial or withdrew early, were requested to return for assessment at 12 months. Patients were also required to return at 15 months for safety and efficacy assessments.
The principal clinical outcome variable was the MRSS, which was determined at 0, 4, 8, 12, and 15 months. Secondary outcomes included 1) the forced vital capacity (FVC) and the diffusing capacity for carbon monoxide (DLco), both expressed as the percent predicted and determined at 0 and 12 months; 2) the serum creatinine level, determined at 0, 4, 8, and 12 months; and 3) the disability index of the Health Assessment Questionnaire (26,27), physician’s and patient’s global assessments of health and patient’s pain assessment on 100-mm visual analog scales (VAS), patient’s blood pressure (BP) and weight, and the 36-item Short Form (SF-36) health survey (28), each determined at 0, 4, 8, 12, and 15 months. The 8-scale SF-36 version 2 with standard (4-week) recall period was used (28). In the SF-36, the physical component summary score and mental component summary score are standardized to the values in the general US population (a mean of 50 and an SD of 10). Our main outcome for measuring efficacy was change in the MRSS from baseline to 1 year in the CI-treated group compared with the placebo-treated group, calculated by subtracting the MRSS values at baseline from the values at the 12-month visit.
The MRSS is an assessment of the extent of skin thickness in 17 body areas, on a scale of 0–3 (i.e., 0 = normal, 1 = mild skin thickness, 2 = moderate skin thickness, and 3 = severe skin thickness; maximum score 51). A prestudy training session was held with all examiners to standardize the techniques used to measure the MRSS. The documented coefficient of variation for the MRSS is 12% for intraobserver reliability and 25% for interobserver variability (29,30). Pulmonary function tests were conducted by pulmonologists who met the American Thoracic Society (ATS) 1978 recommendations for pulmonary function laboratory directors, which require that the pulmonary function technician be certified by the National Board of Respiratory Care or meets the ATS recommendations for personnel qualification. All equipment and procedures used to assess pulmonary function were in accordance with the ATS 1994 criteria for standardization of spirometry (31,32). The FVC was measured until the best agreement between 2 measurements, i.e., having a value within 5% of each other, was reached. The DLco was determined until results were within 10% (±3 ml CO) of each other, according to the ATS 1995 guidelines (31,32).
Safety monitoring was performed at screening, baseline, 4, 8, 12, and 15 months. In addition to determining the occurrence of adverse events from patients’ responses to open-ended questions, we carried out laboratory tests, including a complete blood cell count and measurements of serum albumin, globulin, alkaline phosphatase, alanine leucine transaminase, alanine serine transaminase, bilirubin, cholesterol, creatinine, blood urea nitrogen, glucose, calcium, phosphate, sodium, chloride, potassium, and bicarbonate. A urine test for pregnancy was performed in all fertile female patients at baseline and at the 12-month visit.
Preparation of native bovine CI and placebo
Native bovine CI, which was isolated from the skin of fetal calves, and placebo (HAc) were prepared under US FDA guidelines (Investigational New Drug 6575) as we have previously described (13). The native CI was maintained in HAc and stored at −80°C until used. The homogeneity of CI was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, which showed an α1(I):α2(I) ratio of 2:1 with no contaminating type III or type V collagens.
Study participants were required to store the CI preparation or placebo in a 4–8°C refrigerator. For each daily treatment, the patients were required to withdraw 2 ml into a sterile, individually packaged, polypropylene, 2-ml calibrated pipette. The treatment was then added to 4–6 ounces of cold orange juice just prior to ingestion, each morning before eating breakfast.
Statistical analysis
The primary outcome variable was change in the MRSS following treatment with CI or placebo from baseline to 12 months. When there were significant differences in an outcome measure at baseline between the 2 groups, subsequent change score differences and P values were obtained using analysis of covariance. Secondary analyses of the MRSS included analysis of subgroups by disease duration categories, i.e., early-phase dcSSc versus late-phase dcSSc, and analysis of change in the MRSS at 15 months. The 15-month data were collected for safety monitoring after patients had been in the trial for 12 months; efficacy was also determined at the 15-month time point.
The change scores for the MRSS were normally distributed, and therefore all analyses of this outcome were based on a normal distribution; all other continuous change scores were compared using the rank sum test. No adjustments were made for multiple comparisons, because the main outcome was the mean change in the MRSS at 12 months between the 2 groups, and all other analyses were treated as secondary or exploratory. All change scores were computed by subtracting the baseline score from the 12-month score or 15-month score, with results expressed as the mean ± SD change in MRSS.
Several analyses of the primary outcome were performed. Our basic analyses included intent-to-treat (ITT) and modified ITT approaches as well as an analysis of the available data, the latter of which included patients who dropped out but returned for the 12- or 15-month visits. If data were missing at 12 or 15 months, the ITT approach was utilized, which involved carrying forward the last observation to month 12 or month 15 for all patients randomized to a treatment group. As per protocol, the ITT analysis was the initial and primary statistical approach. The modified ITT approach was the same as the ITT approach, except that the last observation carried forward was only for patients who were in the trial for at least 6 months. The modified ITT approach was thought to be a reasonable way to analyze the data, because clinical responses were expected to be delayed due to the mechanism of action of the CI treatment (13).
We also used a variety of mixed effects models to analyze the primary outcome. Mixed effects models allow all data points to be included in the analysis and are appropriate to use when data are missing at random. Results produced from such models are also relatively robust to data that are not missing at random. Our outcome variable was the change in MRSS in all mixed effects models, and we incorporated a random intercept for subject effect or both a random intercept and a random slope. Analyses were performed for the total patient population and, separately, for the early-phase and late-phase dcSSc groups, with and without a linearity assumption, with time, group, and a time-by-group interaction term as covariates. We also explored analyses under the assumption that data were not missing at random. Along with the mixed effects models, a simple imputation model was used, with time, group membership, and the current MRSS as covariates.
Finally, we analyzed change in the MRSS as a binary outcome (regardless of whether the patient’s MRSS had improved by 25%). Correlative analyses were performed to compare change in the MRSS with change in other clinical parameters from baseline to 12 months in each treatment group. Chi-square tests were used to analyze all differences related to adverse events.
RESULTS
Demographic and clinical characteristics at baseline
An overview of the study design and distribution of patients, including enrollment, dropouts, and the number of data points available for analyzing our primary outcome (the MRSS), is given in Figure 1. The characteristics of the enrolled patients were examined at baseline according to treatment group (i.e., total population of CI-treated versus placebo-treated patients) and according to phase of the disease (i.e., early phase versus late phase). Table 1 shows the baseline summary data on the demographic and clinical characteristics of the 168 patients.
Figure 1.
Overall summary of study enrollment and dropouts among patients with diffuse cutaneous systemic sclerosis randomized to receive either oral bovine type I collagen or placebo, with subgroups according to disease duration (early phase ≤3 years, late phase >3–10 years). * = available data analysis, involving patients with data at baseline and at 12 or 15 months, including dropouts who returned for the 12- or 15-month visit; ** = intent-to-treat (ITT) analysis, involving last observation carried forward to the 12- or 15-month visit; *** = modified ITT analysis, involving last observation carried forward but including patients who were in the trial for at least 6 months. MRSS = modified Rodnan skin thickness score.
Table 1.
Characteristics of the 168 patients in the oral CI and placebo groups at baseline*
| All |
Early-phase dcSSc |
Late-phase dcSSc |
||||
|---|---|---|---|---|---|---|
| CI | Placebo | CI | Placebo | CI | Placebo | |
| Sex, no. | ||||||
| Male | 17 | 18 | 11 | 10 | 6 | 8 |
| Female | 66 | 67 | 41 | 39 | 25 | 28 |
| Total | 83 | 85 | 52 | 49 | 31 | 36 |
| Age, years | ||||||
| Mean ± SD | 50.7 ± 12.4 | 50.9 ± 12.1 | 50.64 ± 11.68 | 49.74 ± 10.27 | 50.80 ± 13.68 | 52.38 ± 14.21 |
| Maximum | 78.97 | 80.80 | 70.56 | 75.07 | 78.97 | 80.80 |
| No. of patients | 83 | 85 | 52 | 49 | 31 | 36 |
| Disease duration, years | 3.15 ± 2.37† | 3.81 ± 2.89 | 1.63 ± 0.76 | 1.74 ± 0.68 | 5.71 ± 1.89 | 6.63 ± 2.3 |
| No. of patients | 83 | 85 | 52 | 49 | 31 | 36 |
| Baseline MRSS | 26.5 ± 7.8 | 26.0 ± 7.5 | 27.1 ± 7.5 | 26.5 ± 7.5 | 25.4 ± 8.3 | 25.3 ± 7.6 |
| No. of patients | 83 | 85 | 52 | 49 | 31 | 36 |
| DLco, % predicted | 69.8 ± 19.3 | 66.1 ± 21.8 | 71.9 ± 19.8 | 69.8 ± 20.6 | 66.2 ± 18.2 | 61.2 ± 22.7 |
| No. of patients | 82 | 84 | 52 | 48 | 30 | 36 |
| FVC, % predicted | 86.7 ± 20.8 | 84.0 ± 15.0 | 89.9 ± 21.8 | 88.0 ± 14.2 | 81.1 ± 18.2 | 78.5 ± 14.5 |
| No. of patients | 83 | 85 | 52 | 49 | 31 | 36 |
| SF-36 physical component summary score | 34.0 ± 12.0 | 35.1 ± 10.1 | 35.6 ± 11.7 | 34.1 ± 10.6 | 31.3 ± 12.2‡ | 36.4 ± 9.3 |
| No. of patients | 80 | 83 | 50 | 47 | 30 | 36 |
| SF-36 mental component summary score | 49.7 ± 11.8 | 47.7 ± 11.5 | 49.3 ± 12.4 | 47.9 ± 12.1 | 50.4 ± 11.0 | 47.5 ± 10.7 |
| No. of patients | 80 | 83 | 50 | 47 | 30 | 36 |
| SF-36 index component summary score | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.14 | 0.6 ± 0.2 | 0.7 ± 0.1 |
| No. of patients | 79 | 82 | 49 | 46 | 30 | 36 |
| Weight, lbs | 152.1 ± 35.9 | 156.6 ± 39.0 | 153.1 ± 36.1 | 159.0 ± 41.8 | 150.5 ± 36.1 | 153.3 ± 35.2 |
| No. of patients | 79 | 81 | 49 | 47 | 30 | 34 |
| Systolic BP, mm Hg | 117.7 ± 18.4 | 120.2 ± 22.4 | 116.4 ± 17.8 | 117.5 ± 16.6 | 119.9 ± 19.6 | 123.8 ± 28.3 |
| No. of patients | 80 | 82 | 50 | 47 | 30 | 35 |
| Diastolic BP, mm Hg | 70.4 ± 10.2 | 71.4 ± 13.5 | 69.9 ± 10.7 | 70.4 ± 9.5 | 71.2 ± 9.5 | 72.7 ± 17.6 |
| No. of patients | 80 | 82 | 50 | 47 | 30 | 35 |
| Creatinine, mg/dl | 0.77 ± 0.34 | 0.78 ± 0.37 | 0.75 ± 0.37 | 0.74 ± 0.41 | 0.80 ± 0.28 | 0.84 ± 0.30 |
| No. of patients | 77 | 80 | 49 | 46 | 28 | 34 |
| HAQ DI score | 1.17 ± 0.74 | 1.28 ± 0.70 | 1.13 ± 0.74 | 1.33 ± 0.69 | 1.22 ± 0.76 | 1.19 ± 0.70 |
| No. of patients | 83 | 85 | 52 | 49 | 36 | 31 |
| Physician’s global assessment VAS score | 44.84 ± 23.2 | 42.40 ± 22.1 | 44.76 ± 24.1 | 46.74 ± 21.3 | 44.97 ± 22.1 | 36.49 ± 22.0 |
| No. of patients | 81 | 85 | 50 | 49 | 31 | 36 |
| Patient’s global assessment VAS score | 35.84 ± 27.8 | 39.81 ± 29.5 | 34.46 ± 29.0 | 46.79 ± 31.07 | 38.23 ± 25.9 | 30.30 ± 24.6 |
| No. of patients | 82 | 85 | 52 | 49 | 30 | 36 |
| Patient’s pain assessment VAS score | 36.97 ± 27.9 | 40.12 ± 27.9 | 35.25 ± 27.6 | 46.49 ± 28.0 | 39.85 ± 28.5 | 31.63 ± 25.8 |
| No. of patients | 83 | 84 | 52 | 48 | 31 | 36 |
Except where indicated otherwise, values are the mean ± SD. CI= type I collagen; dcSSc = diffuse cutaneous systemic sclerosis; MRSS= modified Rodnan skin thickness score; DLco= diffusing capacity for carbon monoxide; FVC= forced vital capacity; SF-36 = Short Form 36; BP= blood pressure; HAQ DI= Health Assessment Questionnaire disability index; VAS= visual analog scale (0–100 mm).
P < 0.02 versus placebo, by rank sum test using available data.
P < 0.05 versus placebo, by rank sum test using available data.
There were no significant differences in the characteristics between treatment groups or between subgroups formed after controlling for disease status (early phase or late phase), except that the total population of CI-treated patients had a mean ± SD disease duration of 3.15 ± 2.37 years compared with 3.81 ± 2.89 years in the total group of placebo-treated patients (P < 0.02). Moreover, among all patients with late-phase dcSSc, the CI-treated patients had lower SF-36 physical component summary scores than did the placebo-treated patients (mean ± SD 31.3 ± 12.2 versus 36.4 ± 9.3; P < 0.05), denoting poorer quality of life in the CI-treated patients at baseline (Table 1).
Dropouts
During the 15-month trial, there were 55 dropouts (Figure 1). Patients in the placebo- and CI-treated groups spent a mean ± SD 12.47 ± 4.97 months and 11.65 ± 5.73 months in the trial, respectively (P not significant [NS]). Among the patients with early-phase dcSSc, the mean ± SD time to dropout was 11.39 ± 6.15 months for the placebo-treated patients and 11.39 ± 5.74 months for the CI-treated patients. Among those with late-phase dcSSc, the mean ± SD time to dropout for the placebo-treated and CI-treated groups was 13.95 ± 3.21 months and 12.09 ± 5.01 months, respectively (P NS). At the time of dropout, the change in MRSS from baseline was not statistically significantly different between the CI- and placebo-treated patients with early-phase dcSSc who dropped out or between the CI- and placebo-treated patients with late-phase dcSSc who dropped out (results not shown).
Effect of oral CI on the MRSS
The primary outcome variable of interest was the mean change in the MRSS in each of the treatment arms (CI versus placebo) at 12 months after treatment assignment. Results of the study based on ITT analysis of the total cohort of 168 patients showed no statistically significant differences in the mean change in the MRSS from baseline to month 12 between the CI-treated and placebo-treated patients (mean ± SD change in MRSS −2.4 ± 7.3 versus −1.8 ± 7.4; P = 0.563). Similar results were obtained in the modified ITT analysis and in the analysis of available data at 12 months. Similarly, at 15 months among the total cohort, there were no statistically significant differences in the mean change in MRSS between the 2 treatment groups, regardless of the basic method of analysis.
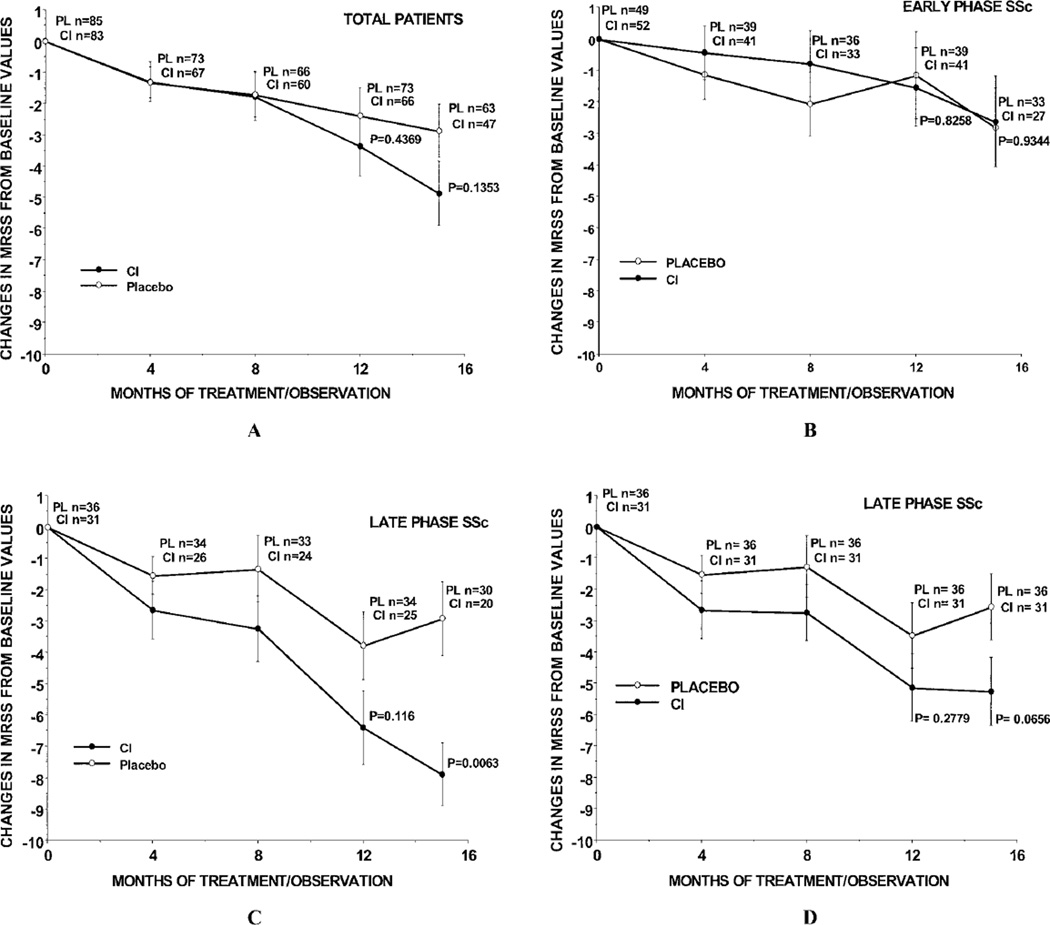
We then performed ITT, modified ITT, and available data subanalyses at 12 and 15 months according to disease duration status, i.e., early-phase dcSSc (≤3 years’ duration) versus late-phase dcSSc (>3–10 years’ duration). Figures 2A, B, and C show the values for the mean change in MRSS from baseline in analyses using available data in the total, early-phase dcSSc, and late-phase dcSSc groups of placebo-treated and CI-treated patients. Figure 2D shows the results from ITT analysis of the MRSS changes among the patients with late-phase dcSSc in each treatment group. In some groups, the numbers of patients at the 8-month visit were fewer than at the 12-month visit, since some patients who dropped out before 8 months returned for the 12-month assessment.
Figure 2.
Mean change in the MRSS from baseline to month 4, month 8, month 12, and month 15 in A, the total population, B, patients with early-phase diffuse cutaneous systemic sclerosis (dcSSc), and C, patients with late-phase dcSSc in the bovine type I collagen (CI)–treated and placebo (PL)–treated groups using available data. At 15 months, the median MRSS value for the CI-treated group of patients with late-phase dcSSc was substantially lower than the median values in the other groups, indicating that these patients experienced the greatest improvement in the MRSS. D, Mean change in the MRSS from baseline to each time point in patients with late-phase dcSSc using ITT analysis. Bars show the mean ± SD. See Figure 1 for other definitions.
For both the total cohort and the early-phase dcSSc group, there were no significant differences in the mean change in MRSS between the 2 treatment groups at any time point (Figures 2A and B). However, among the patients with late-phase dcSSc, there was a very noticeable and significant decrease in the MRSS from baseline to 15 months (P = 0.0063) (Figure 2C), with a mean change in MRSS of −7.9 in the 20 CI-treated patients with late-phase dcSSc compared with a mean change of −2.9 in the 30 placebo-treated patients with late-phase dcSSc (Figure 2C). Visually, this difference became apparent after 8 months of treatment (Figure 2C). In the ITT analysis, the mean decrease in MRSS from baseline to 15 months was −2.6 in the placebo-treated patients and −5.3 in the CI-treated patients (P = 0.0656) (Figure 2D).
These interesting findings regarding the differences in the change in MRSS between treatment groups at months 12 and 15 were based on the available data from 139 patients at 12 months and 110 patients at 15 months. We recognize that the results from the 15-month assessment may not have truly represented the results for the whole cohort, since the data would have been biased in favor of the less sick patients who remained in the trial. However, when we carried forward to month 15 the month 12 MRSS values for those patients who did not return for the month 15 MRSS determination, we found that the mean decrease in the MRSS in the CI-treated patients with late-phase dcSSc was still significantly different from that in the placebo group of patients with late-phase disease (change in MRSS −6.56 for CI-treated patients versus −3.17 for placebo-treated patients; P = 0.0187).
All analyses of the change in MRSS with mixed effects models produced results similar to those obtained in analyses using 2-sample independent t-tests of available data. In the mixed effects model, the mean change in MRSS at 12 months was not significantly different between the 2 treatment groups, whether in patients with early-phase dcSSc or in the total cohort. For patients with late-phase dcSSc, only the analysis using the random intercept model with linear mean structure produced a P value of 0.04, with the significant difference in change scores favoring the CI treatment group at 12 months. There was no statistically significant difference in the mean change in MRSS at 15 months between the 2 treatment groups of patients with early-phase dcSSc or between the 2 treatment groups from the whole cohort. However, among patients with late-phase disease, the mean change in MRSS at 15 months was significantly different between the 2 treatment groups, and this finding remained consistent across all of the models examined (P values ranging from 0.001 to 0.0096).
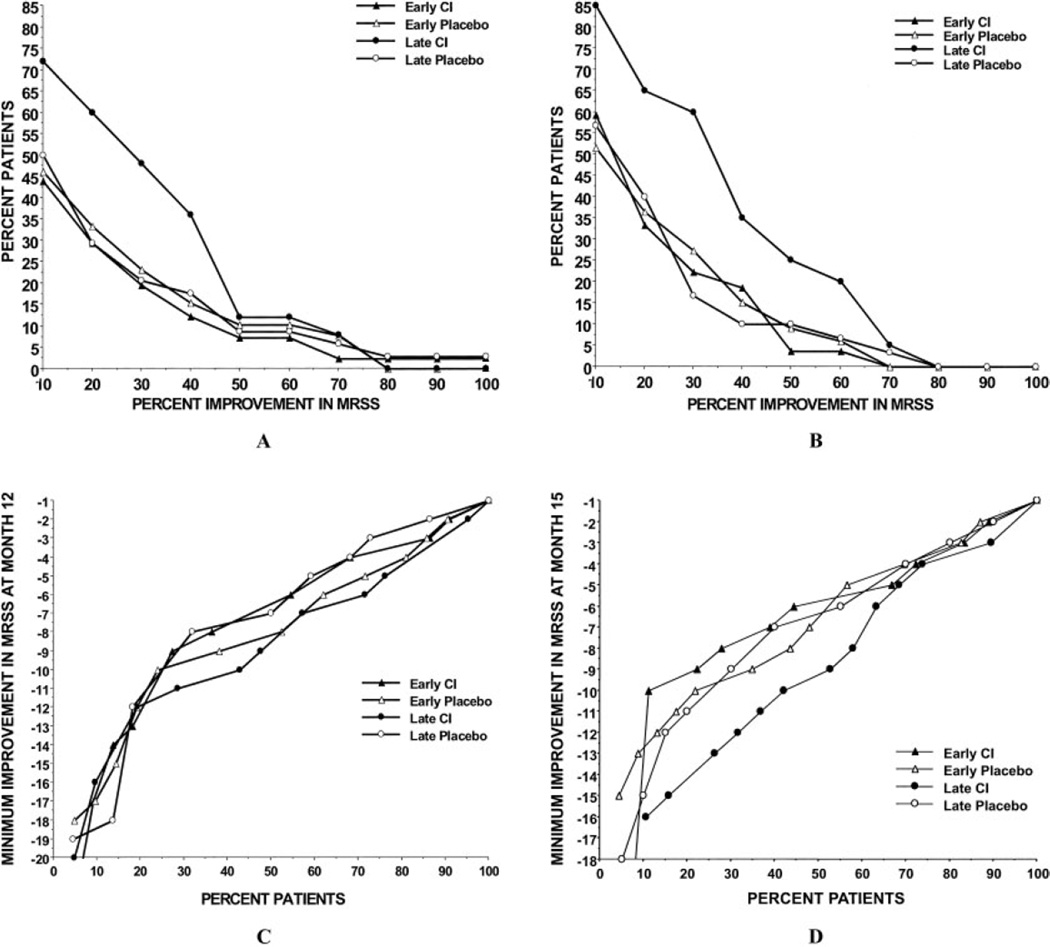
We also analyzed the mean change in MRSS, in analyses based on available data, according to the percentage of patients who showed various degrees of skin improvement (i.e., those who experienced a decrease in MRSS values) at 12 months (Figure 3A) and at 15 months (Figure 3B). Each graph plots the percentage of the cohort in each of the 4 subgroups (early-phase and late-phase CI-treated and placebo-treated patients) who experienced different degrees of improvement in the MRSS. For instance, in Figure 3B, ~65% of CI-treated patients in the late-phase dcSSc group experienced a reduction of 25% in the MRSS at 15 months, while ~40% of patients in each of the other 3 subgroups experienced a similar level of improvement.
Figure 3.
Percentage of patients achieving percentage levels of improvement in the MRSS at month 12 (A) and month 15 (B) in the bovine type I collagen (CI)–treated and placebo-treated groups. Among the patients with late-phase diffuse cutaneous systemic sclerosis (dcSSc) receiving oral CI, ~50% had a 25% reduction in the MRSS at 12 months, but only 19% of the patients in the early-phase dcSSc group experienced similar improvements in the MRSS at 12 months (A). At month 15, the patients with late-phase dcSSc receiving oral CI had the greatest improvement in the MRSS (B). Also shown are the percentage of patients achieving minimum improvement in the MRSS according to incremental units of change scores at month 12 (C) and month 15 (D). See Figure 1 for other definitions.
Both Figure 3A and Figure 3B clearly show that patients with late-phase disease benefited the most from collagen treatment as compared with the levels of improvement in the other 3 subgroups (P = 0.021). Among the CI-treated patients, chi-square tests confirmed that a significantly higher proportion of the patients with late-phase dcSSc, compared with those with early-phase dcSSc, had an improvement in the MRSS of at least 25% from baseline to 15 months.
Similar findings were obtained with the use of either the ITT approach or the modified ITT approach, with the late-phase dcSSc collagen treatment group showing a definite and consistent improvement in the MRSS as compared with the other 3 subgroups. Moreover, there were somewhat lower percentages of improvement in the MRSS in all subgroups using the ITT and modified approaches as compared with the levels of improvement obtained in analyses of all subgroups using available data. In Figures 3C and D, the percentages of patients in each subgroup who experienced various units of reduction in the MRSS from baseline to 12 months and from baseline to 15 months are plotted.
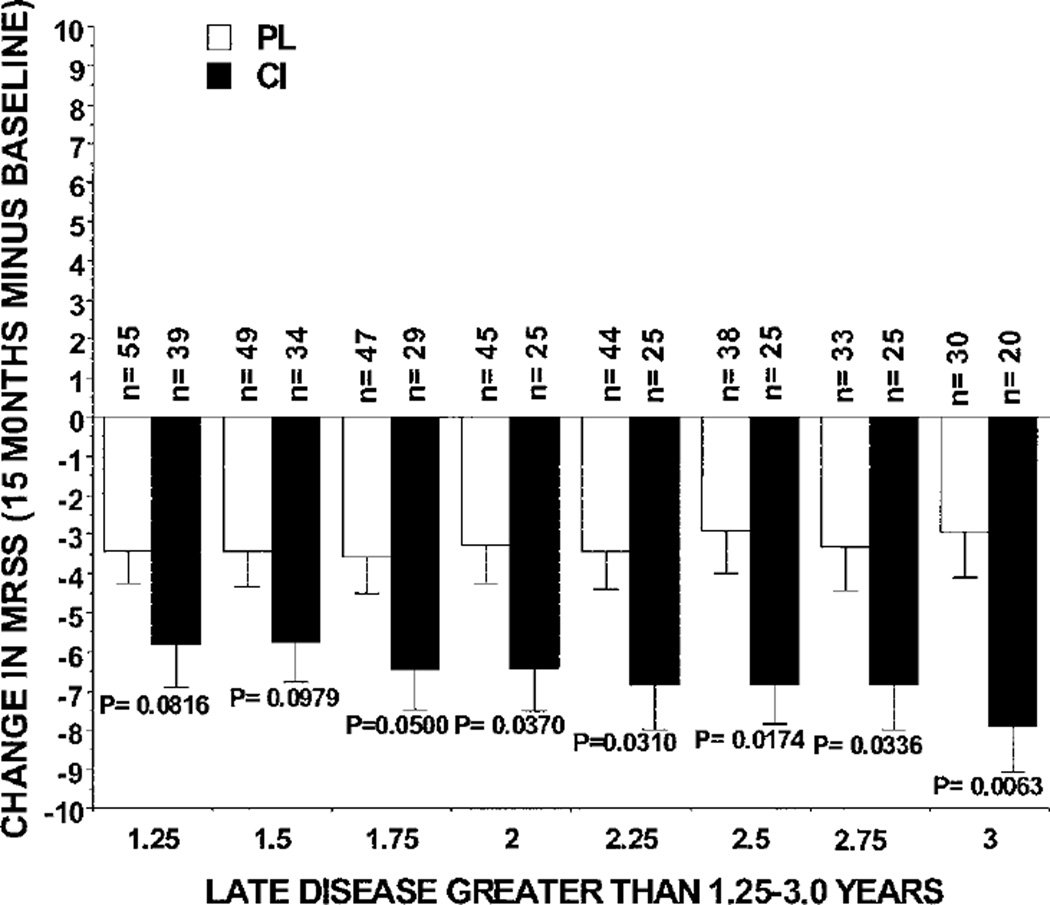
To determine whether patients with disease onset earlier than 3 years could be included in the late-phase dcSSc group and still retain statistically significant differences in the mean change in MRSS between the CI and placebo treatment groups using available data, we analyzed changes in the MRSS from baseline to 15 months in patients with a disease duration of >1.25–10 years up to >3–10 years according to 0.25-year increments (Figure 4), to compare the findings with those obtained in patients with late disease defined as a disease duration of >3–10 years. All patients treated with CI whose disease duration was >1.75–10 years, >2–10 years, >2.25–10 years, >2.5–10 years, >2.75–10 years, and >3–10 years had significantly larger reductions in the MRSS compared with the changes observed in placebo-treated patients with these same increments of disease duration (Figure 4).
Figure 4.
Change in the MRSS from baseline to 15 months using available data from the patients with late-phase diffuse cutaneous systemic sclerosis in the bovine type I collagen (CI) and placebo (PL) treatment groups, defined according to disease duration groups in 0.25-year increments from >1.25–10 years up to >3–10 years. Bars show the mean and SD. P values for comparisons between treatment groups were determined by Student’s 2-sample t -test. See Figure 1 for other definitions.
Effect of oral CI on other clinical parameters
The changes in most of the other clinical parameters were not significantly different between the CI- and placebo-treated groups, with the exception of changes in the SF-36 index component summary score, weight, diastolic BP, and patient’s VAS pain score (detailed data available online at http://www.utmem.edu/ctr/). The results from available data analyses indicated that both the total group of CI-treated patients and the group of CI-treated patients with late-phase dcSSc experienced worsening in the SF-36 index component summary score at 12 months as compared with that in both the total and late-phase dcSSc groups of placebo-treated patients (P < 0.05), but these differences were not clinically significant (Table 2) (33). However, changes in the SF-36 index component summary score at 12 or 15 months were not significantly different between the groups according to ITT or modified ITT analyses.
Table 2.
Changes in clinical parameters at 12 months and 15 months using available data*
| All |
Early-phase dcSSc |
Late-phase dcSSc |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ 12 mos. | 95% CI | Δ 15 mos. | 95% CI | Δ 12 mos. | 95% CI | Δ 15 mos. | 95% CI | Δ 12 mos. | 95% CI | Δ 15 mos. | 95% CI | |
| MRSS | ||||||||||||
| Total CI | −3.4 ± 7.6 | −5.2 to −1.5 | −4.9 ± 7.1 | −6.9 to −2.8 | −1.5 ± 8.0 | −4.0 to −1.0 | −2.6 ± 7.5 | −5.6 to 0.4 | −6.4 ± 5.8 | −8.8 to −4.0 | −7.9 ± 53† | −10.4 to −5.4 |
| No. of patients | 66 | 47 | 41 | 27 | 25 | 20 | ||||||
| Total placebo | −2.4 ± 7.7 | −4.1 to −0.5 | −2.9 ± 6.8 | −4.6 to −1.1 | −1.1 ± 8.6 | −3.9 to 1.7 | −2.8 ± 7.3 | −5.4 to −.2 | −3.8 ± 6.3 | −6.0 to −1.5 | −2.9 ± 6.5 | −5.4 to −0.5 |
| No. of patients | 73 | 63 | 39 | 33 | 34 | 30 | ||||||
| SF-36 index component summary score |
||||||||||||
| Total CI | 0.02 ± 0.1‡ | 0 to 0.04 | −0.011 ± 0.12 | −0.05 to 0.03 | 0.01 ± 0.11 | −0.02 to 0.05 | −0.03 ± 0.13 | −0.08 to 0.02 | −0.03 ± 0.07‡ | 0.01 to 0.06 | 0.01 ± 0.11 | −0.04 to 0.03 |
| No. of patients | 64 | 49 | 40 | 29 | 24 | 20 | ||||||
| Total placebo | −0.01 ± 0.1 | −0.03 to 0.01 | −0.02 ± 0.11 | −0.05 to 0.01 | −0.02 ± 0.1 | −0.05 to 0.02 | −0.05 ± 0.12 | −0.09 to 0 | 0 ± 0.10 | −0.04 to 0.03 | 0.01 ± 0.10 | −0.03 to 0.04 |
| No. of patients | 71 | 62 | 39 | 33 | 34 | 29 | ||||||
| Weight, lbs | ||||||||||||
| Total CI | −3.0 ± 7.8‡ | −4.9 to 1.0 | −2.4 ± 9.7 | −5.2 to 0.4 | −2.6 ± 7.3 | −5.0 to −0.26 | −0.6 ± 7.2 | −3.4 to 2.1 | −3.5 ± 8.7 | −7.1 to 0.1 | −5.0 ± 12.2 | −10.7 to 0.8 |
| No. of patients | 64 | 49 | 39 | 29 | 25 | 20 | ||||||
| Total placebo | −0.2 ± 93 | −2.4 to 2.0 | 0.8 ± 10.4 | −1.9 to 3.4 | 0.9 ± 11.0 | −2.7 to 4.5 | 3.0 ± 12.2 | −13 to 7.4 | −1.4 ± 6.8 | −3.8 to 1.0 | −1.73 ± 7.4 | −4.5 to 1.0 |
| No. of patients | 71 | 63 | 38 | 33 | 33 | 30 | ||||||
| Diastolic BP, mm Hg | ||||||||||||
| Total CI | 0.92 ± 10.8 | −1.8 to 3.6 | −0.67 ± 11.4 | −4.0 to 2.6 | 0.73 ± 9.9 | −2.4 to 3.8 | −4.07 ± 8.8§ | −7.4 to 0.7 | 1.25 ± 12.4 | −4.0 to 6.5 | 4.53 ± 13.1 | −1.8 to 10.9 |
| No. of patients | 65 | 48 | 41 | 29 | 24 | 19 | ||||||
| Total placebo | 2.6 ± 13.9 | −0.7 to 5.9 | 5.4 ± 20.6 | 0.2 to 10.6 | 1.98 ± 10.3 | −1.3 to 5.3 | 6.79 ± 23.1 | −1.4 to 1.5 | 3.36 ± 17.5 | −2.9 to 9.6 | 3.93 ± 17.7 | −2.7 to 10.6 |
| No. of patients | 73 | 63 | 40 | 33 | 33 | 30 | ||||||
| Patient’s pain assessment VAS |
||||||||||||
| Total CI | −3.60 ± 24.4 | −9.6 to 2.4 | −4.8 ± 21.4 | −10.9 to 1.3 | −1.66 ± 24.5§ | −9.5 to 6.2 | −4.42 ± 18.4 | −113 to 2.5 | −6.69 ± 24.3 | −16.7 to 3.4 | −5.44 ± 25.7 | −17.5 to 6.6 |
| No. of patients | 65 | 50 | 40 | 30 | 25 | 20 | ||||||
| Total placebo | 8.89 ± 25.4 | −14.8 to −3.0 | −4.08 ± 25.2 | −10.4 to 2.3 | −13.3 ± 21.0 | −20.1 to −6.5 | −6.32 ± 24.5 | −15.0 to 2.4 | −3.82 ± 29.14 | −14.0 to 6.4 | −1.62 ± 26.2 | −11.4 to 8.2 |
| No. of patients | 73 | 63 | 39 | 33 | 34 | 30 | ||||||
Except where indicated otherwise, values are the mean ± SD. Only clinical parameters showing significant differences between the CI- and placebo-treated patients in ≥1 of the groups are shown. Clinical parameters showing no significant differences between the CI- and placebo-treated patients in any of the patient groups are listed online at http://www.utmem.edu/ctr/. 95% CI = 95% confidence interval (see Table 1 for other definitions).
P < 0.01 versus placebo, by rank sum test using available data.
P < 0.05 versus placebo, by rank sum test using available data.
P < 0.02 versus placebo, by rank sum test using available data.
The mean change in weight at 12 months in the total CI-treated group (mean ± SD change −3.0 ± 7.8 lbs) was significantly different from the mean change in weight at 12 months in the total placebo-treated group (−0.2 ± 9.3 lbs) (Table 2). Although this difference between the groups was found to be statistically significant in both ITT and modified ITT analyses, the difference in weight change was not clinically significant. At 15 months, the CI-treated patients with early-phase dcSSc had a significant decrease in diastolic BP (−4.07 ± 8.8 mm Hg), compared with an increase in diastolic BP in the placebo-treated patients with early-phase dcSSc (+6.79 ± 23.1 mm Hg) (Table 2). However, this difference in the mean change in diastolic BP was not statistically significant in ITT or modified ITT analyses. Furthermore, among the patients with early-phase dcSSc, the CI-treated patients had less improvement in the VAS pain score at 12 months compared with the placebo-treated patients (P < 0.02), and this was significantly different between these groups in all 3 analyses (Table 2).
There were notable significant correlations at 12 months between changes in some of the other clinical parameters and changes in the MRSS in the CI-treated patients, but no correlations were observed in the placebo-treated patients. First, in the total group of CI-treated patients, a positive correlation was observed between the mean change in MRSS and the mean change in patient’s VAS pain scores (r = 0.3039, P = 0.0139; n = 65), but this correlation was not observed in the total placebo-treated group. Second, in the CI-treated patients with late-phase dcSSc, positive correlations were observed between the mean change in MRSS and the mean change in patient’s VAS pain scores (r = 0.5611, P = 0.0035; n = 25), patient’s global VAS scores (r = 0.4895, P = 0.0178; n = 23), and SF-36 physical component summary scores (r = 0.3690, P = 0.0063; n = 23), but none of these correlations was observed in the placebo-treated patients with late-phase dcSSc. In addition to the correlations observed with the use of available data, these same correlations (with the exception of patient’s global assessment of health in the CI-treated patients with late-phase dcSSc) were also significant with the use of the ITT and modified ITT analyses.
Adverse events
There was a large number of adverse events in the trial, and many patients had multiple adverse events. Among the CI-treated patients, there was a mean 2.36 adverse events per patient, while there was a mean 2.55 adverse events per patient among the placebo-treated patients (P NS). There were no differences between groups in the distribution of adverse events according to each body system examined, except for adverse events in the central nervous system (CNS) and in the hematologic and gastrointestinal (GI) systems.
Among the adverse events occurring in the CNS, headaches were observed less frequently in the CI-treated patients than in the placebo-treated patients (P = 0.012 for the total group, P = 0.02 for the late-phase dcSSc group). Although there were more instances of adverse events in the hematologic system (decreases in hemoglobin level, white blood cell count, or thrombocytopenia) among the CI-treated patients (16 events compared with 7 events in the placebo-treated group; P = 0.03), these occurred in only 9 CI-treated patients, whereas 7 of these events occurred in 6 placebo-treated patients.
Adverse events in the GI system occurred inconsistently among the subgroups; differences were observed both between the early-phase dcSSc and late-phase dcSSc groups and between the placebo-treated and CI-treated patients. The number of adverse events in the GI system was either increased or decreased in these groups, which is probably a reflection of chance differences (see detailed comparisons online at http://www.utmem.edu/ctr/).
Although there were 22 serious adverse events in 14 CI-treated patients and 21 serious adverse events in 18 placebo-treated patients, there were no statistically significant differences overall or by body system between the 2 groups (P = 0.532). Several patients had serious adverse events that affected multiple systems (e.g., 1 patient had involvement of the pulmonary, renal, cardiovascular, and GI systems during sepsis), which would account for the differences between the instances of serious adverse events and the number of patients affected by serious adverse events.
There were 5 deaths in each treatment group among the patients with early-phase disease (P NS). There was 1 death in each of the treatment groups among the patients with late-phase disease. The causes of death for each patient in the early-phase dcSSc CI-treated group were attributable to the following conditions: pulmonary hypertension in 1 patient, respiratory failure, renal failure, and GI bleeding in 1 patient, adenocarcinoma of the lung/pneumonia in 1 patient, renal crisis in 1 patient, and scleroderma-related GI disease in 1 patient. The causes of death for each patient in the early-phase dcSSc placebo-treated group were attributable to the following conditions: renal crisis in 1 patient, cardiopulmonary failure in 1 patient, progression of scleroderma in 1 patient, pulmonary hypertension in 1 patient, and cardiopulmonary and GI disease in 1 patient. Among the late-phase dcSSc group, the causes of death in the CI-treated patients were pulmonary hypertension, respiratory failure, and renal failure, whereas progression of scleroderma was responsible for the death of the placebo-treated patient with late-phase disease.
The higher incidence of deaths that was observed among the patients with early-phase dcSSc is expected, since the early phase of the disease is associated with more rapid progression and a higher mortality rate. In essence, patients with late-phase dcSSc represent survivors who live beyond the first 3 years of the disease, and therefore the death rate is lower among these survivors. All deaths were deemed by a blinded observer to be related to the disease rather than to the treatment (a more detailed discussion of these data is available online at http://www.utmem.edu/ctr/).
With regard to the effects of treatment on laboratory parameters, no significant changes in liver function parameters or levels of creatine kinase, creatinine, or cholesterol were observed in either treatment group at any time (results not shown). However, as discussed above, changes did occur in the levels of hematocrit and hemoglobin among the patients in each treatment group.
DISCUSSION
Patients with dcSSc having a disease duration from 1 year to 10 years and having received treatment with oral CI for 12 months (either among the total population or among those with early-phase disease) did not show a significant improvement in the MRSS at 12 or 15 months when analyzed in an ITT or modified ITT approach or using available data. Analysis of the data using ITT and modified ITT approaches also did not show significant differences in the change in MRSS between the CI- and placebo-treated patients with late-phase disease. However, analyses using the available data (i.e., patients who received treatment for 1–12 months and then returned for the 12-month or 15-month assessment) showed a significant reduction in the MRSS at 15 months among CI-treated patients with late-phase dcSSc as compared with placebo-treated patients with late-phase dcSSc.
Findings from the exploratory analyses suggested that patients with dcSSc having a disease duration as short as 1.75 years may respond beneficially to treatment with oral CI. Additional support for a specific effect of oral CI in patients with late-phase dcSSc was provided by the correlation analyses, which showed statistically significant associations in this group between improvement in the MRSS and changes in the patient’s pain score on VAS, patient’s global assessment on VAS, and SF-36 physical component summary score. The improvement in the MRSS in these patients with late-phase dcSSc reached statistical significance as well as clinically important significance, as compared with that in the placebo-treated patients with late-phase dcSSc, at month 15 of the study, 3 months after oral CI was stopped. This suggests that oral CI may have a delayed effect on the skin of patients with dcSSc, perhaps attributable to the length of time needed to attain full tolerance to CI and to the impairment in production of type I collagenase by SSc fibroblasts, which could slow degradation of deposited collagen (13,34,35).
The differences noted in the analysis of available data reflect the result of actually evaluating the patients at the designated time per protocol. Neither the ITT analysis nor the modified ITT analysis demonstrated this effect, perhaps because patients dropped out before an effect could be observed (ITT) or because there were random baseline dropouts occurring for reasons such as moving out of the area (modified ITT). Oral native bovine CI administered at 500 µg/day was well tolerated and was not associated with any clinical differences, in terms of adverse events, compared with that in the placebo control group.
The reasons for the differential clinical response between the early-phase and late-phase dcSSc groups cannot be ascertained from this study, although testable hypotheses have been presented. Perhaps components of the immune system that mediate fibrosis in the early phase of the disease are less susceptible to modulation by orally administered CI than are those in the late phase of the disease.
After the first few years of the disease, fibrosis of the skin and internal organs tends to stabilize or improve, but can also worsen in some patients with dcSSc (27,36–38). However, many patients with late-phase dcSSc still have an elevated MRSS (27,37,38). In this study, the mean MRSS at baseline in patients with a disease duration of >3–10 years was high, at an MRSS of 25. The results of this study suggest that oral CI may accelerate the rate of improvement in the MRSS in patients with late-phase disease.
Although the results of this trial did not meet the primary outcome goals, the findings from secondary analyses suggested, for the first time, a possible effect of this treatment in patients with “late” dcSSc. There was a beneficial, albeit delayed, effect of oral bovine CI, without significant toxicity, in patients with late-phase dcSSc. However, given that this finding was obtained in a secondary analysis, it can only be regarded as hypothesis generating and will need to be corroborated with additional studies.
ACKNOWLEDGMENTS
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Allergy and Infectious Diseases (contract N01-AR-902242), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Scleroderma SCOR P50-AR-044890-55), and the US Department of Veterans Affairs. Funding was also provided by the National Center for Research Resources and the General Clinical Research programs at Boston University (M01-RRO-00533), Georgetown University (M01-RR-020359), Medical University of South Carolina (M01-RR-01070), University of Alabama at Birmingham (M01-RR-00032), University of Connecticut Health Sciences Center (M01-RR-06192), University of Texas, Houston (UL-1-RR-024148), and University of Tennessee, Memphis (M01-RR-00211). Drs. Postlethwaite, Wong, and Furst’s work was supported in part by a Senior Investigator grant awarded to Dr. Wong in 2006 and 2007 by the Scleroderma Foundation. Dr. Merkel’s work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K24-AR-2224-01A1).
Dr. Postlethwaite has received consulting fees, speaking fees, and/or honoraria (more than $10,000) from arGentis Pharmaceuticals and owns stock or stock options in arGentis Pharmaceuticals. The University of Tennessee Research Foundation owns a patent and licensing rights for use of type I collagen as an oral tolerogen. Drs. Postlethwaite and Kang contributed data and intellectual property that supported the patent application; arGentis has obtained licensing rights for this patent. Dr. Mayes has received consulting fees, speaking fees, and/or honoraria (less than $10,000 each) from Actelion and Novartis.
The authors want to acknowledge the excellent contributions of Jesse Ingels, Janie Freeman, Virginia Geer, Jacquelyn Fountain, Diane Weisfeld, Patricia Wheller, Emma Hasan, Debbie Granner, Ronika Alexander, Christina Armenta-Burger, Karen Barrow, Kattie Caldwell, Sandy Enuha, Iresha Abeynayahe, Edrick Forbes, Frances Ingenito, Melynn Nuite, Kim Tobin, Adriana Ortiz, Tina Parkhill, Paula McKenzie, Cynthia Parks, Samantha Jordan, Claudia Focks, Adrienne Marshall, Elena Breen, June Arnold, Amanda Mondt, April Thurman, Erika Hull, Karen Wilson, Anise Cary, Ali Farrokh, William Wagner, Phyllis Mikula, Gwen Leatherman, Millie Sterz, Zora Injie, and Vickie Griffin.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Postlethwaite had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Postlethwaite, Clements, Furst.
Acquisition of data. Postlethwaite, Clements, Chatterjee, Fessler, Korn, Mayes, Merkel, Molitor, Moreland, Rothfield, Simms, Smith, Spiera, Steen, Warrington, White, Wigley, Furst.
Analysis and interpretation of data. Postlethwaite, Wong, Clements, Furst.
Manuscript preparation. Postlethwaite, Wong, Clements, Furst.
Statistical analysis. Wong
Preparation of collagen. Kang.
REFERENCES
- 1.White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 1996;22:695–708. doi: 10.1016/s0889-857x(05)70296-9. [DOI] [PubMed] [Google Scholar]
- 2.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–263. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 3.Roumm AD, Whiteside TL, Medsger TA, Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis: quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27:645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 4.Wells AU, Lorimer S, Majumdar S, Harrison NK, Corrin B, Black CM, et al. Fibrosing alveolitis in systemic sclerosis: increase in memory T-cells in lung interstitium. Eur Respir J. 1995;8:266–271. doi: 10.1183/09031936.95.08020266. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmajer R, Perlish JS, Reeves JR. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977;20:975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- 6.Sakkas LI, Xu B, Artlett CM, Lu S, Jimenez SA, Platsoucas CD. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J Immunol. 2002;168:3649–3659. doi: 10.4049/jimmunol.168.7.3649. [DOI] [PubMed] [Google Scholar]
- 7.Claman HN. Mast cell changes in a case of rapidly progressive scleroderma-ultrastructural analysis. J Invest Dermatol. 1989;92:290–295. doi: 10.1111/1523-1747.ep12276876. [DOI] [PubMed] [Google Scholar]
- 8.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42:1168–1178. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Atamas SP, White B. Interleukin 4 in systemic sclerosis: not just an increase. Clin Diagn Lab Immunol. 1999;6:658–659. doi: 10.1128/cdli.6.5.658-659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 2003;14:537–550. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 11.Molteni M, Della Bella S, Mascagni B, Bazzi S, Zulian C, Compasso S, et al. Increased interferon-γ (IFN-γ) levels produced in vitro by alloactivated T lymphocytes in systemic sclerosis and Raynaud’s phenomenon. Clin Exp Immunol. 1999;11:164–168. doi: 10.1046/j.1365-2249.1999.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart JM, Postlethwaite AE, Kang AH. Evidence for cell-mediated immunity to collagen in progressive systemic sclerosis. J Lab Clin Med. 1976;88:601–607. [PubMed] [Google Scholar]
- 13.McKown KM, Carbone LD, Bustillo J, Seyer JM, Kang AH, Postlethwaite AE. Induction of immune tolerance to human type I collagen in patients with systemic sclerosis by oral administration of bovine type I collagen. Arthritis Rheum. 2000;43:1054–1061. doi: 10.1002/1529-0131(200005)43:5<1054::AID-ANR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Gurram M, Pahwa S, Frieri M. Augmented interleukin-6 secretion in collagen-stimulated peripheral blood mononuclear cells from patients with systemic sclerosis. Ann Allergy. 1994;73:493–496. [PubMed] [Google Scholar]
- 15.Hawrylko E, Spertus A, Mele CA, Oster N, Frieri M. Increased interleukin-2 production in response to human type I collagen stimulation in patients with systemic sclerosis. Arthritis Rheum. 1991;34:580–587. doi: 10.1002/art.1780340510. [DOI] [PubMed] [Google Scholar]
- 16.Huffstutter JE, DeLustro FA, LeRoy EC. Cellular immunity to collagen and laminin in scleroderma. Arthritis Rheum. 1985;28:775–780. doi: 10.1002/art.1780280708. [DOI] [PubMed] [Google Scholar]
- 17.Miller EJ. Chemistry of the collagens and their distribution. In: Piez KA, AH Reddi, editors. Extracellular matrix biochemistry. New York: Elsevier; 1984. pp. 41–82. [Google Scholar]
- 18.Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 19.Nussenblatt RB, Whitcup SM, Smet MD, Caspi RR, Kozhich AT, Weiner HL, et al. Intraocular inflammatory disease (uveitis) and the use of oral tolerance: a status report. Ann N Y Acad Sci. 1996;778:325–337. doi: 10.1111/j.1749-6632.1996.tb21140.x. [DOI] [PubMed] [Google Scholar]
- 20.Myers LK, Higgins GC, Finkel TH, Reed AM, Thompson JW, Walton RC, et al. Juvenile arthritis and autoimmunity to type II collagen. Arthritis Rheum. 2001;44:1775–1781. doi: 10.1002/1529-0131(200108)44:8<1775::AID-ART313>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.McKown KM, Carbone LD, Kaplan SB, Aelion JA, Lohr KM, Cremer MA, et al. Lack of efficacy of oral bovine type II collagen added to existing therapy in rheumatoid arthritis. Arthritis Rheum. 1999;42:1204–1208. doi: 10.1002/1529-0131(199906)42:6<1204::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Barnett ML, Combitchi D, Trentham DE. A pilot trial of oral type II collagen in the treatment of juvenile rheumatoid arthritis. Arthritis Rheum. 1996;39:623–628. doi: 10.1002/art.1780390413. [DOI] [PubMed] [Google Scholar]
- 23.Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 24.Sieper J, Kary S, Sorensen H, Alten R, Eggens U, Huge W, et al. Oral type II collagen treatment in early rheumatoid arthritis: a double-blind, placebo-controlled, randomized trial. Arthritis Rheum. 1996;39:41–51. doi: 10.1002/art.1780390106. [DOI] [PubMed] [Google Scholar]
- 25.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the Stanford Health Assessment Questionnaire functional disability index in patients with rheumatoid arthritis. J Rheumatol. 1988;15:1480–1488. [PubMed] [Google Scholar]
- 27.Clements PJ, Furst DE, Wong WK, Mayes M, White B, Wigley F, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42:1194–1203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405–413. doi: 10.1023/a:1012588218728. [DOI] [PubMed] [Google Scholar]
- 29.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–1896. [PubMed] [Google Scholar]
- 30.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 31.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique: 1995 update. Am J Respir Crit Care Med. 1995;152(6 Pt 1):2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 33.Strand V, Tugwell P, Bombardier C, Maetzel A, Crawford B, Dorrier C, et al. on behalf of the Leflunomide Rheumatoid Arthritis Investigators Group. Function and health-related quality of life: results from a randomized controlled trial of leflunomide versus methotrexate or placebo in patients with active rheumatoid arthritis. Arthritis Rheum. 1999;42:1870–1878. doi: 10.1002/1529-0131(199909)42:9<1870::AID-ANR11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994;103:359–363. doi: 10.1111/1523-1747.ep12394936. [DOI] [PubMed] [Google Scholar]
- 35.Hasty KA, Kanangat S, Postlethwaite AE. Collagenase expression is inhibited in fibroblasts from fibrotic, but not non-fibrotic, scleroderma skin: the role of intracellular interleukin 1 receptor antagonist [abstract] Arthritis Rheum. 2007;56(Suppl 9):S77. [Google Scholar]
- 36.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;43:2445–2454. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Steen VD, Medsger TA., Jr Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44:2828–2835. doi: 10.1002/1529-0131(200112)44:12<2828::aid-art470>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 38.Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum. 2007;56:2422–2431. doi: 10.1002/art.22721. [DOI] [PubMed] [Google Scholar]