Abstract
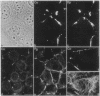
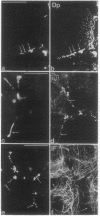
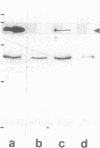
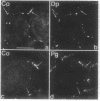
By transfecting epithelial cells with gene constructs encoding chimeric proteins of the transmembrane part of the gap junction protein connexin 32 in combination with various segments of the cytoplasmic part of the desmosomal cadherin desmocollin 1a, we have determined that a relatively short sequence element is necessary for the formation of desmosome-like plaques and for the specific anchorage of bundles of intermediate-sized filaments (IFs). Deletion of as little as the carboxyl-terminal 37 aa resulted in a lack of IF anchorage and binding of the plaque protein plakoglobin, as shown by immunolocalization and immunoprecipitation experiments. In addition, we show that the sequence requirements for the recruitment of desmoplakin, another desmosomal plaque protein, differ and that a short (10 aa) segment of the desmocollin 1a tail, located close to the plasma membrane, is also required for the binding of plakoglobin, as well as of desmoplakin, and also for IF anchorage. The importance of the carboxyl-terminal domain, homologous in diverse types of cadherins, is emphasized, as it must harbor, in a mutually exclusive pattern, the information for assembly of the IF-anchoring desmosomal plaque in desmocollins and for formation of the alpha-/beta-catenin- and vinculin-containing, actin filament-anchoring plaque in E- and N-cadherin.
Full text
PDF

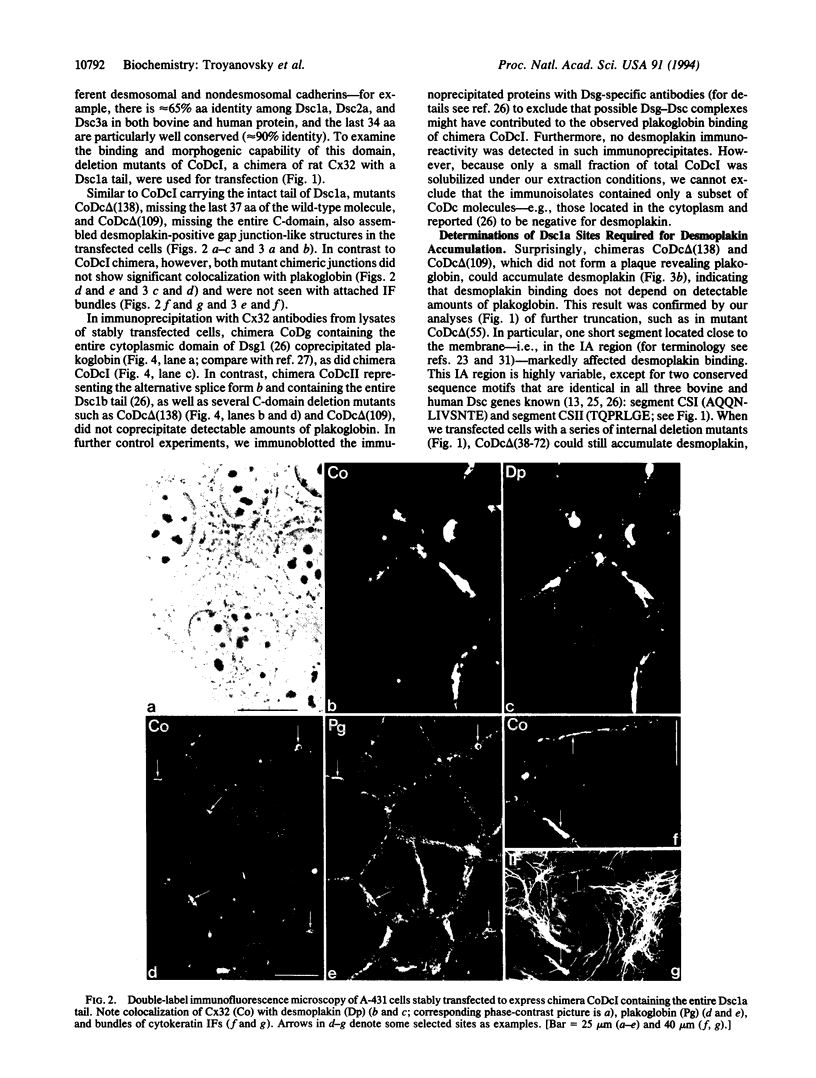
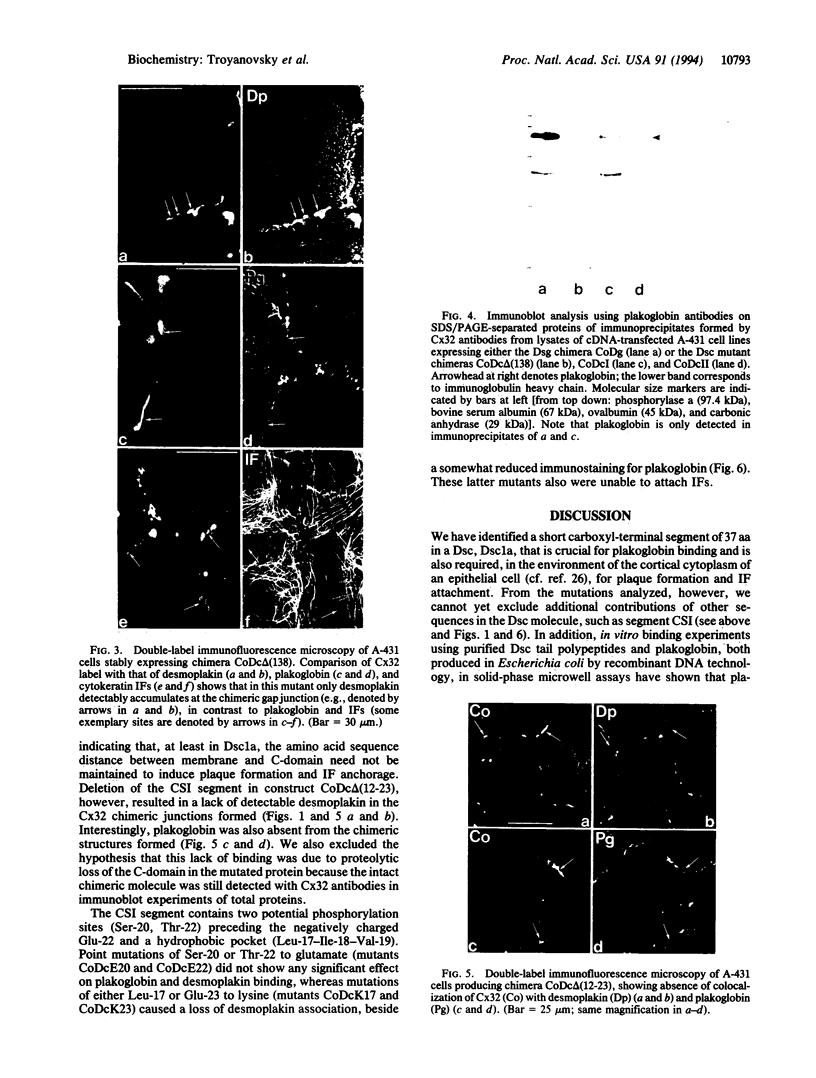

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxton R. S., Cowin P., Franke W. W., Garrod D. R., Green K. J., King I. A., Koch P. J., Magee A. I., Rees D. A., Stanley J. R. Nomenclature of the desmosomal cadherins. J Cell Biol. 1993 May;121(3):481–483. doi: 10.1083/jcb.121.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S., Magee A. I. Structure and interactions of desmosomal and other cadherins. Semin Cell Biol. 1992 Jun;3(3):157–167. doi: 10.1016/s1043-4682(10)80012-1. [DOI] [PubMed] [Google Scholar]
- Collins J. E., Legan P. K., Kenny T. P., MacGarvie J., Holton J. L., Garrod D. R. Cloning and sequence analysis of desmosomal glycoproteins 2 and 3 (desmocollins): cadherin-like desmosomal adhesion molecules with heterogeneous cytoplasmic domains. J Cell Biol. 1991 Apr;113(2):381–391. doi: 10.1083/jcb.113.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W., Tamkun J., Hynes R. O. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986 Sep 26;46(7):1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W. The complement of desmosomal plaque proteins in different cell types. J Cell Biol. 1985 Oct;101(4):1442–1454. doi: 10.1083/jcb.101.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Troyanovsky S. M., Koch P. J., Troyanovsky R., Fouquet B., Leube R. E. Desmosomal proteins: mediators of intercellular coupling and intermediate filament anchorage. Cold Spring Harb Symp Quant Biol. 1992;57:643–652. doi: 10.1101/sqb.1992.057.01.070. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., von Overbeck J., Gudat F., Heitz P. U., Stähli C. Identification of the conserved, conformation-dependent cytokeratin epitope recognized by monoclonal antibody (lu-5). Virchows Arch A Pathol Anat Histopathol. 1987;411(2):137–147. doi: 10.1007/BF00712737. [DOI] [PubMed] [Google Scholar]
- Garrod D. R. Desmosomes and hemidesmosomes. Curr Opin Cell Biol. 1993 Feb;5(1):30–40. doi: 10.1016/s0955-0674(05)80005-5. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993 Oct;5(5):797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron. 1993 Oct;11(4):551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Kemler R. Classical cadherins. Semin Cell Biol. 1992 Jun;3(3):149–155. doi: 10.1016/s1043-4682(10)80011-x. [DOI] [PubMed] [Google Scholar]
- King I. A., Arnemann J., Spurr N. K., Buxton R. S. Cloning of the cDNA (DSC1) coding for human type 1 desmocollin and its assignment to chromosome 18. Genomics. 1993 Nov;18(2):185–194. doi: 10.1006/geno.1993.1453. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992 Apr 17;69(2):225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Wheelock M. J. Plakoglobin, or an 83-kD homologue distinct from beta-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol. 1992 Aug;118(3):671–679. doi: 10.1083/jcb.118.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P. J., Goldschmidt M. D., Walsh M. J., Zimbelmann R., Schmelz M., Franke W. W. Amino acid sequence of bovine muzzle epithelial desmocollin derived from cloned cDNA: a novel subtype of desmosomal cadherins. Differentiation. 1991 May;47(1):29–36. doi: 10.1111/j.1432-0436.1991.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Koch P. J., Goldschmidt M. D., Zimbelmann R., Troyanovsky R., Franke W. W. Complexity and expression patterns of the desmosomal cadherins. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):353–357. doi: 10.1073/pnas.89.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P. J., Walsh M. J., Schmelz M., Goldschmidt M. D., Zimbelmann R., Franke W. W. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990 Oct;53(1):1–12. [PubMed] [Google Scholar]
- Krutovskikh V., Mazzoleni G., Mironov N., Omori Y., Aguelon A. M., Mesnil M., Berger F., Partensky C., Yamasaki H. Altered homologous and heterologous gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer. 1994 Jan 2;56(1):87–94. doi: 10.1002/ijc.2910560116. [DOI] [PubMed] [Google Scholar]
- Mechanic S., Raynor K., Hill J. E., Cowin P. Desmocollins form a distinct subset of the cadherin family of cell adhesion molecules. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4476–4480. doi: 10.1073/pnas.88.10.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A. E., Wheeler G. N., Arnemann J., Pidsley S. C., Ataliotis P., Thomas C. L., Rees D. A., Magee A. I., Buxton R. S. Desmosomal glycoproteins II and III. Cadherin-like junctional molecules generated by alternative splicing. J Biol Chem. 1991 Jun 5;266(16):10438–10445. [PubMed] [Google Scholar]
- Piepenhagen P. A., Nelson W. J. Defining E-cadherin-associated protein complexes in epithelial cells: plakoglobin, beta- and gamma-catenin are distinct components. J Cell Sci. 1993 Mar;104(Pt 3):751–762. doi: 10.1242/jcs.104.3.751. [DOI] [PubMed] [Google Scholar]
- Schmelz M., Duden R., Cowin P., Franke W. W. A constitutive transmembrane glycoprotein of Mr 165,000 (desmoglein) in epidermal and non-epidermal desmosomes. I. Biochemical identification of the polypeptide. Eur J Cell Biol. 1986 Dec;42(2):177–183. [PubMed] [Google Scholar]
- Schwarz M. A., Owaribe K., Kartenbeck J., Franke W. W. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Koch P. J., Franke W. W. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res. 1994 Apr;211(2):391–399. doi: 10.1006/excr.1994.1103. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Troyanovsky S. M., Heid H. W., Eshkind L. G., Koch P. J., Franke W. W. Cytoskeletal architecture and epithelial differentiation: molecular determinants of cell interaction and cytoskeletal filament anchorage. C R Acad Sci III. 1993 Nov;316(11):1316–1323. [PubMed] [Google Scholar]
- Stappenbeck T. S., Green K. J. The desmoplakin carboxyl terminus coaligns with and specifically disrupts intermediate filament networks when expressed in cultured cells. J Cell Biol. 1992 Mar;116(5):1197–1209. doi: 10.1083/jcb.116.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis D. G., Koch P. J., Franke W. W. Differential synthesis of type 1 and type 2 desmocollin mRNAs in human stratified epithelia. Int J Dev Biol. 1993 Mar;37(1):101–110. [PubMed] [Google Scholar]
- Troyanovsky S. M., Eshkind L. G., Troyanovsky R. B., Leube R. E., Franke W. W. Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell. 1993 Feb 26;72(4):561–574. doi: 10.1016/0092-8674(93)90075-2. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Itoh M., Nagafuchi A., Yonemura S., Tsukita S. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol. 1993 Dec;123(5):1049–1053. doi: 10.1083/jcb.123.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Nagafuchi A., Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992 Oct;4(5):834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]