Abstract
Phosphodiesterases (PDEs) are a superfamily of intracellular second messenger cyclic nucleotide hydrolyzing enzymes composed of 12 families. The Pde4 family has been implicated in depression and cognition and PDE4 inhibitors have been evaluated as antidepressants and possible cognitive enhancers. Pde4d−/− mice show an antidepressant phenotype and learning enhancement on some tests, but not others as do mice treated with PDE4 inhibitors. Here we report for the first time the behavioral phenotype of a new Pde4d knock-down (KD) rat model of PDE4D deficiency. Consistent with other data on PDE4D deficiency, Pde4d KD rats showed depression resistance in the Porsolt forced swim test and hyperreactivity of the acoustic startle response with no differential response on prepulse inhibition, suggesting no sensorimotor gating defect. Pde4d KD rats also exhibited a small exploratory activity reduction but no difference following habituation, and no enhanced spatial learning or reference memory in the Morris water maze. A selective improvement in route-based learning in the Cincinnati water maze was seen as well as enhanced contextual and cued fear conditioning and a more rapid rate of cued extinction from the higher freezing level that declined to WT levels only after ~20 extinction trials. The rat model confirms Pde4d’s role in depression but not in spatial learning or memory enhancement and shows for the first time higher fear conditioning and altered extinction compared with controls. The new model provides a tool by which to better understand the role of PDE4D in neuropsychiatric disorders and for the development of alternate treatment approaches.
Keywords: Phosphodiesterase, Pde4, Pde4d, Morris water maze, acoustic startle, locomotor activity, fear conditioning, Cincinnati water maze, F344 rats
Introduction
Phosphodiesterases (PDEs) are a superfamily of intracellular second messenger cyclic nucleotide hydrolyzing enzymes consisting of 12 families (Conti et al., 2003;Houslay, 2001). Each family has isoforms and some have post-transcriptional splice variants (Houslay, 2001). PDE4A and 4D are expressed in cortex, olfactory bulb, hippocampus, and brainstem (area postrema and n. tractus solitarius) (Perez-Torres et al., 2000). PDE4A,B,D are expressed in neurons whereas 4C is not (Zhang, 2009). PDEs are regulators of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) signaling and are implicated in some neurological and neuropsychiatric disorders (Siuciak, 2008). Accordingly, they have become potential therapeutic targets (Siuciak, 2008;Zhang, 2009). PDE4 inhibitors such as rolipram show preclinical and clinical antidepressant efficacy (Zhang et al., 2002;Li et al., 2009;Zhang, 2009), and preclinical evidence as cognitive (Burgin et al., 2010;Zhang, 2009) and noradrenergic enhancers (Nishi et al., 2008). The PDE4s are cAMP-specific and act via protein kinase A (PKA)-phophoso-cyclic AMP-response binding (pCREB) protein and related cascades (Zhang, 2009). Chronic treatment with PDE4 inhibitors increase brain BDNF and neurogenesis, as do established antidepressants. Although PDE4 inhibitors show antidepressant efficacy clinically (Fleischhacker et al., 1992;Hebenstreit et al., 1989;Zeller et al., 1984;Bobon et al., 1988), they have problematic side-effects (nausea) (Robichaud et al., 2002b;Robichaud et al., 2002a;Robichaud et al., 2001).
Pde4d−/− mice (Jin et al., 1999;Zhang et al., 2008;Li et al., 2011;Zhang et al., 2002;Hansen et al., 2000;Dlaboga et al., 2006) and rolipram-treated mice and rats show some overlapping effects. Rolipram-treated and Pde4d−/− mice show increased time in the target quadrant on Morris water maze (MWM) probe trials, increased novel object recognition (NOR), and increased passive avoidance latency. MicroRNA (miRNA)-induced Pde4d inhibition induces effects similar to those seen in Pde4d−/− and rolipram-treated mice (Li et al. 2011). Pde4d−/− mice also show antidepressant effects on tail-suspension (Zhang et al., 2002) and forced swim tests (FST) (Zhang et al., 2002;Zhang et al., 2008;Zhang, 2009) and rolipram-treated rats show increased responding on a differential low-rates of response (DRL) schedule (Zhang et al., 2006). In addition, PDE4 inhibitors (rolipram and HT0712) are able to reverse novel object memory deficits in CREB binding protein-deficient (CBP+/−) mice (Bourtchouladze et al., 2003), and rolipram reverses MK-801-induced radial-arm and passive avoidance memory deficits (Zhang et al., 2000), and reverses MEK-ERK (MAP-ERK kinase-extracellular related kinase) inhibitor-induced radial-arm maze (RAM) memory deficits (Zhang et al., 2004).
There are also inconsistencies. Pde4d−/− mice show no differences in fear conditioning 1 h and impaired retention at 24 h after unconditioned stimulus-conditioned stimulus (US-CS) pairing (Rutten et al., 2008). In the MWM, rats treated with the PDE4 inhibitors rolipram or DC-TA 46, show impaired probe trial performance with no differences on hidden or visible platform learning (Giorgi et al., 2004) in contrast to KO mice (Li et al., 2011). Pde4d−/− mice show no change in working memory in the radial-arm maze and fewer late (but not early) reference memory errors (Li et al., 2011). PDE4 inhibitors induce anxiolytic effects in rats (Silvestre et al., 1999) but no change (Imaizumi et al., 1994) or anxiogenic effects in mice (Zhang et al., 2008) and dogs (Heaslip and Evans, 1995).
To address such differences an alternate model was developed: a new PDE4D genetically modified rat, F344-Pde4dTn(sb-T2/Bart3)2.285Mcwi.
Materials and Methods
Animals and housing
The Pde4d KD rat was generated by Transposagen Biopharmaceuticals Pde4dTn(sb-T2/Bart3)2.285Mcwi (Lexington, KY) on a F344 background using a single gene trap insertion method based on approaches using the sleeping beauty transposable element (Lu et al., 2007). After germline transmission was verified, rats carrying the mutation were transferred to the Cincinnati Children’s Research Foundation’s vivarium where a breeding colony was established. Rats were housed in a 22 ± 1°C environment at 50 ± 10% humidity with a 14/10 h light/dark cycle (lights on at 600 h). Each polycarbonate cage (46 × 24 × 20 cm) contained woodchip bedding, ad libitum food and water, and was equipped with a stainless steel enclosure for environmental enrichment (Vorhees et al., 2008). The Institutional Animal Care and Use Committee approved the research protocol. The vivarium is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Genotyping
Genotyping was by PCR analysis of ear DNA. The genotyping primers used were 5′-AAA ATG GTG TGT TTC CGT GTG A-3′, 5′- GAG AGA CAC GTT ACC CTT CAA AAA T-3′, and 5′- CTG ACC TAA GAC AGG GAA TT-3′. PCR conditions were 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 40 s. The PCR assay generated a 918 bp fragment for the wild type allele and a 416 bp fragment for the Pde4d knock-down (KD) allele.
RT-PCR Gene Expression
Brain tissue from three wild-type and homozygous rats for the Pde4dtm1Mct targeted knockout-mutation were isolated and immediately homogenized in 1 ml of Prep Easy RNA Spin Kit RA1 buffer (USB, Cleveland, OH), lysates were cleared using PrepEase Filter Units (USB, Cleveland, OH), and RNA was isolated following the Prep Easy RNA Spin Kit (USB, Cleveland, OH) including DNase I treatment. RNA was quantified using a BioRad spectrophotometer at 260 nm and the 260/280 nm ratio was calculated for each sample. All sample A260:A280 ratios were approximately 1:90. The first strand cDNA was synthesized using USB 1st Strand cDNA Synthesis Kit (USB, Cleveland, OH). For the quantitative PCR, 10 μL of Kapa Sybr Fast qPCR Kit 2x Sybr Mastermix (Kappa Biosystems, Woburn, MA) was mixed with 0.4 μL of the 10 μM forward and reverse primers (5′-GCGAAGCGAATCAGAGAAC-3′ and 5′-CGCTGATGTGCCATTGTCC-3′ for the rat Pde4d and 5′-GCACAGTCAAGGCTGAGAATG-3′ and 5′-ATGGTGGTGAAGACGCCAGTA-3′ for rat Gapdh), 3 μL of the first strand cDNA template, 0.4 μL ROX dye (Kappa Biosystems, Woburn, MA) and 5.8 μL of DEPC treated water per sample. The 96 well plate was cycled using an Eppendorf Realplex 2.2 Mastercycler for 1 cycle at 95°C for 3 min; 40 cycles for an initial step at 95°C for 3 s, an annealing step at 58°C for 20 s, and an extension step at 72°C for 20 s. After the final cycle a 10 min dissociation curve protocol was performed. Melting curve analysis resulted in one peak for each product, indicating specificity of the PCR primers and acceptability of intercalating dye quantification. CT values were determined using the Eppendorf Realplex 2.2 Mastercycler software generated threshold and the Livak comparative CT method (Livak and Schmittgen, 2001) was used to determine fold-change. Fold-change was normalized to the endogenous control and wild type (WT) was set at one. All manufacture’s instructions were followed for the kits.
Offspring lineage
Only heterozygotes were bred to generate Pde4d KD and Pde4d+/+ offspring for testing. Dams were removed from their litters on P28 and pups were housed in groups of four of the same sex until P42 at which time they were housed 2 per cage throughout testing.
Behavioral Methods
Behavioral testing began at the ages indicated below. Male and female WT and KD rats were tested. Rats of each genotype were subdivided such that half received one set of tests (Arm-A) and the other half a second set (Arm-B) in order to reduce the number of tests each rat received. Order for each test arm was from least to more stressful. However, the last three tests of Arm-B were intended to constitute Arm-C but litter sizes were too small to accommodate this design, therefore, these tests were added at the end of Arm-B.
Arm-A
Elevated zero maze (EZM): P60
Straight channel swimming: P61
Cincinnati water maze (CWM): P62–79
Fear conditioning and extinction: P80–82
Arm-B
Light-dark exploration (LD test): P60
Straight channel swimming: P61
Morris water maze (MWM) hidden platform: P62–82
MWM cued: P83–84
Open-field locomotor activity: P88
Forced swim test (FST): P96–97
Acoustic startle response (ASR) with prepulse inhibition (PPI): P99
EZM
The first test for animals in Arm-A was the EZM as previously described (Shepherd et al., 1994) with minor modification (Braun et al., 2011). The apparatus is a circular runway (105-cm diameter), 72 cm above the floor, with a 10-cm wide path divided in equal quadrants. Two quadrants have 28-cm walls and two have 1.3-cm clear acrylic curbs. Mice were tested for 5 min and scored in real-time via an overhead camera connected to a monitor that was located outside the testing room for time spent in open quadrants, head-dips over the edge of open quadrants, number of open zone entries, and latency to first open zone entry. The test room was dimly lit and each animal was brought directly from its home cage and placed in a closed quadrant at which point the experimenter exited the room.
Straight Channel swimming
One day following EZM, animals were tested in a 15 × 244 × 50 cm straight swimming channel filled to a depth of 25 cm with room temperature water (21 ± 1°C) with a platform submerged 1.5 cm below the surface at one end. Each rat received four consecutive trials on the test day. On each trial, the rats were placed at one end facing the wall and timed until they climbed on the platform at the opposite end (maximum time = 2 min/trial).
CWM
The CWM, a test of route-based egocentric learning, began the day following straight channel swimming. The maze, as described elsewhere (Vorhees, 1987;Vorhees et al., 1991), consists of a series of nine T-shaped cul-de-sacs that branch from a central circuitous channel. The width of the channel throughout is 15 cm with walls that are 51 cm high, and the maze was filled half-way with water. Water was replaced at least twice per week and allowed to equilibrate overnight to room temperature (21 ± 1 °C) prior to daily testing. Testing was conducted under infrared light using infrared light emitters and a near infrared sensitive camera placed above the maze that was connected to a closed circuit TV monitor located in an adjacent room where the experimenter recorded performance (Vorhees et al., 2011). Administering the test under infrared light eliminates extramaze cues and prevents animals from using distal cues by which to navigate. Animals were started facing the wall at one end of the channel and were allowed 5 min per trial to find the escape platform with a 5 min intertrial interval if they failed to locate the goal on the first trial of the day. Animals were given two daily trials for 18 days. Errors and latency to escape were recorded. An error was counted when an animal digressed from the central channel into a stem or when they entered an arm of one of the T-shaped cul-de-sacs. Repetitive errors within a T were counted separately. Occasionally an animal did not find the escape despite many trials and stopped searching. This resulted in few errors but maximum time compared with animals that escaped. To adjust for this, error scores for these animals were corrected to the number of errors committed by the poorest performing successful animal + 1.
Fear conditioning with extinction
Fear conditioning began the day following CWM. On each day, animals were placed in test chambers (Coulbourn Instruments, Allentown, PA) fitted with a speaker and grid floor connected to a scrambled foot shock source with a video camera mounted in the ceiling that was connected to Freeze Frame software (Coulbourn Instruments, Allentown, PA). Test chambers were placed inside larger sound-attenuating enclosures. On day 1, animals were placed in the test chambers for 10 min prior to tone presentation and then given 3 tone-footshock pairings (82 dB, 2 kHz, 30 s duration). Each pairing consisted of the 30 s tone accompanied during the last second by footshock (0.5 mA). Tone-shock pairings were separated by 3 min. One day later, animals were returned to the same test chamber for 6 min to test for contextual freezing. Activity records were later scored for minor movement occurrences >4 s. Twenty-four h following contextual testing, animals were tested for cued fear. For this they were returned to the test chambers with a different floor (grid rather than bars). Animals were exposed to a 3 min baseline interval then to 3 min of continuous tone presentation and scored as above. Following this, animals were given 30 additional trials. Each trial lasted 60 s and consisted of 30 s of tone followed by 30 s of no-tone. Data for contextual conditioning were analyzed as percentage of time freezing when returned to the test chamber on day-2. Data for cued conditioning were analyzed as percentage of time freezing on day-3 after tone presentation. Data for extinction trials were scored on successive tone presentations.
Arm-B
LD test
For animals in Arm-B, the first test was the LD test. Rats were placed in locomotor activity chambers made of a clear acrylic (~40 × 40 cm; Accuscan Electronics, Columbus, OH) fitted with a dark enclosure that occupied 50% of the interior space. Rats were placed in the corner of the lighted side and the number of transitions from light to dark (dark entries), latency to first entry to the dark side, and time spent in the light side were recorded for 10 min (Crawley and Goodwin, 1980). The room was illuminated with overhead fluorescent lights. The chambers were cleaned with a 70% ethanol solution in between animals.
Straight Channel swimming
Straight channel swimming was assessed on P62 as above for Arm-A animals.
MWM
Spatial learning and memory were assessed in the MWM using procedures described previously (Vorhees and Williams, 2006). Testing began on P63 and was performed in a 210 cm diameter circular tank. Animals were trained in 3 phases of hidden platform learning (acquisition (platform in SW position), reversal (platform in NE position), and shift (platform in NW position)) followed by cued learning. For hidden platform training, animals received 4 trials per day for 6 days followed by a 30 s probe trial on day 7 with the platform removed. Each phase used a platform of a different size (10, 7, and 5 cm diameter, respectively). Following the hidden platform phases, cued learning was tested to ensure that the rats could use proximal cues and swim at comparable speeds. For this procedure, the submerged 10 cm platform was used to which a plastic ball mounted on a rod was added that protruded above the surface of the water to mark the platform’s location. Curtains were closed around the maze to minimize extramaze cues and the animals were given four trials per day for two days with the platform and start positions changed for every trial such that spatial cues could not be used to find the platform. During hidden platform trials, a video camera and tracking software were used to monitor each animal’s performance (AnyMaze, Stoelting Instruments, Wood Dale, IL). On platform trials, latency, cumulative distance, path length, and swim speed were analyzed. On probe trials, platform site crossovers, swim speed, and average distance from the target were analyzed. For cued trials, latency was recorded manually and analyzed.
Open-field locomotor activity
Locomotor activity was assessed the day following MWM in 76 × 76 × 48 cm black polypropylene chambers for 60 min. Total distance, peripheral distance (i.e., within 5 cm from the wall), and center distance were analyzed using Anymaze (Stoelting Company, Wood Dale, IL) in 5 min intervals.
FST
Forced swim was assessed 7–14 days after the completion of locomotor activity. Chambers were made of clear acrylic with an inside diameter of 19 cm and height of 60 cm. Each of 4 chambers were separated from the next by black dividers such that one animal could not see the next. Water was filled to a depth of 30 cm (rats were not able to touch the bottom). On test day-1, animals were placed in the chambers for 15 min with no scoring. On test day-2, animals were again placed in the chambers for 5 min while being scored by an observer who was blinded to treatment group for latency to first bout of immobility and total time spent immobile. Water was drained and the apparatus cleaned with 70% EtOH between animals.
ASR/PPI
This test was conducted 1–7 days following FS. Acoustic startle reactivity (ASR) with reflex modified inhibition by prepulse stimulation (PPI) was measured in four SR Lab test chambers (San Diego Instruments, San Diego, CA). Each test chamber was calibrated using the manufacturer’s guidelines and sensitivity was verified using a mechanical oscillator to ensure consistent sensitivity. At the start of each test, animals were placed in acrylic cylindrical tubes mounted atop piezoelectric force transducers and positioned inside sound attenuated test chambers. Background white noise was set at 70 dB. The test paradigm was similar to that of (de Jong et al., 2006). Each test session consisted of a 5 min acclimation period followed by a 5 × 5 Latin square sequence of trials of 5 different types: no stimulus, startle signal alone, startle signal with prepulse 3 dB above background (73 dB), startle signal with prepulse 5 dB above background (75 dB), and startle signal with prepulse 10 dB above background (80 dB). Each animal received each trial type once in each of 5 test orders, and the entire Latin square sequence was repeated; hence, each animal received a total of 50 trials (10 of each type). Trials of the same type were averaged together for analysis. The intertrial intervals ranged from 10–20 s. The interstimulus interval was 100 ms (from prepulse onset to startle signal onset). The startle signal was a 120 dB mixed frequency white noise burst lasting 20 ms. The recording window was 100 ms. Prepulses lasted for 20 ms. Stimulus intensity was measured using a Quest sound level meter (SPL scale) with the meter placed in the test chamber in the center of the test stage with door closed and the microphone directed toward the speaker. Response amplitude (Vmax = maximum voltage change) was recorded in units of voltage (mV). Test chambers were cleaned with 70% ethanol between animals.
Statistical Analysis
Data were analyzed using completely randomized block analyses of variance (ANOVA) mixed linear models (SAS v9.2, Proc Mixed, SAS Institute, Cary, NC). The Kenward-Roger adjusted degrees of freedom method was used. Interactions were analyzed using the slice-effect ANOVA method at each level of the within-subject factor. A posteriori group comparisons were analyzed using the Hochberg step-up method. Significance was accepted at p ≤ 0.05 and data are presented as least square (LS) mean ± SEM.
Results
Quantitative RT-PCR of each genotype with WT rats expressed as unity resulted in expression levels for WT = 1.0 ± 0.14 compared with Pde4d KD = 0.28 ± 0.04. Hence, a gene dosage effect of the mutation is evident but complete absence of Pde4d in mutants did not occur, hence, it is a genetic known-down (KD).
Arm-A Results
Elevated Zero Maze
There were no significant genotype differences observed for time-in-open quadrants, number of head dips, number of open zone entries, or latency to first open quadrant entry (not shown).
Straight Channel Swimming
A genotype x trial ANOVA showed no significant genotype effect; nor did a separate analysis on the fastest trail, suggesting no motoric or motivation deficits to escape from water (not shown).
Cincinnati Water Maze
No significant genotype differences in latency to reach the goal or errors were observed. However, trends were seen on both measures. For errors there was a genotype main effect trend (F(1,58.8) = 3.17, p ≈ 0.08) and a genotype x day trend (F(17,810) = 1.6, p ≈ 0.06). Learning curves for errors are shown in Fig. 1A. As can be seen, learning in this maze consists of essentially three phases: multiple but unsuccessful search trials to find the escape (trials 1–4), finding the escape and improving (trials 5–12), and mastery to reach asymptotic performance (trials 13–18). Based on these components, additional analyses were conducted on each learning phase. There were no significant genotype main effects or genotype-related interactions on the first or third phase of learning. However, the analysis of the middle or improvement phase (trials 5–12) showed a significant genotype main effect (F(1,56) = 4.57, p < 0.04). As can be seen, the KD group made significantly fewer errors than WT controls during the mid-portion of the learning curve.
Fig. 1.
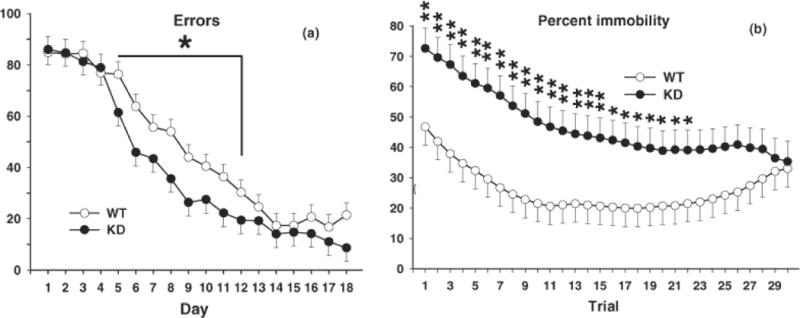
CWM and Fear Extinction. A, Total errors committed during CWM testing for 18 consecutive days, 2 trials per day. There was a significant genotype effect during the middle portion of maze learning with KD rats making fewer errors than WT rats. Group sizes were = Total (males/females): WT = 32 (16/16), KD = 26 (13/13); sex did not interact with genotype. B, Fear extinction. Following conditioned fear training, rats were tested for contextual fear 24 h after training (see Fig. 2) and cued fear 48 h after training, including with repeated cue presentations (extinction), i.e., rats were given 30 trials consisting of alternate 30 s periods of tone and 30 s of no-tone (no foot-shock). From extinction trials 1–22 Pde4d KD rats were more immobile than WT controls (i.e., they showed less extinction than controls). Group sizes are as in Fig. 2. *P < 0.05; **P < 0.01 vs. WT.
Fear Conditioning
On day-1, there were no differences in movement patterns during the pre-CS-US interval. Similarly, on day-1 there were no differences in freezing during the 3 tone-shock pairings or during the intervals between CS-US presentations (data not shown). On day-2 (contextual conditioning) there was a genotype effect on immobility (F(1,45) = 4.11, p < 0.05). The KD group spent more time immobile than the WT group (Fig. 2A). On day-3 (cued conditioning), immobility during the tone was not significantly different between genotypes but a genotype main effect trend was seen (F(1,45) = 2.96, p ≈ 0.10) (Fig. 2B). During the extinction phase, there was a genotype (F(1,45.8) = 6.2, p < 0.02) and genotype x trial (F(1,45.8) = 1.71, p < 0.01) interaction. KD animals spent more time immobile during extinction overall (Fig. 2C) and especially on trials 1–22 (Fig. 1B). To determine there was a difference in the net extinction irrespective of the higher time spent freezing at the beginning of extinction in the KD group, we compared the difference between the amount of freezing on the first and last extinction trials for the two groups by two-tailed t-test for independent samples. The difference was significant (t(47) = 2.05, p < 0.05). The mean percent freezing time declined in the KD group by 39.6 ± 9.6 whereas it declined by only 14.6 ± 7.7 in the WT group.
Fig. 2.
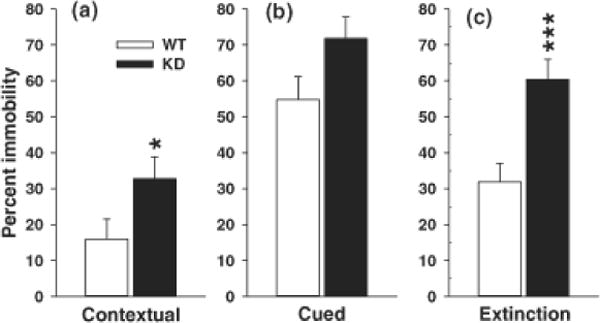
Conditioned Fear Learning. Conditioned fear consisted of training (no differences in time spent moving vs. not moving were seen during training. 24 h after training, rats were tested for conditioned fear, assessed as percent time immobile/freezing to the same context or in response to the conditioning tone. (A) contextual fear (percent time spent freezing when placed back in the original test chamber), (B) cued fear (percent time spent freezing in test chamber with new floor after presentation of the tone), and (C) cued extinction (percent time spent freezing as in (B) with repeated tone presentations). There were no genotype differences in immobility during training or cued retention. There were significant genotype differences during contextual retention and cued extinction. Panel C showed extinction averaged across trials (see Fig. 1 for the extinction curve by trial). Group sizes were = Total (males/females): WT = 26 (14/12), KD = 23 (14/9); sex did not interact with genotype. *P < 0.05; ***P < 0.001 vs. WT.
Arm-B Results
Light/Dark exploration
No genotype differences in the number of transitions from light to dark (dark entries), latency to first dark side entry, or total time spent in the light side was found (not shown).
Straight Channel swimming
No genotype differences in latency to swim the straight channel were obtained in the genotype x trial ANOVA or on a separate ANOVA on fastest trial, suggesting no motoric or motivational problem for water escape (not shown).
Morris Water Maze
No differences in hidden platform learning were observed for any of the three test phases, i.e., no differences in path length during acquisition, reversal, or shift platform learning (Fig. 3).
Fig. 3.
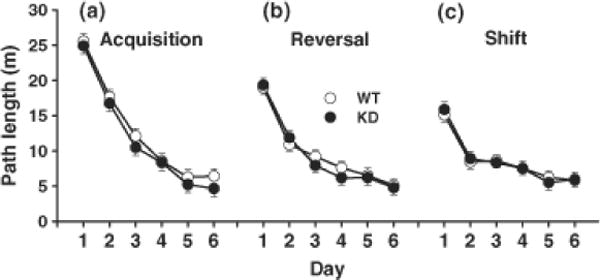
Morris water maze (MWM) hidden platform learning. Data are mean ± SEM of 4 trials per day for path length (m) to reach the goal during 6 days of training during (A) acquisition, (B) reversal, and (C) shift trials with progressively smaller platforms (10, 7, and 5 cm in diameter). No differences in hidden platform learning were observed. Group sizes were = Total (males/females): WT = 30 (15/15), KD = 27 (15/12); sex did not interact with genotype.
There were effects of genotype on probe trials (Fig. 4). On the probe trial given after acquisition, there was a genotype x sex effect for number of crossovers (F(1,54) = 7.04, p < 0.01), however a posteriori comparisons between males and females of each genotype failed to reveal significant differences. For swim speed, there was a significant genotype main effect during acquisition-probe only (F(1,54) = 6.31, p < 0.02) with the KD group swimming slower than the WT group. No effects of genotype were obtained on other measures of acquisition probe performance or on any measure of reversal-probe or shift-probe.
Fig. 4.
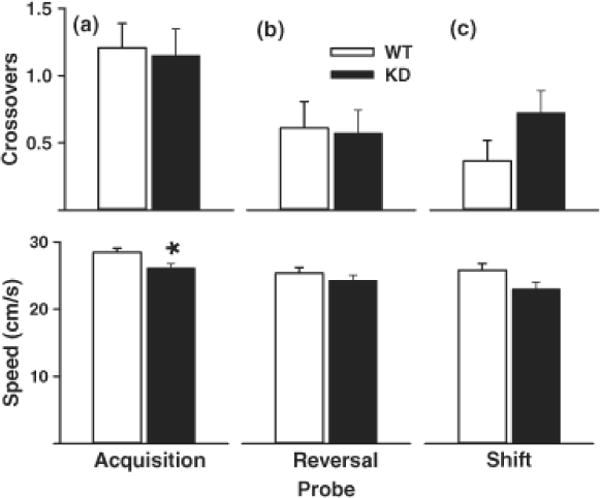
MWM Probe trials. 24 h after the last training trial for each phase shown in Fig. 5, rats were given a single 30 s probe trial with the platform removed. Top, mean ± SEM platform site crossovers; Bottom, mean ± SEM swim speed (cm/s). (A) Acquisition-probe, (B) reversal-probe, and (C) shift-probe. KD rats swam significantly slower on acquisition-probe compared with WT rats. Group sizes are as in Fig. 5. *P < 0.05 vs. WT.
On the cued version of the MWM, there were no genotype differences in latency to find the randomly positioned cued platform (not shown).
Locomotor Activity
For total distance traveled, there was a genotype main effect (F(1,41.9) = 6.56, p < 0.02) with the KD group traveling less than the WT group. There was also a genotype x sex x interval interaction (F(11,396) = 2.26, p < 0.02). To examine exploration, the first 5 min interval was analyzed using a slice-effect ANOVA which showed that KD females were less active than WT females. During the first 30 min, both KD males and females were less active than WT animals, whereas during the last 30 min, there were no genotypic differences (Fig. 5).
Fig. 5.
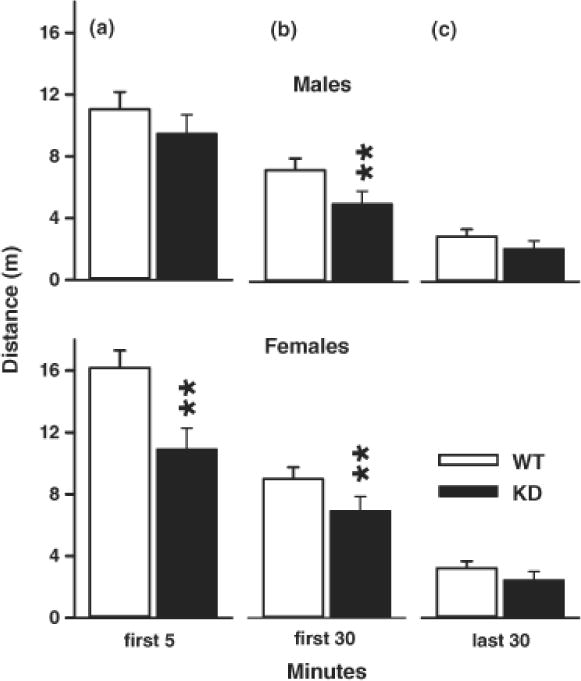
Locomotor Activity. Locomotor activity was tested for 60 min and analyzed for (A) exploration (first 5 min), (B) habituation (first 30 min), (C) baseline (last 30 min). KD males and females were significantly less active during the first 30 min compared with same sex controls. Females were less active during the first 5 min compared with female WT rats. Top: males; Bottom, females. Group sizes were = Total (males/females): WT = 26 (13/13), KD = 20 (11/9). **P < 0.01 vs. WT.
Forced Swim
There was a main effect of genotype for immobility time (F(1,40) = 5.94, p < 0.02) during the forced swim test. Rats in the KD group (regardless of sex) spent less time immobile than those in the WT group (WT: 207.3 ± 9.2 compared with KD: 174.2 ± 10.0. There was no significant effect observed for latency to first immobility bout (WT: 17.5 ± 4.7 s; KD: 26.3 ± 5.1 s).
Startle/PPI
There was a significant genotype main effect on ASR (F(1,40) = 10.01, p < 0.01) and a genotype x prepulse interaction (F(1,40) = 4.53, p < 0.02). For prepulses of 0 and 70 dB, KD animals had higher ASR amplitude than WT animals with a trend at 76 dB (Fig. 6).
Fig. 6.
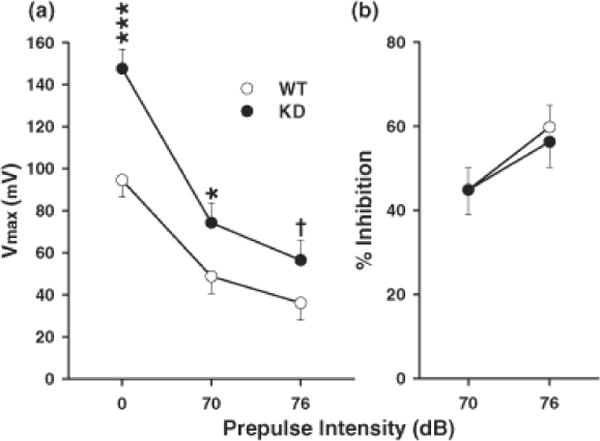
Acoustic startle response with prepulse inhibition (ASR/PPI) amplitude (Vmax: measured in mV). Data are mean ± SEM of 10 trials of each type averaged together. Trial order was by Latin square design. Trials of no stimulus were included as an internal control to ensure system integrity. (A) Average startle response for each prepulse intensity level (0, 70, and 76 db). No effects were seen on the no stimulus trials (not shown). KD rats showed significant hyperreactivity to the basic startle signal and in the presence of a 70 dB prepulse, but at 76 db prepulse the effect was marginal compared with WT rats. (B) Percent prepulse inhibition as a function of response on no-prepulse trials. Group sizes were = Total (males/females): WT = 25 (12/13), KD = 19 (10/9). Sex did not interact with genotype. †P<0.10; *P < 0.05 vs. WT; ***P < 0.001 vs. WT.
Discussion
The PDE4 family has been implicated in depression and multiple lines of evidence support this role. These include studies in mice using gene deletion, enzyme inhibitors, and miRNA to knock-down the protein. Pde4d−/− mice show an antidepressant phenotype in the tail-suspension (Zhang et al., 2002) and forced swim tests (Zhang et al., 2002;Zhang et al., 2008;Zhang, 2009). Treatment of WT mice with PDE4 inhibitors: rolipram, piclamilast, CPD840, MEM1018, and MEM1091 induced an antidepressant phenotype in the forced swim test, but only rolipram and picolamilast decreased responses and increased reinforcement rates in an operant DRL paradigm (Zhang et al., 2006). In the present experiment, using an entirely different model of PDE4D deficiency, the Pde4d KD F344 rat, we confirmed an antidepressant phenotype in the forced swim test. Hence, across two species, two genetic models, and multiple pharmacological models, PDE4D reduction reliably induces immobility resistance which is predictive of antidepressant activity and related changes in DRL testing. Moreover, human trials of PDE4 inhibitors show clinical antidepressant efficacy, however, this is accompanied by the complication of nausea which makes these drugs unacceptable until further molecular differentiation of the structure of such inhibitors can be designed to eliminate this untoward effect. Hence, the rat Pde4d deficient model provides convergent evidence that PDE4D is a potentially important pharmacotherapeutic target that represents a new class of antidepressants that would benefit some of the many patients refractory to the therapeutic effects of existing antidepressants.
The Pde4d−/− mouse also shows evidence of cognitive enhancement. For example, these mice have been shown to exhibit greater target quadrant preference on memory trials in the MWM, increased preference for a new object over a familiar one in the novel object recognition test, increased latency to remain on an elevated platform in the step-down inhibitory avoidance test, and fewer reference memory errors for constantly unbaited arm entries on the last 2 days out of 14 in the radial arm maze with no differences on trial-dependent performance in the daily constantly baited arms in an 8-arm apparatus; several of these effects were also seen in rolipram-treated mice (Li et al., 2011). On the other hand, another study showed no differences in Pde4d−/− mice on another type of learning, fear conditioning (Rutten et al., 2008), and rats treated with rolipram show impaired MWM learning and reference memory on probe trials (Giorgi et al., 2004). For the present data in the genetic Pde4d KD rat, we find a mixed pattern of cognitive effects. We tested the MWM more extensively than in any of the previous studies, testing the animals in three progressively more challenging phases of the task, acquisition with a 10 cm platform, reversal with a 7 cm platform, and shift with a 5 cm platform. In addition, we used a maze that was proportionately more challenging than that used in the mouse and rat studies (Li et al., 2011;Giorgi et al., 2004). The mouse pool was 95 cm in diameter with an 8.5 × 15.5 cm platform for a search area ratio of 53.8:1. Moreover, impaired probe performance in rats treated with PDE4D inhibitors was observed in the MWM in a 180 cm pool with a 13 × 15 cm platform for a search area ratio of 130.5:1. By contrast, we used a 210 cm diameter pool with a 10 cm diameter platform during acquisition for a search area ratio of 441:1, and this ratio became even larger on reversal (900:1), and still larger on shift (1764:1). Despite this, we found no differences on any phase of the hidden platform learning trials. Nor did we find any reference memory change on probe trials given 24 h after each of the three learning phases for average distance to the platform site, crossovers, or percent time or percent distance in the target quadrant. Hence, we found no evidence of enhanced or impaired spatial learning or spatial reference memory.
As mentioned, one study in Pde4d−/− mice showed no changes in 1 h contextual or cued fear conditioning, but showed impaired conditioning at 24 h in both contextual and cued fear (Rutten et al., 2008). In the present experiment with Pde4d KD rats, we did not conduct a 1 h fear conditioning test and tested at 24 h for contextual fear conditioning and at 48 h for cued fear conditioning. In contrast to Pde4d−/− mice, Pde4d KD rats showed enhanced contextual fear conditioning 24 h post training. During the 48 h cued conditioning, Pde4d KD rats showed no only a trend on the first retention trial. The most striking finding in the Pde4d KD rats was on subsequent cued extinction trials, in which the KD group showed higher percent time spent freezing with a prolonged retention of the immobility response compared with WT. However the rate of extinction from the first to the last trial, was greater in the KD group than in the WT group, requiring 20 trials to decline to WT levels. Hence, the issue of whether PDE4D enhances or impairs conditioned fear is not easily resolved, but the present data support enhancement of conditioning with a prolonged extinction of the response. This may have implications for the way PDE4D inhibitors might affect patients with depression but the precise clinical implications cannot be predicted based on this finding.
No other data are available on route-based egocentric learning after PDE4D reduction. In the present experiment, we tested this form of learning using the CWM. We did not find an overall change in route-based learning in the absence of distal cues in Pde4d KD rats but we did see fewer errors in the mid-portion of the learning curve and this effect was significant. In this mid-range, the Pde4d KD rats learned to eliminate errors at a faster rate than WT even though WT rats later caught up and the groups ended the test at the same performance level.
We also found Pde4d KD rats to be slightly less exploratory in an open-field during the first 5 and first 30 min but not after they had become fully habituated to the apparatus during the last 30 min of the 60 min test period. Pde4d−/− mice also show reduced open-field activity during a 5 min exploration test (Zhang et al., 2008). However, a subsequent experiment did not replicate this finding and showed no differences in 5 min of open-field exploration in Pde4d−/− mice (Li et al., 2011). Because the mouse studies did not test for longer periods, further comparisons on locomotor exploration and habituation are not possible.
We also found that Pde4d KD rats had a marked enhancement of the acoustic startle reflex with no indication that prepulse inhibition was differentially affected. Although the magnitude of the startle facilitation effect observed in the Pde4d KD group was reduced in the presence of the 70 dB prepulse compared with no prepulse, and further reduced in the presence of the 76 db prepulse, further analysis in terms of percent inhibition revealed no differential response, i.e., the WT group showed a 48.4% startle inhibition at 70 dB versus the Pde4d KD group that showed a 49.7% inhibition at this prepulse level. At the 76 dB prepulse level, the WT group showed a 61.8% startle inhibition and the Pde4d KD group a 61.7% startle inhibition; essentially identical degrees of inhibition. This indicates that despite the Pde4d KD rats being hyper-reactive to the startle signal, they do not show a deficit in sensorimotor gating.
The Pde4d KD rat shows a clear behavioral phenotype and this should prove useful in research to further understand the role of PDE4D in brain function, especially in depression. This new model may also be valuable for understanding the role of PDE4D in the retention of conditioned fear responses that may have therapeutic value when used appropriately. While the Pde4d KD rat is not a complete gene deletion, it should nonetheless be a valuable model in further analyses of this protein and can be used in conjunction with the Pde4d−/− mouse and pharmacological inhibitors to more fully elucidate how this important enzyme may be manipulated for treatment purposes.
Acknowledgments
Supported by National Institutes of Health grants DA006733 (CVV) and training grant ES007051 (TLS and MRS).
Footnotes
Author Conflict of Interest Statement
TLS, AAB, RMAK, MTW, and CVV declare no conflict of interest. EO is President and CEO of Transposagen Biopharmaceutical Company that provided the gene-targeted animals for this project.
References
- Bobon D, Breulet M, Gerard-Vandenhove MA, Guiot-Goffioul F, Plomteux G, Hernandez M, Schratzer M, Troisfontaines B, von FR, Wachtel H. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. doi: 10.1007/BF00381071. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Snaphaan LJ, Pattij T, Veening JG, Waldinger MD, Cools AR, Olivier B. Effects of chronic treatment with fluvoxamine and paroxetine during adolescence on serotonin-related behavior in adult male rats. Eur Neuropsychopharmacol. 2006;16:39–48. doi: 10.1016/j.euroneuro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dlaboga D, Hajjhussein H, O’Donnell JM. Regulation of phosphodiesterase-4 (PDE4) expression in mouse brain by repeated antidepressant treatment: comparison with rolipram. Brain Res. 2006;1096:104–112. doi: 10.1016/j.brainres.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wolf R, Gerlach W, Jaklitsch H, Hernandez M. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154:99–106. doi: 10.1016/j.bbr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Hansen G, Jin S, Umetsu DT, Conti M. Absence of muscarinic cholinergic airway responses in mice deficient in the cyclic nucleotide phosphodiesterase PDE4D. Proc Natl Acad Sci U S A. 2000;97:6751–6756. doi: 10.1073/pnas.97.12.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip RJ, Evans DY. Emetic, central nervous system, and pulmonary activities of rolipram in the dog. Eur J Pharmacol. 1995;286:281–290. doi: 10.1016/0014-2999(95)00457-2. [DOI] [PubMed] [Google Scholar]
- Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, Hernandez M, Schony W, Schratzer M, Soukop W. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22:156–160. doi: 10.1055/s-2007-1014599. [DOI] [PubMed] [Google Scholar]
- Houslay MD. PDE4 cAMP-specific phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 2001;69:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- Imaizumi M, Miyazaki S, Onodera K. Effects of a non-xanthine adenosine antagonist, CGS 15943, and a phosphodiesterase inhibitor, Ro 20-1724, in a light/dark test in mice. Methods Find Exp Clin Pharmacol. 1994;16:717–721. [PubMed] [Google Scholar]
- Jin SL, Richard FJ, Kuo WP, D’Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci U S A. 1999;96:11998–12003. doi: 10.1073/pnas.96.21.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-F, Cheng Y-F, Huang Y, Conti M, Wilson SP, O’Donnell JM. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, Zhang HT. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology. 2009;34:2404–2419. doi: 10.1038/npp.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–346. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–374. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Lachance N, Jolicoeur P, Rasori R, Chan CC. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002a;135:113–118. doi: 10.1038/sj.bjp.0704457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–269. doi: 10.1016/s0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002b;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, Wallace TL. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci. 2008;28:625–632. doi: 10.1111/j.1460-9568.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze“ as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Fernandez AG, Palacios JM. Effects of rolipram on the elevated plus-maze test in rats: a preliminary study. J Psychopharmacol. 1999;13:274–277. doi: 10.1177/026988119901300309. [DOI] [PubMed] [Google Scholar]
- Siuciak JA. The role of phosphodiesterases in schizophrenia : therapeutic implications. CNS Drugs. 2008;22:983–993. doi: 10.2165/0023210-200822120-00002. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/−methylenedioxymethamphetamine, (+)-amphetamine and +/−fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2011;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Hernandez M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–190. doi: 10.1055/s-2007-1017435. [DOI] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Crissman AM, Dorairaj NR, Chandler LJ, O’Donnell JM. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, O’Donnell JM. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O’Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Deng C, Hopper AT, De VM, Rose GM, O’Donnell JM. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology (Berl) 2006;186:209–217. doi: 10.1007/s00213-006-0369-4. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O’Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]


