Abstract
Background
Co-culture of mesenchymal stem cells (MSCs) from the retropatellar fat pad and peripheral blood has been shown to stimulate anterior cruciate ligament (ACL) fibroblast proliferation and collagen production in vitro. Current techniques of bio-enhanced ACL repair in animal studies involve adding a biologic scaffold, in this case an extracellular matrix based scaffold saturated with autologous whole blood, to a simple suture repair of the ligament. Whether the enrichment of whole blood with MSCs would further improve the in vivo results of bio-enhanced ACL repair was investigated.
Hypothesis/Purpose
The hypothesis was that the addition of MSCs derived from adipose tissue or peripheral blood to the blood-extracellular matrix composite, which is used in bio-enhanced ACL repair to stimulate healing, would improve the biomechanical properties of a bio-enhanced ACL repair after 15 weeks of healing.
Study Design
Controlled laboratory study.
Methods
Twenty-four adolescent Yucatan mini-pigs underwent ACL transection followed by: 1) bio-enhanced ACL repair, 2) bio-enhanced ACL repair with the addition of autologous adipose-derived MSCs and 3) bio-enhanced ACL repair with the addition of autologous peripheral blood derived MSCs. After fifteen weeks of healing, structural properties of the ACL (yield & failure load, linear stiffness) were measured. Cell and vascular density were measured in the repaired ACL via histology, and its tissue structure was qualitatively evaluated using the Advanced Ligament Maturity Index.
Results
After fifteen weeks of healing, there were no significant improvements in the biomechanical or histological properties with the addition of adipose-derived MSCs. The only significant change with the addition of peripheral blood MSCs was an increase in knee anteroposterior (AP) laxity when measured at 30 degrees of flexion.
Conclusions
These findings suggest that the addition of adipose or peripheral blood MSCs to whole blood prior to saturation of an extracellular matrix carrier with the blood did not improve the functional results of bio-enhanced ACL repair after 15 weeks of healing in the pig model.
Clinical Relevance
Whole blood represents a practical biologic additive to ligament repair, and any other additive (including stem cells) should be demonstrated to be superior to this baseline before clinical use is considered.
Keywords: Bio-enhanced ACL Repair, Blood, MSCs, Biomechanical Properties, Ligament Maturity Index
INTRODUCTION
The tear of the anterior cruciate ligament (ACL) is one of the more common knee ligament injuries, with an especially high prevalence in active adolescent athletes.36 The current standard treatment is the surgical restoration of the mechanical stability of the knee joint by replacing the torn ACL with a tendon graft; however, recent reports of increased failure rates in adolescents,51 and the high rate of progression to premature osteoarthritis,1 has stimulated interest in alternative treatments of this injury, including bio-enhanced ACL repair. Bio-enhanced ACL repair involves augmenting a suture repair of a ligament with placement of an extracellular matrix scaffold containing a biologic additive in the ligament wound site to stimulate ligament healing. This method, using whole blood or platelet-rich plasma as the biologic additive, has recently demonstrated reasonable results when evaluated in a large animal model of ACL injury.30, 48 In these models, while the use of a scaffold alone has been found to be ineffective at improving the mechanics of a suture repair,14 the combination of the scaffold and platelets (either in the form of platelet-rich plasma or whole blood) has been found to significantly improve the mechanical properties of a suture repair of the ACL,22 as well as the mechanical properties of an ACL graft.15
The healing of the injured ACL using bio-enhanced repair is primarily achieved by fibroblasts, which migrate from the ACL into the wound site.34 Once within the wound site, key processes including cell proliferation and collagen production result in the formation of a fibrovascular scar, which then remodels into tissue with a structure closer to that of the normal ligament.32–35 A recent in vitro study demonstrated that adding MSCs obtained from the adipose tissue or peripheral blood stimulated cellular proliferation and collagen production by ACL cells,40 and thus, we hypothesized that the stimulation of these behaviors with MSCs may lead to improved healing in vivo.
Other investigators have looked at the utility of adipose tissue-derived stem cells (ADSCs) for ligament engineering. They found that human ADSCs could be pushed toward a ligament fibroblast phenotype by culturing them on a ligament-derived extracellular matrix scaffold,25 though not by culture with EGF or bFGF.11 Co-culture of ACL fibroblasts and ADSCs led not only to an upregulation of collagen type I and type III gene expression in ACL fibroblasts but also in ADSCs when tested in vitro,40 a further indication that these cells have the potential to modulate the behavior of surrounding cells through secretion of cytokines4 as well as differentiate down the fibroblast differentiation pathway.11 ADSCs have also been found to be helpful for regenerating periodontal tissue when combined with platelets and studied in vitro,42 and in small animal models when combined with a PLGA scaffold.2 These positive effects of MSCs in vivo may also be a result of MSCs functioning to suppress both transient and perpetual immune surveillance systems and creating an ideal healing environment by secreting factors and altering the local microenvironment.3
Peripheral blood mononuclear cells have also become of recent interest as a potential source of mesenchymal stem cells. A comparison of peripheral blood mononuclear cell derived (PBSC) and bone marrow derived mesenchymal stem cells demonstrated similar ability for self-renewal and tri-lineage differentiation of the two cell sources.8 In a study utilizing co-culture of ACL fibroblasts and PBSCs, PBSCs had increased collagen type I gene expression, an indicator of their potential to differentiate into the fibroblast lineage.40 PBSCs have also been shown to enhance the anabolic effects of platelet-rich plasma on ACL fibroblasts53 as well as stimulate the proliferation and collagen gene expression in these cells by co-culture.40
The current method of using either ADSCs or PBSCs involves obtaining the cells, expanding them in culture and then surgically reimplanting them. This series of procedures, while technically possible, represents significant health care costs and regulatory hurdles. The regulatory pathway for these biologic additives requires not only addressing all of the standard concerns of sterility and biocompatibility for biologics, but also addressing the issues of exposure to the cells to potentially hazardous materials during processing (bacteria, bovine serum, other infectious agents), sterility at the time of implantation, and rigorous tracking of the identification of each sample throughout the culture process. Because of the additional costs and risks for the patient, particularly for diseases that are typically not life-threatening, these types of biologic additives need strong evidence of efficacy in preclinical models before any translation to clinical trials should be attempted.
Our central hypothesis was that the enrichment of whole blood with autologous ADSCs or PBSCs to the extracellular matrix scaffold for bio-enhanced ACL repairs would enhance the biomechanical outcomes of the repair in the pig.
MATERIALS AND METHODS
Experimental design
Approvals from the Institutional Animal Care and Use Committee were obtained prior to the start of this study. Based on an a priori sample size calculation, twenty-four male Yucatan mini-pigs in late adolescence with closed femoral and tibial physes [age (mean ± SD): 20.2 ± 2.25 months, weight: 56.9 ± 8.3 kg] were randomized to one of three experimental groups (Figure 1); 1) bio-enhanced ACL repair using a bioactive scaffold with autologous whole blood (BLOOD), 2) bio-enhanced ACL repair using the same bioactive scaffold with autologous whole blood and the addition of autologous adipose-derived stem cells from the retro-patellar fat pad (ADSC), and 3) bio-enhanced ACL repair using the same bioactive scaffold with autologous whole blood and the addition of autologous peripheral blood stem cells (PBSC). All animals were housed for 15 weeks after surgery.
Figure 1. Surgical technique flow chart.
Time flow chart of ACL-repair methods used in this study. 1) BLOOD; A - requires blood draw during surgery to soak the ECM scaffold after implantation ; 2) ADSC; B -Initially, tissue sample from the retropatellar fat pad is harvested from which ADSCs are derived via adhesion cell culture; C – after 14 days ADSCs are harvested, mixed with blood during surgery and injected in the ECM scaffold after implantation; 3) PBSC; D - requires PBSC isolation from peripheral blood sample by Ficoll density gradient separation and adhesion cell culture; E – after 14 days PBSCs are harvested, mixed with blood during surgery and injected in the ECM scaffold after implantation.
BLOOD – bioenhanced ACL Repair
ADSC – bioenhanced ACL Repair with addition of adipose tissue derived stromal cells
PBSC - bioenhanced ACL Repair with addition of peripheral blood derived mononuclear cells
ECM –extracellular matrix
ADSC – adipose tissue derived stem cells
PBSC – peripheral blood mononuclear cell derived stem cells
Retropatellar Adipose Tissue-Derived Stem Cells (ADSC)
The ADSCs were harvested as previously described.40 Two weeks ahead of the ACL surgery, animals from group ADSC had a small portion of their retropatellar fat pad from the left knee [weight (mean ± SD): 1.1 ± 0.21 g)] removed through a small medial arthrotomy. This tissue was minced, digested with collagenase (Worthington Biochemical, Lakewood Township, NJ, USA), and filtered through a cell strainer (BD Falcon, Franklin Lakes, NJ, USA). Cells were resuspended in the growth medium [DMEM and Ham’s F-12, 50/50 medium (Mediatech, Manassas, VA, USA), 10% fetal calf serum (FCS; Sigma, St. Louis, MO, USA), 0.2 mM L-Glutamine (Sigma, St. Louis, MO, USA), 100 IU/ml Penicillin and 100 μg/ml Streptomycin (Sigma, St. Louis, MO, USA)] and seeded at a density of 3×105/cm2 in a 150 cm2 cell culture flask (Greiner Bio-One, Monroe, NC, USA). Non plastic-adherent cells were taken off after 48 hours, and adherent cells were washed twice with PBS and expanded (Figure 1B).
Peripheral Blood Mononuclear Cell-Derived Stem Cells (PBSCs)
PBSCs were isolated as previously described.49 Two weeks before ACL repair, the animals from group PBSC (Figure 1D) had 50ml of blood taken from the external jugular vein, which was placed on Percoll-Paque (1.077 g/ml; GE Healthcare Biosciences, Upsalla, SE) and centrifuged. The cells from the lymphocyte/monocyte layer of the Percoll-Paque density gradient were resuspended in growth medium and seeded at a density of 3×106/cm2 in a 150 cm2 cell culture flask. Non plastic-adherent cells were taken off after 48 hours, and the adherent cells were washed twice with PBS and expanded (Figure 1D).
Fluorescent labeling of ADSCs and PBSCs with lentiviral vector and FAC-Sorting
ADSCs and PBSCs were infected with a lentiviral vector containing the DNA of yellow fluorescent protein (YFP) which stably integrated the YFP gene into the cell DNA. Three days after labeling, the cells were sorted using a FACSAria cell sorter (BD Biosciences, San Jose, CA) to obtain 100% YFP expressing cell lines. Cells were then resuspended in growth medium and seeded at a density of 3×106/cm2for further expansion. First passage cells were used for transplantation during the bio-enhanced ACL repair procedure. Cultures of labeled cells were kept for 15 weeks to control for YFP expression. Determination of cell densities was conducted using a Cellometer Auto T4 Cell Counter (Nexcelom Bioscience, Lawrence, MA, USA).
Preparation of the extra-cellular matrix scaffold
The extracellular matrix scaffolds (Name removed for review only) were aseptically manufactured as previously described.31 Bovine fascia was solubilized using pepsin and the resulting extracellular matrix digest lyophilized in molds to create a cylindrical scaffold which was able to absorb 3 ml of autologous whole blood or 3 ml of autologous whole blood with added autologous ADSCs or PBMCs. The scaffold itself was non-autologous.15 Scaffolds were stored at room temperature until surgery.
Determination of Scaffold Pore Size
Six scaffolds were cut transversely at variable locations. Cut surfaces were recorded using a Keyence VHX 2000 Digital Microscope (Keyence, Itasca, IL, USA). Image J (NIH, Bethesda) MD, USA) was used to determine the average of 10 pores per slice.
Bio-enhanced ACL repair procedure
To expose the ACL, the fat-pad was partially resected [weight of resected fat pad (mean ± SD): 6.6 ± 2.2 g] through a medial arthrotomy. The ACL was cut between the proximal and middle thirds using a scalpel. All knees had the same level of gross AP instability after complete ACL transection as verified by joint subluxation with manual Lachman testing. Subsequently, the bio-enhanced ACL repair was performed as previously described.31 Briefly, a button loaded with three suture loops was fixed to the lateral distal femur. The scaffold was threaded onto two of the sutures and slid up into the femoral notch next to the femoral stump of the ACL. The two sutures were then thread through a pre-bored tunnel in the tibia where they were fixed extracortically over a second button with the knee in maximum extension (30 degrees). A Kessler stitch using a #1 Vicryl suture was placed in the distal tibial stump of the ACL and the remaining suture coming from the femoral button was knotted to it (Figure 1 – Column “Bio-enhanced ACL repair”).13 Three ml of autologous blood were drawn from the left femoral vein without addition of anticoagulant and immediately used to saturate the scaffold in group BLOOD (Figure 1A). In group ADSC 3×106 ADSCs and in group PBSC 3×106 PBSCs were mixed with three ml of autologous blood, drawn from the left femoral vein without addition of anticoagulant, before saturation of the scaffold with the cell blood mixture (Figure 1C/E, respectively). After the addition of the blood to the scaffold, all animals were kept under anesthesia for sixty minutes to minimize joint movement until coagulation of the blood within the scaffold was completed.48
After surgery, all animals were housed for four weeks in individualized pens and were then shipped to a farm (Coyote Consulting Corporation Inc, Douglas, MA). At 15 weeks after surgery, the animals were euthanized with pentobarbital, the limbs harvested, and the knees were frozen at −20°C until mechanical testing. Euthanasia at 15 weeks was performed based on the results of a previous study that showed a minimum strength between 6 and 9 weeks after bio-enhanced ACL repair and 15 weeks is a time point at which tissue maturation and generation of biomechanical strength are increasing.22
Biomechanical testing
Biomechanical testing was performed as previously described.15 A servohydraulic load frame with custom fixtures (MTS Systems Corporation, Eden Prairie, MN) was used to perform the biomechanical testing procedures (i.e. anteroposterior (AP) knee laxity and the ligament structural properties).15 During specimen testing, all investigators were blinded to the treatment group. The AP laxity of the knee and the structural properties of the ligaments were analyzed16. Briefly, AP laxity testing was done at 30, 60, and 90 degrees of knee flexion by applying fully reversed, sinusoidal anterior–posterior directed shear loads of ±40 N at 0.0833 Hz for 12 cycles as previously described.26 AP-laxity tests were performed with axial rotation locked in a neutral position, while varus–valgus angulation and the coronal plane translations were left unconstrained. After AP laxity testing, the capsule, collateral ligaments, menisci, and posterior cruciate ligament was resected and specimen was placed in the tensile test fixtures. Then the femur was lowered until the load across the joint surface was +5 N of compression. The ACL was then stretched at a rate of 20 mm/min while the load-displacement data were recorded at 100 Hz.24 Yield failure load, maximum failure load, and linear stiffness were determined from the load-displacement data.
Histology
After biomechanical testing, the repaired ACL was dissected free from the tibial and femoral insertion site and fixed in neutral buffered formalin. The ACL tissue was dehydrated, embedded in paraffin, and sagittal sections stained with hematoxylin and eosin (H&E), α-smooth muscle actin (SMA), or YFP antibodies.22 The slides were analyzed by an examiner blinded to the specimen number and treatment groups. Analysis was performed using bright-field microscopy for five regions of the ligament; the region 1mm away from the femoral insertion site, the region 1 mm away from the tibial insertion site, and three regions in between those two regions, with the exclusion of the zone deformed by the biomechanical testing. The synovium was excluded from the analysis.
Advanced Ligament Maturity Index (LMI)
A mixed qualitative/quantitative analysis using the LMI (Table 1) was performed. The LMI is comprised of three sub-scores determining cellular, collagen, and vascular organization. The cellular sub-score assesses the presence of inflammatory cells, nuclear aspect ratio and orientation of the fibroblasts relative to the collagen fibers as well as fibroblast cell density (determined for individual visual fields of Hematoxylin & Eosin stained slides at 400× magnification). The fibroblast cell density was determined at high magnification on H&E slides for individual visual fields. H&E is superior to SMA for this task because the cell and cell core morphologies are easily detectable (red cytoplasm, blue cell core). Based on the cell core morphology, fibroblasts are distinguishable from inflammatory cells because they are predominantly fusiform with elongated nuclei oriented along the length of the ligament. No inflammatory cells were detected in the specimens. Collagen sub-score evaluates crimp appearance and collagen bundle orientation (determined for individual visual fields of Hematoxylin & Eosin stained slides at 40× magnification using polarized light). Vascular sub-score determines orientation of blood vessels relative to the collagen fibers, maturity as well as density of blood vessels (determined for individual visual fields of SMA stained slides at 100× magnification). A maximum combined score of 26 was given to a healthy ligament.39
Table 1.
Criteria used to generate the advanced Ligament Maturity Index for healing ligaments and graft tissue
| Cell Sub-Score (total = 8 pts) | Collagen Sub-Score (total = 12 pts) | Vessel Sub-Score (total = 6 pts) | |||
|---|---|---|---|---|---|
|
| |||||
| Criteria | pts | Criteria | pts | Criteria | pts |
| Presence of inflammatory cells | Width of bundles | Density of blood vessels | |||
| Necrosis | 0 | No bundles | 0 | None present | −1 |
| Polymorphonuclear cells | 1 | Width less than 50 μm | 2 | more than 200% present | 0 |
| No inflammatory cells | 2 | Width greater than 50 μm | 4 | 150%–200% present | 1 |
| Less than 150% present | 2 | ||||
| Nuclear aspect ratio (NAR) of fibroblasts | Bundle orientation with long axis of ligament | Vessel orientation with long axis of ligament | |||
| No cells | −1 | No bundles | −2 | ||
| Average NAR less than 2 (round) | 0 | less than 50% oriented | 0 | No vessels oriented | −2 |
| Average NAR 2–4 | 1 | 50%–75% oriented | 2 | Less than 30% oriented | −1 |
| Average NAR greater than 4 (elongated) | 2 | 75%–100% oriented | 4 | less than 50% oriented | 0 |
| 50%–75% oriented | 1 | ||||
| Nucleus of fibroblast aligned with fascicles and long axis of ligament | Collagen Crimp | 75%–100% oriented | 2 | ||
| No crimp | −2 | ||||
| less than 25% crimp | 0 | Vessel maturity | |||
| No cells | −2 | 25%–75% crimp | 2 | No vessels seen | 0 |
| Less than 30% of cells oriented | −1 | Crimp with normal length present | 4 | Capillaries only present | 1 |
| 30%–50% oriented | 0 | Arterioles present | 2 | ||
| 50%–75% oriented | 1 | ||||
| 75%–100% oriented | 2 | ||||
| Number of fibroblasts | |||||
| None | −1 | ||||
| Greater than 2× normal | 0 | ||||
| Between 1.5× and 2× normal | 1 | ||||
| Less than 1.5× normal | 2 | ||||
Statistical Methods
The sample size for this study was selected according to an a priori power calculation that used data from earlier studies 21, 22, 31, 48 to detect a 20% difference in yield load, maximum failure load and linear stiffness between treatment groups with a standard deviation of 10% and an α (P-value) of 5% with a minimum power of 95%.
General estimating equations were used to model the biomechanical parameters as a function of limb within animal and treatment condition. The within-subject error was modeled as correlated with a heterogeneous compound symmetry variance-covariance matrix structure, grouped block-diagonal by treatment condition. This permitted different variances to be modeled in surgical and contralateral control limbs along with the within-subject covariance, and for groups to have different sets of values within this structure. The biomechanical parameters were modeled as either Gaussian (AP laxity) or log-normally (yield load, maximum failure load, linear stiffness) distributed, based on visual inspection of the distribution of each model’s Pearson residuals. The maximum-likelihood estimators of the models were adjusted for any model misspecification using sandwich-estimation. Mean differences with 95% confidence intervals (95% CI) were expressed as absolute (Gaussian) or fold (back-transformed log-normal) for dissemination. A priori comparisons were made between affected and contralateral limbs within each treatment condition, and the magnitude of these differences were compared between the treatment conditions.
Differences between treatment groups in cell and vessel densities as well as LMI and its’ sub-scores as well as cell and vessel densities were assessed using a one-way analysis of variance. Bonferroni correction was utilized for multiple comparisons. An adjusted alpha value of 5% was considered significant. All data is presented as geometrical mean with a 95% confidence interval.
RESULTS
Animal welfare
All animals recuperated well after surgery. No wound infection, signs of local inflammation or general sickness of the animals was seen during the 15 week period.
Scaffold Pore Size
The mean scaffold pore size was 208 ± 68 μm.
Knee Biomechanics
The mean structural properties for the surgical and control knees and the mean normalized structural properties (relative to the contralateral leg) after 15 weeks of healing are presented in Figure 2. Statistically significant lower biomechanical properties were detected between the intact contralateral control knees and three treatment groups (BLOOD, ADSC, PBSC) (Padj< .05 for all comparisons between intact and treatment groups).
Figure 2. Biomechanical Properties.
A – Yield failure load normalized to the contralateral intact ACL (treatment / intact);
B – Maximum failure load normalized to the contralateral intact ACL (treatment / intact);
C – Linear stiffness normalized to the contralateral intact ACL (treatment / intact);
D – Difference between treatment and contralateral knee AP-laxity at 30 degrees flexion [mm].
▴Statistically significant difference between BLOOD and PBSC (P< 0.05);
E – Difference between treatment and contralateral knee AP-laxity at 60 degrees flexion [mm];
F – Difference between treatment and contralateral knee AP-laxity at 90 degrees [mm].
▴Statistically significant difference between treatment groups (P< .05)
AP-Laxity – Anterior-posterior knee laxity
BLOOD – bioenhanced ACL Repair
ADSC – bioenhanced ACL Repair with addition of adipose tissue derived stem cells
PBSC - bioenhanced ACL Repair with addition of peripheral blood mononuclear cell derived stem cells
Normalized values of yield load, maximum failure load and linear stiffness were calculated by division of treatment by intact side from each individual animal. Comparison of the means of normalized yield load, maximum load and linear stiffness did not yield a statistically significant difference between the treatment groups (Figure 2A/B/C, Padj= 1.0 for yield load, .104> Padj> .972 for maximum failure load, Padj= 1.0 for linear stiffness).
Normalized values of AP-laxity at 30, 60 and 90 degrees were calculated by subtraction of treatment from intact side from each individual animal. Comparison of the means of normalized AP laxity at 30, 60 and 90 degrees only showed a statistically significant difference in AP laxity at 30 degrees between BLOOD and PBSC (Figure 2D, Padj< .05). Treatment groups were neither significantly different in AP laxity at 60 nor 90 degrees (Figure 2E/F, Padj= 1.0 AP-laxity at 60 degrees, .529< Padj< .737 for AP-laxity at 90 degrees).
Histology
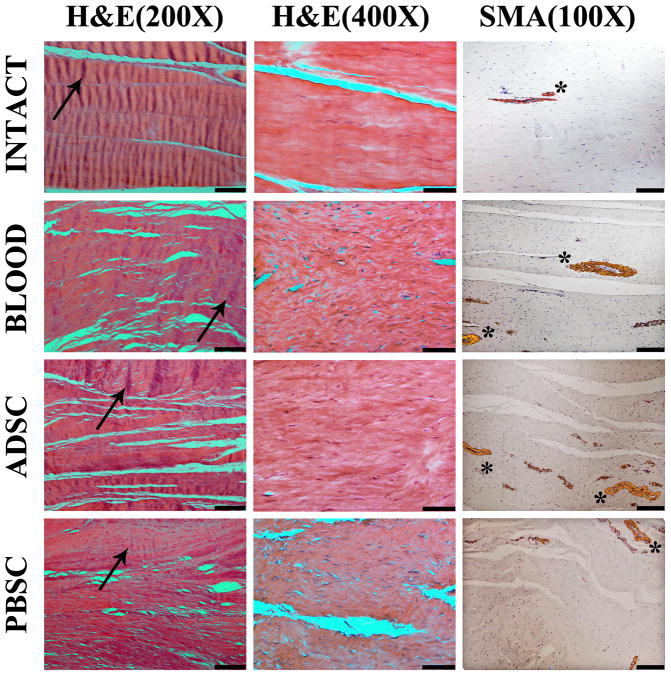
The repaired ligaments in all groups had an increased number of cells per visual field, collagen bundles which were smaller and less organized, collagen crimp with a higher crimp length and lower crimp height, and more vessels per visual field when compared to the intact ligaments (Figure 3).
Figure 3. Micrographs.
First column - hematoxylin and eosin histology at 200× magnification using polarized light to visualize collagen crimp (black arrows point to single crimp wave, black bar = 100 μm); second column - hematoxylin and eosin histology at 400× magnification using to visualize fibroblasts (black bar = 100 μm); third column – immunohistochemistry for alpha-smooth muscle actin at 100× magnification to visualize cells and vessels (stars adjacent to positively stained cells surrounding vessels, black bar = 200 μm).
H&E – Hematoxylin and Eosin
SMA – alpha smooth muscle actin
BLOOD – bioenhanced ACL Repair
ADSC – bioenhanced ACL Repair with addition of adipose tissue derived stem cells
PBSC - bioenhanced ACL Repair with addition of peripheral blood mononuclear cell derived stem cells
LMI
The intact samples yielded a mean of 25.5 ± 0.3 of 26 possible points for the combination of cellularity, collagen and vessel sub-scores. The repaired ligaments yielded statistically significant lower mean LMI values (15.4 to 17.5 points) than the intact ACLs (Figure 4A, Padj< .001). Mean LMI scores did not differ significantly between the treatment groups (Figure 4A, .373< Padj ≤ 1.0).
Figure 4. Histological Properties.

A – Total advanced ligament maturity index score and its sub-scores (cellularity, collagen, vessel) for intact and treatment groups. ▴ Statistically significant difference in total LMI and all sub-scores between intact and treatment groups (P< .05); B - Cell density for intact and treatment groups per mm2 (determined in an area of 0.08 mm2). ▴ Statistically significant difference between intact and treatment groups (P< .05); C – Vessel density for intact and treatment groups per mm2 (determined in an area of 1.4 mm2). ▴ Statistically significant difference between intact and treatment groups (P< .05);
▴Statistically significant difference (P< .05) between intact and treatment groups
LMI – advanced ligament maturity index
BLOOD – bioenhanced ACL Repair
ADSC – bioenhanced ACL Repair with addition of adipose tissue derived stem cells
PBSC - bioenhanced ACL Repair with addition of peripheral blood mononuclear cell derived stem cells
Cellularity
Cell sub-scores were statistically significantly higher in the intact group than in the treatment groups, but not different between the treatment groups themselves (Figure 4A, Padj< .001 and .540< Padj ≤ 1.0, respectively). There were no inflammatory cells detected in either intact or treatment groups. Fibroblast cell and nuclear morphology in the intact ACLs was predominantly fusiform with elongated nuclei oriented along the length of the ligament. In all treatment groups, there were more areas containing cells with round shaped nuclei and lower levels of fibroblast cell alignment than were present in the intact ligament (Figure 3, Column “H&E(400×)”).
Fibroblast cell density was significantly increased in the treated ACLs when compared with the intact ACLs. Fibroblast cell density did not differ significantly between the treatment groups (Figure 3, Column “H&E(400×)”, Figure 4B, Padj< .001 and Padj= 1.0, respectively). Mean fibroblast cell density in the intact ACLs was 394 cells/mm2, whereas the mean cell density in the treatment groups ranged from 1129 to 1273 cells/mm2.
Collagen Organization
At 15 weeks, Collagen sub-scores were statistically significantly higher in the intact group than in the treatment groups, but not different between the treatment groups themselves (Figure 4A, Padj< .001 and .542< Padj ≤ 1.0, respectively). Collagen bundles were generally smaller in the treatment groups than in the intact ACLs (Figure 3, Column “H&E (200×)”). However, in both the intact and treated ligaments, these bundles were predominantly aligned with the long axis of the ligaments. There were scattered areas of crimped fibers in the treated ligaments and in those areas, the crimp was organized longitudinally. However, this appearance was not uniform as seen in the intact ligaments (Figure 3, Column “H&E (200×)”).
Vascularity
The vessel sub-score of the treatment groups at 15 weeks was significantly lower than in the intact ACLs, but there was no statistically significant difference detected between the treatment groups themselves (Figure 4A, Padj< .001 and .987< Padj ≤ 1.0, respectively). Most of the vessels in the treated ligaments had aligned with the collagen bundles and the long axis of the ligament. In all treated ligaments, mature arterioles that have multiple layers of SMA-positive smooth muscle cells (compared to capillaries which are lined by a single endothelial cell layer), were detectable. (Figure 3, Column “SMA (100×)”).
The vessel density was higher in the repaired ligaments than in the intact ligaments but did not differ significantly between the treatment groups themselves (Figure 3, Column “SMA (100×)”, Figure 4C, Padj< .001 and .642< Padj ≤ 1.0, respectively).
YFP-labeled cells
Control in-vitro cell culture of YFP labeled ADSCs and PBSCs showed positive fluorescence at 15 weeks. Neither dark field fluorescence microscopy nor immunohistochemical staining against YFP of formalin fixed ACL sections showed cells expressing YFP.
DISCUSSION
In this study, we hypothesized that enrichment of the whole blood used in a bio-enhanced repair technique with stem cells would result in additional improvements in healing of the injured ACL. However, after 15 weeks of healing, we found no evidence to support our hypothesis. Neither ADSCs nor PBSCs led to a statistically significant improvement of the bio-enhanced ACL repair in the mean normalized yield and maximum failure loads, linear stiffness or AP laxity. In addition, we were unable to detect any significant differences in cellular and vascular densities or ligament maturity scores when either type of MSCs was added.
The biomechanical results of the present study are in conformance with the previous studies of bio-enhanced ACL repair.27, 31, 48 Yield and maximum failure load in this study averaged 25% and 23% of the intact ACL values, while they previously had been measured at 21% to 26% and 22% to 27% at 15 weeks after bio-enhanced ACL repair, respectively. Linear stiffness values of 32% of the intact ACL values in this study were comparable to earlier results that averaged between 20% and 33%.28, 31, 40 The linear stiffness results are encouraging as this is the parameter that tissue engineers attempt to replicate as ligaments and tendons routinely operate in the range that is less than 10% of the failure load when patients perform activities of daily living.6
In addition, we were not able to detect any fluorescent cells in the bio-enhanced ACL repair groups treated with ADSCs or PBSCs. While the MSCs maintained in parallel in vitro cultures could be visualized, suggesting the cells are able to continue to express the flourescent protein for 15 weeks, no flourescent cells were detected in the ACL tissue. Possible reasons in the present study include the process of freeze/thaw, or histologic processing that may have rendered the YFP label inactive. However this finding could also indicate that the ADSCs and PBMCs did not survive the entire 15 weeks after transplantation into the knee. This would be consistent with prior reports using MSCs in the knee joint, where few, if any, cells are found in the repair sites of cartilage or ligament 3 weeks after implantation.46 It would also be consistent with studies in multiple other tissues, where MSC cell survival after transplant is typically less than four weeks in liver,52 brain,9 and heart.38, 44, 47 However, unlike these prior studies which demonstrated some functional improvements after MSC injection, even with the observed early cell death, in this study we found no functional contribution to the biomechanical healing with the addition of ADSCs or PBSCs to the whole blood in the scaffold. This suggests that the trophic effects previously observed in other studies of MSCs may also be provided by the cells present in whole blood (platelets, white blood cells, red blood cells).
There are several limitations to this study. This study was performed in non-human knees. We chose the porcine model because its knee anatomy and size and blood characteristics are very close to that of a human. It also exhibits similar biomechanical function and ACL dependence.5 However, it is a quadruped rather than a biped. Another limitation was the single freeze-thaw cycle the knee specimens experienced which could have potentially influenced the biomechanical outcomes. To minimize variability due to this treatment between the groups the freeze-thawing protocol was consistent for all specimens.
There are multiple possible etiologies for the observed failure of the implanted MSCs to impact the functional outcome of bio-enhanced ACL repair in this model, including early MSC death, the MSCs actively migrating out of the scaffold or a harmful effect of the autologous whole blood on the autologous MSCs in vivo. Regarding the possible harmful effect of whole blood, the peripheral blood MSCs are derived from whole blood. Therefore, PBMCs are unlikely to be adversely effected by replacing them into their native environment. In addition, ADSCs are commonly administered via intravenous injection in experimental models with good functional effect in other organs.10, 41, 45 This intravenous injection would be directly into autologous whole blood. Based on the results of these prior reports, a harmful effect of autologous whole blood on the MSCs is less likely. Another possible etiology is that the trophic factors released by MSCs in the wound site, which are thought to be the main mechanism by which MSCs improve functional outcomes,29 are similar to those released from the platelets in the whole blood after collagen activation. The trophic factors thought to be released by MSCs after implantation include VEGF,43 EGF,28 and FGF-2,28 all factors known to be released from platelets after activation with collagen.19, 37, 50 In addition, activated platelets also release HGF, IGF-1, PDGF-AB, PDGF-BB, TGF-b1, which are also thought to be important in wound healing.12 Thus, it is possible that the observed failure of the implanted MSCs to impact the functional outcome of bio-enhanced ACL repair in this model may be that the trophic effects of the platelets in the whole blood preparation serve to camouflage the trophic effects of the added MSCs.
Another limitation of this study was that it did not use FDA approved protocols for cell harvest and culture. Thus, use of these sources of stem cells would require additional study and development of appropriate manufacturing protocols prior to proceeding to a clinical study.
Also limiting in the current study was the use of lentiviral vectors to label the cells. Current standard transduction methods using lentiviral vectors are known to inhibit MSC proliferation rate and potentially the differentiation potential of the transduced cells. However, we have previously demonstrated the multilineage capabilities and proliferation capacity of both the ADSCs and PBMCs after lentiviral transduction in this porcine model.40
Another limitation was that the histological analyses were performed after biomechanical testing had been conducted. In performing testing for yield and maximum load, the collagen crimp will first flatten reversibly. This phase is followed by inelastic deformation.18 In this study, the irreversible changes in the collagen were kept minimal by utilizing a servohydraulic load frame. Thus, testing was performed in a very controlled manner, stopping right after the drop in load occurred to minimize gross tissue damage. Furthermore, most ligaments failed in the midsubstance, with a visible focal defect. The region of failure was excluded from the histologic analysis, because the evaluation of crimp length in this passage and thus the collagen sub-score, could have been altered. Moreover, all specimens were tested identically, making it likely that any changes in the collagen sub-scores would have occurred uniformly.
Whole blood as a biologic adjunct for soft tissue healing has only recently received attention. It has been previously appreciated that platelets are an important growth factor delivery system.19 Whole blood also contains leukocytes, lymphocytes and macrophages, which are involved in the signaling and regulation of the wound healing.17 Whole blood also contains red blood cells, which have been demonstrated to stimulate collagen production by ACL fibroblasts 20, possibly due to their ability to serve as an oxygen source before sufficient blood supply is established within the wound site.20 And as shown before, whole blood contains stem cells that have the potential to differentiate into the fibroblast cell line.7, 8, 23, 40
This study compares the outcomes of MSCs with whole blood rather than an empty carrier. The use of whole blood as a control is important, as whole blood is inexpensive, can be “harvested” at the time of surgery and does not need to be cultured and then re-implanted in a second procedure. Thus, whole blood represents a practical biologic adjunct to ligament repair and could potentially be used as a baseline for comparisons of other adjuncts before clinical use of more complicated constructs is contemplated. The results of this study support the biologic utility of whole blood in wound healing, and suggest that the more intense and costly procedures of stem cell harvest, culture and re-implantation may not be effective in further enhancing ligament repair.
CONCLUSION
The data suggest that in this study there is little functional benefit gained with the enrichment of whole blood with MSCs from either the retropatellar fat pad or the peripheral blood for bio-enhanced ACL repair. The use of whole blood alone was equally effective in supporting biomechanical healing of the ACL in the current experimental setting.
What is known about the subject
Bio-enhanced ACL repair is a new technology where an extracellular matrix scaffold containing some sort of biologic additive is placed between the torn ligament ends to enhance ligament healing. A recent in vitro study demonstrated that mesenchymal stem cells could be harvested from the retropatellar fat pad and the peripheral blood and that they stimulate ACL fibroblast proliferation and collagen production - two functions important in ligament healing. Therefore, it seems plausible that a bio-enhanced ACL repair that is augmented with MSCs may improve ACL healing.
What this study adds to existing knowledge
The majority of MSC studies are performed in vitro or in small animal models. This study looks at the performance of two types of MSCs in a previously validated large animal model of ACL injury. While prior studies have reported on bio-enhanced ACL repair, where an extracellular matrix based scaffold saturated with autologous whole blood has been used to enhance the outcomes of a suture repair of the ACL, this study evaluated the further addition of MSCs from adipose tissue or peripheral blood to the biologic scaffold. The use of whole blood as a control is important, as whole blood is inexpensive, can be “harvested” at the time of surgery and does not need to be cultured and then re-implanted in a second procedure. Thus, whole blood represents a practical biologic adjunct to ligament repair, and any other adjunct should be demonstrated to be superior to this baseline, rather than an empty carrier or untreated group, before clinical use is considered. Indeed, this study demonstrates the biologic utility of using whole blood in ligament repair and suggests that more intense and costly procedures of stem cell harvest, culture and re-implantation may not be necessary to enhance ligament repair.
Acknowledgments
The authors thank Elise Magarian, Linda Chao, and Ryu Yoshida for their assistance in surgery and tissue collection, as well as the ARCH staff, Dr Arthur Nedder, Kathryn E. Donovan, Dana Bolgen and Courtney White, for their assistance and care in handling the animals. They also thank David Paller, Alison Biercevicz, and Sarath Koruprolu for the conduction of the biomechanical testing.
M.M.M. and B.C.F received support by the National Institutes of Health under NIAMS (2R01-AR054099, 1R01-AR056834 and 1R01-AR056834S1) and the Lucy Lippitt Endowment. C.M.H. received support by the Ruth L. Kirschstein National Research Service Award (f32-AR061186. Dr. Matthew Warman, the Children’s Orthopaedic Surgery Foundation, and Harvard Medical School. The article content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or NIAMS.
References
- 1.Ajuied A, Wong F, Smith C, et al. Anterior Cruciate Ligament Injury and Radiologic Progression of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Am J Sports Med. 2013 doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 2.Akita D, Morokuma M, Saito Y, et al. Periodontal tissue regeneration by transplantation of rat adipose-derivedstromal cells in combination with PLGA-based solid scaffolds. Biomed Res. 2014;35(2):91–103. doi: 10.2220/biomedres.35.91. [DOI] [PubMed] [Google Scholar]
- 3.Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. 2012;1(3):200–205. doi: 10.5966/sctm.2011-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaber SP, Webster RA, Hill CJ, et al. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172. doi: 10.1186/1479-5876-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J Orthop Res. 2011;29(5):641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 6.Butler DL, Lewis JL, Frank CB, et al. Evaluation criteria for musculoskeletal and craniofacial tissue engineering constructs: A conference report. Tissue Eng Part A. 2008;14(12):2089–2104. doi: 10.1089/ten.tea.2007.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng MT, Liu CL, Chen TH, Lee OK. Comparison of potentials between stem cells isolated from human anterior cruciate ligament and bone marrow for ligament tissue engineering. Tissue Eng Part A. 2010;16(7):2237–2253. doi: 10.1089/ten.TEA.2009.0664. [DOI] [PubMed] [Google Scholar]
- 8.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30(4):634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 9.Detante O, Valable S, de Fraipont F, et al. Magnetic resonance imaging and fluorescence labeling of clinical-grade mesenchymal stem cells without impacting their phenotype: study in a rat model of stroke. Stem Cells Transl Med. 2012;1(4):333–341. doi: 10.5966/sctm.2011-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du G, Liu Y, Dang M, et al. Comparison of administration routes for adipose-derived stem cells in the treatment of middle cerebral artery occlusion in rats. Acta Histochem. 2014;116(6):1075–1084. doi: 10.1016/j.acthis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Eagan MJ, Zuk PA, Zhao KW, et al. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.474. [DOI] [PubMed] [Google Scholar]
- 12.Evanson JR, Guyton MK, Oliver DL, et al. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil Med. 2014;179(7):799–805. doi: 10.7205/MILMED-D-13-00336. [DOI] [PubMed] [Google Scholar]
- 13.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anterioposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28(6):703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37(8):1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank CB. Ligament structure, physiology and function. J Musculoskelet Neuronal Interact. 2004;4(2):199–201. [PubMed] [Google Scholar]
- 17.Glim JE, van Egmond M, Niessen FB, Everts V, Beelen RH. Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen. 2013;21(5):648–660. doi: 10.1111/wrr.12072. [DOI] [PubMed] [Google Scholar]
- 18.Goulam Houssen Y, Gusachenko I, Schanne-Klein MC, Allain JM. Monitoring micrometer-scale collagen organization in rat-tail tendon upon mechanical strain using second harmonic microscopy. J Biomech. 2011;44(11):2047–2052. doi: 10.1016/j.jbiomech.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. 2011;39(4):729–734. doi: 10.1177/0363546511401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison SL, Vavken P, Murray MM. Erythrocytes inhibit ligament fibroblast proliferation in a collagen scaffold. J Orthop Res. 2011;29(9):1361–1366. doi: 10.1002/jor.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haslauer CM, Elsaid KA, Fleming BC, Proffen BL, Johnson VM, Murray MM. Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2013;21(12):1950–1957. doi: 10.1016/j.joca.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37(12):2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann Surg. 2011;254(6):1066–1074. doi: 10.1097/SLA.0b013e3182251559. [DOI] [PubMed] [Google Scholar]
- 24.Katsuragi R, Yasuda K, Tsujino J, Keira M, Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28(1):47–56. doi: 10.1177/03635465000280012001. [DOI] [PubMed] [Google Scholar]
- 25.Little D, Guilak F, Ruch DS. Ligament-derived matrix stimulates a ligamentous phenotype in human adipose-derived stem cells. Tissue Eng Part A. 2010;16(7):2307–2319. doi: 10.1089/ten.tea.2009.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastrangelo AN, Magarian EM, Palmer MP, Vavken P, Murray MM. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28(5):644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29(7):1002–1007. doi: 10.1002/jor.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda JP, Filipe E, Fernandes AS, et al. The human umbilical cord tissue-derived MSC population UCX(R) promotes early motogenic effects on keratinocytes and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when transplanted in vivo. Cell Transplant. 2013 doi: 10.3727/096368913X676231. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27(5):639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 34.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 35.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24(4):820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 36.Myer GD, Ford KR, Hewett TE. Rationale and Clinical Techniques for Anterior Cruciate Ligament Injury Prevention Among Female Athletes. J Athl Train. 2004;39(4):352–364. [PMC free article] [PubMed] [Google Scholar]
- 37.Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) Thromb Haemost. 2002;88(5):834–842. [PubMed] [Google Scholar]
- 38.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 39.Proffen BL, Fleming BC, Murray MM. Histologic predictors of maximum failure loads differ between the healing ACL and ACL grafts after 6 and 12 Months in vivo. Orthop J Sports Med. 2013;(1):1–11. doi: 10.1177/2325967113512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proffen BL, Haslauer CM, Harris CE, Murray MM. Mesenchymal Stem Cells from the Retropatellar Fat Pad and Peripheral Blood Stimulate ACL Fibroblast Migration, Proliferation, and Collagen Gene Expression. Connect Tissue Res. 2012 doi: 10.3109/03008207.2012.715701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu X, Villalta J, Ferretti L, et al. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med. 2012;9(7):1834–1841. doi: 10.1111/j.1743-6109.2012.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Requicha JF, Viegas CA, Munoz F, et al. A Tissue Engineering Approach for Periodontal Regeneration Based on a Biodegradable Double-Layer Scaffold and Adipose-Derived Stem Cells. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seebach E, Freischmidt H, Holschbach J, Fellenberg J, Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh AY, Huber BC, Narsinh KH, et al. In vivo functional and transcriptional profiling of bone marrow stem cells after transplantation into ischemic myocardium. Arterioscler Thromb Vasc Biol. 2012;32(1):92–102. doi: 10.1161/ATVBAHA.111.238618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun M, Wang S, Li Y, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80. doi: 10.1186/scrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Buul GM, Siebelt M, Leijs MJ, et al. Mesenchymal stem cells reduce pain but not degenerative changes in a mono-iodoacetate rat model of osteoarthritis. J Orthop Res. 2014;32(9):1167–1174. doi: 10.1002/jor.22650. [DOI] [PubMed] [Google Scholar]
- 47.van der Bogt KE, Schrepfer S, Yu J, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87(5):642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28(5):672–680. doi: 10.1016/j.arthro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 50.Wartiovaara U, Salven P, Mikkola H, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80(1):171–175. [PubMed] [Google Scholar]
- 51.Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. doi: 10.1177/0363546513517540. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, Li J, Pang P, et al. Polymeric vector-mediated gene transfection of MSCs for dual bioluminescent and MRI tracking in vivo. Biomaterials. 2014;35(28):8249–8260. doi: 10.1016/j.biomaterials.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida R, Murray MM. Peripheral blood mononuclear cells enhance the anabolic effects of platelet-rich plasma on anterior cruciate ligament fibroblasts. J Orthop Res. 2013;31(1):29–34. doi: 10.1002/jor.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]