Abstract
Pain assessment in animal models of osteoarthritis is integral to interpretation of a model’s utility in representing the clinical condition, and enabling accurate translational medicine. Here we describe two methods for behavioral pain assessments available for use in animal models of experimental osteoarthritic pain: Von Frey filaments and spontaneous activity monitoring.
Keywords: Pain, Animal models, Rats, Mice, Rodents, Methods, Osteoarthritis, Assessment, Von Frey, Spontaneous activity
1 Introduction
Animal models of osteoarthritis (OA) include those that develop spontaneously or are surgically or nonsurgically (chemically) induced, all of which can provide insights into the molecular, pathological, or biochemical progression of changes in the joint during OA. Chronic pain is a hallmark of OA, and its evaluation in any animal model is integral to assessing the relevance and utility of that model in translation research.
Small animals (primarily mice and rats but also rabbits and guinea pigs) are used extensively in OA research, and a large repository of historical data, especially in rats and mice, exists to which research data can be compared. Their small size and typically lower cost for purchase and maintenance compared with large animals make them attractive animals in which to model OA. Some small animals, such as mice, can be genetically altered to enable the study of specific modulators in the development of OA, and while others, such as the Dunkin Hartley guinea pig, can spontaneously develop OA.
Osteoarthritis pain is typically localized and related to movement or weight-bearing of the affected joint(s), which in animal models are typically the knee and/or hip joint. Pain is difficult to evaluate objectively in humans because of the inherent variability in the individual’s interpretation of the sensory input. This variability represents the emotional and cognitive components of pain perception. In addition, little correlation exists between the objective measures of OA (e.g., radiologic or pathologic changes) and the degree of chronic pain experienced by the individual.
Methods used to assess pain in rodents include those that are mechanically, anatomically, or chemically based. The most critical part in any testing method is to ensure that the animal is calm and relaxed before the testing. Many assessments of pain in OA animal models are behaviorally based, and may require that the animals be acclimatized to any apparatus before testing. These behavioral animal tests can usually be carried out at room temperature. Measures for assessing pain in animals can be direct or indirect. Indirect measures include static or dynamic weight-bearing, foot posture, gait analysis, and spontaneous movement. Direct measures include hind limb withdrawal test to mechanical/thermal/cold stimulation, knee compression force, struggle threshold angle of knee extension, knee tissue edema, vocalizations after stimulation of the affected knee, and brain imaging. Here, we describe two methods for the measurement of pain that can be used for experimental OA models in rodents: mechanical sensitivity by use of Von Frey filaments and spontaneous activity.
2 Materials
2.1 Mechanical Sensitivity
Von Frey filaments: We will describe the force units in grams, but the units written on the handles of the calibrated Von Frey filaments are in log units.
Stainless steel instrument tray stand with grid support.
Clear plastic rodent cage.
Timer.
2.2 Assessment of Spontaneous Behavior
Photobeam activity system to measure open-field activity.
Laboratory Animal Behavior Observation Registration and Analysis System (LABORAS, Metris, The Netherlands) for behavior pattern recognition.
Scale (with chamber and lid) to measure body weight.
3 Methods
3.1 Von Frey Filaments
Place a wire mesh (see Note 1) across the top of a stainless steel instrument tray holder, from which the solid tray has been removed. Many animal facilities already have such meshes (at least for rats) as part of their standard animal caging.
Place the rat or mouse on top of the wire mesh, and place the clear plastic rodent cage over the animal (see Note 2).
Allow the animal to explore its surroundings and acclimatize to the test area (see Note 3).
Collect your set of calibrated Von Frey filaments [0.028–5.5 g for mice (2.44–4.74 log units); 0.41–15 g for rats (3.61–5.18 log units)].
After 10–15 min, once exploratory behavior has ceased, begin the testing. Using an intermediated value of Von Frey hair [0.4 g in mice (3.61 log unit), 2.0 g in rats (4.31 log unit)], touch the tip of the filament at a right angle to the bottom (midplantar) surface of the rodent’s hind foot through the mesh floor until the filament bends. Continued advancement produces more bending but not more force (Fig. 1) (see Note 4).
Wait for at least 5 s, record the response [foot withdrawal (mark X) or no foot withdrawal (mark 0)], and then repeat, using the next higher successive Von Frey filament if there was no response to the previous filament, or the next lower successive Von Frey filament if there was a response to the previous filament.
Continue testing until four stimuli have been applied following the first response reversal [i.e., a change from foot withdrawal to no foot withdrawal (X to 0), or, a change from no foot withdrawal to foot withdrawal 0 to X)]. In theory, as many as nine stimuli may be required. If no filament in the set produces any withdrawal then use the default values of 0.02 g in mice and 0.3 g in rats; if all filaments in the set produce withdrawal then use the default values of 6 g in mice and 15 g in rats.
-
Calculate the threshold force corresponding to 50 % withdrawal using the above up-down iterative method [1, 2].
The equation for rats is
where k is obtained from the pattern of Xs and 0s (using the table in Appendix 1 of ref. 1).The equation for mice is
where k is obtained from the pattern of Xs and 0s (using the table in Appendix 1 of ref. 1).
Fig. 1.
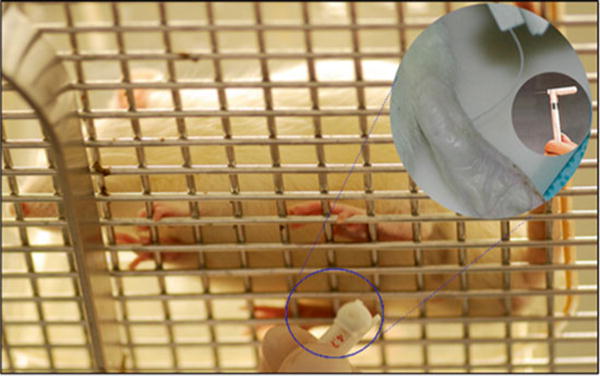
Mechanical allodynia (Von Frey filament testing). Photograph from below showing rat resting on wire platform and Von Frey filament testing of rat’s hind paw. Inset shows close-up of filament bending against plantar surface of rat’s paw. Inset within inset shows close-up of Von Frey filament apparatus
3.2 Photobeam Activity System: Open-Field System
Adjust the upper set of photobeams to 5 cm above ground for testing mice and 11 cm for testing rats (see Note 5).
Place the animal within the testing chamber with only a minimal amount of bedding (see Note 6).
Run the software package to monitor open-field activity for rearing (vertical photobeam crossings) and ambulatory activity (horizontal photobeam crossings) (Fig. 2) (see Note 7).
Fig. 2.
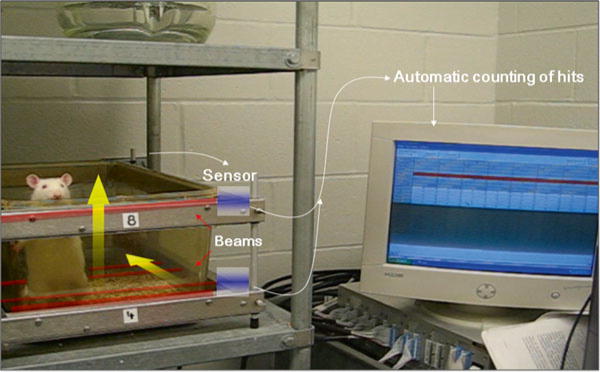
Spontaneous photobeam activity system for rearing (vertical photobeam crossings) and ambulatory movement (horizontal photobeam crossings)
3.3 Behavior Pattern Recognition: LABORAS
Weigh the animal. Enter this datum into the LABORAS system (see Note 8).
Place the animal within the rodent cage portion of the LABORAS system.
Start data acquisition.
Fig. 3.
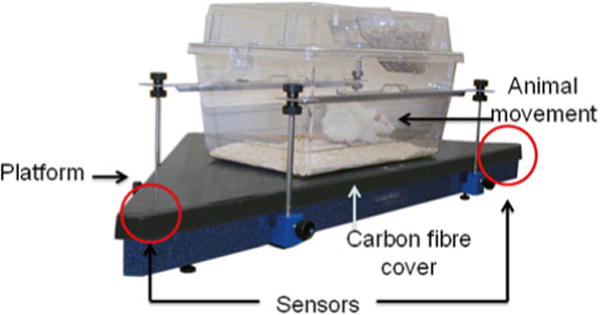
Spontaneous behavior pattern recognition system by LABORAS. Vibration made by the rat is measured by ultrasensitive sensors located in two corners of the triangular platform
Acknowledgments
This work was supported by NIH NIAMS R01 grants to HJI from AR053220, AR062136 and VA BLD&R Merit Award.
Footnotes
Ensure that the wire mesh surface used for the testing is consistent between all testing sessions. The threshold for pain withdrawal can be influenced by the surface on which the rodents are placed [3]. Sanitize the wire mesh and shoebox cage between animals so that olfactory signals do not distract the animal from becoming quickly acclimated. On any day, use a different set of wire meshes for male versus female rodents to reduce distracting olfactory signals.
Placing a clear plastic shoebox-like cage over the animal serves to contain it within the testing area and prevents it from escaping or from falling off the surface. In addition, it is easy to visualize the animal throughout the testing period.
Ensure that the room in which the testing is occurring is quiet, warm, and environmentally stable. Sudden unexpected noises can startle animals and affect reliability or reproducibility of test results. Similarly, changes in room temperature can adversely affect the animal’s ability to acclimatize to the testing area. Von Frey filaments are composed of nylon plastic, which can be adversely affected by heat and humidity and lose calibration. Newer type filaments can be found made of optical glass fibers, which may obviate these potential problems [4].
To minimize observer bias and improve reproducibility, one individual should be responsible for testing all animals. Alternatively, an automated system can be employed. Use of automated systems may be more reliable in evaluating pain in rodents in some circumstances [5]. An electronic Von Frey apparatus (Ugo Basile, Italy) can automatically record the animal’s response to user-controlled application of force rate. A touch stimulator transducer is placed on the midplantar surface of the rodent’s hind foot, until the foot is lifted by the animal. A display then gives the operator a summary of the results of the force and time corresponding to the response. Software helps in consistent application of force at the desired rate. A dynamic plantar aesthesiometer (Ugo Basile, Italy) is an automated system that allows measurement of the sensitivity threshold in one test with high repetitiveness. It typically consists of a moveable touch-simulator unit, a framed metal mesh, an animal enclosure, and a microprocessor-controlled electronic unit. The animal moves freely within the enclosure positioned on the metal mesh. After the animal has acclimatized to the apparatus and stopped any exploratory behavior, the operator places the touch simulator below the animal’s paw. The unit then automatically raises the filament at a preset force until a signal is received that the animal has either moved its paw or the greatest preset force has been met. Latency to paw withdrawal and force exerted are recorded.
Adjacent beams at any height used are 5 cm apart and beam interruptions are recorded automatically. One set of photobeams is set at foot level to measure ambulation (i.e., movement from one beam to another), and an upper set of photobeams is adjusted above ground to measure rearing (i.e., beam breaks in the vertical direction).
Activity is monitored in a low-lit room for a predetermined time, typically 60 min. The animal should not be acclimatized to the chamber, since spontaneous exploratory behavior (e.g., rearing) will lessen given prior exposure to the new environment.
Photobeam crossings will be recorded automatically. It is best if the investigator leaves the room. Rearing may be affected by the presence of OA.
The animal must be accurately weighed before each session. Once the body weight is entered into the software program, the machine automatically calibrates the system. The LABORAS measures behavior based on analysis of vibration and force signals picked up by sensors in the platform on which the cage is placed (Fig. 3). Pattern recognition software then determines and quantifies behaviors that may have been changed by OA-induced pain, including hind limb licking, scratching, wet dog shakes, head shakes, head twitches, purposeless chewing, grooming, locomotion, climbing, immobility, and feeding. Position tracking information is also monitored and quantified, including position (X, Ƴ) and position distribution, speed, and traveled distance. Changed behavioral parameters in an experimental OA model can be interpreted to be knee OA-induced pain response [6, 7].
References
- 1.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 2.Osikowicz M, Mika J, Makuch W, et al. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117–126. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Methods. 1999;87:185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 4.Fruhstorfer H, Gross W, Selbmann O. Von Frey hairs—new materials for a new design. Eur J Pain. 2001;5:341–342. doi: 10.1053/eujp.2001.0250. [DOI] [PubMed] [Google Scholar]
- 5.Nirogi R, Goura V, Shanmuganathan D, et al. Comparison of manual and automated filaments for evaluation of neuropathic pain in rats. J Pharmacol Toxicol Methods. 2012;66:8–13. doi: 10.1016/j.vascn.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Inglis JJ, Notley C, Essex D, et al. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of TNF blockade. Arthritis Rheum. 2007;56:4015–4023. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- 7.Miller RE, Tran PB, Das R, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109:20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]


