Summary
Aim
To determine neuropsychological tests likely to predict cognitive decline.
Methods
A sample of nonconverters (n = 106) was compared with those who declined in cognitive status (n = 24). Significant univariate logistic regression prediction models were used to create multivariate logistic regression models to predict decline based on initial neuropsychological testing.
Results
Rey–Osterrieth Complex Figure Test (RCFT) Retention predicted conversion to mild cognitive impairment (MCI) while baseline Buschke Delay predicted conversion to Alzheimer’s disease (AD). Due to group sample size differences, additional analyses were conducted using a subsample of demographically matched nonconverters. Analyses indicated RCFT Retention predicted conversion to MCI and AD, and Buschke Delay predicted conversion to AD.
Conclusion
Results suggest RCFT Retention and Buschke Delay may be useful in predicting cognitive decline.
Keywords: Alzheimer’s disease, cognitive decline, conversion, memory disorder, mild cognitive impairment
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that causes a host of cognitive and other behavioral changes and afflicts an estimated 5.2 million Americans [1]. Based on 2012 data from the Center for Disease Control and Prevention, AD was reported as the underlying cause of death for 83,637 people, making it the sixth leading cause of death in the USA [2]. Aggregate payments for healthcare, long-term care and hospice for people with AD and related dementias in 2014 are estimated at US$214 billion, with Medicare and Medicaid covering about 70% of the costs of care [1].
Healthcare professionals may identify neurocognitive changes early in the disease course, allowing for potential intervention opportunities. In particular, early identification of mild cognitive impairment (MCI), oftentimes considered as a ‘pre-dementia’ stage, has received a lot of attention. Studies indicate that as many as 3–22% of people age 65 and older experience MCI [3–5]. Approximately 10–15% of individuals diagnosed with MCI convert to AD every year [6], and those with primarily amnestic features are more likely to develop AD [6–8]. Although some people with MCI (primarily those without memory problems) experience improvement in cognition or revert to normal cognitive status [8], nearly a third of all people with MCI symptoms develop AD in 3 or 4 years [9]. Therefore, it is important for healthcare professionals to identify those who experience and exhibit signs of cognitive decline in order to slow the disease trajectory.
The identification of age-related cognitive conditions occurs through a collective approach comprised of neuroimaging, neuropsychological tests, laboratory tests, as well as collaborative and self-reported symptoms [10]. Of particular relevance, neuropsychological measures are considered one of the primary means in monitoring cognitive status. One study concluded that select neuropsychological measures more efficiently monitored the disease progression than MRI [11].
Deficits in three main cognitive domains are sensitive in predicting conversion to AD: episodic verbal and visuospatial memory, executive function and language. Most research has focused on tests of verbal memory (e.g., list-learning, recall for contextual information), including, the California Verbal Learning Test [12–15], Rey Auditory Verbal Learning Test [16], Buschke Selective Reminding Test (SRT) [17–21], Wechsler Memory Scale’s Verbal Paired Associates [22] and Wechsler Memory Scale’s Logical Memory [13]. Visual memory tests that have received attention in the literature include Wechsler Memory Scale’s Visual Reproduction [15] and the Rey–Osterrieth Complex Figure Test (RCFT) [23–25]. Measures of executive and language functioning that may predict conversion include Trail Making Test B (TMT B) [15,25–29], Wechsler Adult Intelligence Test-Revised digit symbol coding [21], Stroop Color Naming [30], Wechsler Memory Scale-Revised digit span [31], semantic fluency [32,33] and the Boston Naming Test (BNT) [34,35].
Of the aforementioned memory tests, the SRT is capable of evaluating several facets of learning and memory, including acquisition, storage, retention and retrieval [36,37]. Despite the ability to comprehensively evaluate memory functioning, few studies have used the Buchke SRT to predict conversion to AD [17,21]; however, the results of these studies have suggested the Buchke SRT does have the capacity to do so. Additionally, the RCFT [38] measuring visuospatial and visuoconstructional abilities, perceptual organization and planning (executive functioning) and visual memory has been shown to be sensitive in discerning early stage AD [39] as well as predicting conversion [24,40–41]. The TMT B, a test of cognitive flexibility, is a popular measure of executive functioning and has been found to be a powerful predictor of conversion to AD [42,43]. In terms of language functioning, verbal semantic fluency is impaired in presymptomatic AD patients [44]. Moreover, the BNT may possess particular predictive ability due to its assessment of long-term memory. A couple of studies have found that the BNT effectively predicts conversion to AD [35,45].
It is apparent that specific neuropsychological measures may possess adequate utility in predicting cognitive decline. Although the above-mentioned studies have examined the ability for neuropsychological measures to predict conversion to a more severe cognitive disorder, rigorous diagnostic methods for determining conversion status, employment of neuropsychological tests from each cognitive domain, and in some cases, stringent statistical processes, were often lacking. The aim of the current study is to investigate the use of the Buschke SRT, RCFT, TMT B, BNT and semantic fluency tests to predict change in cognitive status. To the best of these authors’ knowledge, this is the first study to examine these measures together with a rigorous diagnostic approach to determining conversion (e.g., clinical consensus, imaging and full neuropsychological battery). We hypothesized that these measures would predict conversion to a more severe cognitive status (e.g., MCI and AD) 2 years prior to conversion.
Materials & methods
• Design & setting
Recruitment through advertisements and physician referral emphasized middle-aged and older people with memory complaints. Any subjects with a neurological, medical or psychiatric condition that could affect memory or other cognitive processing were excluded. Written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board. Subjects were asked to return for a 2-year (mean [M] = 2.4; standard deviation [SD] = 1.3 years) follow-up re-evaluation and then classified depending on assessment results.
Sample population
A convenience sample based on the availability of follow-up neuropsychological data was evaluated. Individuals were drawn from a larger longitudinal study of age-related memory loss designed to determine neuropsychological, neuroimaging, and genetic predictors of subsequent cognitive decline. Standardized laboratory screening tests for a dementia evaluation and MRI scans were performed to uncover potentially treatable causes of cognitive impairment. DNA was obtained from blood samples, and APOE genotypes were determined with the use of standard techniques. Investigators blind to the genetic findings performed all of the clinical procedures. Those with specific neurological and medical disorders were excluded from participation. A family history of subjects’ relatives was obtained and corroborated by medical records. A positive family history was defined as one or more first-degree relatives (parent and sibling) with documented AD. A negative family history was defined as no first- or second-degree relative with a history of dementia. Participants with ambiguous family histories were excluded.
Predictive neuropsychological measures
A comprehensive neuropsychological test battery was administered to quantify cognitive performance. For the present study, we selected neuropsychological tests that are widely used in research on normal aging and that also have demonstrated sensitivity to the types of cognitive changes associated with conversion to a more severe cognitive disorder. Tests included measures of verbal memory (Buschke SRT) nonverbal memory and visuospatial functioning (RCFT), executive functioning (TMT B) and confrontation naming (BNT). The participant’s raw scores were used for all analyses.
Conversion outcome
Participants were classified at the initial neuropsychological assessment as either normal (no cognitive diagnosis) or MCI. Classification was determined via rigorous diagnostic methods, including multiple sources of diagnosis (MRI scan, clinical consensus of neurology, geriatric psychiatry, neuropsychology and radiology staff). Neuropsychology determined diagnosis based on a full neuropsychological assessment battery and clinical interview that consisted of over 20 neuropsychological measures, four of which were examined in the current study. To diagnose mild cognitive impairment, we used standard diagnostic criteria. These include the subject’s awareness of a memory problem, preferably as confirmed by another person; memory impairment detected with the use of standard assessment tests; normal overall thinking and reasoning skills and the ability to perform normal activities of daily living [6]. The diagnosis was corroborated by clinical judgment; to increase the specificity in detecting impairments, we included only subjects with mild cognitive impairment who had a score of 1 SD or more below the age-corrected norms on at least two neuropsychological tests in one of the five cognitive domains assessed. Subjects with Alzheimer’s disease met the standard diagnostic criteria of memory impairment, impairment in at least one other cognitive domain, gradual onset and progressive decline and impaired occupational or social functioning or both [46,47]. Conversion outcomes were dichotomously coded as ‘stable’ or ‘converted’ and additionally coded as either ‘normal’, ‘MCI’ or ‘AD’. Upon follow-up evaluation, participants were independently re-classified as either normal, MCI or AD via the same standards used during initial assessment.
Statistical analyses
SPSS 17 was used for all analyses conducted to determine which neuropsychological measures predict conversion from normal or MCI to MCI or probable AD. The database was screened for missing scores, and participants who were not given the measures of interest were eliminated from the study. Frequency analyses were completed in order to determine demographic characteristics within the study sample. χ2 and t-tests were conducted in order to determine differences between converters and nonconverters with regards to age, gender and education. Univariate logistic regression analysis was used to determine conversion to MCI and AD for all neuropsychological tests. Multivariate models were constructed based on significant univariate predictors after applying the Bonferroni correction. To correct for error caused by group size discrepancy between converters and nonconverters, a second set of univariate binary regressions was conducted using a demographically matched (gender, age, education and ethnicity) subset of nonconverters; however, multivariate models could not be constructed due to the limited sample size and power.
Results
• Demographic data
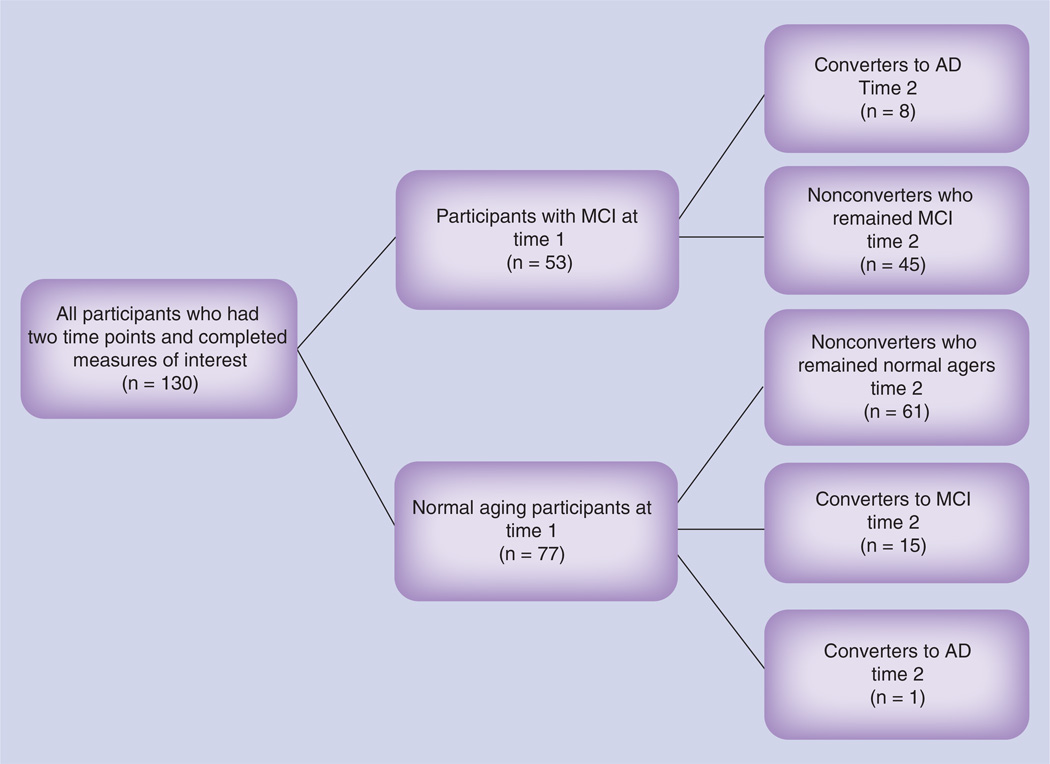
Data were examined from 130 individuals (see Figure 1). In total, 130 participants were examined at both time points; 53 were initially classified with MCI and 77 were initially classified as normal aging. Of the 53 who began the current study with MCI, eight developed probable AD and 45 remained classified as MCI. Of the 77 who began the current study as normal aging, one converted to AD, 15 converted to MCI and 61 remained classified as normal aging. The sample was mostly Caucasian 87% with a smaller number of Latinos (3%), Asian–Americans (5%) and African–Americans (5%). Additionally, 60% of the participants were women, the mean age (at the initial evaluation) was 61.4 years (SD = 11.3) and average years of education was 16.4 (SD = 3.0). Groups (converters and nonconverters) did not significantly differ based on age, gender and education (Tables 1–3).
Figure 1. Quantity of participants at each cognitive status at time 1 and time 2.
AD: Alzheimer’s disease; MCI: Mild cognitive impairment.
Table 1.
Age and education: nonconverters versus converters to mild cognitive impairment (n = 76).
| Variable | Nonconverters | Converters to AD | T | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | |||
| Age | 61 | 60.84 | 10.76 | 15 | 65.2 | 11.42 | −1.341 | 0.194 |
| Education | 61 | 16.67 | 2.94 | 15 | 15.73 | 3.24 | 1.086 | 0.281 |
Not significant after Bonferroni correction.
AD: Alzheimer’s disease; M: Mean; SD: Standard deviation; T: T-test.
Table 3.
Gender: nonconverters versus converters.
| Variable | Nonconverters | Converters | Total | Pearson χ2 | df | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| MCI Groups | 23 | 38 | 7 | 8 | 76 | 0.405 | 1 | 0.525 |
| AD Groups | 48 | 73 | 6 | 3 | 130 | 2.514 | 1 | 0.629 |
Not significant after Bonferroni correction.
AD: Alzheimer’s disease; MCI: Mild cognitive impairment.
• Conversion to MCI
The individual univariate logistical regressions indicated that the Buschke SRT (Total, Delay and Recognition), RCFT Retention and TMT B individually predicted conversion to MCI (Table 4). The multivariate logistic regression model, based on the significant results from the univariate analysis indicated the RCFT Retention test as the only significant predictor of conversion to MCI (Table 5). This model correctly classified 88.2% of participants (96.7% nonconverters and 53.3% converters). Specifically, individuals who could retain 30% (RCFT Retention score M = 30.7; SD = 15.8) of visual information after a delay were more likely to be classified as MCI at follow-up than individuals who could retain 54% (RCFT Retention score M = 54.4; SD = 16.8) or more of the same visual information.
Table 4.
Univariate binary logistic regression predicting conversion from normal aging to mild cognitive impairment (61 nonconvertors and 15 converted to mild cognitive impairment).
| Variable | Wald (df = 1) | p-value | Odds ratio |
|---|---|---|---|
| Buschke Total | 8.508 | 0.004* | 0.944 |
| Buschke Delay | 13.439 | 0.000* | 0.645 |
| Buschke Recognition | 5.812 | 0.016 | 0.256 |
| RCFT Copy | 0.003 | 0.959 | 1.005 |
| RCFT Delay | 0.220 | 0.639 | 0.983 |
| RCFT Retention | 13.663 | 0.000* | 0.913 |
| RCFT Recognition | 1.070 | 0.301 | 3.080 |
| Boston Naming Test – 2 | 4.518 | 0.034 | 0.824 |
| TMT B | 9.604 | 0.002* | 1.049 |
| Animals | 0.389 | 0.533 | 0.961 |
p < 0.005 level (after applying the Bonferroni correction method).
Table 5.
Multivariate logistic regression predicting conversion from normal aging to mild cognitive impairment (61 nonconvertors and 15 converted normal to mild cognitive impairment).
| Variable | Wald (df = 1) | p-value | Odds ratio |
|---|---|---|---|
| Buschke Total | 0.380 | 0.538 | 1.027 |
| Buschke Delay | 3.108 | 0.078 | 0.659 |
| RCFT Retention | 8.063 | 0.005* | 0.925 |
| TMT B | 1.648 | 0.199 | 1.026 |
p < 0.005.
• Conversion to AD
The individual univariate logistical regressions indicated that the Buschke SRT (Total, Delay and Recognition), RCFT (Copy, Delay and Retention) and TMT B individually predicted conversion to AD (Table 6). The multivariate logistic regression model indicated that only the Buschke SRT Delay predicted conversion to AD (Table 7), correctly classifying 96.2% of participants (98.3% nonconverters and 66.7% converters). Specifically, individuals who were not demented initially but recalled only approximately 2 out of 12 words from a list (Buschke SRT Delay score M = 1.9; SD = 2.7) were more likely to develop dementia at follow-up than individuals who were able to recall approximately 8 out of 12 words on this same task (Buschke SRT Delay score M = 8.4; SD = 3.2).
Table 6.
Univariate binary logistic regression predicting conversion from normal aging/mild cognitive impairment to probable Alzheimer’s disease (121 nonconvertors and 9 converted to probable Alzheimer’s disease)
| Variable | Wald (df = 1) | p-value | Odds ratio |
|---|---|---|---|
| Buschke Total | 12.883 | 0.000* | 0.927 |
| Buschke Delay | 12.578 | 0.000* | 0.550 |
| Buschke Recognition | 12.012 | 0.001* | 0.382 |
| RCFT Copy | 9.786 | 0.002* | 0.722 |
| RCFT Delay | 8.234 | 0.004* | 0.780 |
| RCFT Retention | 6.717 | 0.010 | 0.936 |
| Boston Naming Test – 2 | 0.862 | 0.353 | 0.943 |
| TMT B | 7.142 | 0.008 | 1.018 |
| Animals | 3.171 | 0.075 | 0.859 |
p < 0.005 (after applying the Bonferroni correction method).
Table 7.
Multivariate logistic regression predicting conversion from normal aging/mild cognitive impairment to probable Alzheimer’s disease (121 nonconvertors and 9 converted to probable Alzheimer’s disease).
| Variable | Wald (df = 1) | p-value | Odds ratio |
|---|---|---|---|
| Buschke Total | 0.662 | 0.416 | 1.037 |
| Buschke Delay | 5.689 | 0.017* | 0.537 |
| Buschke Recognition | 0.331 | 0.565 | 0.765 |
| RCFT Copy | 2.931 | 0.087 | 0.781 |
| RCFT Delay | 0.480 | 0.488 | 0,924 |
p < 0.05 level.
• Demographically matched sample
To correct for error caused by group size discrepancy between converters and nonconverters, a second set of univariate binary logistic regressions was conducted using a demographically matched (gender, age, education and ethinicity) subset of nonconverters.
• Conversion to MCI
These tests indicated that the RCFT Retention test significantly predicted conversion to MCI. Specifically, individuals who were only able to retain approximately 31% (RCFT Retention score M = 30.7; SD = 15.8) of visual information after a delay were more likely to develop MCI at follow-up than individuals who were able to retain approximately 51% (RCFT Rentention score M = 50.9; SD = 21.2) or more of the same visual information (Table 8). This test correctly classified 66.7% of participants (53.3% nonconverters and 80.0% converters). None of the other univariate models predicted conversion within the small demographically matched sample.
Table 8.
Univariate binary logistic regression predicting conversion from normal aging to mild cognitive impairment (15 nonconvertors and 15 converted to mild cognitive impairment).
| Variable | Wald (df = 1) | p-value | Odds ratio |
|---|---|---|---|
| Buschke Total | 0.421 | 0.516 | 0.988 |
| Buschke Delay | 1.839 | 0.175 | 0.864 |
| Buschke Recognition | 0.393 | 0.531 | 0.815 |
| RCFT Copy | 0.617 | 0.432 | 1.085 |
| RCFT Delay | 0.001 | 0.980 | 0.999 |
| RCFT Retention | 5.520 | 0.019* | 0.940 |
| RCFT Recognition | 0.357 | 0.550 | 2.154 |
| Boston Naming Test – 2 | 0.200 | 0.655 | 1.030 |
| TMT B | 0.355 | 0.551 | 1.046 |
| Animals | 0.355 | 0.551 | 1.046 |
p < 0.05 level.
• Conversion to AD
Furthermore, the results revealed that the Buschke SRT Delay and the RCFT Retention tests were significant univariate predictors of conversion to AD (Table 9). Specifically, individuals who were not demented at initial evaluation but only retained approximately 30% (RCFT Retention score M = 29.8; SD = 14.3) of visual information after a delay were more likely to develop dementia at follow-up than individuals who were able to retain approximately 51% (RCFT Retention score M = 50.6; SD = 17.3) or more of the same visual information. This test correctly classified 77.8% of participants (77.8% nonconverters and 77.8% converters). Furthermore, those individuals who were not demented at the initial evaluation but recalled only approximately 2 out of 12 words from a list (Buschke SRT Delay score M = 2.0; SD = 2.7) were more likely to develop dementia by follow-up than individuals who were able to recall approximately 7 out of 12 words on this same task (Buschke SRT Delay score M = 7.0; SD = 4.1). This test correctly classified 72.2% of participants (66.7% nonconverters and 77.8% converters). None of the other univariate models significantly predicted conversion within the small matched sample.
Table 9.
Univariate binary logistic regression predicting conversion from normal/mild cognitive impairment to Alzheimer’s disease (9 nonconvertors and 9 converted to Alzheimer’s disease).
| Variable | Wald (df = 1) | p-value | Odds ratio |
|---|---|---|---|
| Buschke Total | 3.267 | 0.071 | 0.944 |
| Buschke Delay | 4.910 | 0.027* | 0.675 |
| Buschke Recognition | 1.932 | 0.165 | 0.534 |
| RCFT Copy | 3.392 | 0.066 | 0.767 |
| RCFT Delay | 3.603 | 0.058 | 0.755 |
| RCFT Retention | 3.897 | 0.048* | 0.899 |
| RCFT Recognition | 0.233 | 0.630 | 0.625 |
| Boston Naming Test – 2 | 0.097 | 0.755 | 0.976 |
| TMT B | 0.889 | 0.346 | 1.010 |
| Animals | 0.986 | 0.321 | 0.903 |
p < 0.05 level
Discussion/Conclusion
The current study revealed that two measures served as predictive indicators of conversion to a more severe cognitive status (e.g., MCI and AD) within a 2-year time-frame. When assessing the entire study sample, the RCFT Retention score predicted conversion from normal aging to MCI; however, it is important to note that this analysis generated a small effect (Table 5). As for those who went onto develop dementia of the Alzheimer’s type, the Buschke Delay score predicted conversion to AD (Table 7). Because only nine participants converted to probable AD, any statements made regarding this effect, are done so with caution.
Given the disparity in the sizes of the two groups (converters and nonconverters), the analyses were also conducted with a subsample of participants who were demographically matched to the converter groups (e.g., MCI and AD). Notably, conversion to MCI or AD was predicted by lower retention scores from the complex figure (RCFT; Tables 8 & 9). As it did in the full sample, the Buschke Delay score predicted conversion to AD in the demographically matched subsample (Table 9).
To the authors’ knowledge, this is one of the first studies to examine these measures together with a rigorous diagnostic approach to determining conversion (e.g., clinical consensus, imaging and full neuropsychological battery). The existing literature corroborates the finding that tests of visual and verbal memory predicted conversion. Select measures of language and executive functioning have also been reported as sensitive in predicting conversion to a more severe memory disorder [15,21,25–28,30–35]. However, these findings were not upheld in the present study. Research suggests that tau protein tangles and amyloid plaques spread in a predictable, nonrandom manner beginning in the entorhinal region (‘relay station’ between the hippocampus and neocortex), spreading to the hippocampus (memory center) and neocortex (responsible for higher functions such as sensory perception, conscious thought and language [48]). The entorhinal-hippocampus system plays an important role in autobiographical, declarative and episodic memories and in particular spatial memories including memory formation, memory consolidation and memory optimization in sleep. Because this entorhinal region is one of the first areas impacted by tau tangles – accumulating and eventually causing neuronal death – it is expected that the tests measuring functions of this region would predict conversion before tests measuring functions of domains impacted later in the disease process (e.g., language and executive functioning).
Additionally, this discrepancy between past research and the current findings may be attributed to our implementation of a more rigorous diagnostic method for determining conversion status. For instance, the current study utilized multiple sources of diagnosis (e.g., clinical consensus by neurology, geriatric psychiatry, neuropsychology and radiology), employment of neuropsychological tests from each cognitive domain, and in some cases, multiple tests within a domain and stringent statistical processes (examined the impact of age, education and gender on conversion and utilized statistical corrections when running multiple tests). Other studies have used diagnostic methods such as imaging when assessing for conversion [18,23,25,32,41,49–52], but the majority of studies did not examine the Buschke SRT, BNT or the RCFT. Few studies have been found that matched the current study’s diagnostic rigor and assessment measures. Participants within the Jacobs et al. [34] study did not undergo imaging as way of confirming conversion, and the Tabert et al. [21] study included imaging as part of their conversion determination, but did not include the RCFT within their neuropsychological battery.
A limitation of this study is the relatively small sample size, especially the individuals who converted to MCI or AD. Other similar studies with larger sample sizes have reported somewhat different findings. For instance, in a community-based sample of approximately 443 people, Jacobs et al. [34] found that, in addition to the immediate recall on the selective reminding test, the BNT–2 and the WAIS-III Similarities subtest were predictive of conversion to dementia. Additionally, in a large sample of approximately 600 individuals, Chen et al. [25] reported word list delayed recall and the TMT B to predict conversion to dementia. We believe this study, although small in sample size, has strength in other areas such as diagnostic rigor in terms of conversion status and statistical methods in utilizing correction methods. It is our hope that future research will replicate this study on larger samples.
Additional caveats were noted within the methodology. First, the measures used for this study were also aided in diagnosis of MCI and AD; therefore, any inherent bias or error could have confounded the results. Furthermore, the authors acknowledge that this study examined one particular group of participants and no conformational analysis was done with a separate group of individuals to confirm the finding. Further, it has been suggested that preselection of candidate variables via univariate modeling may not be the best approach; however, given our limited sample size and power we felt it would be best to use this method in combination with the Bonferroni correction in order to decrease type one error and develop the most robust models available provided our power limitations. Given the limited and varied published data on prediction of conversion of MCI and AD, we did not have sufficient a priori hypotheses that would have allowed for alternative methods (e.g., hierarchical regression).
Another limitation of the study is ethnicity and education diversity. Our sample consisted of mainly Caucasian and college-educated individuals. Individuals of diverse demographics may differ with regards to cognitive degeneration (e.g., higher education may buffer against a cognitive degenerative disease diagnosis [3]) and the impact of this difference on predicting conversion is not known.
The findings of the current study suggest that two neuropsychological measures can predict conversion to a more severe cognitive status. If similar results are found with more diverse samples, neuropsychologists may use such measures to determine whether a patient may later convert to a more severe memory disorder, with the goal of intervening to delay progression [5,53–58]. Furthermore, it is critical to study those who develop AD at the earliest stages to better understand the progression to facilitate the development of preventative treatments.
Although it is essential to determine whether an individual will convert to a more severe cognitive diagnosis, it is also imperative to recognize that the job of the provider is not simply to inform our patient of their likelihood of converting. Rather, our goal is to help inform the patient of their cognitive strengths and weaknesses in relation to their daily functioning to inform coping strategies, as well as to intervene via cognitive training, diet, exercise, among others. Therefore, although our findings may help to predict later cognitive decline, these tests should be used along with a full neuropsychological battery, particularly when a decline in patient functioning is reported. Using these tests alone may inhibit the provider from gathering measurable cognitive strengths and weaknesses from each cognitive domain and more importantly, offering relevant coping strategies and recommendations related to these findings.
In summary, the current study sought to determine which neuropsychological measures best predict future cognitive decline. Among the current study’s sample, the Buschke SRT and the RCFT are sensitive in predicting conversion to a more severe cognitive disorder (e.g., MCI and probable AD) 2 years prior to conversion. These findings are in line with the majority of past research demonstrating verbal and visual memory tasks to be the most predictive of conversion. However, a few other studies have also reported that tests of executive functioning and language can predict conversion. The current study examined the Buschke SRT and RCFT (among others: BNT, TMT B and Animals) because of the unique capability of these tests to measure multiple cognitive resources (e.g., Buschke SRT: verbal memory, working memory and executive functioning; RCFT: visual memory and executive functioning). Future studies may seek to carry out the current methodology among more diverse samples to determine the sensitivity of the current study’s measures among individuals of various demographics. This is one of the first studies to examine these measures together with a rigorous diagnostic approach to determining conversion (e.g., clinical consensus, imaging and full neuropsychological battery). These findings may assist clinicians and researchers in detecting individuals who may convert to either MCI or probable AD.
Future perspective
In the upcoming decade, the aging research community, particularly those examining preclinical markers for pathological cognitive decline, expects to better understand what measures individually or collaboratively best predict conversion to a more severe cognitive status and specifically, what measures predict future functional impairment. It is anticipated that future studies will work to build upon the current study’s findings and determine if these results are found in varied samples. Particularly, it is our hope that future studies will test the current study’s hypotheses with advanced methodological rigor (e.g., larger sample size, include an independent test sample, examine alternative MCI and dementia subtypes). Ultimately, researchers will use this information to further examine the effectiveness of interventions in slowing or halting the development of memory disorders (e.g., dementia).
Table 2.
Age and education: nonconverters versus converters to Alzheimer’s disease (n = 130).
| Variable | Nonconverters | Converters to AD | T | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | |||
| Age | 121 | 60.69 | 11.15 | 9 | 68.56 | 9.98 | −2.056 | 0.042 |
| Education | 121 | 16.45 | 3.03 | 9 | 15.78 | 2.77 | 0.642 | 0.522 |
Not significant after Bonferroni correction.
AD: Alzheimer’s disease; M: Mean; SD: Standard deviation.
Practice points.
The current study’s investigators sought to determine which neuropsychological tests (e.g., Buschke Selective Reminding Test [SRT] and Rey–Osterrieth Complex Figure Test [RCFT]) are more likely to predict an individual’s cognitive decline (i.e., normal to mild cognitive impairment [MCI] and MCI to Alzheimer’s disease [AD]).
A sample of nonconverters compared with those who convert in cognitive status (i.e., MCI or AD) was examined. Univariate binary logistic regression was performed to test the predictive values neuropsychological tests. Multivariate analyses were built based on the univariate findings.
The results established the use of the RCFT and Buschke SRT in predicting cognitive decline to MCI and AD.
Clinically, these findings suggest two measures may predict cognitive decline, and may be important for clinicians/researchers to monitor.
Acknowledgments
This work was supported by an NIH NRSA 1F31AG035438-01 and by NIH grants P01-AG025831, AG13308, P50 AG 16570, MH/AG58156, MH52453; AG10123; M01-RR00865, General Clinical Research Centers Program; the Fran and Ray Stark Foundation Fund for Alzheimer’s Disease Research; and the Ahmanson Foundation. GW Small reports having served as an advisor and/or having received lecture fees from Actavis, Cogniciti, Herbalife, Janssen, Novartis, Pfizer, and Forum Pharmaceuticals. GW Small also reports having received stock options from TauMark, LLC and a grant from Pom Wonderful.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Association Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2014;10(2):e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Miniño AMS, Xu J, Kochanek K. National Vital Statistics Reports. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. Deaths: final data for 2008. http://www.cdc.gov/nchs/data/nvsr/nvsr59. [Google Scholar]
- 3.Hanninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol. Scand. 2002;106(3):148–154. doi: 10.1034/j.1600-0404.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Lopez OL, Jagust WJ, Dekosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch. Neurol. 2003;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 8.Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch. Neurol. 2011;68(6):761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 10.Mckhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging – Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmand B, Rienstra A, Tamminga H, et al. Responsiveness of magnetic resonance imaging and neuropsychological assessment in memory clinic patients. J. Alzheimers Dis. 2014;40(2):409–418. doi: 10.3233/JAD-131484. [DOI] [PubMed] [Google Scholar]
- 12.Beck IR, Gagneux-Zurbriggen A, Berres M, Taylor KI, Monsch AU. Comparison of verbal episodic memory measures: consortium to establish a registry for Alzheimer’s disease – Neuropsychological Assessment Battery (CERAD-NAB) versus California Verbal Learning Test (CVLT) Arch. Clin. Neuropsychol. 2012;27(5):510–519. doi: 10.1093/arclin/acs056. [DOI] [PubMed] [Google Scholar]
- 13.Rabin LA, Pare N, Saykin AJ, et al. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2009;16(3):357–376. doi: 10.1080/13825580902825220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silva D, Guerreiro M, Maroco J, et al. Comparison of four verbal memory tests for the diagnosis and predictive value of mild cognitive impairment. Dement. Geriatr. Cogn. Dis. Extra. 2012;2(1):120–131. doi: 10.1159/000336224. • It was one of very few studies that examined the ability of neuropsychological measures to predict conversion to mild cognitive impairment (MCI); the majority of tests examined conversion to dementia only.
- 15.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J. Int. Neuropsychol. Soc. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 16.Tierney MC, Yao C, Kiss A, Mcdowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64(11):1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 17.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol. Psychiatry. 2008;64(10):871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 19.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44(8):1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 20.Sarazin M, Berr C, De Rotrou J, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69(19):1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 21.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch. Gen. Psychiatry. 2006;63(8):916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 22.Venneri A, Gorgoglione G, Toraci C, Nocetti L, Panzetti P, Nichelli P. Combining neuropsychological and structural neuroimaging indicators of conversion to Alzheimer’s disease in amnestic mild cognitive impairment. Curr. Alzheimer Res. 2011;8(7):789–797. doi: 10.2174/156720511797633160. [DOI] [PubMed] [Google Scholar]
- 23.Borroni B, Anchisi D, Paghera B, et al. Combined 99mTc-ECD SPECT and neuropsychological studies in MCI for the assessment of conversion to AD. Neurobiol. Aging. 2006;27(1):24–31. doi: 10.1016/j.neurobiolaging.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis. Assoc. Disord. 2009;23(3):253–259. doi: 10.1097/WAD.0b013e3181999e92. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Ratcliff G, Belle SH, Cauley JA, Dekosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55(12):1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch. Gen. Psychiatry. 2007;64(12):1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewers M, Walsh C, Trojanowski JQ, et al. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol. Aging. 2012;33(7):1203–1214. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou B, Nakatani E, Teramukai S, Nagai Y, Fukushima M. Risk classification in mild cognitive impairment patients for developing Alzheimer’s disease. J. Alzheimers Dis. 2012;30(2):367–375. doi: 10.3233/JAD-2012-112117. [DOI] [PubMed] [Google Scholar]
- 29.Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging – Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: the power of errors in Stroop color naming. Psych. Aging. 2010;25(1):208–218. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurt P, Yener G, Oguz M. Impaired digit span can predict further cognitive decline in older people with subjective memory complaint: a preliminary result. Aging Ment. Health. 2011;15(3):364–369. doi: 10.1080/13607863.2010.536133. [DOI] [PubMed] [Google Scholar]
- 32.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 33.Lonie JA, Herrmann LL, Tierney KM, et al. Lexical and semantic fluency discrepancy scores in aMCI and early Alzheimer’s disease. J. Neuropsychol. 2009;3(Pt 1):79–92. doi: 10.1348/174866408X289935. [DOI] [PubMed] [Google Scholar]
- 34. Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer’s disease. Neurology. 1995;45(5):957–962. doi: 10.1212/wnl.45.5.957. •• Although this study did not examine conversion to MCI, it did use methodologically sound means to examine conversion to Alzheimer’s disease (AD).
- 35.Kraut MA, Cherry B, Pitcock JA, et al. The Semantic Object Retrieval Test (SORT) in amnestic mild cognitive impairment. Cogn. Behav. Neurol. 2007;20(1):62–67. doi: 10.1097/WNN.0b013e3180335f7d. [DOI] [PubMed] [Google Scholar]
- 36.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary (2nd Edition) NY, USA: Oxford University Press; 1998. [Google Scholar]
- 37.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment (4th Edition) NY, USA: Oxford University Press; 2004. [Google Scholar]
- 38.Meyers JE, Bayless JD, Meyers KR. Rey complex figure: memory error patterns and functional abilities. Appl. Neuropsychol. 1996;3(2):89. doi: 10.1207/s15324826an0302_8. [DOI] [PubMed] [Google Scholar]
- 39.Fujimori M, Imamura T, Yamashita H, et al. Age at onset and visuocognitive disturbances in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998;12(3):163–166. doi: 10.1097/00002093-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Alladi S, Arnold R, Mitchell J, Nestor PJ, Hodges JR. Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol. Med. 2006;36(4):507–515. doi: 10.1017/S0033291705006744. [DOI] [PubMed] [Google Scholar]
- 41.Perri R, Serra L, Carlesimo GA, Caltagirone C. Amnestic mild cognitive impairment: difference of memory profile in subjects who converted or did not convert to Alzheimer’s disease. Neuropsychology. 2007;21(5):549–558. doi: 10.1037/0894-4105.21.5.549. [DOI] [PubMed] [Google Scholar]
- 42.Chapman RM, Mapstone M, Mccrary JW, et al. Predicting conversion from mild cognitive impairment to Alzheimer’s disease using neuropsychological tests and multivariate methods. J. Clin. Exp. Neuropsychol. 2011;33(2):187–199. doi: 10.1080/13803395.2010.499356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozzini L, Chilovi BV, Conti M, et al. Conversion of amnestic mild cognitive impairment to dementia of Alzheimer type is independent to memory deterioration. Int. J. Geriatr. Psychiatry. 2007;22(12):1217–1222. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- 44.Adlam AL, Bozeat S, Arnold R, Watson P, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2006;42(5):675–684. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- 45.Howieson DB, Camicioli R, Quinn J, et al. Natural history of cognitive decline in the old old. Neurology. 2003;60(9):1489–1494. doi: 10.1212/01.wnl.0000063317.44167.5c. [DOI] [PubMed] [Google Scholar]
- 46.Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 47.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Edition), Text Revision (DSM-IVTR) Washington, DC, USA: American Psychiatric Association; 2000. [Google Scholar]
- 48.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 49.Artero S, Tierney MC, Touchon J, Ritchie K. Prediction of transition from cognitive impairment to senile dementia: a prospective, longitudinal study. Acta Psychiatr. Scand. 2003;107(5):390–393. doi: 10.1034/j.1600-0447.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 50.Estevez-Gonzalez A, Kulisevsky J, Boltes A, Otermin P, Garcia-Sanchez C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: comparison with mild cognitive impairment and normal aging. Int. J. Geriatr. Psychiatry. 2003;18(11):1021–1028. doi: 10.1002/gps.1010. [DOI] [PubMed] [Google Scholar]
- 51.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2008;14(2):266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rami L, Gomez-Anson B, Sanchez-Valle R, et al. Longitudinal study of amnesic patients at high risk for Alzheimer’s disease: clinical, neuropsychological and magnetic resonance spectroscopy features. Dement. Geriatr. Cogn. Dis. 2007;24(5):402–410. doi: 10.1159/000109750. [DOI] [PubMed] [Google Scholar]
- 53.Blasko I, Hinterberger M, Kemmler G, et al. Conversion from mild cognitive impairment to dementia: influence of folic acid and vitamin B12 use in the VITA cohort. J. Nutr. Health Aging. 2012;16(8):687–694. doi: 10.1007/s12603-012-0051-y. [DOI] [PubMed] [Google Scholar]
- 54.Cheng ST, Chow PK, Song YQ, et al. Mental and physical activities delay cognitive decline in older persons with dementia. Am. J. Geriatr. Psychiatry. 2013;22(1):63–74. doi: 10.1016/j.jagp.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 55.Lee LK, Shahar S, Rajab N, Yusoff NA, Jamal RA, Then SM. The role of long chain omega-3 polyunsaturated fatty acids in reducing lipid peroxidation among elderly patients with mild cognitive impairment: a case–control study. J. Nutr. Biochem. 2013;24(5):803–808. doi: 10.1016/j.jnutbio.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 56. Miller KJ, Siddarth P, Gaines JM, et al. The memory fitness program: cognitive effects of a healthy aging intervention. Am J. Geriatr. Psychiatry. 2012;20(6):514–523. doi: 10.1097/JGP.0b013e318227f821. •• Presents a cognitive fitness program that may be effective in increasing memory functioning. These programs may be of particular importance as we become more adept in determining who is at risk for converting/developing MCI or AD.
- 57. Roberts RO, Roberts LA, Geda YE, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimers Dis. 2012;32(2):329–339. doi: 10.3233/JAD-2012-120862. •• Investigated the impact of nutrition on MCI and dementia. The impact of diet on the development of MCI or dementia may be of increasing importance as we become more adept in determining who is at greater risk for converting/developing a cognitive disorder as this may inform clinician recommendation.
- 58.Wenisch E, Cantegreil-Kallen I, De Rotrou J, et al. Cognitive stimulation intervention for elders with mild cognitive impairment compared with normal aged subjects: preliminary results. Aging Clin. Exp. Res. 2007;19(4):316–322. doi: 10.1007/BF03324708. [DOI] [PubMed] [Google Scholar]