Abstract
Antenatal synthetic glucocorticoids promote fetal maturation in pregnant women at risk of preterm delivery and their mechanism of action may involve other endocrine systems. This study investigated the effect of maternal dexamethasone treatment, at clinically relevant doses, on components of the renin-angiotensin system (RAS) in the pregnant ewe and fetus. From 125 days of gestation (term, 145 ± 2 d), 10 ewes carrying single fetuses of mixed sex (3 female, 7 male) were injected twice im, at 10–11 pm, with dexamethasone (2 × 12 mg, n = 5) or saline (n = 5) at 24-hour intervals. At 10 hours after the second injection, maternal dexamethasone treatment increased angiotensin-converting enzyme (ACE) mRNA levels in the fetal lungs, kidneys, and heart and ACE concentration in the circulation and lungs, but not kidneys, of the fetuses. Fetal cardiac mRNA abundance of angiotensin II (AII) type 2 receptor decreased after maternal dexamethasone treatment. Between the two groups of fetuses, there were no significant differences in plasma angiotensinogen or renin concentrations; in transcript levels of renal renin, or AII type 1 or 2 receptors in the lungs and kidneys; or in pulmonary, renal or cardiac protein content of the AII receptors. In the pregnant ewes, dexamethasone administration increased pulmonary ACE and plasma angiotensinogen, and decreased plasma renin, concentrations. Some of the effects of dexamethasone treatment on the maternal and fetal RAS were associated with altered insulin and thyroid hormone activity. Changes in the local and circulating RAS induced by dexamethasone exposure in utero may contribute to the maturational and tissue-specific actions of antenatal glucocorticoid treatment.
In clinical practice, synthetic glucocorticoids, such as dexamethasone, are administered routinely to pregnant women at risk of preterm delivery in order to promote fetal maturation and neonatal survival (1, 2). These drugs mimic the normal rise in endogenous glucocorticoids seen in the fetus near term by promoting structural and functional changes in fetal tissues in preparation for life after birth (3). Over the last 40 years, antenatal glucocorticoid therapy has improved survival of the premature infant and has reduced markedly the incidence of many disorders associated with preterm delivery, such as respiratory distress syndrome (2, 4). The clear beneficial effects of maternal glucocorticoid treatment have been offset, however, by evidence showing adverse consequences for growth and long-term blood pressure (BP) control, especially in infants exposed to multiple doses in utero (5–7). It is therefore important to understand the mechanisms of glucocorticoid action in the control of fetal growth and maturation.
Some of the effects of the glucocorticoids on the development of fetal tissues are mediated, in part, by other endocrine systems (8, 9). For example, in fetal sheep, endogenous and synthetic glucocorticoids stimulate the production of the active thyroid hormone, T3, and in turn, T3 promotes hepatic glycogen deposition and gluconeogenic enzyme activity in preparation for blood glucose control at birth (10–13). The renin-angiotensin system (RAS) is functional in the fetus from relatively early in gestation (14, 15) and is known to have an important role in the growth and development of specific tissues, as well as in the regulation of renal and cardiovascular function in utero (16–18). A number of maturational changes are observed in the fetal RAS near term, some of which are regulated by the prepartum surge in endogenous glucocorticoids (19–22). In addition, direct administration of dexamethasone to the sheep fetus increases both pulmonary and circulating concentrations of angiotensin-converting enzyme (ACE) in association with a rise in fetal arterial BP (23). However, the effect of maternal dexamethasone treatment, in a regime similar to that used in clinical practice, on the components of the RAS in the pregnant mother and fetus during late gestation is unknown in any species.
Therefore, the aim of this study was to investigate the acute effect of maternal dexamethasone treatment, in clinically relevant doses, on various components of the RAS in the pregnant ewe and fetus. The study hypothesized that synthetic glucocorticoid administration to the pregnant ewe would stimulate components of both the maternal and fetal RAS with potential consequences for fetal development.
Materials and Methods
Animals
Ten Welsh Mountain ewes carrying singleton fetuses of known gestational age were used in this study. There were 3 female and 7 male fetuses. The ewes were maintained on 200 g kg−1 concentrates daily (sheep nuts number 6; 18% protein and 10 MJ/kg; H & C Beart Ltd) with free access to hay, water and a salt-lick block. All experimental procedures were carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and approved by the research ethics committee at the University of Cambridge.
Experimental procedures
From 125 days of gestation, all of the ewes were injected twice im with either dexamethasone (2 × 12 mg in 2-mL 0.9% NaCl, n = 5) or saline (2-mL 0.9% NaCl, n = 5) at 24-hour intervals. The experimental regime of dexamethasone treatment was similar to that recommended in human clinical practice by the Royal College of Obstetricians and Gynaecologists (24). At 10 hours after the second injection, the fetuses were delivered by caesarean section under general anesthesia (20 mg kg−1 sodium pentobarbitone iv). This time point was chosen so that data were obtained when the fetal dexamethasone concentration was comparable with previous studies that examined the cardiovascular effects of 1) maternal dexamethasone treatment and 2) direct fetal dexamethasone infusion in chronically catheterized fetuses (25, 26). The plasma dexamethasone concentration in the sheep fetus at this time point was approximately one-fifth of that measured in umbilical arterial blood samples taken from human infants at caesarean section after maternal dexamethasone treatment (27). Before anesthesia at between 9 and 10 am, 10-mL blood samples were obtained from the ewes by jugular venepuncture. At delivery, 10-mL blood samples were taken by venepuncture of the umbilical artery, and a number of tissues were collected from the ewes and fetuses after the administration of a lethal dose of barbiturate (200 mg kg−1 sodium pentobarbitone iv). Samples of lung, kidney, and heart from the fetus, and lung and kidney, but not heart, from the ewe, were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Biochemical analyses
Plasma hormone concentrations
All blood samples were immediately placed into EDTA-containing tubes and centrifuged for 5 minutes at 1000g and 4°C. The plasma aliquots were stored at −20°C until analysis. Plasma angiotensinogen and renin concentrations were measured by RIA as described previously (28, 29). The lower limits of detection were 0.01 μg mL−1 for angiotensinogen and 0.5 pg mL−1 h−1 for renin. Plasma concentrations of cortisol, T4, T3, and insulin were measured by RIA or ELISA as detailed and published in these animals previously (12, 30).
RAS protein concentrations
Tissue and plasma ACE concentrations (as a proxy measure of activity) were determined by a spectrophotometric enzyme assay as described previously (20, 22). Tissue ACE concentration was expressed as nanomoles of hippurate generated per min per mg protein, whereas plasma ACE concentration was measured in U l−1 where 1 U equals 1 μmol of hippurate generated in 1 minute. Protein levels of the angiotensin (AII) type 1 receptor (AT1R) and AT2R subtypes were determined in maternal lung and renal cortex, and fetal lung, heart, and renal cortex, by Western blotting as detailed previously (31). The primary antibodies used were both rabbit polyclonal antibodies to epitopes on the human AT1R (0.2-μg/mL 306, sc-579; Santa Cruz Biotechnology, Inc) and the human AT2R (0.04-μg/mL H-143, sc-9040; Santa Cruz Biotechnology, Inc) (Supplemental Table 1). Membranes were analyzed with Ponceau S to normalize for protein loading as validated previously (32). Proteins were quantified using ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/ij/), and ratios of protein content were arcsine transformed before statistical analysis.
RAS mRNA abundance
Tissue mRNA abundance of renin, ACE, AT1R, and AT2R were measured by TaqMan quantitative RT-PCR. Frozen samples of tissue (15 mg) were placed in Lysing Matrix-D tubes (MP Biomedicals) with 170-μL lysis/binding solution from a MagMax96 Total RNA Isolation kit (Life Technologies) and 0.75-μL β-mercaptoethanol, and homogenized using a FastPrep-24 (MP Biomedicals). After homogenisation, 106-μL 100% isopropanol was added to each sample. Samples were placed into a MagMAX96 system (Applied Biosystems) where RNA was isolated and deoxyribonuclease (DNase)-treated (TURBO DNase) using the MagMAX96 Total RNA Isolation kit (Life Technologies). Sample RNA yields and purities were assessed by a Nanodrop (Thermo Fisher). Ratios of absorption (260/280nm) of all preparations were between 1.8 and 2.0.
Reverse transcription of mRNA was carried out using a PCR Express machine (Thermo Fisher) and materials from Promega and Invitrogen. For each sample, 5 μL of DNase-treated RNA was mixed with 1-μL random primers, 1-μL deoxyribonucleotide triphosphate mix, and 5-μL ribonuclease-free water, and incubated at 65°C for 2 minutes. A master reverse transcription mix was made, consisting of 4-μL first strand buffer, 2-μL dithiothreitol, 1-μL RNaseOUT, and 1-μL Superscript II enzyme. The samples were incubated at room temperature for 5 minutes, at 42°C for 50 minutes and at 70°C for 15 minutes.
TaqMan quantitative real-time polymerase chain reaction (qRT-PCR) was performed to measure mRNA abundance of target genes in tissue samples. Samples were analyzed using a TaqMan 7900HT and data were acquired and processed with Sequence Detector version 2.3 software (Applied Biosystems). TaqMan Master Mix (5 μL), 0.5-μL target gene probe and primer set, and 3.5-μL water, were added to each well of a 96-well high-throughput plate (Applied Biosystems). In addition, 1-μL tissue cDNA at 1:20 dilution was added to each well apart from the nontemplate controls, where 1 μL of water was added. The sequences of the TaqMan qRT-PCR probes for renin, ACE, AT1R, and AT2R are listed in Table 1. Each tissue sample was measured in triplicate and normalized to the geometric mean of 2 housekeeping genes, glyceraldehyde 3-phosphate dehydrogenase, and cyclophilin A (Table 1). The mRNA levels of these housekeeping genes were not affected by maternal dexamethasone treatment. For each assay, a negative control without cDNA was included to ensure that amplicon contamination had not occurred in the reaction. Cycle thresholds determined by qRT-PCR were analyzed by the delta-delta-cycle threshold method as all standard curves were linear and parallel.
Table 1.
Primer and Reporter Sequences Used for TaqMan qRT-PCR in the Sheep
| Gene | Forward Primer Sequence | Reverse Primer Sequence | Reporter Sequence | Reporter Dye |
|---|---|---|---|---|
| Renin | GGATCTGGGAAGGTCAAAGGTTTC | CGCCAAAGGTCTGTGTGACT | CCGCCCACAGTCACC | FAM |
| ACE | CCTTCCCGCTACAACTATGACT | GGACAACCGGAGGACAGATC | ATACTTGGTTCGAAGATACC | FAM |
| AT1R (bovine) | TaqMan gene expression assays (Assay ID Bt03213473_m1; part number 4331182; reporter sequence AGGTCTGCATCCAGGTGCATTTGGC) | FAM | ||
| AT2R | CTGTCATTTACCCCTTTCTGTCTCA | CAGACAAGCCATACACCAAACAAG | TTGCCAGGGATTTCT | FAM |
| Glyceraldehyde 3-phosphate dehydrogenase | GCTACACTGAGGACCAGGTT | AGCATCGAAGGTAGAAGAGTGAGT | CTCCTGCGACTTCAAC | FAM |
| Cyclophilin A | GGTTCCTGCTTTCACAGAATAATTCC | GTACCATTATGGCGTGTGAAGTCA | CACCCTGGCACATAAA | FAM |
Statistical analyses
A sample size of 5 animals was calculated in order to find a 2-fold difference in fetal pulmonary ACE concentration, assuming a SD of 0.26, and to achieve 99% power at the 5% significance level. This sample size calculation was based on mean and SD values measured in previous studies from this laboratory examining the effects of fetal dexamethasone treatment on pulmonary ACE concentration (23) (Sigmastat 3.5; Systat Software, Inc). All data are presented as mean ± SEM. The distributions of data for plasma and tissue measurements were assessed for normality by the Kolmogorov-Smirnov test, and compared between the treatment groups by Student's unpaired t test (parametric) or Mann-Whitney test (nonparametric), as appropriate. Relationships between the variables measured were determined by Pearson correlation and partial correlation analyses. The null hypothesis was rejected where P < .05.
Results
Fetus
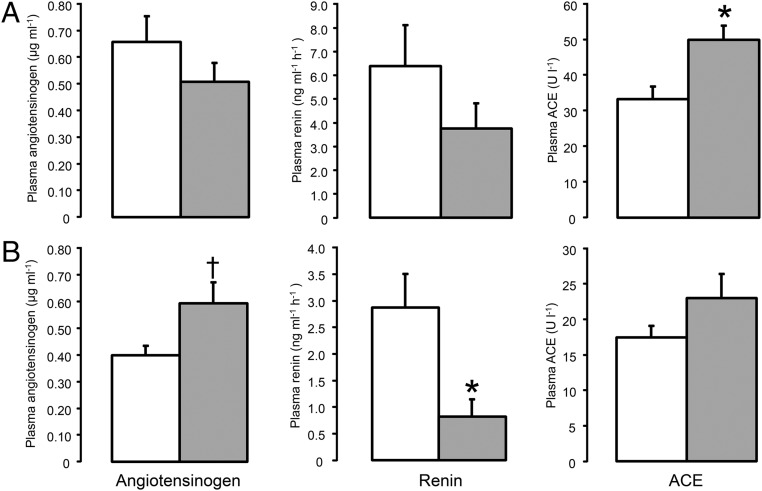
Within 10 hours of the second maternal injection of dexamethasone, the fetal plasma concentration of ACE increased significantly (P < .05) (Figure 1A). Plasma cortisol decreased, and T3 and insulin concentrations increased in the fetuses exposed to dexamethasone compared with those from the saline-treated ewes (P < .05) (Table 2). There were no significant differences in plasma concentrations of angiotensinogen, renin or T4 between the 2 groups of fetuses (Table 2 and Figure 1A).
Figure 1.
Plasma concentrations of angiotensinogen, renin, and ACE in the fetuses (A) and ewes (B) sampled at 10 hours after the second daily injection of either saline (□, n = 5) or dexamethasone ( , n = 5). Data are presented as mean values (±SEM). Significant difference from saline-treated group: *, P < .05; †, P = .06.
, n = 5). Data are presented as mean values (±SEM). Significant difference from saline-treated group: *, P < .05; †, P = .06.
Table 2.
Plasma Concentrations of Cortisol, Thyroid Hormones (T4 and T3), and Insulin in the Ewes and Fetuses at 10 Hours After Saline or Dexamethasone Treatment
| Ewe |
Fetus |
|||
|---|---|---|---|---|
| Saline | Dexamethasone | Saline | Dexamethasone | |
| Cortisol (ng mL−1) | 57.8 ± 12.2 | 3.2 ± 0.1a | 16.1 ± 2.8 | 10.0 ± 1.4a |
| T3 (ng mL−1) | 1.13 ± 0.08 | 0.69 ± 0.06a | 0.28 ± 0.06 | 0.70 ± 0.08a |
| T4 (ng mL−1) | 52.1 ± 10.6 | 21.8 ± 5.3a | 132.5 ± 11.7 | 115.0 ± 21.3 |
| Insulin (ng mL−1) | 0.19 ± 0.08 | 0.27 ± 0.05 | 0.25 ± 0.05 | 1.35 ± 0.31a |
Data are presented as mean values (±SEM).
Significantly different from saline-treated group, P < .05.
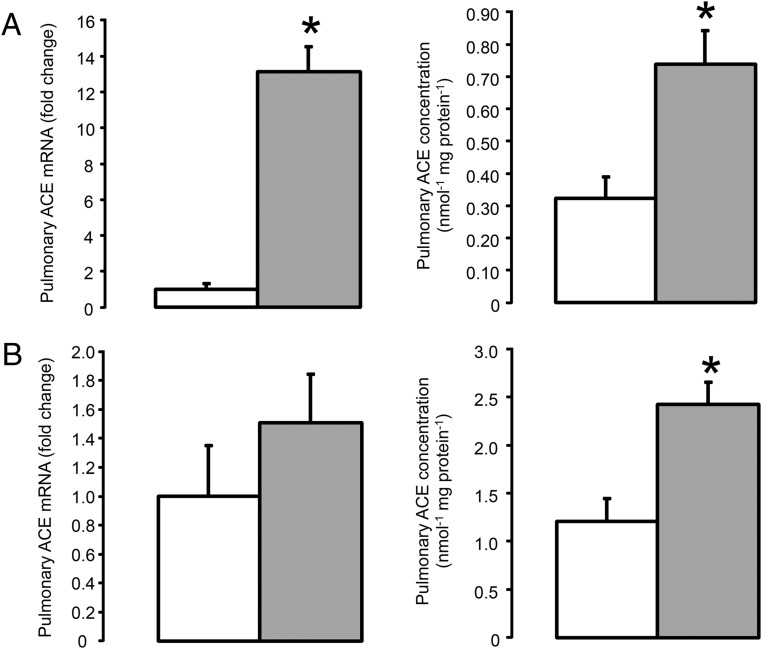
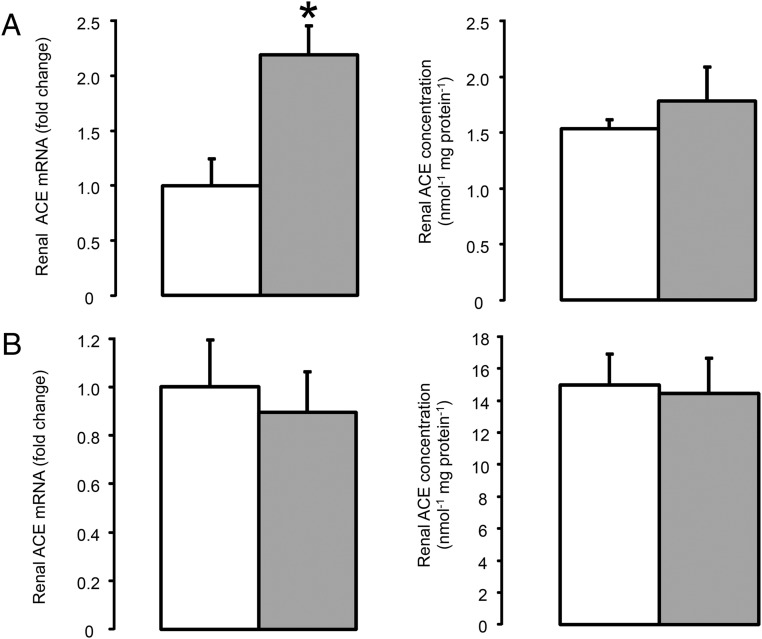
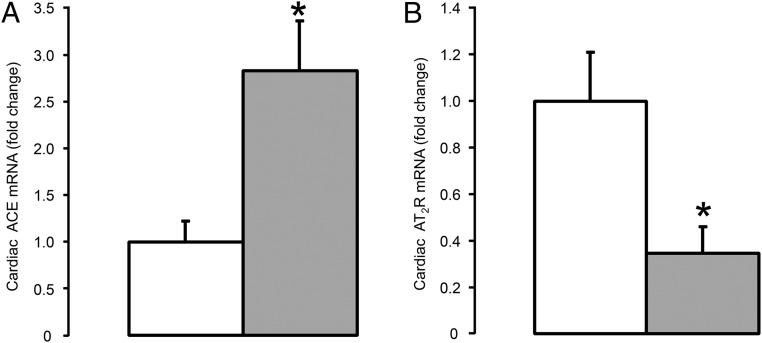
Pulmonary ACE mRNA and ACE concentration were significantly greater in the fetuses exposed to dexamethasone compared with the control fetuses (P < .05) (Figure 2A). The mRNA levels of ACE in the fetal kidney and heart were also elevated by maternal dexamethasone treatment (P < .05) (Figures 3A and 4A), although renal ACE concentration was unchanged and cardiac ACE concentration was below the limit of assay detection in both groups of fetuses (0.10 nmol min−1 per mg protein−1) (Figure 3A).
Figure 2.
Pulmonary ACE mRNA and concentration in the fetus (A) and ewe (B) at 10 hours after saline (□, n = 5) or dexamethasone ( , n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
, n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
Figure 3.
Renal ACE mRNA and concentration in the fetus (A) and ewe (B) at 10 hours after saline (□, n = 5) or dexamethasone ( , n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
, n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
Figure 4.
Cardiac ACE (A) and AT2R (B) mRNA levels in the fetus at 10 hours after saline (□, n = 5) or dexamethasone ( , n = 5) treatment. Data are presented as mean fold changes (±SEM) relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
, n = 5) treatment. Data are presented as mean fold changes (±SEM) relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
Neither relative mRNA abundance nor protein content for AT1R and AT2R in the lungs and kidneys were significantly different between fetuses of mothers treated with dexamethasone or saline (Table 3). Renal renin mRNA abundance was also unchanged by maternal dexamethasone treatment (Table 3). In the fetal heart, no significant changes in AT1R mRNA level, or protein content of AT1R or AT2R, were seen after maternal dexamethasone treatment, although cardiac AT2R mRNA abundance was reduced in the fetuses exposed to dexamethasone (Table 3 and Figure 4B; P < .05).
Table 3.
Relative Protein and mRNA Levels of Renin, AT1R, and AT2R in Fetal and Maternal Lung and Kidney at 10 Hours After Saline or Dexamethasone Treatment
| Ewe |
Fetus |
|||
|---|---|---|---|---|
| Saline | Dexamethasone | Saline | Dexamethasone | |
| Lung mRNA | ||||
| AT1R | 1.00 ± 0.29 | 1.69 ± 0.50 | 1.00 ± 0.25 | 1.57 ± 0.25 |
| AT2R | 1.00 ± 0.79 | 2.21 ± 1.15 | 1.00 ± 0.22 | 1.56 ± 0.43 |
| Lung protein | ||||
| AT1R | 1.00 ± 0.33 | 1.09 ± 0.26 | 1.00 ± 0.05 | 1.19 ± 0.09 |
| AT2R | 1.00 ± 0.08 | 1.03 ± 0.15 | 1.00 ± 0.15 | 1.15 ± 0.17 |
| Kidney mRNA | ||||
| Renin | 1.00 ± 0.24 | 0.53 ± 0.19 | 1.00 ± 0.33 | 1.37 ± 0.50 |
| AT1R | 1.00 ± 0.51 | 0.76 ± 0.29 | 1.00 ± 0.43 | 1.11 ± 0.49 |
| AT2R | 1.00 ± 0.59 | 0.21 ± 0.06 | 1.00 ± 0.40 | 0.57 ± 0.23 |
| Kidney protein | ||||
| AT1R | 1.00 ± 0.08 | 0.99 ± 0.07 | 1.00 ± 0.11 | 0.95 ± 0.10 |
| AT2R | 1.00 ± 0.08 | 1.16 ± 0.08 | 1.00 ± 0.10 | 1.21 ± 0.13 |
| Heart mRNA | ||||
| AT1R | NA | NA | 1.00 ± 0.28 | 1.20 ± 0.19 |
| AT2R | NA | NA | 1.00 ± 0.16 | 1.23 ± 0.09 |
| Heart protein | ||||
| AT1R | NA | NA | 1.00 ± 0.10 | 1.15 ± 0.07 |
| AT2R | NA | NA | 1.00 ± 0.21 | 0.34 ± 0.11a |
Data are presented as mean fold changes (±SEM) relative to the saline-treated group. NA, not available.
a Significant difference from saline-treated group, P < .05.
When observations from all of the fetuses were considered, a significant positive correlation was observed between pulmonary ACE concentration and circulating ACE levels (r = +0.77, P < .001, n = 10). Significant negative correlations were observed between plasma cortisol concentration and both pulmonary ACE mRNA abundance and plasma ACE concentration (Table 4). Plasma renin concentration was negatively correlated with plasma ACE concentration in the fetuses (Table 4). In addition, plasma T3 correlated with circulating and pulmonary ACE concentrations, and with ACE mRNA abundance in the fetal lungs, kidneys, and heart (Table 4). Significant positive relationships were also seen between plasma insulin concentration and various components of the fetal RAS (Table 4). Partial correlation analyses showed that pulmonary ACE concentration and renal ACE mRNA were positively associated with plasma T3 concentration (r = +0.67, P < .05, n = 10 and r = +0.72, P < .05, n = 10, respectively), independent of plasma insulin; all other partial correlations failed to identify a single significant independent factor when multiple hormones correlated with RAS components.
Table 4.
Correlation Coefficients From Relationships Between Plasma Hormone Concentrations in the Fetuses, and ACE mRNA and Concentrations in the Fetal Circulation and Tissues
| Plasma ACE | Lung ACE mRNA | Lung ACE Concentration | Kidney ACE mRNA | Kidney ACE Concentration | Heart ACE mRNA | |
|---|---|---|---|---|---|---|
| Cortisol | −0.71a | −0.67a | NS | NS | NS | NS |
| T3 | +0.63a | +0.81b | +0.82b | +0.84b | NS | +0.88b |
| Insulin | +0.75a | +0.80a | +0.66a | +0.65a | +0.69a | NS |
| Renin | −0.72a | NS | NS | NS | NS | NS |
NS, not significant. Pearson correlation, n = 10.
P < .05.
P < .005.
Ewe
In the ewes treated with dexamethasone, plasma concentrations of cortisol, renin, T3, and T4 were suppressed, and plasma insulin increased, within 10 hours of the second injection (Table 2 and Figure 2B; P < .05). Plasma angiotensinogen concentration was increased by dexamethasone administration, but this just failed to reach statistical significance (P = .059) (Figure 1B). Maternal dexamethasone treatment had no significant effect on plasma ACE concentration (Figure 1B).
Pulmonary ACE concentration was increased in the dexamethasone-treated ewes compared with those treated with saline (P < .05) (Figure 2B). However, there was no significant effect of dexamethasone administration on ACE mRNA abundance in the maternal lungs (Figure 2B). Renal ACE mRNA and ACE concentrations were also unchanged by maternal dexamethasone treatment (Figure 3B). Maternal dexamethasone treatment had no significant effect on the gene transcript or protein levels of the AII receptors in the lungs and kidneys, or renin mRNA abundance in the kidneys of the pregnant ewes (Table 3).
Using data from all ewes, significant inverse correlations were observed between pulmonary ACE concentration and plasma concentrations of both cortisol (r = −0.89, P < .001, n = 10) and T4 (r = −0.80, P < .005, n = 10). Partial correlation analyses showed that pulmonary ACE concentration was inversely associated with plasma T4 concentration (r = −0.74, P < .05, n = 10), independent of plasma cortisol concentration. Plasma renin concentration was correlated with renal renin mRNA abundance (r = +0.70, P < .05, n = 10) in the pregnant ewes. Significant positive relationships were also seen between plasma T3 concentration and both renal renin mRNA abundance (r = +0.67, P < .05, n = 10) and plasma renin concentration (r = +0.75, P < .01, n = 10).
Discussion
Effects of maternal dexamethasone treatment on the RAS in utero
The present study demonstrates for the first time that maternal dexamethasone treatment, at a dose equivalent to that used in clinical practice, alters various components of the RAS in both the pregnant ewe and fetus. Administration of the synthetic glucocorticoid up-regulated ACE mRNA abundance in a variety of fetal tissues; it also increased pulmonary ACE concentration in both the pregnant ewe and fetus and ACE concentration in the fetal circulation. In the pregnant ewe, plasma angiotensinogen tended to increase in response to dexamethasone administration, and plasma renin was suppressed, which suggested negative feedback control by activation of the AT1R in the kidney.
In the present study, the increments in circulating and pulmonary ACE concentrations observed in the fetuses after maternal dexamethasone treatment were similar to those seen previously in sheep fetuses infused directly with the synthetic glucocorticoid (23). In addition, both fetal and maternal routes of dexamethasone administration had no significant effect on renal ACE concentration in the sheep fetus (23). The present findings indicate that maternal dexamethasone treatment elevated pulmonary and circulating ACE concentration in utero, at least in part, by increasing ACE mRNA abundance in the lungs and other fetal tissues. At 10 hours after the second injection of dexamethasone to the pregnant ewe, ACE mRNA levels in the fetal kidney and heart had increased without any change in enzyme concentration. The duration of exposure to the synthetic glucocorticoid, and/or the timing of tissue collection, however, may have been too short to observe significant effects on protein translation in these fetal organs.
The changes in plasma and pulmonary ACE concentration, and cardiac ACE mRNA, induced in utero by maternal dexamethasone treatment were similar to the normal maturational changes seen in sheep fetuses close to term (22, 33). In the lungs, ACE is localized to the vascular endothelium and for most of gestation, both pulmonary blood flow and ACE concentration in the fetal lungs are relatively low. However, pulmonary ACE concentration in the fetus increases near term and this appears to be driven by the prepartum glucocorticoid surge as part of the preparation for extrauterine life (22). Neonatal plasma AII concentrations after vaginal delivery are much higher than after caesarean section in human infants (34), suggesting that the capacity to convert AI to AII is activated by exposure to endogenous glucocorticoids before birth in preparation for pulmonary vasodilatation and increased pulmonary blood flow and delivery of AI after birth.
Developmental changes in AII receptor expression are also seen in the fetus towards term. The relative expression of AII receptor subtypes in a variety of fetal tissues shifts with gestational age from widespread AT2R abundance to tissue-specific and predominant localization of AT1R (35). In the heart of the sheep fetus, mRNA abundance and receptor density of the AT2R receptor are high from at least midgestation and decrease over the perinatal period (36, 37). The reduction in cardiac AT2R mRNA level induced by dexamethasone in the present study indicates that the normal developmental decline in AT2R expression seen in the fetal heart near term may be a glucocorticoid-dependent event.
Mechanisms of glucocorticoid action on the RAS
The mechanisms of glucocorticoid action on the RAS in the pregnant ewe and fetus may be direct and/or indirect involving coincident changes in other endocrine systems, such as insulin and the thyroid hormones. The effects of antenatal glucocorticoid treatment on the RAS observed in the present study are unlikely to be the consequence of fetal hypoxaemia or hypotension. In the chronically catheterized pregnant ewe and fetus, the same protocol of maternal dexamethasone treatment does not influence fetal blood gas status and causes a small, but significant, rise in arterial BP (26, 38).
Dexamethasone may have direct effects on the genes for angiotensinogen, ACE and the AT2R. A glucocorticoid-response element in the angiotensinogen gene is an important regulator of angiotensinogen synthesis (39); thus, the near-significant rise in maternal plasma angiotensinogen may have been directly stimulated by dexamethasone treatment. Glucocorticoid-response elements have also been identified close to a promoter region in the murine and human ACE gene (40), and dexamethasone has been shown previously to promote ACE mRNA abundance and enzyme activity in rabbit alveolar macrophages, bovine pulmonary artery endothelial cells and rat cardiac fibroblasts studied in vitro (41–43). Moreover, dexamethasone increases ACE mRNA abundance and enzyme activity in cultured rat aortic smooth muscle cells by stabilization of mRNA as well as enhanced gene transcription (44). In rats, multiple glucocorticoid-response elements are localized near to the regulatory region of the AT2R gene, which have inhibitory influences on promoter activity and AT2R gene expression (45). Indeed, AT2R mRNA and protein levels in hearts isolated from fetal rats are suppressed by 48 hours of dexamethasone treatment in vitro (45).
The present study was part of a larger project examining the effects of maternal dexamethasone treatment on fetal growth and development and in which plasma concentrations of insulin and the thyroid hormones were measured (12, 30). Significant associations were observed between circulating concentrations of T3 in the fetus and plasma and pulmonary ACE concentrations, and the transcript levels of ACE in the fetal lungs, kidneys, and heart. These findings support the suggestion that T3 may have an important role in mediating the regulatory effects of glucocorticoids on tissue and circulating ACE expression in utero (46). Indeed, in fetal sheep, experimental thyroid hormone deficiency prevents the normal developmental rise in pulmonary and renal ACE concentration near term, and exogenous T3 infusion has been shown to increase ACE concentration in the fetal lungs, but not kidneys (46). Previous studies, however, have shown that maternal dexamethasone treatment has differential effects on the thyroid hormone axis in the pregnant ewe and fetus (12). In the sheep fetus, synthetic and endogenous glucocorticoids activate the production of T3, whereas in the mother, the thyroid hormone axis is suppressed (11, 12). Therefore, the rise in pulmonary ACE concentration seen in the pregnant ewe treated with dexamethasone may be the direct consequence of glucocorticoid, rather than T3, action. Alternatively, dexamethasone and/or T3 may have different and tissue-specific effects on ACE expression in the fetus and mother. In adult rats, dexamethasone treatment causes a rise in ACE concentration in the lungs, but not renal cortex or medulla, and T3 administration reduces pulmonary ACE, while increasing renal and circulating ACE, concentrations (47).
Implications of altered RAS activity induced by maternal dexamethasone treatment
Changes in the activity of the RAS in utero after maternal dexamethasone treatment may contribute to the maturation of fetal tissues induced clinically by synthetic glucocorticoids. In addition, alterations in fetal RAS activity may have both local and endocrine effects on growth and maturation before birth (48). Although a limitation of this study was that circulating and tissue concentrations of AII, and downstream signaling pathways, were not determined, the increase in pulmonary ACE concentration is likely to result in enhanced production of AII locally in the lungs of the ewe and fetus. Local production of AII in the fetal lungs has been shown to promote maturation of pulmonary structure, including vascularization and airway branching (49, 50), and may mediate, in part, some of the beneficial effects of antenatal synthetic glucocorticoids on the developing lungs.
Activation of ACE mRNA, and suppression of AT2R mRNA, abundances in the fetal heart by dexamethasone may influence the development of cardiac structure and function in utero, if the mRNA levels were to translate to altered protein expression in the longer term. The developmental processes of growth and differentiation in fetal cardiomyocytes are sensitive to glucocorticoids and thyroid hormones (51), and the mechanisms of hormone action may involve changes in local AII activity (52, 53). It is increasingly recognized that, whereas antenatal synthetic glucocorticoid treatment can be life-saving for the infant when delivery occurs preterm, there may also be adverse sequelae reaching into adulthood (54). Indeed, dexamethasone-induced changes in the fetal RAS, especially within the heart and kidney, may have consequences for the regulation of arterial BP in both fetal and postnatal life. In fetal sheep, exposure to dexamethasone either by direct fetal infusion or by maternal treatment causes an increase in arterial BP and modifies the cardiovascular responses to hypoxaemia induced experimentally in utero (23, 26, 38). Previously, arterial BP was found to correlate with pulmonary ACE concentration in sheep fetuses infused with either dexamethasone or saline (23). Pulmonary ACE is responsible both for the production of vasoconstrictive AII and for the degradation of the vasodilator bradykinin, and the RAS is known to have an important role in the control of fetal BP by peripheral and central mechanisms (55–57). Furthermore, the RAS has been implicated in the developmental programming of hypertension in sheep and rodent offspring exposed to glucocorticoids in utero (58–60). In conclusion, antenatal dexamethasone treatment stimulates components of the maternal and fetal RAS, and suppresses fetal cardiac AT2R mRNA levels, in the sheep. These changes may influence maturation of the developing lungs, heart, and kidney and may have acute and long-term consequences for the regulation of arterial BP.
Acknowledgments
We thank Peter Marsters at Queen's Medical Centre, University of Nottingham, and the members of the Department of Physiology, Development and Neuroscience, University of Cambridge, who have assisted with this study.
This work was supported by the Biotechnology and Biological Sciences Research Council and by Tommy's, the baby charity.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACE
- angiotensin-converting enzyme
- AII
- angiotensin
- AT1R
- AII type 1 receptor
- BP
- blood pressure
- DNase
- deoxyribonuclease
- qRT-PCR
- quantitative real-time polymerase chain reaction
- RAS
- renin-angiotensin system.
References
- 1. Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. [DOI] [PubMed] [Google Scholar]
- 2. Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454. [DOI] [PubMed] [Google Scholar]
- 3. Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–122. [DOI] [PubMed] [Google Scholar]
- 4. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 5. French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. [DOI] [PubMed] [Google Scholar]
- 6. Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci. 2000;98:137–142. [PubMed] [Google Scholar]
- 7. Murphy KE, Hannah ME, Willan AR, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–2151. [DOI] [PubMed] [Google Scholar]
- 8. Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. [DOI] [PubMed] [Google Scholar]
- 9. Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol. 2014;221:R87–R103. [DOI] [PubMed] [Google Scholar]
- 10. Forhead AJ, Poore KR, Mapstone J, Fowden AL. Developmental regulation of hepatic and renal gluconeogenic enzymes by thyroid hormones in fetal sheep during late gestation. J Physiol. 2003;548:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forhead AJ, Curtis K, Kaptein E, Visser TJ, Fowden AL. Developmental control of iodothyronine deiodinases by cortisol in the ovine fetus and placenta near term. Endocrinology. 2006;147:5988–5994. [DOI] [PubMed] [Google Scholar]
- 12. Forhead AJ, Jellyman JK, Gardner DS, et al. Differential effects of maternal dexamethasone treatment on circulating thyroid hormone concentrations and tissue deiodinase activity in the pregnant ewe and fetus. Endocrinology. 2007;148:800–805. [DOI] [PubMed] [Google Scholar]
- 13. Forhead AJ, Cutts S, Matthews PA, Fowden AL. Role of thyroid hormones in the developmental control of tissue glycogen in fetal sheep near term. Exp Physiol. 2009;94:1079–1087. [DOI] [PubMed] [Google Scholar]
- 14. Schütz S, Le Moullec JM, Corvol P, Gasc JM. Early expression of all the components of the renin-angiotensin-system in human development. Am J Pathol. 1996;149:2067–2079. [PMC free article] [PubMed] [Google Scholar]
- 15. Wintour EM, Alcorn D, Albiston A, et al. The renin-angiotensin system and the development of the kidney and adrenal in sheep. Clin Exp Pharmacol Physiol Suppl. 1998;25:S97–S100. [DOI] [PubMed] [Google Scholar]
- 16. Lumbers ER, Boyce AC, Joulianos G, et al. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol. 2005;288:R567–R574. [DOI] [PubMed] [Google Scholar]
- 17. Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18:123–137. [DOI] [PubMed] [Google Scholar]
- 18. Gubler MC, Antignac C. Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int. 2010;77:400–406. [DOI] [PubMed] [Google Scholar]
- 19. Broughton Pipkin F, Lumbers ER, Mott JC. Factors influencing plasma renin and angiotensin II in the conscious pregnant ewe and its foetus. J Physiol. 1974;243:619–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forhead AJ, Melvin R, Balouzet V, Fowden AL. Developmental changes in plasma angiotensin-converting enzyme concentration in fetal and neonatal lambs. Reprod Fertil Dev. 1998;10:393–398. [DOI] [PubMed] [Google Scholar]
- 21. Forhead AJ, Broughton Pipkin F, Fowden AL. Effect of cortisol on blood pressure and the renin-angiotensin system in fetal sheep during late gestation. J Physiol. 2000;526(pt 1):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forhead AJ, Gillespie CE, Fowden AL. Role of cortisol in the ontogenic control of pulmonary and renal angiotensin-converting enzyme in fetal sheep near term. J Physiol. 2000;526(pt 2):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmermann H, Gardner DS, Jellyman JK, Fowden AL, Giussani DA, Forhead AJ. Effect of dexamethasone on pulmonary and renal angiotensin-converting enzyme concentration in fetal sheep during late gestation. Am J Obstet Gynecol. 2003;189:1467–1471. [DOI] [PubMed] [Google Scholar]
- 24. Royal College of Obstetricians and Gynaecologists. Antenatal corticosteroids to prevent neonatal morbidity and mortality. Green-top guideline 2010; No.7. Retrieved from https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg7/ July 8, 2015.
- 25. Fletcher AJ, Goodfellow MR, Forhead AJ, et al. Low doses of dexamethasone suppress pituitary-adrenal function but augment the glycemic response to acute hypoxemia in fetal sheep during late gestation. Pediatr Res. 2000;47:684–691. [DOI] [PubMed] [Google Scholar]
- 26. Jellyman JK, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep. J Physiol. 2005;567:673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kream J, Mulay S, Fukushima DK, Solomon S. Determination of plasma dexamethasone in the mother and the newborn after administration of the hormone in a clinical trial. J Clin Endocrinol Metab. 1983;56:127–133. [DOI] [PubMed] [Google Scholar]
- 28. Broughton Pipkin F, Hunter JC, Turner SR, O'Brien PM. The effect of prostaglandin E2 upon the biochemical response to infused angiotensin II in human pregnancy. Clin Sci. 1984;66:399–406. [DOI] [PubMed] [Google Scholar]
- 29. Tetlow HJ, Broughton Pipkin F. The effect of changes in blood gas tension upon the renin-angiotensin system of the newborn infant. Br J Obstet Gynaecol. 1983;90:898–903. [DOI] [PubMed] [Google Scholar]
- 30. Franko KL, Giussani DA, Forhead AJ, Fowden AL. Effects of dexamethasone on the glucogenic capacity of fetal, pregnant, and non-pregnant adult sheep. J Endocrinol. 2007;192:67–73. [DOI] [PubMed] [Google Scholar]
- 31. Forhead AJ, Jellyman JK, Gillham K, Ward JW, Blache D, Fowden AL. Renal growth retardation following angiotensin II type 1 (AT1) receptor antagonism is associated with increased AT2 receptor protein in fetal sheep. J Endocrinol. 2011;208:137–145. [DOI] [PubMed] [Google Scholar]
- 32. Romero-Calvo I, Ocón B, Martínez-Moya P, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. [DOI] [PubMed] [Google Scholar]
- 33. Reini SA, Wood CE, Keller-Wood M. The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr Patterns. 2009;9:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Broughton Pipkin F, Symonds EM. Factors affecting angiotensin II concentrations in the human infant at birth. Clin Sci Mol Med. 1977;52:449–456. [DOI] [PubMed] [Google Scholar]
- 35. Shanmugam S, Sandberg K. Ontogeny of angiotensin II receptors. Cell Biol Int. 1996;20:169–176. [DOI] [PubMed] [Google Scholar]
- 36. Samyn ME, Petershack JA, Bedell KA, Mathews MS, Segar JL. Ontogeny and regulation of cardiac angiotensin types 1 and 2 receptors during fetal life in sheep. Pediatr Res. 1998;44:323–329. [DOI] [PubMed] [Google Scholar]
- 37. Burrell JH, Hegarty BD, McMullen JR, Lumbers ER. Effects of gestation on ovine fetal and maternal angiotensin receptor subtypes in the heart and major blood vessels. Exp Physiol. 2001;86:71–82. [DOI] [PubMed] [Google Scholar]
- 38. Jellyman JK, Gardner DS, Fowden AL, Giussani DA. Effects of dexamethasone on the uterine and umbilical vascular beds during basal and hypoxemic conditions in sheep. Am J Obstet Gynecol. 2004;190:825–835. [DOI] [PubMed] [Google Scholar]
- 39. Menard J, Clauser E, Bouhnik J, Corvol P. Angiotensinogen: biochemistry. In: Robertson JIS, Nicholls MG, eds. The Renin-Angiotensin System. London, New York: Gower Medical Publishing; 1993:8.1–8.10. [Google Scholar]
- 40. Shai SY, Langford KG, Martin BM, Bernstein KE. Genomic DNA 5′ to the mouse and human angiotensin-converting enzyme genes contains two distinct regions of conserved sequence. Biochem Biophys Res Commun. 1990;167:1128–1133. [DOI] [PubMed] [Google Scholar]
- 41. Friedland J, Setton C, Silverstein E. Angiotensin converting enzyme: induction by steroids in rabbit alveolar macrophages in culture. Science. 1977;197:64–65. [DOI] [PubMed] [Google Scholar]
- 42. Dasarathy Y, Lanzillo JJ, Fanburg BL. Stimulation of bovine pulmonary artery endothelial cell ACE by dexamethasone: involvement of steroid receptors. Am J Physiol. 1992;263:E745–E753. [DOI] [PubMed] [Google Scholar]
- 43. Barreto-Chaves MLM, Aneas I, Krieger JE. Glucocorticoid regulation of angiotensin-converting enzyme in primary culture of adult cardiac fibroblasts. Am J Physiol. 2001;280:R25–R32. [DOI] [PubMed] [Google Scholar]
- 44. Fishel RS, Eisenberg S, Shai SY, Redden RA, Bernstein KE, Berk BC. Glucocorticoids induce angiotensin-converting enzyme expression in vascular smooth muscle. Hypertension. 1995;25:343–349. [DOI] [PubMed] [Google Scholar]
- 45. Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forhead AJ, Fowden AL. Effects of thyroid hormones on pulmonary and renal angiotensin-converting enzyme concentrations in fetal sheep near term. J Endocrinol. 2002;173:143–150. [DOI] [PubMed] [Google Scholar]
- 47. Michel B, Grima M, Coquard C, Welsch C, Barthelmebs M, Imbs JL. Effects of triiodothyronine and dexamethasone on plasma and tissue angiotensin converting enzyme in the rat. Fundam Clin Pharmacol. 1994;8:366–372. [DOI] [PubMed] [Google Scholar]
- 48. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. [DOI] [PubMed] [Google Scholar]
- 49. Morrell NW, Grieshaber SS, Danilov SM, Majack RA, Stenmark KR. Developmental regulation of angiotensin converting enzyme and angiotensin type 1 receptor in the rat pulmonary circulation. Am J Respir Cell Mol Biol. 1996;14:526–537. [DOI] [PubMed] [Google Scholar]
- 50. Nogueira-Silva C, Carvalho-Dias E, Piairo P, et al. Local fetal lung renin-angiotensin system as a target to treat congenital diaphragmatic hernia. Mol Med. 2012;18:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thornburg K, Jonker S, O'Tierney P, et al. Regulation of the cardiomyocyte population in the developing heart. Prog Biophys Mol Biol. 2011;106:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol. 2003;548:881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Norris AW, Bahr TM, Scholz TD, Peterson ES, Volk KA, Segar JL. Angiotensin II-induced cardiovascular load regulates cardiac remodeling and related gene expression in late-gestation fetal sheep. Pediatr Res. 2014;75:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Forhead AJ, Fowden AL. The consequences for preterm infants of antenatal glucocorticoid treatment. In: Ebtehaj F, Herring J, Johnson MH, Richards M, eds. Birth Rites and Rights. Chap 7 Oxford, UK: Hart Publishing; 2011:129–149. [Google Scholar]
- 55. Jones OW, 3rd, Cheung CY, Brace RA. Dose-dependent effects of angiotensin II on the ovine fetal cardiovascular system. Am J Obstet Gynecol. 1991;165:1524–1533. [DOI] [PubMed] [Google Scholar]
- 56. Xu Z, Shi L, Hu F, White R, Stewart L, Yao J. In utero development of central ANG-stimulated pressor response and hypothalamic fos expression. Brain Res Dev Brain Res. 2003;145:169–176. [DOI] [PubMed] [Google Scholar]
- 57. Shi L, Mao C, Zeng F, Hou J, Zhang H, Xu Z. Central angiotensin I increases fetal AVP neuron activity and pressor responses. Am J Physiol. 2010;298:E1274–E1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans. 1999;27:88–93. [DOI] [PubMed] [Google Scholar]
- 59. Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40:729–734. [DOI] [PubMed] [Google Scholar]
- 60. O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol. 2004;287:E863–E870. [DOI] [PubMed] [Google Scholar]