Abstract
Glucocorticoids act rapidly at the paraventricular nucleus (PVN) to inhibit stress-excitatory neurons and limit excessive glucocorticoid secretion. The signaling mechanism underlying rapid feedback inhibition remains to be determined. The present study was designed to test the hypothesis that the canonical glucocorticoid receptors (GRs) is required for appropriate hypothalamic-pituitary-adrenal (HPA) axis regulation. Local PVN GR knockdown (KD) was achieved by breeding homozygous floxed GR mice with Sim1-cre recombinase transgenic mice. This genetic approach created mice with a KD of GR primarily confined to hypothalamic cell groups, including the PVN, sparing GR expression in other HPA axis limbic regulatory regions, and the pituitary. There were no differences in circadian nadir and peak corticosterone concentrations between male PVN GR KD mice and male littermate controls. However, reduction of PVN GR increased ACTH and corticosterone responses to acute, but not chronic stress, indicating that PVN GR is critical for limiting neuroendocrine responses to acute stress in males. Loss of PVN GR induced an opposite neuroendocrine phenotype in females, characterized by increased circadian nadir corticosterone levels and suppressed ACTH responses to acute restraint stress, without a concomitant change in corticosterone responses under acute or chronic stress conditions. PVN GR deletion had no effect on depression-like behavior in either sex in the forced swim test. Overall, these findings reveal pronounced sex differences in the PVN GR dependence of acute stress feedback regulation of HPA axis function. In addition, these data further indicate that glucocorticoid control of HPA axis responses after chronic stress operates via a PVN-independent mechanism.
The paraventricular nucleus (PVN) of the hypothalamus serves as the chief integrator of the hypothalamic-pituitary-adrenal (HPA) axis. Excitation of CRH neurons housed in the parvocellular domain of the PVN triggers a cascade of neuroendocrine events, ultimately leading to the synthesis and release of glucocorticoids (cortisol or corticosterone) from the zona fasiculata of the adrenal gland (1). Glucocorticoids play an important fast negative feedback role, acting at the PVN to rapidly inhibit activation of CRH neurons, thereby constraining ACTH release (2, 3). Recent studies indicate that fast feedback effects on CRH neuronal excitability are mediated at the level of the membrane, acting to mobilize endocannabinoids and inhibit presynaptic glutamate release (4–6). Subsequent studies demonstrate that this same mechanism is present in vivo, because local injection of the glucocorticoid receptor (GR) agonist dexamethasone causes an endocannabinoid-dependent inhibition of ACTH and corticosterone release at the level of the cell membrane (3). Although many studies have queried to the role of PVN GR in acute stress responses, the role of the PVN GR in regulation of chronic stress reactivity is unknown. Chronic stress induces marked cellular and morphological alterations in the PVN, suggestive of HPA axis excitability, including increases in CRH and/or arginine vasopressin (AVP) mRNA expression (7, 8), increased glutamatergic innervation of CRH neurons (9), and enhanced ACTH and corticosterone responses to novel stressors. Hyperactivity of the PVN may be due to deficits in glucocorticoid feedback in upstream limbic circuits or at the level of the PVN. For example, chronic stress decreases GR mRNA expression in the medial prefrontal cortex, hippocampus, and PVN (7, 10, 11).
Of note, work regarding the role of GR on HPA axis regulation has been almost exclusively performed in male subjects. This is particularly problematic, given prominent sex differences in HPA axis reactivity and the strong female bias toward stress-related psychopathologies (12–15). Previous work from our laboratory indicates sex differences in the necessity of forebrain (medial prefrontal cortex, hippocampus, basolateral amygdala) GR to modulate HPA axis regulation and depression-like behavior (16). Although the loss of forebrain GR induces marked HPA axis dysregulation and increased depression-like behaviors in males, these same neuroendocrine and behavioral outcomes are not observed in females (16–18). These findings suggest that the loss of forebrain GR is relatively inconsequential for HPA axis regulation and mood-like behaviors in intact, adult females. Importantly, GR signaling remained intact in the PVN, the “motor arm” of the HPA axis in FBGRKO mice.
As such, the goal of the present study was to 1) examine the necessity of the PVN GR for fast feedback inhibition of the HPA axis, and 2) determine whether the PVN GR plays an important role in mediating HPA axis hyperactivity seen under conditions of chronic stress. To address the important question of sex differences, these questions were addressed in both male and female subjects. To test the role of the PVN GR, we employed a genetic approach using a mouse model lacking GR in the PVN, sparing GR signaling in other HPA axis modulatory limbic regions, including the medial prefrontal cortex, hippocampus, and amygdala. Transgenic mice expressing Cre recombinase under the control of the sim1 promoter were crossed with homozygous GR flox mice to target PVN GR. The sim1 gene product is a ligand-modulated basic helix-loop-helix/Per-ARNT-Sim transcription factor that is highly expressed in the PVN, supraoptic nucleus, and anterior periventricular nucleus and is necessary for development of magno- and parvocellular neurons in the PVN (19). Our data suggest that the PVN GR is required for normal shutoff of HPA axis stress responses in male subjects, which is supported by recent electrophyiological (20). Moreover, the present study suggests that alternative feedback mechanisms are required for HPA axis regulation in females, as well as for individuals of both sexes after chronic stress.
Materials and Methods
Animals
PVN GR knockdown (KD) was accomplished by crossing mice engineered with lox P sites flanking exons 1C and 2 of the mouse GR gene (GRloxP) with mice expressing Cre recombinase driven by the Sim1Cre promoter (Sim1Cre) on a C57BL6 × 129 background. Male and female PVN GR KD mice (homozygous for GR flox [GR flox/flox] and expressing Sim1Cre), here denoted as GRflox/floxSim1cre+, and littermate control mice (GRflox/floxSim1cre−) containing only the GR flox allele (no Sim1-Cre transgene) were used for all studies and were 8–10 weeks old at the beginning of each experiment. To generate litters expressing both of these genotypes, the female breeders were GRflox/floxSim1cre−, whereas the male breeders were GRflox/floxSim1cre+. This breeding scheme was employed to obviate possible effects of hypothalamic GR deletion on maternal behavior.
Animals were housed in a temperature-controlled vivarium with a 12-hour light, 12-hour dark cycle, with lights on at 6 am. Ad libitum access to food and water was provided throughout the study. When more than 1 experiment was performed in the same set of mice, at least 1 week intervened between studies (Experiments 1a and 1b). All protocols were reviewed and approved by the Institutional Animal Care and Use Committees and were in accordance to the National Institutes of Health Council on Animal Care guidelines involving vertebrate animals in biomedical research.
HPA axis regulation
Experiment 1a. Basal HPA axis regulation and stress-related neuropeptides
Tail blood samples from GRflox/floxSim1cre− controls (n = 8 males, n = 14 females) and GRflox/floxSim1cre+ PVN GR KD (n = 9 males, n = 15 females) mice were taken on 2 occasions during the morning (between 8 and 9 am) and evening (between 2 and 3 pm) to assess circadian nadir and peak corticosterone concentrations. One week intervened between the morning and evening blood sample collection. Approximately 15–20 μL of blood were collected via a small tail nick during the sampling period. If necessary, sterile gauze was applied to the base of the tail after the sampling period. All tail blood samples for this study were collected within 2 minutes from the initial handling of the animal's cage.
Experiment 1b. Acute restraint stress regulation of HPA axis activity (single time point)
To assess neuroendocrine (ie, ACTH and corticosterone) responses to an acute novel restraint challenge, GRflox/flox Sim1cre− control (n = 9 males, n = 10 females) and GRflox/flox Sim1cre+ PVN GR KD mice (n = 10 males, n = 10 females) were placed in well-ventilated 50-mL conical plastic tubes for 15 minutes. Immediately after restraint stress exposure, mice underwent rapid decapitation and trunk blood (∼500 μL) was collected, stored, and processed; detailed later in “ACTH and corticosterone RIA”. The restraint stress exposure for this particular experiment was limited to 15 minutes to allow us to capture peak stress-induced ACTH concentrations. The collection of endocrine measurements for ACTH analyses began at 8 am and ended at approximately 10 am.
Experiment 2. Acute restraint stress regulation of HPA axis (time-course study)
A separate set of GRflox/flox Sim1cre− littermate control (n = 17 males, n = 16 females) and GRflox/floxSim1cre− PVN GR KD mice (n = 14 males, n = 15 females) was used to assess neuroendocrine responses to an acute novel restraint challenge. Mice were placed in well-ventilated 50-mL conical tubes for 30 minutes. Tail blood samples were taken at 0 (prestress), 30, 60, and 120 minutes. The 60- and 120-minute time points were free-bleed poststress measures, because the mice had been removed from the restraint tubes and were placed back into the home cages. The collection of endocrine measurements for corticosterone analyses began at 8 am and ended at approximately 11:30 am. Note that the amount of plasma obtained was not sufficient to assay ACTH. After endocrine collection, animals were overdosed with sodium pentobarbital and perfused for immunohistochemistry (GR and neuronal nuclei [NeuN[ immunolabeling) and in situ hybridization (CRH and AVP) analyses. Immunohistochemistry for GR was done at the completion of all studies as a secondary verification of genotype.
Experiment 3. HPA axis and behavioral responses after chronic stress
Chronic variable stress regimen
A separate set of GRflox/flox Sim1cre− controls (n = 15 males, n = 12 females) and GRflox/floxSim1cre+ GR KD (n = 13 males, n = 12 females) mice underwent a 2-week chronic variable stress (CVS) regimen previously described by (16, 18). Briefly, mice were exposed to random morning and evening presentations of stressors, including rotation stress (orbital shaker at 100 rpm for 1 h), cold exposure (4°C for 15 min in clean cages without bedding), hypoxia (8% oxygen, 92% nitrogen for 30 min), open-field stress (group housed under bright light for 15 min), and restraint (30 min in well-ventilated plastic tube). Body weight and food intake were assessed on a weekly basis.
Chronic stress-induced HPA axis regulation and forced swim testing
HPA axis regulation
On the morning after CVS (d 15) Mice were placed in a 2-L beaker half-filled with water (23 ± 2°C). This level of water prevents the mice from reaching the bottom of the container. Each session was 10 minutes and was videotaped. Tail blood samples were collected at 15, 30, 60, and 120 minutes after FST onset in freely moving mice in the same manner as previously described. The behavioral testing and collection of endocrine measures were conducted between 8 am and 12:30 pm. After endocrine collection, animals were overdosed with sodium pentobarbital and perfused for immunohistochemistry and in situ hybridization analyses as indicated above.
Behavioral analyses
All behaviors in the FST were scored in 5-second intervals by 2 independent observers who were unaware of the genotype of each mouse. The total counts of mobility behaviors (including swimming, climbing, and diving) and immobility were summed for each animal and averaged for each group. The behaviors scored included: 1) climbing-rapid movement of limbs in and out of the water with the body parallel to the apparatus, 2) diving, 3) swimming-moving limbs in an active manner and making circular movements around the beaker, and 4) immobility-no active movements or floating in the water without struggling. We used a modified one-day version of the forced swim test, similar to that used in our previous studies (16, 21, 22).
ACTH and corticosterone RIA
Tail blood was collected into chilled EDTA coated tubes. Plasma was separated by centrifugation at 4°C 6000 rpm for 15 minutes and stored at −20°C until RIA. Plasma corticosterone concentrations were determined using 125I RIA kits (MP Biomedicals, Inc). Plasma ACTH concentrations were determined by an RIA using a specific antiserum generously donated by Dr William Engeland (University of Minnesota, Minneapolis, MN) at a dilution of 1:120 000, with 125I ACTH (Amersham Biosciences) as a labeled tracer (23). All samples were run in duplicate and all samples were run in the same assay. The intraassay coefficient of variation was less than 10% for both assays.
Tissue collection for immunohistochemistry and in situ hybridization
Mice were given an overdose of sodium pentobarbital and perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate buffered saline. Brains were postfixed in the same fixative overnight for 24 hours, and then stored in 30% sucrose in diethylpyrocarbonate-treated water at 4°C. Brains were then serially sectioned on a freezing microtome (Leica) in 30-μm coronal sections and stored at −20°C in sterile, diethylpyrocarbonate-treated cryoprotectant solution until processing for immunohistochemistry or in situ hybridization.
GR immunohistochemistry
All sections were washed in PBS (5× for 5 min) endogenous peroxidases quenched with 1% H2O2, washed in PBS before immunolabeling (10 min). After rinsing in PBS (5× for 5 min), sections were blocked in incubation solution for 1 hour at room temperature (2% BSA and 0.1% Triton X-100 [Sigma-Aldrich] in PBS), then incubated with a well-characterized polyclonal rabbit antibody directed against the GR (GR-M20, 1:5000; Santa Cruz Biotechnologies, Inc) (16, 18) in incubation solution overnight at room temperature. Sections were then rinsed with PBS (5× for 5 min) and incubated for 1 hour at room temperature with biotinylated antirabbit (1:500; Vector Laboratories), rinsed with PBS, incubated with Vectastain ABC (1:800; Vector Laboratories), rinsed with PBS (5× for 5 min), and incubated in diaminobenzidine in 0.05% H2O2 in PBS, rinsed with PBS, and mounted. Slides were dehydrated through a graded ethanol series, cleared in xylene, and coverslipped with Distyrene Plasticizer Xylene mountant.
NeuN immunohistochemistry
Sections were washed in PBS (5× for 5 min) endogenous peroxidases quenched with 1% H2O2, washed in PBS before immunoloabeling (10 min). After rinsing in PBS (5× for 5 min), sections were blocked in incubation solution for 1 hour at room temperature (4% normal goat serum and 0.3% Triton X-100 in PBS), then incubated with a well-characterized monoclonal mouse antibody directed against NeuN (1:1000; Chemicon) (24, 25) in incubation solution overnight at room temperature. Sections were rinsed with PBS (5× for 5 min) and incubated for 1 hour at room temperature with biotylinated antimouse (1:500; Vector Laboratories). All other subsequent steps were identical to the steps performed for the immunolabeling for the GR (Table 1).
Table 1.
Antibody Table
| Peptide/protein target | Name of antibody | Manufacturer, catalog number, and/or name of individual providing the antibody | Species raised in; monoclonal or polyclonal | Dilution used |
|---|---|---|---|---|
| GR | Santa Cruz Biotechnology, Inc; M-20 | Rabbit polyclonal | 1:5000 | |
| NeuN | NeuN | Chemicon | Mouse monoclonal | 1:1000 |
Image analysis for immunohistochemistry
For analysis of GR- and NeuN-immunoreactive nuclei, digital images of each side of the PVN in GRflox/flox Sim1cre− controls (n = 8) and GRflox/flox Sim1cre+ PVN GR KD mice (n = 9) as defined by the Paxinos and Franklin mouse brain atlas second edition) were captured at ×10 magnification with a Carl Zeiss Imager Z.1 (Carl Zeiss Microimaging) and set at a threshold to subtract the background optical density, and the numbers of cell nuclei above the background were counted by using Scion Software Image. The software allowed us to determine the number of immunoreactive nuclei (defined as nuclei with optical densities exceeding threshold) within a defined region of interest. Approximately 3 sections per animal were used in the analyses.
In situ hybridization
Adjacent brain sections were used to assess CRH and AVP mRNA expression in GRflox/flox Sim1cre− male (n = 6) and female (n = 6) control and GRflox/floxSim1cre+ male (n = 10) and female (n = 10) PVN GR KD mice using previously reported procedures (16, 25). Briefly, riboprobes complementary to CRH and AVP mRNA were generated by in vitro transcription using 35S-labeled uridine 5c-triphosphate (UTP). The AVP (exon C) DNA construct is a 161-bp insert in a pCR4 TOPO vector, which was linearized with Notl restriction enzyme, and transcribed using T3 RNA polymerase. The CRH DNA construct is a 765-bp fragment cloned into pGEM3 vector, which was linearized with HindIII and transcribed using T7 RNA polymerase. Each 15-μL riboprobe transcription reaction was made from 1.0 to 2.5 μg of linearized DNA fragment, 62.5 μCi of [35S]UTP, 330μM ATP, 330μM GTP, 330μM CTP, 10μM cold UTP, 1× transcription buffer, 66.6 dithiothreitol, 40 U of RNase inhibitor, and 20 U of the appropriate RNA polymerase. Before hybridization, slides were washed in potassium PBS, acetylated, delipidated in chloroform, and dehydrated through a graded ethanol series. Each riboprobe was diluted (1.0 × 106 cpm/50-μL buffer) in hybridization buffer (50% formamide, 1× Denhardt's solution, 10% dextran sulfate, 200-μg/mL fish sperm single-stranded DNA, 100-μg/mL yeast tRNA, and 20mM dithiothreitol). Coverslipped slides were placed in hybridization chambers over blotting paper soaked in 50% formamide and incubated overnight at 55°C. The next day, coverslips were removed, and slides were washed in 2× saline sodium citrate (SSC). Slides were subsequently incubated in 100-μg/mL RNase A for 30 minutes at 37°C, washed 3 times in 0.2× SSC, 1 more in 0.2× SSC for 1 hour at 65°C, and finally dehydrated through a graded ethanol series.
Image analysis for In situ hybridization
Hybridized slides were exposed on Eastman Kodak Biomax MR film (16 h for AVP and 14 d for CRH). Film images of brain sections were captured by a digital video camera. Semiquantitative analyses of autoradiograph images were performed using Scion Image software, and hybridization signal was expressed as gray level units. The gray level signal of the hybridized tissue region of interest (PVN) was corrected by subtracting the gray level signal over a nonhybridized tissue region of interest was corrected by subtracting the gray level signal over a nonhybridized area of tissue and expressed as integrated gray level (14). C standards were also measured using Scion Image and transferred to Assay Zap (Biosoft and P.L. Taylor; West Markham) to generate a standard curve to verify that all measured gray levels were on the linear range of the film.
RNA isolation, cDNA synthesis, and real-time quantitative PCR
Given that the pituitary is a prime peripheral target area for GR-mediated feedback, the pituitary was extracted from GRflox/floxSim1cre− male (n = 7) and female (n = 8) controls and GRflox/flox Sim1cre+ male (n = 9) and female (n = 8) PVN GR KD mice to assess GR mRNA content from a separate set of animals under basal conditions between 8 and 10 am. Total RNA from pituitary extracts was isolated with an RNeasy kit, according to manufacturer protocol (QIAGEN). The RNA quality and quantity were determined with NanoDrop ND-1000 UV Vis Spectrophotometer (NanoDrop Technologies). The RNA was treated with Turbo DNA-free to remove genomic DNA (Ambion) and reverse transcribed with an iScript cDNA synthesis kit according to manufacturer's recommendations (Bio-Rad). Real-time quantitative polymerase chain reaction analyses were carried out in iCycler iQ multicolor real-time polymerase chain reaction detection system (Bio-Rad). Primers for GR mRNA (forward, 5′-CCACTGCAGGAGTCTACAA-3′ and reverse, 5′ACTGCTGCAAT-CACTTGACG-3′) and the housekeeping gene L32 (forward, 5′-CATCGTAGAAAGAGCAGCAC-3′ and reverse, 5′-GCACACAAGC-CATCTATTCAT-3′) were used (Integrated DNA Technologies). Quantification for cDNA was determined with iQ SYBR Green Supermix (Bio-Rad). L32 was used as the internal standard for gene expression data, because it has been demonstrated to be stable under various physiological conditions (ie, chronic variable stress, site specific manipulation of GR) that are similar to the present study (26–28). Threshold cycle reading for each of the unknown samples was used, and the results were transferred and calculated in Excel using the delta delta cycle threshold method (29). Negative reverse transcriptase samples were included to rule out DNA contamination.
Estrous cycle determination in females
Although we did not have enough female mice to do a thorough staging of the estrous cycle before the initiation of neuroendocrine and behavioral studies (as is the case with many genetic knockout mouse studies), we determined the phase of the cycle via vaginal lavage after the end of each experiment. However, there was not a sufficient sample size in any given stage to include estrous cycle as a factor in the statistical analyses. As such, data for females include randomly cycling females categorized with respects to genotype.
Statistical analyses
Data are expressed as mean ± SEM. Data were analyzed using independent sample t test, ANOVA, and repeated measures ANOVA where appropriate. Note, the neuroendocrine, neuropeptide expression, and behavioral were analyzed such that the impact PVN GR signaling on the aforementioned endpoints is determined within and not between sex (ie, female PVN GR KD vs female littermate controls). Time-course hormonal data were analyzed using two-way repeated measures ANOVA (genotype × time), with time as the repeating measure. Fisher's least significant differences were used for a priori planned comparison for each specific time point for the time-course study (ie, 15, 30, 60, and 120 min). Significance for all statistical analyses was set at P ≤ .05. Outliers were defined by standardized scored greater than 1.96 of the standard deviation and 1.5 × the interquartile range (30). When data were not homogenously distributed, log or square root transformations were performed.
Results
Verification of hypothalamic GR deletion
GR and NeuN immunohistochemistry analyses
Loss of PVN GR was assessed by cell counts of GR-immunoreactive neurons. There was a clear qualitative difference in GR expression between GRflox/flox Sim1cre− control and GRflox/flox Sim1cre+ PVN GR KD mice (Figure 1, A and B). We then quantified the extent of GR KD between the 2 mouse lines. GRflox/flox Sim1cre+ PVN GR KD mice had approximately 60% reduction in the number of PVN GR-expressing neurons compared with GRflox/flox Sim1cre− controls (t15 = 8.9, P < .01) (Figure 1C). In contrast, there was no quantitative difference in number or NeuN-immunoreactive neurons within the PVN between GRflox/flox Sim1cre− controls and GRflox/flox Sim1cre+ PVN GR KD mice (Figure 1, D–F). These findings suggest that depletion of PVN GR does not lead to a loss of neuronal viability. Notably, GR immunoreactivity was comparable in the medial prefrontal cortex (Figure 2, A and B), hippocampus (Figure 2, C and D) and amgydala (Figure 2, E and F) between the 2 groups. This finding illustrates that the loss of GR signaling is primarily confined to the PVN and not other GR-mediated negative feedback brain regions (31). The pattern of GR expression and percentage of GR KD in the PVN was similar in males and females.
Figure 1.
Photomicrographs of coronal forebrain sections illustrating expression of GR immunoreactivity in the PVN of GRflox/floxSim1cre− control (n = 8) (A) and GRflox/floxSim1cre+ PVN GR KD representative male mice (n = 8) (B). C, Male GRflox/floxSim1cre+ PVN GR KD mice have significantly less GR immunoreactivity in the PVN compared with GRflox/floxSim1cre− control mice; P < .05. Photomicrographs of NeuN immunoreactivity in the PVN of GRflox/floxSim1cre− control (D) and PVN GR KD male mice (E). F, GRflox/floxSim1cre+ PVN GR KD mice have similar NeuN immunoreactivity in the PVN compared with GRflox/floxSim1cre− control mice; P > .05. Scale bar, 50 μm. Data are represented as mean ± SEM.
Figure 2.
Photomicrographs of coronal forebrain sections illustrating expression of GR in the medial prefrontal cortex (A and B), hippocampus (C and D), and amygdala (E and F) in GRflox/floxSim1cre− control (n = 8) and GRflox/floxSim1cre+ PVN GR KD male mice (n = 9), respectively. GRflox/floxSim1cre+ PVN GR KD and GRflox/floxSim1cre− control mice have similar GR immunoreactivity within these forebrain regions. Scale bar, 50 μm. fmi, forceps minor.
Pituitary GR mRNA content
Given that the pituitary is the primary peripheral target for GR-mediated negative feedback, we quantified pituitary GR mRNA expression. As determined by RT-qPCR, there was no difference in GR mRNA content between the 2 genotypes in males (Figure 3A) or females (P > .05) (Figure 3B).
Figure 3.
A, Male GRflox/floxSim1cre+ PVN GR KD mice (n = 9) have similar pituitary GR mRNA expression relative to GRflox/floxSim1cre− control mice (n = 7); P > .05. B, Female GRflox/floxSim1cre+ PVN GR KD mice (n = 8) have similar pituitary GR mRNA expression relative to GRflox/floxSim1cre− control mice (n = 8); P > .05. Data were normalized to the housekeeping gene L32 and expressed as percentage of control mice (GR flox). Data are represented as mean ± SEM.
Depletion of PVN GR differentially regulates circadian HPA axis activity and responsiveness to acute stress in males and females
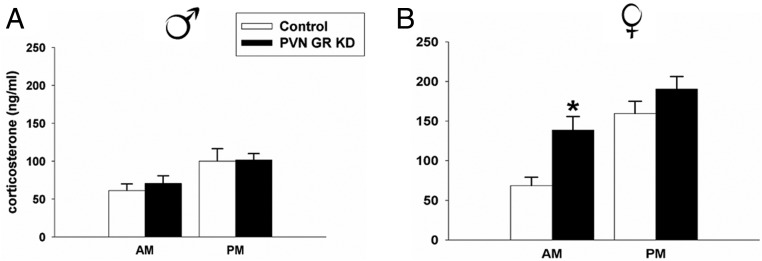
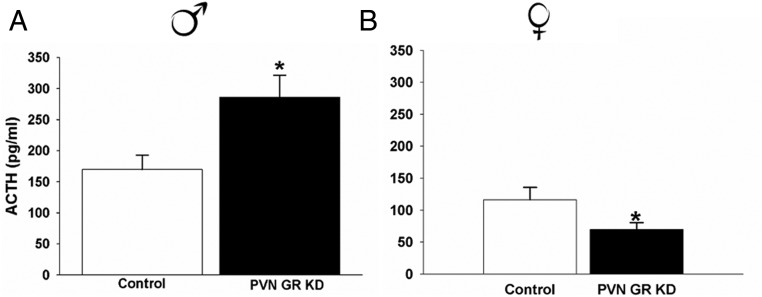
Male GRflox/floxSim1cre− controls and GRflox/flox Sim1cre+ PVN GR KD mice had similar nadir and peak corticosterone concentrations (P > .05), suggesting that GR signaling within the PVN is not critical for normal HPA axis tone in males (Figure 4A). However, there was a significant difference in stress-induced ACTH responses to restraint stress (t17 = 2.69, P < .01). Male GRflox/flox Sim1cre+ PVN GR KD mice had significantly higher ACTH concentrations relative to GRflox/flox Sim1cre− control mice 15 minutes after restraint onset (Figure 5A). There was no significant difference in corticosterone concentrations in these same animals at the 15-minute time point (data not shown), consistent with the time-lag between initiation of ACTH and corticosterone secretion. In a separate set of animals we completed a time-course study to evaluate stress-induced corticosterone responses to restraint stress. KD of PVN GR in males significantly increased corticosterone responses to restraint stress at the 60-minute time point (F3,71 = 2.42, P < .05) (Figure 6A). In addition, GRflox/flox Sim1cre+ PVN GR KD mice secreted more corticosterone over time in response to stress as indicated by the integrated corticosterone response (area under the curve) (t21 = 2.42, P < .05) (Figure 6B).
Figure 4.
Nadir and peak corticosterone concentration in (A) male GRflox/floxSim1cre− control (n = 8) and GRflox/floxSim1cre+ PVN GR KD male mice (n = 9) and (B) female GRflox/floxSim1cre− control (n = 14) and GRflox/floxSim1cre+ PVN GR KD female mice (n = 15). There is no difference in circadian rhythmicity of corticosterone between male GRflox/floxSim1cre+ PVN GR KD and GRflox/floxSim1cre− male control mice; P > .05. However, female GRflox/floxSim1cre+ PVN GR KD have significantly higher morning corticosterone concentrations relative to control female mice; P < .05. Data are represented as mean ± SEM.
Figure 5.
Plasma ACTH responses to an acute restraint challenge at 15 minutes after restraint onset in (A) male GRflox/floxSim1cre− control mice (n = 9) and male GRflox/floxSim1cre+ PVN GR KD mice (n = 10) and (B) female GRflox/floxSim1cre− control (n = 10) and female GRflox/floxSim1cre+ PVN GR KD mice (n = 10). Male GRflox/floxSim1cre− PVN GR KD mice secreted significantly more ACTH in response to restraint stress compared with GRflox/floxSim1cre− control mice; P < .05., Female GRflox/floxSim1cre− PVN GR KD mice secrete significantly less ACTH in response to restraint stress compared with female GRflox/floxSim1cre− control mice; P < .05. Data are represented as mean ± SEM.
Figure 6.
Time-course (A) and integrated (B) corticosterone response to a 30-minute restraint challenge in male GRflox/floxSim1cre− control (n = 17) and male GRflox/floxSim1cre+ PVN GR KD male mice (n = 14). Time-course (C) and integrated area under the curve (AUC) (D) corticosterone response in female GRflox/floxSim1cre− control (n = 15) and female GRflox/floxSim1cre+ PVN GR KD mice (n = 16). Male GRflox/floxSim1cre+ PVN GR KD mice secrete significantly more corticosterone at the 60-minute time point relative to male GRflox/floxSim1cre− control mice; P < .05. Male GRflox/floxSim1cre+ PVN GR KD mice also have a higher integrated stress response to restraint stress relative to control GRflox/floxSim1cre− mice; P < .05. Female GRflox/floxSim1cre+ PVN GR KD mice have significantly higher basal corticosterone (0 min) relative to female GRflox/floxSim1cre− control mice; P < .05. Integrated corticosterone response to an acute restraint challenge is similar in control and PVN GR KD female mice. Data are represented as mean ± SEM.
KD of PVN GR induced a different HPA axis phenotype in females. Female GRflox/flox Sim1cre+ PVN GR KD mice had significantly higher nadir circadian corticosterone concentrations compared with GRflox/floxSim1cre− controls (t27 = −3.364, P < .01) (Figure 4B). Female GRflox/flox Sim1cre+ PVN GR KD mice secreted significantly less ACTH in response to an acute restraint challenge compared with GRflox/floxSim1cre− control mice (t18 = −2.017, P = .05) (Figure 5B). Unlike the observed HPA axis hyperactivity to restraint stress in male GRflox/flox Sim1cre+ PVN GR KD mice, depletion of PVN GR in females did not modulate corticosterone responses to an acute restraint challenge (P > .05) (Figure 6, C and D). However, consistent with our previous experiment, females with depletion of PVN GR exhibited higher prestress corticosterone concentrations (0-min time point) relative to female GRflox/floxSim1cre− controls, P < .05.
Depletion of PVN GR does not significantly alter neuroendocrine responses after chronic stress exposure in males or females
Deficits in PVN GR signaling did not exacerbate HPA axis responses to chronic stress, because there were no genotype-specific differences in corticosterone concentrations to an acute novel forced swim test after a history of chronic stress in either males or females (P > .05) (Figure 7, A–D).
Figure 7.
Time-course (A) and integrated area under the curve (AUC) (B) corticosterone response to a novel stressor (forced swim test) after a history of chronic stress exposure in male GRflox/floxSim1cre−control (n = 13) and male GRflox/floxSim1cre+ PVN GR KD mice (n = 15). Time-course (C) and integrated (D) stress response to forced swim test after a history of chronic stress in female GRflox/floxSim1cre−control (n = 12) and female GRflox/floxSim1cre+ PVN GR KD (n = 12) mice. Data are represented as mean ± SEM.
Depletion of PVN GR does not impact CRH and AVP mRNA expression in males or females
Despite significant differences in acute neuroendocrine stress responses, there were no differences observed in stress-related neuropeptide mRNA content expression between the 2 genotypes in males or females under basal or stress-induced conditions (P > .05) (data not shown).
Depletion of PVN GR does not significantly alter neuroendocrine and behavioral responses after chronic stress exposure in males or females
Loss of PVN GR signaling did not impact immobility or mobility (ie, swimming, climbing) in the FST of either genotype in males or females (P > .05) (data not shown).
Discussion
Our results indicate that sim1-cre-mediated GR KD inhibits feedback inhibition of ACTH and corticosterone to acute stress in males, but not females. Work from our collaborators indicates that deletion of GR in CRH neurons blocks fast nongenomic glucocorticoid inhibition of glutamate release in a hypothalamic slice preparation (20). In combination with our in vivo data, these results suggest that the potentiation of stress responses observed in GRflox/floxSim1cre+ mice is mediated by parvocellular PVN GR deletion. The exact mechanism for GR-dependence of the fast glucocorticoid feedback is not known. There are no obvious structural properties associated with the GR that would target membranes. Recent findings in cultured cells indicate that GR is trafficked to the membrane in response to corticosterone exposure, but that this does not appear to be dependent on the palmitoylation of GR (32). Consequently, fast effects of glucocorticoids on stress-induced neuroendocrine responses in males may be mediated by “near membrane” interactions (33, 34).
The importance of the PVN GR appears to be sex-dependent. Although male GRflox/flox Sim1cre+ PVN GR KD mice have exaggerated HPA axis responses to acute stress, in terms of both ACTH and corticosterone secretion relative to male GRflox/floxSim1cre− controls, females have elevated nadir corticosterone and a suppressed ACTH response to acute stress compared with female GRflox/flox Sim1cre− controls. The lack of a pronounced effect of PVN GR on corticosterone responses to acute restraint stress indicates that females may not require the PVN GR for glucocorticoid regulation of the HPA axis. These data are consistent with our findings in female forebrain GR knockout mice (16), and together, these data suggest that glucocorticoid signaling mechanisms differ substantially between the sexes, with cycling, adult females being less sensitive to rapid signaling effects of glucocorticoids via the GR, although see Ref. 35. It is possible that the reduced ACTH response to stress in female GRflox/flox Sim1cre+ PVN GR KD mice is driven by an enhanced tonic signal mediated by elevated basal glucocorticoids which is compensated at the level of the adrenal. Alternatively, it is possible that females are able to compensate for loss of PVN GR by alternative regulatory mechanisms, perhaps due to enhanced estrogen receptor (ER)β signaling at the level of the PVN (36). For example, activation of ERβ with diaprylpropionitrile or 5α-androstan-3β, 17β-diol implants aimed slightly dorsal to the PVN modulates neuroendocrine stress responses (eg, ACTH) in gonadectomized males. Further, ERβ agonists block the deleterious effects of enhanced central amygdalar GR signaling on neuroendocrine and behavioral (anxiety-like behavior) stress responses (37). These data suggest a role for estradiol signaling via ERβ in modulating HPA axis-related endpoints attributed to GR. Finally, it is possible that HPA axis feedback responses in females occurs via other forebrain (bed nucleus of stria terminalis, medial preoptic area) or brainstem (nucleus of solitary tract) regions (38).
Given the widely documented role of gonadal hormones as modulators of neuroendocrine and behavioral stress responses (39), the estrous cycle stage was determined at the end of each experiment. Even though the estrous cycle was not considered as a factor in the statistical analyses (due to small sample size), female GRflox/floxSim1cre+ PVN GR KD mice exhibited a distinct neuroendocrine phenotype (morning glucocorticoid hypersecretion and suppressed stress-induced ACTH) from female GRflox/floxSim1cre− controls. These findings suggest that this neuroendocrine phenotype in females is primarily attributed to loss of PVN GR and less likely due to activational effects of gonadal hormones. However, future studies may include an in depth assessment of the role of gonadal hormones in neuroendocrine stress responses in both male and female GRflox/flox Sim1cre+ PVN GR KD mice and GRflox/floxSim1cre− controls to directly test this postulate.
There was no significant effect of PVN GR KD on HPA axis responses to chronic stress in either sex. These data suggest that acute and chronic stress regulatory mechanisms are mediated by distinct neural mechanisms. Previous studies from our group and others suggest that chronic stress recruits neural pathways that can enhance HPA axis drive (9, 24, 40). Thus, it is possible the chronic stress-recruited pathways override rapid glucocorticoid effects in the PVN, perhaps as a mechanism to maintain response capacity in the face of an increased feedback signal.
In brain, expression of Sim-1 is most pronounced in the PVN and supraoptic nucleus of the hypothalamus (SON), consistent with its pivotal role in development of these structures (19). Previous report GR expression in magnocellular neurosecretory neurons in the SON (41) but very low (to absent) GR expression in magnocellular neurons in the PVN (41, 42). However, HPA axis responses are largely controlled by parvocellular hypophysiotrophic neurons, making it unlikely that KD of GR in magnocellular neurons in these nuclei is responsible for the observed increases in stress-induced ACTH and corticosterone. Sim1 is sparsely expressed in areas outside of the PVN and SON, including the sporadic expression in other hypothalamic nuclei (lateral hypothalamus, posterior hypothalamus) (43). Although we cannot completely rule out a role for GR signaling within these regions on HPA axis regulation, we did not observe a difference in GR immunoreactivity in these regions between the GRflox/flox Sim1cre+PVN GR KD and GRflox/floxSim1cre− control mice. In addition, we did not observe a difference in GR immunoreactivity and/or mRNA expression in primary HPA axis regulatory regions, including the central amygdaloid nucleus, basolateral amygdaloid nucleus, ventral hippocampus, and medial prefrontal cortex. Finally, Sim1-dependent GR KD-mediated enhancement of ACTH and corticosterone release in vivo agrees with evidence from in vitro studies, where GR deletion in PVN neurons blocks fast feedback neuronal inhibition (20). Outside of the brain, Sim1 expression is reported in the kidneys and spinal cord (44). Importantly, Sim1 expression appears to be absent in other critical peripheral targets, including the adrenals (43), and there was no difference in pituitary GR expression between GRflox/flox Sim1cre+PVN GR KD and GRflox/floxSim1cre− control mice. Collectively, these observations provide strong evidence that our results are driven by PVN GR KD, rather than Sim-1 promoter-mediated gene deletion in other brain regions, adrenals, or pituitary gland.
Although Sim1-mediated GR targeting clearly affects parvocellular cell populations in the PVN, we only observed approximately a 60% loss in neuron counts. Because GR is richly localized in nonneurosecretory neurons, it is possible that the remaining neurons do not express Sim1, and are perhaps related to autonomic drive or interneuronal signaling. Additional GR signal may come from glial elements such as astrocytes and microglia, which also express GR (45).
A recent study suggests that Sim-1 promoter-mediated deletion of GR exon 3, which contains the zinc finger of the DNA binding domain, results in a Cushingoid phenotype (ie, basal glucocorticoid hypersecretion, impaired dexamethasone feedback, elevated PVN CRH mRNA, and marked impairments in metabolic function) (46). These data raise the possibility that the exon 2 deletion constitutes a KD, rather than a complete deletion of the PVN GR. Regardless of the phenotypic difference across mouse lines, it is apparent that the Sim-1-mediated GR KD in the PVN is of sufficient magnitude to block fast feedback inhibition (in males) and promotes morning corticosterone hypersecretion in females.
Sim1-driven GR KD did not alter immobility in the forced swim test after chronic stress, further suggesting that a full complement of PVN GR is not required for appropriate behavioral responses to chronic stressor exposure. One potential limitation in the interpretation of this finding is the omission of non-CVS animals as controls. However, similar studies using mice with Sim1 Cre-driven deletion of GR exon 1c-2 or 3 did not result in behavioral phenotypes characteristic of depression-like behavior (46). These data are also consistent with previously published findings whereby local administration of mifepristone (GR and PR antagonist) in the PVN induced HPA axis disinhibition, but did not impact immobility in the forced swim test (47). Together, these data suggest that extra-PVN regions (medial prefrontal cortex, hippocampus, amygdala) may be responsible for effects of glucocorticoids on stress-related behaviors, consistent with previous studies documenting changes in sucrose preference and forced swim tests (16, 17, 48)
Collectively, our data highlight a role for PVN GR in regulating acute neuroendocrine responses to stress, and further highlight the potential for differential engagement of brain regions regulating HPA axis responses under acute vs chronic stress conditions. In addition, these data indicate that rapid inhibition of the HPA axis, a critical step toward limiting the magnitude and duration of glucocorticoid stress responses, is governed in large part by local, probably nongenomic glucocorticoid actions. Finally, and perhaps more importantly, the data suggest that alternative HPA axis regulatory mechanisms exist in females, and raise the possibility that failure of these mechanisms (rather than a GR deficit) contribute to sex differences in HPA axis-associated pathologies.
Acknowledgments
We thank Dr Joel Elmquist at University of Texas Southwestern for kindly providing the Sim1-cre mice. We also thank Dr Eduardo Carvalho-Netto, Benjamin Packard, and Charles Dolgas for assistance with some of the experiments.
This work was supported by National Institutes of Health Grants K12HD051953 (to M.B.S.); MH069725, MH069860, and MH049698 (to J.P.H.); and MH066958 (to J.G.T.).
Disclosure Summary: The authors have nothing to disclose.
- AVP
- arginine vasopressin
- CVS
- chronic variable stress
- ER
- estrogen receptor
- GR
- glucocorticoid receptor
- HPA
- hypothalamic-pituitary-adrenal
- KD
- knockdown
- NeuN
- neuronal nuclei
- PVN
- paraventricular nucleus
- RNase
- Ribonuclease
- SSC
- saline sodium citrate
- SON
- supraoptic nucleus of the hypothalamus.
References
- 1. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. [DOI] [PubMed] [Google Scholar]
- 3. Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151(10):4811–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23(12):4850–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tasker JG, Di S, Malcher-Lopes R. Rapid central corticosteroid effects: evidence for membrane glucocorticoid receptors in the brain. Integr Comp Biol. 2005;45(4):665–671. [DOI] [PubMed] [Google Scholar]
- 6. Di S, Popescu IR, Tasker JG. Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. J Neurosci. 2013;33(46):18331–18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136(8):3299–3309. [DOI] [PubMed] [Google Scholar]
- 8. Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61(2):180–190. [DOI] [PubMed] [Google Scholar]
- 9. Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517(2):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119(3):887–897. [DOI] [PubMed] [Google Scholar]
- 11. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):112–119. [DOI] [PubMed] [Google Scholar]
- 12. Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205(5):807–815. [DOI] [PubMed] [Google Scholar]
- 13. Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. [DOI] [PubMed] [Google Scholar]
- 14. Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5(1):1–23. [PubMed] [Google Scholar]
- 15. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2–3):85–96. [DOI] [PubMed] [Google Scholar]
- 16. Solomon MB, Furay AR, Jones K, et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyle MP, Brewer JA, Funatsu M, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102(2):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149(11):5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duplan SM, Boucher F, Alexandrov L, Michaud JL. Impact of Sim1 gene dosage on the development of the paraventricular and supraoptic nuclei of the hypothalamus. Eur J Neurosci. 2009;30(12):2239–2249. [DOI] [PubMed] [Google Scholar]
- 20. Nahar J, Haam J, Chen C, et al. Rapid non-genomic glucocorticoid actions in male mouse hypothalamic neuroendocrine cells are dependent on the nuclear glucocorticoid receptor. Endocrinology. 2015;156:2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35(7):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;261(5 pt 2):R1257–R1268. [DOI] [PubMed] [Google Scholar]
- 24. Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149(2):818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang R, Packard BA, Tauchi M, D'Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci USA. 2009;106(14):5913–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang R, Jankord R, Flak JN, Solomon MB, D'Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci. 2010;30(44):14907–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Kloet AD, Krause EG, Solomon MB. Adipocyte glucocorticoid receptors mediate fat-to-brain signaling. Psychoneuroendocrinology. 2015;56:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 30. McClave JTaD, Dietrich FH., II Statistics. 6th ed New York, NY: Dellen-MacMillan; 1994. [Google Scholar]
- 31. Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. [DOI] [PubMed] [Google Scholar]
- 32. Deng Q, Waxse B, Riquelme D, Zhang J, Aguilera G. Helix 8 of the ligand binding domain of the glucocorticoid receptor (GR) is essential for ligand binding. Mol Cell Endocrinol. 2015;408:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malcher-Lopes R, Di S, Marcheselli VS, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26(24):6643–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci. 2009;29(2):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor β activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10(3–4):371–394. [DOI] [PubMed] [Google Scholar]
- 39. Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97(2):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4(2):62–69. [DOI] [PubMed] [Google Scholar]
- 41. Kiss JZ, Van Eekelen JA, Reul JM, Westphal HM, De Kloet ER. Glucocorticoid receptor in magnocellular neurosecretory cells. Endocrinology. 1988;122(2):444–449. [DOI] [PubMed] [Google Scholar]
- 42. Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26(3):235–269. [DOI] [PubMed] [Google Scholar]
- 43. Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. [DOI] [PubMed] [Google Scholar]
- 44. Fan CM, Kuwana E, Bulfone A, et al. Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Mol Cell Neurosci. 1996;7(6):519. [DOI] [PubMed] [Google Scholar]
- 45. Yan P, Xu J, Li Q, et al. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J Neurosci. 1999;19(21):9355–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laryea G, Arnett MG, Wieczorek L, Muglia LJ. Site-specific modulation of brain glucocorticoid receptor and corticotropin-releasing hormone expression using lentiviral vectors. Mol Cell Endocrinol. 2013;371(1–2):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47(2):109–115. [DOI] [PubMed] [Google Scholar]
- 48. McKlveen JM, Myers B, Flak JN, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]