This study established an experimental strategy to identify effective factors for cell transplantation in Parkinson’s disease (PD) based on comparison of several brain environments. Neurexophilin 3 was identified as a new supportive factor for cell transplantation and may be a useful biomarker for PD patients. These findings are expected to promote in vivo and in vitro applications of stem cell technology.
Keywords: Neurexophilin 3; Brain environment; Cell transplantation therapy; Parkinson’s disease; Induced pluripotent stem cells; 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Abstract
Successful cell transplantation for Parkinson’s disease (PD) depends on both an optimal host brain environment and ideal donor cells. We report that a secreted peptide, neurexophilin 3 (NXPH3), supports the survival of mouse induced pluripotent stem cell-derived (iPSC-derived) dopaminergic (DA) neurons in vitro and in vivo. We compared the gene expression profiles in the mouse striatum from two different environments: a supportive environment, which we defined as 1 week after acute administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and a nonsupportive environment, defined as 8 weeks after chronic administration of MPTP. NXPH3 expression was higher in the former condition and lower in the latter compared with untreated controls. When we injected mouse iPSC-derived neural cells along with NXPH3 into the mouse striatum, the ratio of tyrosine hydroxylase-positive DA neurons per graft volume was higher at 8 weeks compared with cell injections that excluded NXPH3. In addition, quantitative polymerase chain reaction analyses of the postmortem putamen revealed that the expression level of NXPH3 was lower in PD patients compared with normal controls. These findings will contribute to optimizing the host brain environment and patient recruitment in cell therapy for PD.
Significance
This study identified neurexophilin 3 (NXPH3), a secreted peptide, through comparison of gene expression profiles in the mouse striatum from various environments generated by different doses of dopaminergic (DA) neuron toxin. When mouse induced pluripotent stem cell-derived neural cells along with NXPH3 were injected into the mouse striatum, the ratio of DA neurons per graft volume was higher at 8 weeks compared with cell injections without NXPH3. In addition, quantitative polymerase chain reaction analyses of the postmortem putamen revealed that the expression level of NXPH3 was lower in patients with Parkinson’s disease (PD) compared with controls without PD. These findings contribute to optimization of the host brain environment and patient recruitment in cell therapy.
Introduction
Human induced pluripotent stem cells (iPSCs) provide a promising source of midbrain dopaminergic (DA) neurons for cell replacement therapy against Parkinson’s disease (PD). Protocols for inducing DA neurons from iPSCs have been developed already [1–3], and midbrain DA progenitors can be enriched [4]. To achieve successful transplantation, however, the grafted cells need to survive, extend neurites, and form synapses with host neurons. Consequently, the outcome of the transplantation can be affected by both the condition of the host brain environment and the quality of the donor cells [5, 6].
The central nervous system has endogenous potential to restore lost neurons and repair neuronal function by recruiting endogenous neural stem/progenitor cells, suggesting that such stem cells are plastic and responsive to alternations in the brain environment [7–9]. In other words, cues from the host brain can alter the survival and maturation of grafted progenitor cells; therefore, the identification of endogenous supportive factors that promote these cues is a strategy for improving transplantation success. Glial cell-derived neurotrophic factor (GDNF), for example, supports the survival of grafted midbrain DA neurons, but clinical application of human recombinant GDNF to treat PD has proven unsuccessful because of the development of binding antibodies against human recombinant GDNF [10]. Alternative factors that support the survival of grafted DA neurons are needed.
To address this issue, we investigated different degrees of host brain environment with different doses of DA neuron toxin. Neuroinflammation plays an important role in the pathogenesis of PD, in which α-synuclein activates microglia to secrete inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6 [11–13]. These cytokines act directly on DA neurons or subsequently activate astrocytes. In addition, allografts inevitably cause inflammation and immune responses in which astrocytes, microglia, and recruited lymphocytes secrete cytokines such as IL-1β and TNF-α [14] or IL-1β, IL-4, and IL-6 [15] at the acute phase. Consequently, inflammation would appear to be a crucial factor in cell transplantation for PD. In the present study, we generated different degrees of inflammation in host brain and compared the survival of grafted mouse iPSC-derived DA neurons in these different conditions. Our observations allowed us to characterize supportive and nonsupportive brain environments for cell grafting. By comparing gene expression profiles between the two brain environments, we found that a synapse-related peptide, neurexophilin 3 (NXPH3), supported the survival of the grafted DA neurons. Furthermore, we demonstrated that single and local administration of NXPH3 increased the number of DA neurons in iPSC-derived grafts. These results suggest that NXPH3 is a candidate factor that supports the survival of iPSC-derived DA neurons.
Materials and Methods
Animals
Male 8-week-old C57BL/6NCrSlc mice were purchased from SLC Inc. (Hamamatsu, Japan, http://www.jslc.co.jp/english/index2.htm) and cared for and handled according to the guidelines for animal experiments of Kyoto University. Experimental protocols were approved by the committee for animal research at Kyoto University.
Human Brain Tissue
Human brain tissues (n = 11; 6 healthy controls, 5 PD patients) were provided by the Brain Bank at the Tokyo Metropolitan Institute of Gerontology (Itabashi, Tokyo, Japan). This research project was approved by ethics committees at Kyoto University and Tokyo Metropolitan Institute of Gerontology.
MPTP Administration
Mice were divided into five groups: two acute groups, 1 week and 8 weeks after acute administration (4 times every 2 hours) of free base 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) HCl (20 mg/kg, 80 mg/kg in total, intraperitoneally; Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com); two chronic groups, 1 week and 8 weeks after chronic administration (once a day for 20 consecutive days) of free base MPTP HCl (4 mg/kg per day, 80 mg/kg in total); and a group 1 week after injection (4 times every 2 hours) of saline (supplemental online Fig. 1). The MPTP administration was performed in accordance with previous reports [16, 17].
Mouse iPSC Culture and Differentiation
Undifferentiated mouse iPSCs (iPS-MEF-Fb/Ng-440A-3) were used in all experiments [18]. Briefly, this cell line (440A-3) was established by plasmid vectors that introduced Oct-3/4, Sox2, Klf4, and c-Myc from mice in which green fluorescent protein (GFP) and the puromycin-resistant gene are driven by the Nanog enhancer and promoter [19]. The 440A-3 line was most likely free from plasmid integration into the host genome [18].
Undifferentiated mouse iPSCs were maintained on mitomycin C-treated mouse embryonic fibroblast (MEF) feeder in Glasgow minimum essential medium (Gibco-Invitrogen, Grand Island, NY, http://www.lifetechnologies.com) supplemented with 1% fetal bovine serum (FBS; JRH Biosciences, Kansas, http://www.bioscreening.com/Details/JRH-Biosciences.html), 5% knockout serum replacement (KSR; Gibco-Invitrogen), 0.1 mM nonessential amino acids (Gibco-Invitrogen), 1 mM pyruvate (Sigma-Aldrich), 0.1 μM 2-mercaptoethanol (Sigma-Aldrich), 2,000 U/ml leukemia inhibitory factor (Chemicon International, Temecula, CA, http://www.emdmillipore.com), and 100 U/ml penicillin and 100 mg/ml streptomycin. The cells were maintained in medium containing 0.75 μg/ml puromycin to eliminate differentiated cells.
For the neural induction of iPSCs, we used the serum-free culture of embryoid body-like aggregates with quick reaggregation (SFEBq) method [20] (supplemental online data).
Cell Transplantation Into Mouse Brain
On day 12, the iPSC-derived aggregates were dissociated into single cells using Accutase (Innovated Cell Technologies, Inc., CA, http://www.innovativecelltech.com) at 37°C for 5 minutes, and a cell suspension of approximately 1.5 × 105 cells/μl was prepared in phosphate-baffered saline ((PBS(−)) containing 30 μM Y-27632 (Wako Pure Chemical Industries, Osaka, Japan, http://www.wako-chem.co.jp/english). Each mouse received a stereotactic injection of 1 μL (1 μl/10 seconds) of the cell suspension into the bilateral striatum (coordinates from the bregma: A +0.5, L and R +2.0, V +3.0, and incisor bar 0) and was observed for 8 weeks without immunosuppression.
To examine the effects of soluble factors, GDNF (1 μg/1 μl; R&D Systems, Minneapolis, MN, http://www.rndsystems.com), neurexophilin 3 (NXPH3; 1μg/1 μl; R&D Systems), or insulin-like growth factor (IGF2; 1μg/1 μl; Wako Pure Chemical Industries) was injected adjacent to the graft (coordinates from the bregma: A +1.0, L and R +1.5, V +3.0, and incisor bar 0). PBS(−) was used as a vehicle control.
Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted using an RNeasy Plus Mini kit (Qiagen, Valencia, CA, http://www.qiagen.com). Then, 1 μg of total RNA was used for reverse transcription by a SuperScript III First-Strand Synthesis System with Oligo(dT)20 primer (Invitrogen). For polymerase chain reaction (PCR) amplification, reactions were performed with Hot Start Taq (Qiagen). The primer sequences and product sizes are shown in supplemental online Table 1.
Quantitative PCR
Quantitative PCR (qPCR) was performed on a StepOne detection system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Data analysis was based on the ∆CT method with normalization of the raw data to Gapdh genes. All PCRs were performed in triplicate. The primer sequences and product sizes are shown in supplemental online Table 2.
Immunocytochemistry
For in vitro multiple immunofluorescence, cells were fixed with 4% paraformaldehyde (PFA) in 100 mM phosphate-buffered saline (PBS) for 30 minutes. Slides were preincubated with PBS containing 0.1% Triton X-100 and 2% skimmed milk for 60 minutes and then incubated at 4°C overnight with primary antibodies (supplemental online Table 3). Samples were then incubated with fluorescent dye-conjugated secondary antibody (diluted 1:500; Alexa Fluor 488, Alexa Fluor 594, or Alexa Fluor 647; Gibco-Invitrogen) for 2 hours at room temperature. In addition, the samples were incubated with 200 ng/ml of 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) for nuclei staining. Fluorescence was then detected using a laser scanning confocal microscope (FV-1000; Olympus, Tokyo, Japan, http://www.olympus-global.com/en). Additional processing of images for contrast, color balance, brightness, and merging was performed as needed with Fluoview software (version 4.01; Olympus) and Adobe Photoshop CS (Adobe Systems, San Jose, CA, http://www.adobe.com).
Immunohistochemistry
Mice were perfused through the aorta with 50 ml of 100 mM PBS, followed by 20 ml of 4% PFA in PBS, under deep anesthesia with sodium pentobarbital (100 mg/kg, i.p.). After perfusion, brains were quickly removed, postfixed for 2 days with 4% PFA in PBS, and then transferred to 30% sucrose solution in PBS at 4°C. Brain pieces were sectioned at 30-μm thickness using a cryostat microtome. DAB staining and immunofluorescence were carried out as described in the supplementary online data.
Evaluation of Histological Analysis
To determine the percentage of positive cells for each marker, immunopositive cells were manually counted for at least three independent samples. The graft volume was determined by identifying venus-positive (venus+) areas in every sixth 30-μm-thick section using BZ-II Analyzer software (Keyence, Osaka, Japan, http://www.keyence.com) and totaling the volumes of whole-tall cylinders according to Cavalieri’s principle. To estimate the number of immunoreactive cells in each graft, the cells were manually counted in every sixth section. Microglial activation in the striatum and substantia nigra pars compacta (SNpc) was evaluated by microglial scores [21].
DNA Microarray
Total RNA was extracted using an RNeasy Plus Mini kit, and 100 ng of total RNA was used for the analysis. Samples were hybridized to GeneChip Mouse Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA, http://www.affymetrix.com), according to the manufacturer’s protocol. Arrays were scanned using the Microarray Scanner System (Agilent Technologies, Santa Clara, CA, http://www.home.agilent.com). The data were analyzed using GeneSpring GX12.1 software (Agilent Technologies). The expression signals of the probe sets were calculated using RMA16. The microarray data are available from the Gene Expression Omnibus (GEO database, http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE60080.
Vector Construction
A pEF1-TetOn-i-Puro/mCherry-i-MCS-3xFLAG vector was constructed from pCMV-Tet3G (Clontech Laboratories, Inc., Mountain View, CA, http://www.clontech.com), pTRE3G-mCherry (Clontech Laboratories, Inc.), linear puromycin marker (Clontech Laboratories, Inc.), and pCS-EF1-venus (a gift from Dr. Hiroyuki Miyoshi, BioResourse Center, RIKEN, Tsukuba, Japan). The 3xFLAG sequence was annealed by GCGGCCGCCTAGCAATCGATAACCGATATCGACTATAAAGACCACGATGGAGACTAC and GTAGTCTCCATCGTGGTCTTTATAGTCGATATCGGTTATCGATTGCTAGGCGGCCGC. The open reading frame of the genes of interest (GOIs) was amplified from cDNAs derived from human putamen total RNA, human brain total RNA, or intact mouse striatum total RNA using PrimeStar (Takara Bio. Inc., Kusatsu, Japan, http://www.takara-bio.com). The pEF1-TetOn-i-Puro/mCherry-i-MCS-3xFLAG vector was digested by NotI and EcoRV, and then each GOI was inserted using InFusion HD (Clontech Laboratories, Inc.).
Establishment of HEK293T Cell Line for In Vitro Screening
Plasmids were transfected into HEK293T cells by FuGENE6 transfection reagent (Roche, Basel, Switzerland, http://www.roche.com), and the cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 2 mM of l-glutamine. After 24 hours, 10 μg/ml of puromycin was added into the culture medium to select drug-resistant cells. The protein expression was induced by 1 μg/ml of doxycycline (DOX), monitored by mCherry fluorescence and confirmed by Western blot analysis using a mouse anti-DDDDK antibody (MBL, Nagoya, Japan, http://www.mbl.co.jp/e). Secretion of the protein was detected by enzyme-linked immunosorbent assay (ELISA) with anti-FLAG high sensitivity, a M2 coated 96-well plate (Sigma-Aldrich) and a rabbit anti-DDDDK-tag pAb-HRP-DirecT (MBL).
In Vitro Screening for Candidate Factors
HEK293T cells with the transgene were replated to a six-well plate. On the next day, iPSC-derived aggregates (day 12) were plated on these cells and maintained in DMEM/Ham’s F-12 medium with N2 and B27 supplements for 7 days. Secretion of the transfected gene product was induced by adding 1 μg/ml of DOX from the first day.
In another evaluation, the iPSC-derived aggregates were dissociated by Accutase and cultured on a dish coated with poly-l-ornithine/fibronection/laminin (OFL) for 7 days with or without GDNF (1, 10, 100 ng/ml; R&D Systems), NXPH3 (1, 10, 100, 500 ng/ml; R&D Systems), procollagen C-endopeptidase enhancer protein (PCOLCE; 1, 10, 100, 500 ng/ml; R&D Systems), CXCL9 (1, 10, 100, 500 ng/ml; R&D Systems), or IGF2 (1, 10, 100, 500 ng/ml; Wako Pure Chemical Industries).
Lentivirus Production and Infection
HEK293T cells were transfected using FuGENE6 transfection reagent with the plasmid combination pCSII-EF-venus, pCMV-VSV-G-Rev, and pCAG-HIVgp. The functional infectious unit of lentiviral supernatant was calculated on HEK293T cells. Differentiating iPSCs were infected with 10 times the number of infectious particles per cell on day 9 of the differentiation process.
Western Blotting
Striatum and ventral midbrain samples were dissected from mouse brain. The tissues were homogenated in CellLytic MT buffer (Sigma-Aldrich) supplemented with PhosSTOP (Roche) and Complete Mini (Roche) as phosphatase inhibitors and protease inhibitors, respectively. Protein concentration was determined using the Bradford protein assay kit. Sodium dodecylsulfate-polyacrylamide gel electrophoresis was carried out, as described in the supplementary online data.
High-Performance Liquid Chromatography Analysis for Dopamine Release
IPSC-derived aggregates (day 12) were washed twice with low-concentration KCl solution (4.7 mM KCl) and incubated in low-concentration KCl solution for 2 minutes. The medium was subsequently replaced with 1 ml of high-concentration KCl solution (60 mM KCl), and the samples were incubated for 15 minutes. High-performance liquid chromatography (HPLC) analysis was performed, as reported previously [4].
Statistical Analysis
Results are given as mean ± SEM. The significance of differences was determined by Student’s t test for single comparisons and by one-way analysis of variance for multiple comparisons. Further statistical analysis for post hoc comparisons was performed using Dunnett’s test or Tukey’s test (Prism 5; GraphPad, San Diego, CA, http://www.graphpad.com).
Results
Induction of DA Neurons From Mouse iPSCs
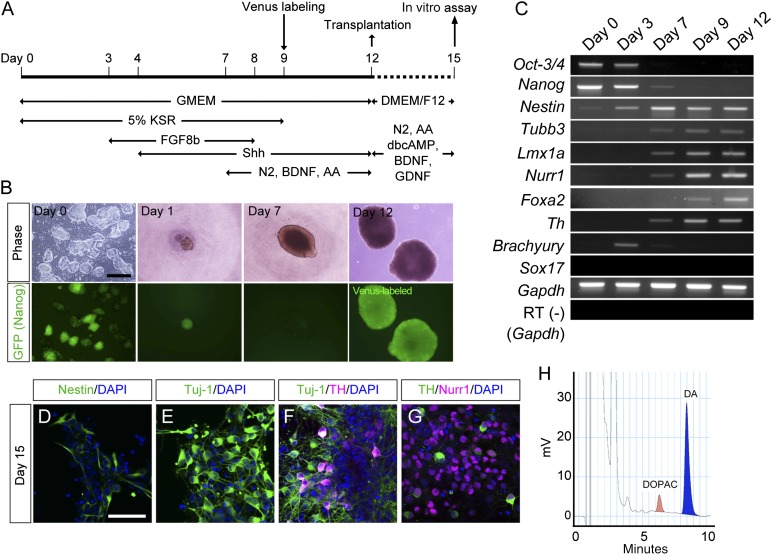
We induced midbrain DA neurons from mouse iPSCs based on the SFEBq method [20] with the addition of FGF8b and Shh (Fig. 1A). Dissociated iPSCs quickly reaggregated in each well of 96-well plates and expanded day by day (Fig. 1B). Nanog-GFP became invisible before day 7 of the culture (Fig. 1B). Reverse transcription PCR analysis showed that the expression of pluripotent cell markers (Oct-3/4, Nanog) gradually decreased until day 7, whereas that of neural and neuronal markers (Nestin, Tubb3) increased after day 3 (Fig. 1C). The markers of midbrain DA neurons (Lmx1a, Nurr1, Foxa2, and Th) also increased from day 7 to 9 (Fig. 1C). In contrast, markers for the mesoderm (Brachyury) and endoderm (Sox17) were never observed throughout the culture (Fig. 1C). Immunofluorescence performed on day 15 revealed that 28.6% ± 4.7% and 65.8% ± 6.2% of the cells expressed Nestin and Tuj-1, respectively (Fig. 1D, 1E). Among the Tuj-1+ neurons, 17.1% ± 4.2% of them expressed TH+ (Fig. 1F), and 48.3% ± 8.2% of these TH+ neurons also expressed Nurr1, a midbrain DA neuron marker (Fig. 1G). HPLC analysis revealed that dopamine was abundantly released into the culture medium of the differentiated cells in response to high potassium-induced depolarization (14.3 ± 0.4 pmol/106 cells) (Fig. 1H). These in vitro results suggested that our protocol generated functional DA neurons from mouse iPSCs.
Figure 1.
DA neuronal differentiation from induced pluripotent stem cells (iPSCs) using serum-free culture of embryoid body-like aggregates with quick reaggregation. (A): Protocol of DA neuronal induction from iPSCs under the feeder-free condition. Transplantation experiments were performed using day 12 cells. Characteristics of differentiated cells were performed using day 15 cells. (B): Phase-contrast (upper) and GFP fluorescence (lower) images during differentiation. Scale bar = 500 μm. (C): Reverse transcription polymerase chain reaction analysis during differentiation of the expression of markers for undifferentiated cells (Oct-3/4, Nanog); neural (Nestin), neuronal (Tubb3), midbrain (Lmx1a, Nurr1, and Foxa2), and DA neurons (Th); and mesoderm (Brachyury), and endoderm (Sox17). (D–G): Immunofluorescence images of the differentiated cells on day 15. Nestin-positive (D) and Tuj-1-positive neurons (E). Double-labeled images for Tuj-1 (green) and TH (magenta) (F) and TH (green) and Nurr1 (magenta) (G). The blue signal is of nuclei stained with DAPI. Scale bar = 50 μm. (H): High-performance liquid chromatography analysis of the DA release into culture medium by high potassium-evoked depolarization on day 15. Abbreviations: AA, ascorbic acid; BDNF, brain-derived neurotrophic factor; DA, dopaminergic; DAPI, 4′,6-diamidino-2-phenylindole; dbcAMP, dibutyryl cyclic AMP; DMEM/F12; Dulbecco’s modified Eagle’s medium nutrient mixture F12; DOPAC, 3,4-dihydroxyphenilacetic acid; GDNF, glial cell-derived neurotrophic factor; GFP, green fluorescent protein; GMEM, Glasgow minimum essential medium; KSR, knockout serum replacement; N2, N2-supplement.
Generation of Parkinsonian Models Exhibiting Different Brain Environments
To identify supportive and nonsupportive host brain environments for the grafted donor cells, we generated several kinds of mouse parkinsonian models using MPTP, from which the magnitude of the pathological conditions can be modified by the dose and dosing schedule [22–24]. We generated four types of MPTP-induced parkinsonian models by changing the injection protocols (high dose over 8 hours vs. low dose over 20 days; total of 80 mg/kg of MPTP for both) and periods (1 week vs. 8 weeks) after the last injection of MPTP. Details of the experimental regimens are described in Materials and Methods. Briefly, the regimens were categorized as (a) 1 week after acute administration (acute-1W), (b) 8 weeks after acute administration (acute-8W), (c) 1 week after chronic administration (chronic-1W), and (d) 8 weeks after chronic administration (chronic-8W) (supplemental online Fig. 1).
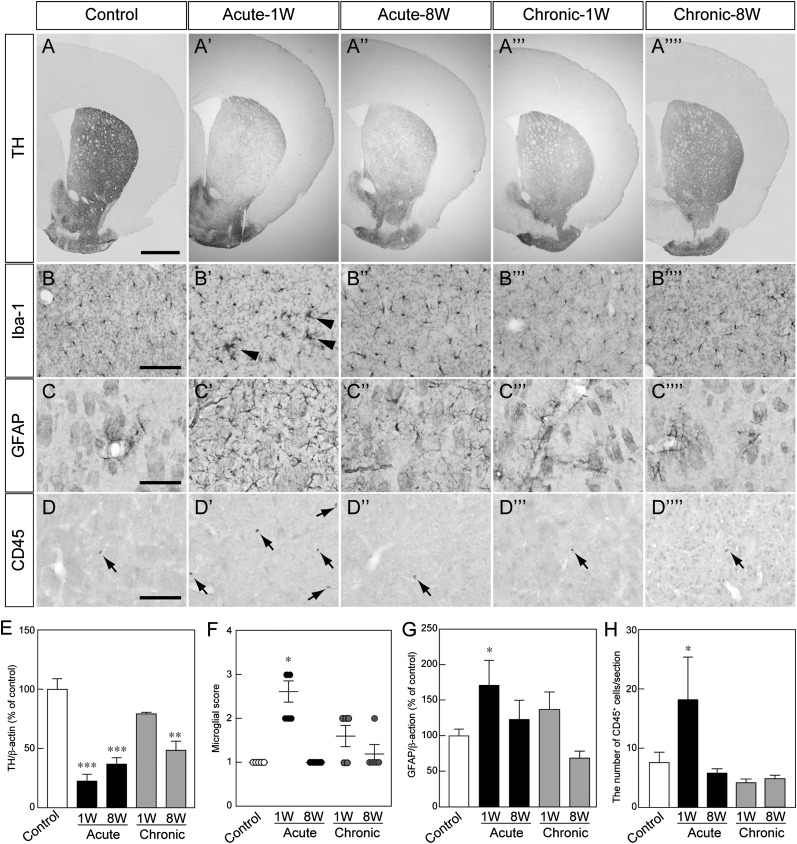
To investigate differences in host brain environments, we performed immunofluorescence of the striatum in MPTP-treated and untreated (control) mice. The degeneration of DA neuron fibers was apparent in the acute MPTP injection groups (Fig. 2A, 2E). The activation of Iba+ microglia (Fig. 2B, 2F) and the emergence of GFAP+ reactive astrocytes (Fig. 2C, 2G) were also seen in acute-1W mice. In addition, the number of CD45+ leukocytes in the striatum increased in acute-1W mice compared with control mice (Fig. 2D, 2H). These results suggested that MPTP-induced inflammation occurred in the striatum of acute-1W mice.
Figure 2.
Histological analysis of the striatum in each MPTP-treated mouse. (A–D): Representative photomicrographs of striatal TH-positive (TH+) fibers (A), Iba1+ cells (arrowheads indicate the accumulation of Iba1+ cells) (B), GFAP+ cells (C), and CD45+ cells (arrows) (D). Scale bars = 500 μm (A) and 100 μm (B–D). (E–H): Quantitative analysis of the protein level of TH (E), microglial activation (F), the protein level of GFAP (G), and the number of CD45+ cells (H). Each value is given as the mean ± SEM (n = 3–5). ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001 versus control group. Abbreviations: 1W, 1 week after administration; 8W, 8 weeks after administration; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Next, we examined the histology of the SNpc among MPTP-treated and untreated mice. The number of TH+ neurons was significantly reduced in all MPTP-treated groups (supplemental online Fig. 2A, 2E), as was immunoreactivity to aromatic amino acid and dopamine transporter (supplemental online Fig. 2A). The activation of microglia and accumulation of reactive astrocytes were also induced in acute-1W mice (supplemental online Fig. 2B, 2C, 2F, 2G). In contrast, the ratio of α-synuclein+ cells per TH+ neuron significantly increased in acute-8W, chronic-1W, and chronic-8W mice (supplemental online Fig. 2D, 2H). These results suggested that inflammation also occurred in the SNpc of acute-1W mice and that degenerative changes, such as α-synuclein accumulation, after MPTP treatment may occur gradually.
Influence of Different Brain Environments on Grafted iPSC-Derived Neural Cells
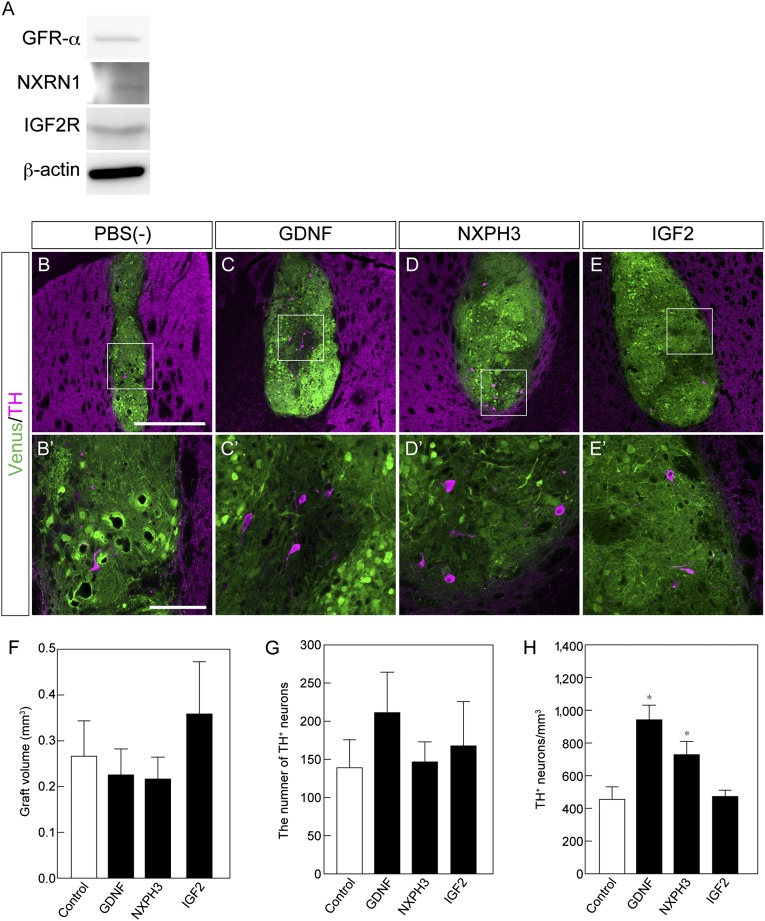
In order to investigate the effects of different conditions in the host brain, iPSC-derived neural cells were grafted into the striatum of MPTP-treated mice. These cells on day 12 of differentiation were composed of neural cells including those committed into DA lineage (Fig. 1C). The grafted cells were prelabeled to express venus (yellow fluorescent protein) by lentiviral transduction, and their survival was compared 8 weeks after transplantation. There were no statistically significant differences in the graft volumes among these animals (Fig. 3A, 3B). Similarly, the number of TH+ neurons in the grafts did not show significant differences (Fig. 3A, 3C). The ratio of TH+ neurons per graft volume significantly increased in acute-1W and acute-8W mice compared with control (Fig. 3D). Furthermore, there was a significant difference between acute-1W and chronic-8W mice. In the clinical situation of cell replacement therapy, both the cell number of surviving TH+ neurons and the graft volume are important parameters for evaluating efficacy and safety: an optimal graft provides a larger number of TH+ neurons in less volume. Consequently, we considered the ratio of TH+ neurons per graft volume as an index of good grafting, which led us to define supportive and nonsupportive brain environments as the acute-1W or chronic-8W brain condition, respectively.
Figure 3.
Histological analysis of induced pluripotent stem cell-derived neural cells 8 weeks after transplantation. (A): Immunofluorescence images of grafts for venus (green) and TH (magenta). Scale bar = 500 μm. (B–D): Quantitative analysis of the graft volume (B), the total number of TH-positive (TH+) neurons (C), and the number of TH+ neurons per graft volume (D). Each value is given as the mean ± SEM (n = 4–6). ∗, p < .05; ∗∗∗, p < .001. (E): Immunofluorescence images of the grafted neural cells for venus (green), GIRK2 (magenta), and TH (cyan). Scale bar = 50 μm. (F): Quantitative analysis of the ratio of GIRK2+/TH+ neurons in each graft. Each value is given as the mean ± SEM (n = 4–6) with no significant difference. (G): Immunofluorescence images of neural cells for venus (green), calbindin (magenta), and TH (cyan). Scale bar = 50 μm. (H): Quantitative analysis of the ratio of calbindin+/TH+ neurons in each graft. Each value is given as the mean ± SEM (n = 4–6) with no significant difference. Abbreviations: 1W, 1 week after administration; 8W, 8 weeks after administration.
There are mainly two subtypes of DA neurons in the midbrain: A9 in the SNpc and A10 in the ventral tegmental area. These two subtypes can be distinguished by the expression of G-protein-gated inwardly rectifying K+ channel subunit (GIRK2) and calbindin, respectively [25]. In all grafts, we found both subtypes (Fig. 3E, 3G), but there was no difference in the ratio of GIRK2+ or calbindin+ cells per TH+ neuron among the acute-1W and chronic-8W conditions (Fig. 3F, 3H).
In order to consider the reason for the difference in the ratio of TH+ neurons between acute-1W and chronic-8W conditions 8 weeks after transplantation, we evaluated early DA differentiation of the grafted cells, name, at 1 week after transplantation (supplemental online Fig. 3). The ratio of TH+ neurons (mature DA neurons) in the graft was not significantly different (supplemental online Fig. 3A, 3B). Similarly, the ratio of Ki67+ Foxa2+ cells per Foxa2+ cells (proliferating DA neuron progenitors) in the graft was approximately 7% in the three brain conditions (supplemental online Fig. 3C, 3D). These results suggested that the increased expansion of DA progenitor cells was unlikely to contribute to the increase of TH+ neurons in the acute-1W condition.
Cell injection may cause inflammation around the graft, and we did not use immunosuppressants; therefore, to investigate inflammation around the graft, we performed immunofluorescence at 1 week after transplantation. Iba-1+ microglia and CD45+ leukocytes were found around the graft, but they were restricted to the marginal area and were small in number. Furthermore, they seemed to have similar distribution that was independent of MPTP treatment (supplemental online Fig. 4). Theses results suggest that even if inflammation was evoked in response to cell injection, the effects did not contribute to differences observed with the different MPTP administration regimens.
Screening of Candidate Genes to Support Grafted DA Neurons
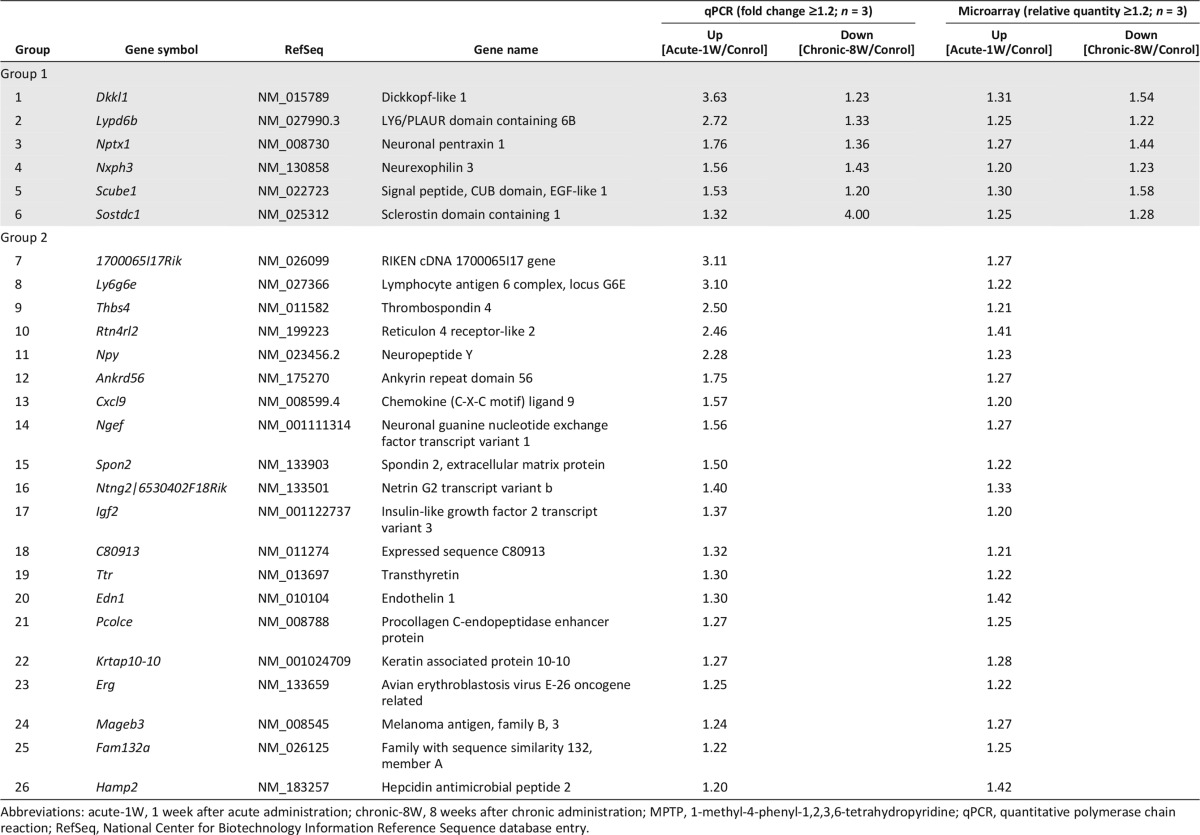
The results above suggested that the host brains of acute-1W mice contain more supportive factors for grafted DA neurons than those of chronic-8W mice. To identify these factors, we compared gene expressions between the two mice (supplemental online Fig. 5). We divided genes into two groups. The first included genes that increased in the acute-1W condition but decreased in the chronic-8W condition compared with control brains (group 1). The second included those that increased in the acute-1W condition but were unchanged in the chronic-8W conditions compared with control brains (group 2). Microarray analysis followed by secretomeP analysis for selecting secreted proteins and a BLAST and UniGene search for conserved genes in humans resulted in the identification of 30 and 270 genes in the two groups, respectively. Finally, we confirmed the gene expression by qPCR and decided on 6 and 20 candidate genes in groups 1 and 2, respectively (Table 1).
Table 1.
Gene screening of secreted factors from comparisons of MPTP mouse models
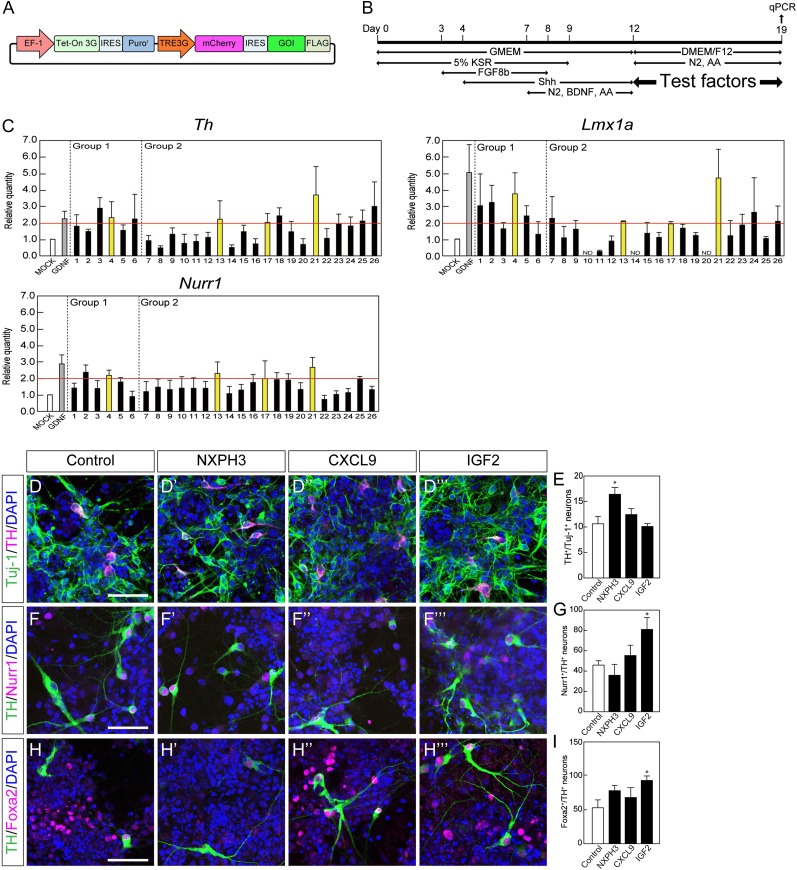
To investigate the function of the products of each candidate, we established expression vectors for each gene and introduced them into HEK293T cells (Fig. 4A). The expression of the gene products was confirmed by Western blot analysis using an antibody for FLAG, and product secretion was confirmed by ELISA (data not shown). GDNF was used as a positive control in this screening assay because it was reported to facilitate the maturation of DA neurons [26]. Next, we cocultured the iPSC-derived aggregates (day 12) with HEK293T cells that expressed candidate gene products for 7 days and evaluated the gene expression levels of Th, Lmx1a, and Nurr1 in differentiated cells on day 19 by qPCR (Fig. 4B). As final candidates, we chose those genes that showed a fold change greater than 2 compared with control cells using a mock vector. The final results led us to consider NXPH3, CXCL9, IGF2, and PCOLCE as well as GDNF.
Figure 4.
Functional screening of candidate genes by in vitro assay. (A): Schematic drawing of the construct of the Tet-on-inducible mCherry and 3xFLAG-tagged gene-of-interest (GOI) expression vector. (B): Experimental procedure for the functional screening. Day 12 induced pluripotent stem cell-derived aggregates were cocultured with GOI-secreting HEK293T cells for 7 days (described in Materials and Methods). (C): The effect of each factor was evaluated by a qPCR-based assay. Each gene number corresponds to Table 1. Each value is given as the mean ± SEM (n = 3–7). (D, F, H): Double-labeled immunofluorescence images for Tuj-1 (green) and TH (magenta) (D), TH (green) and Nurr1 (magenta) (F), TH (green) and Foxa2 (magenta) (H) with or without each soluble factor. Scale bar = 50 μm. (E, G, I): The ratio of TH+ neurons per Tuj-1+ neuron (E), Nurr1+ neurons per TH+ neuron (G) and Foxa2+ neurons per TH+ neurons (I). Each value is given as the mean ± SEM (n = 3). ∗, p < .05 versus control group. (J, L): Double-labeled immunofluorescence images of the striatum of MPTP-treated mice for NXPH3 (green) and NeuN (magenta) (J) and for IGF2 (green) and GFAP (magenta) (L). Scale bar = 50 μm (K, M): Semiquantitative analysis of the protein level of NXPH3 (K) and IGF2 (M) in the striatum. Each value is given as the mean ± SEM (n = 3). ∗, p < .05 versus control group; ††, p < .01 versus acute-1W group. Abbreviations: acute-1W, 1 week after acute administration; AA, ascorbic acid; BDNF, brain-derived neurotrophic factor; chronic-8W, 8 weeks after chronic administration; DAPI, 4′,6-diamidino-2-phenylindole; DMEM/F12; Dulbecco’s modified Eagle’s medium nutrient mixture F12; GDNF, glial cell-derived neurotrophic factor; GMEM, Glasgow minimum essential medium; KSR, knockout serum replacement; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; N2, N2-supplement; qPCR, quantitative polymerase chain reaction.
Because these four proteins are commercially available, we added each at different concentrations to the culture medium of iPSC-derived aggregates during days 12–19 and evaluated the expression level of Th, Lmx1a, and Nurr1 by qPCR (supplemental online Fig. 6). We chose those proteins that showed a fold change greater than 1.5 compared with control vehicle and finally decided to use 100 ng/ml of NXPH3, 100 ng/ml of CXCL9, and 100 ng/ml of IGF2 in the following experiments.
We examined the effect of these three proteins on iPSC-derived DA neurons by immunofluorescence. Aggregates were dissociated and replated onto OFL-coated dishes on day 12 and cultured for another 7 days with each of the three candidates. The ratio of TH+ neurons per Tuj-1+ (a marker for postmitotic neurons) neurons was significantly increased when the cells were treated with 100 ng/ml NXPH3 (Fig. 4D, 4E). When we examined the proportion of midbrain DA neurons, we found that treatment with 100 ng/ml IGF2 increased the ratio of Nurr1+/TH+ neurons and Foxa2+/TH+ neurons (Fig. 4F–4I). Based on these results, we chose NXPH3 and IGF2 as candidates that may support the survival of iPSC-derived DA neurons.
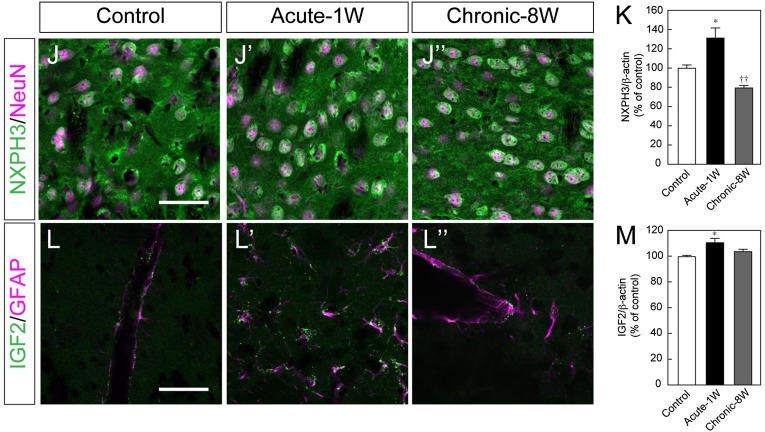
Immunofluorescence of the striatum revealed that NXPH3 was expressed in NeuN+ (another marker for postmitotic neurons) cells, whereas IGF2 was expressed in GFAP+ (a marker for astrocytes) cells (Fig. 4J, 4L). Semiquantitative analysis using Western blot revealed that the protein level in the striatum of NXPH3 was increased in acute-1W mice but decreased in chronic-8W mice and that IGF2 was increased in acute-1W mice (Fig. 5K, 5M).
Figure 5.
Effect of soluble factors on induced pluripotent stem cell-derived (iPSC-derived) dopaminergic neurons in vivo. (A): Western blot analysis of receptors for GFR-α, α-NRXN and IGF2R. (B–E): Histological analysis of iPSC-derived grafts at 8 weeks with the indicated factor. Immunofluorescence images for venus (green) and TH (magenta). Scale bar = 50 μm. (F–H): Quantitative analysis of the graft volume (F), the total number of TH-positive (TH+) neurons (G), and the number of TH+ neurons per graft volume (H). Each value is given as the mean ± SEM (n = 5–6). ∗, p < .05 versus PBS(−) control group. Abbreviation: PBS(−), phosphate-buffered saline.
NXPH3 Increased the Ratio of DA Neurons in the Graft
In order to investigate the effects of NXPH3 and IGF2 in vivo, we grafted iPSC-derived neural cells on day 12 into the striatum of healthy mice, which have a relatively more stable brain condition than that of several MPTP-treated mice, and simultaneously injected the two proteins into adjacent places. In this in vivo screening assay, we used PBS and GDNF as negative and positive controls, respectively. Before transplantation, we confirmed that the receptors α-neurexin (NRXN) for NXPH3 [27], IGF2 receptor (IGF2R) for IGF2 [28], and GDNF family receptor α (GFR-α) for GDNF [29] were expressed by the donor cells (Fig. 5A). At 8 weeks after transplantation, we evaluated the survival of grafted iPSC-derived DA neurons in the striatum. Although there were no significant differences in graft volume or number of TH+ neurons in the grafts (Fig. 5B, 5F, 5G), the number of TH+ neurons per graft volume was increased in mice with GDNF and NXPH3 (Fig. 5B–5H). Taken together, these results indicated that NXPH3 promoted the survival of iPSC-derived DA neurons in the striatum.
Expression of NXPH3 and IGF2 in PD Patients
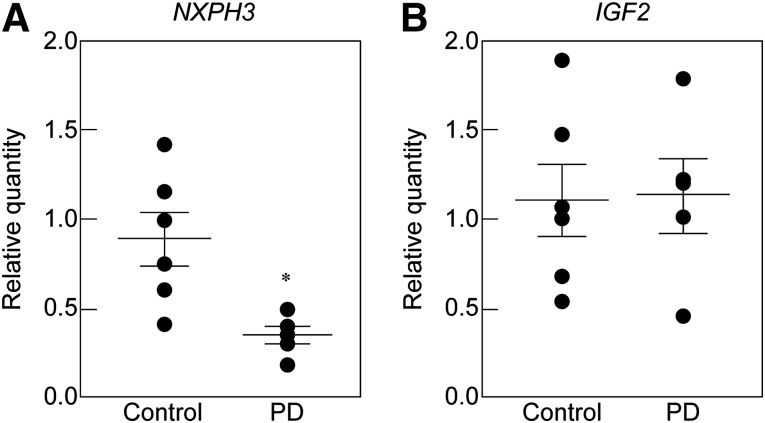
To examine whether the expression of NXPH3 or IGF2 is related to the pathology of PD patients, we analyzed the expression levels of their mRNAs in the postmortem putamen of healthy controls and PD patients by qPCR. Age and sex were adjusted between the two groups (mean age: control, 72.8 ± 1.1 years [n = 6]; PD, 75.0 ± 2.6 years [n = 5]) (supplemental online Table 4). The expression level of NXPH3 was significantly lower in PD patients compared with healthy controls (Fig. 6A). In contrast, the expression of IGF2 was unchanged between the two groups (Fig. 6B).
Figure 6.
Gene expression levels of NXPH3 (A) and IGF2 (B) in the putamen of postmortem healthy controls and PD patients. Each value is given as the mean ± SEM (n = 5–6). ∗, p < .05 versus control group. Abbreviation: PD, Parkinson’s disease.
Discussion
We constructed an experimental approach to identify effective soluble factors for cell grafting by comparing gene expression profiles between supportive and nonsupportive host brain environments (1 week after acute administration of MPTP and 8 weeks after chronic administration). We revealed that NXPH3 increased the ratio of iPSC-derived DA neurons for cell grafting. In addition, NXPH3 treatment increased the ratio of TH+ neurons per Tuj1+ neuron in in vitro neural differentiation. NXPH3 also increased the ratio of TH+ neurons per graft volume 8 weeks after the transplantation of iPSC-derived neural cells into mouse brain. Furthermore, the expression of NXPH3 was lower in the postmortem putamen of PD patients than in healthy counterparts. These results suggest that NXPH3 acts as a supportive factor for the survival of grafted DA neurons in the striatum and can be used as a marker for alternations of the putamen in PD patients.
We observed that the ratio of TH+ neurons per graft volume was increased at 8 weeks when iPSC-derived neural cells were grafted into the acute-1W condition compared with the chronic-8W one (Fig. 3). However, we did not observe a significant difference in the ratio of either mature DA neurons (TH+) or proliferating DA progenitor cells (Ki67+ Foxa2+) cells between these conditions at 1 week after transplantation (supplemental online Fig. 3). In addition, Ki67+ proliferating cells were not observed in any grafts at 4 weeks after transplantation (data not shown). These results suggested that the increase of the TH+ cells was due to the sustained survival of mature DA neurons rather than an increased expansion of DA progenitor cells.
NXPH3 is a secreted peptide that binds to the second LNS domain of synaptic NRXN, a neuronal cell surface receptor [30]. We found that NXPH3 is expressed by striatal neurons, and its expression level altered in response to the host brain environment (Fig. 4J). In addition, donor cells expressed NRXN, a receptor for the NXPH family (Fig. 5A). A previous report showed that NXPH3 is strongly expressed in cortical and vestibulocerebellar neurons in adult brains and Cajal-Retzius cells during development [31]. In addition, Nxph3 knockout mice exhibit a loss of motor coordination and an increase in acoustic startle response, suggesting that NXPH3 is involved in the synaptic transmission of motor and acoustic function [31]. NXPH-NRXN interaction is able to stabilize the synaptic function by contributing to synaptic plasticity [32]. In the neuronal developmental process, stimuli via synaptic formation play a key role in the survival and maturation of newly generated neurons [33]. These reports led us to speculate that upregulation of NXPH3 promoted neuronal plasticity after MPTP-induced brain injury and that the resulting synaptic stability supported the survival of grafted DA neurons. Further work is required to confirm this hypothesis.
Another candidate supportive factor for grafted DA neurons is IGF2, which increased the ratio of TH+ neurons that simultaneously expressed the midbrain markers Nurr1 and Foxa2 during neural differentiation in vitro. IGF2 is a secreted peptide that belongs to the IGF/IGFBP (IGF/IGF binding protein) family, which plays important roles in somatic mitosis, development, tissue repair, and regeneration [34]. IGF2 was also reported to promote DA differentiation from human embryonic stem cells [35]; however, despite our in vitro results and the findings noted, the local and single administration of IGF2 had no effect on the survival of iPSC-derived DA neurons in vivo.
Previous studies on postmortem brains reported that the expression levels of several genes in the SNpc and putamen differ between healthy controls and PD patients [36, 37]. Furthermore, oxidative/nitrosative stress and mitochondrial dysfunction based on changes in 3-nitrtyrosine and glutathione can characterize alternations in the PD brain and thus can act as possible markers for PD [38]. We found that expression of the NXPH3 gene in the postmortem putamen was significantly lower in PD patients. We speculate that the long-term decline of neuronal connections in the putamen of PD patients may lead to the reduced expression of NXPH3. Because the putamen is a target area for cell transplantation in clinical trials [39–41], an expression profile of the putamen may offer important information for predicting whether or not a target area is favorable. In a double-blinded randomized clinical study, transplantation of embryonic DA neurons showed some benefits in PD patients aged 60 years old and younger but not in older patients [42], suggesting that the host brain environment is important for the success of cell replacement therapy for PD. Taking all these findings together, the expression level of NXPH3 in the putamen may be a suitable marker for appropriate patient recruitment in PD treatment.
Conclusion
We established an experimental strategy for identifying effective factors for cell transplantation in PD based on a comparative study of several brain environments. Accordingly, we succeeded in identifying NXPH3 as a new supportive factor for cell transplantation. We also found that the expression level of NXPH3 in the postmortem putamen of PD patients was decreased compared with control, suggesting that NXPH3 may be a useful biomarker for PD patients. These findings are expected to contribute to future human trials using iPSC-derived DA neurons and promote both in vivo and in vitro applications of stem cell technology.
Supplementary Material
Acknowledgments
We thank Dr. Keisuke Okita and Prof. Shinya Yamanaka for providing the induced pluripotent stem cells, Dr. Kazutoshi Takahashi and Dr. Mari Onuki for teaching of the lentiviral transduction, Dr. Takuya Yamamoto and Dr. Masamitsu Sone for helping with the microarray analysis, and Dr. Yuichi Ono (KAN Research Institute) for valuable advice on a differentiation protocol. We also thank Kei Kubota, Tomoka Ashida, and Yusuke Nakajima for technical assistance; Dr. Peter Karagiannis for critical reading of the manuscript; and other laboratory members for helpful discussions. This study was supported by the Highway Project for Realization of Regenerative Medicine from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Network Program for Realization of Regenerative Medicine from the Japan Science and Technology Agency (JST), and an Intramural Research Grant for Neurological and Psychiatric Disorders from the National Center of Neurology and Psychiatry (NCNP).
Author Contributions
K.N.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.M.: provision of study material or patients, data analysis and interpretation; J.T.: supervision, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interests.
References
- 1.Kikuchi T, Morizane A, Doi D, et al. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinsons Dis. 2011;1:395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- 2.Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkeby A, Grealish S, Wolf DA, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Reports. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Doi D, Samata B, Katsukawa M, et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishino H, Hida H, Takei N, et al. Mesencephalic neural stem (progenitor) cells develop to dopaminergic neurons more strongly in dopamine-depleted striatum than in intact striatum. Exp Neurol. 2000;164:209–214. doi: 10.1006/exnr.2000.7426. [DOI] [PubMed] [Google Scholar]
- 6.Mine Y, Hayashi T, Yamada M, et al. Environmental cue-dependent dopaminergic neuronal differentiation and functional effect of grafted neuroepithelial stem cells in parkinsonian brain. Neurosurgery. 2009;65:741–753; discussion 753. doi: 10.1227/01.NEU.0000351281.45986.76. [DOI] [PubMed] [Google Scholar]
- 7.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 8.Höglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 9.Ohira K, Furuta T, Hioki H, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- 10.Tatarewicz SM, Wei X, Gupta S, et al. Development of a maturing T-cell-mediated immune response in patients with idiopathic Parkinson’s disease receiving r-metHuGDNF via continuous intraputaminal infusion. J Clin Immunol. 2007;27:620–627. doi: 10.1007/s10875-007-9117-8. [DOI] [PubMed] [Google Scholar]
- 11.Imamura K, Hishikawa N, Sawada M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 13.Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirza B, Krook H, Andersson P, et al. Intracerebral cytokine profiles in adult rats grafted with neural tissue of different immunological disparity. Brain Res Bull. 2004;63:105–118. doi: 10.1016/j.brainresbull.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Gomi M, Aoki T, Takagi Y, et al. Single and local blockade of interleukin-6 signaling promotes neuronal differentiation from transplanted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2011;89:1388–1399. doi: 10.1002/jnr.22667. [DOI] [PubMed] [Google Scholar]
- 16.Przedborski S, Jackson-Lewis V, Naini AB, et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 18.Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 19.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 21.Pierri M, Vaudano E, Sager T, et al. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–524. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Kurosaki R, Muramatsu Y, Kato H, et al. Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson’s disease. Pharmacol Biochem Behav. 2004;78:143–153. doi: 10.1016/j.pbb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Gibrat C, Saint-Pierre M, Bousquet M, et al. Differences between subacute and chronic MPTP mice models: Investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J Neurochem. 2009;109:1469–1482. doi: 10.1111/j.1471-4159.2009.06072.x. [DOI] [PubMed] [Google Scholar]
- 24.Luchtman DW, Shao D, Song C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson’s disease. Physiol Behav. 2009;98:130–138. doi: 10.1016/j.physbeh.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Thompson L, Barraud P, Andersson E, et al. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci. 2005;25:6467–6477. doi: 10.1523/JNEUROSCI.1676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblad C, Martinez-Serrano A, Björklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75:979–985. doi: 10.1016/0306-4522(96)00343-0. [DOI] [PubMed] [Google Scholar]
- 27.Missler M, Hammer RE, Südhof TC. Neurexophilin binding to alpha-neurexins. A single LNS domain functions as an independently folding ligand-binding unit. J Biol Chem. 1998;273:34716–34723. doi: 10.1074/jbc.273.52.34716. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 29.Paratcha G, Ledda F. GDNF and GFRalpha: A versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beglopoulos V, Montag-Sallaz M, Rohlmann A, et al. Neurexophilin 3 is highly localized in cortical and cerebellar regions and is functionally important for sensorimotor gating and motor coordination. Mol Cell Biol. 2005;25:7278–7288. doi: 10.1128/MCB.25.16.7278-7288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Born G, Breuer D, Wang S, et al. Modulation of synaptic function through the α-neurexin-specific ligand neurexophilin-1. Proc Natl Acad Sci USA. 2014;111:E1274–E1283. doi: 10.1073/pnas.1312112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA. 2005;102:9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkes C, Kar S. The insulin-like growth factor-II/mannose-6-phosphate receptor: Structure, distribution and function in the central nervous system. Brain Res Brain Res Rev. 2004;44:117–140. doi: 10.1016/j.brainresrev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Vazin T, Becker KG, Chen J, et al. A novel combination of factors, termed SPIE, which promotes dopaminergic neuron differentiation from human embryonic stem cells. PLoS One. 2009;4:e6606. doi: 10.1371/journal.pone.0006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hennecke G, Scherzer CR. RNA biomarkers of Parkinson’s disease: Developing tools for novel therapies. Biomarkers Med. 2008;2:41–53. doi: 10.2217/17520363.2.1.41. [DOI] [PubMed] [Google Scholar]
- 37.Bossers K, Meerhoff G, Balesar R, et al. Analysis of gene expression in Parkinson’s disease: Possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathol. 2009;19:91–107. doi: 10.1111/j.1750-3639.2008.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mythri RB, Venkateshappa C, Harish G, et al. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res. 2011;36:1452–1463. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- 39.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 40.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brundin P, Li JY, Holton JL, et al. Research in motion: The enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 42.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.