Abstract
Mesenchymal stem cells and multipotent adult progenitor cells (MAPCs) have been proposed as novel therapeutics for solid organ transplant recipients with the aim of reducing exposure to pharmacological immunosuppression and its side effects. In the present study, we describe the clinical course of the first patient of the phase I, dose-escalation safety and feasibility study, MiSOT-I (Mesenchymal Stem Cells in Solid Organ Transplantation Phase I). After receiving a living-related liver graft, the patient was given one intraportal injection and one intravenous infusion of third-party MAPC in a low-dose pharmacological immunosuppressive background. Cell administration was found to be technically feasible; importantly, we found no evidence of acute toxicity associated with MAPC infusions.
Significance
Liver transplantation is the only definitive treatment for liver failure. However, in order to prevent rejection of the graft, patients must receive lifelong pharmacological immunosuppression, which itself causes clinically significant side effects. This study provides preclinical evidence that mesenchymal stem cells (MSCs) and multipotent adult progenitor cells (MAPCs) can prolong allogeneic solid organ transplant survival in animals by switching the host response toward operational tolerance. To examine the safety and feasibility of MAPC therapy in patients receiving a living-related or dead-before-donation unrelated donor liver graft, the MiSOT-I (Mesenchymal Stem Cells in Solid Organ Transplantation Phase I) trial was designed. The first study patient, a 27-year-old male with liver cirrhosis of unknown etiology, received a living-related adult right liver graft from his brother. MAPC administration in both the operating room (day 0) and intensive care unit (day 2) was feasible, and no evidence was seen of acute complications associated with the cell infusion. The absence of any acute clinical complications of cell infusion is reassuring for the future administration of MAPCs.
Keywords: Multipotent stem cells, Mesenchymal stem cells, Liver transplantation, Immunomodulation, Tolerance, Cell therapy
Introduction
Liver transplantation is the only definitive treatment for liver (function) failure. Grafting of an allogeneic organ usually requires significant immunosuppression of the host. Mesenchymal stem cells (MSCs) [1] and multipotent adult progenitor cells (MAPCs) [2] have been proposed as immune-active treatments for multiple indications. The aim of cell-based immunosuppression in transplantation is to reduce the need for long-term pharmacological immunosuppression and the associated deleterious side effects. We [3], and others [4], have provided preclinical evidence that MSCs and MAPCs can prolong allogeneic solid organ transplant survival in animals by switching the host response toward operational tolerance [5].
In the present study, we present a clinical case report from the MiSOT-I (Mesenchymal Stem Cells in Solid Organ Transplantation Phase I) trial [6, 7]. The patient, a 27-year-old male with liver cirrhosis of unknown etiology, received a living-related adult right liver graft from his brother. The recipient’s medical history included an episode of atrial fibrillation. In addition, the recipient was of the rare Lu(b)-negative blood type, with high serum anti-Lu(b) antibody titers [donor blood type 0, rhesus-negative, Lu(b)+].
Materials and Methods
Trial Design
MiSOT-I [7] is a phase I, dose-escalation, safety, and feasibility study of MAPC therapy after liver transplantation in patients receiving living-related or dead-before-donation unrelated donor grafts (clinicaltrials.gov identifier, NCT01841632; trial details available at: http://www.misot.eu). The primary endpoint is acute toxicity gauged by a previously established toxicity score [6]. The secondary endpoints are the time to biopsy-proven acute rejection and evidence that MAPCs do not cause or promote malignancy.
MAPC Storage
MAPC cryobags (1.5 × 108 cells in 18 ml of PlasmaLyte-A) (Baxter Healthcare Corp., Deerfield, IL, http://www.baxter.com) were shipped to and stored at our clinical site according to validated protocols. Cryopreserved MAPCs remain viable for at least 2 years, allowing MAPC therapy to be integrated into existing dispensing chains with cryostorage facilities [8].
MAPC infusion
MAPCs were injected into the portal vein under direct visualization in the operating room (day 0) and by central venous infusion (day 2).
Immunohistochemistry
Liver biopsy sections were fixed (ice-cold acetone, 8 minutes) and blocked (30 minutes) at room temperature (RT) using 10% heat inactivated species-specific serum and 1:10 anti-human FcR-blocking reagent (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). After washing, the samples were stained for 1 hour at RT in phosphate-buffered saline containing 0.1% bovine serum albumin using 1:100 monoclonal anti-human leukocyte antigen (HLA)-A*03 antibody (Abcam, Cambridge, U.K., http://www.abcam.com). The samples were washed and incubated with goat 1:100 anti-mouse IgG (Abcam) at RT. After washing, the sections were incubated with 1:500 Cy3-labeled donkey anti-goat IgG (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). Images were captured using a Zeiss Axio Observer microscope and Zeiss Axiovision 4.04 (Carl Zeiss, Jena, Germany, http://www.zeiss.com).
Recipient Leukocyte Characterization
Peripheral blood leukocyte frequencies were monitored at frequent intervals after transplantation and MAPC infusion to establish a high-granularity description of the immunological changes that occurred. The standardized methods used for this immune monitoring study have been previously described [9].
Results
MAPC Characterization
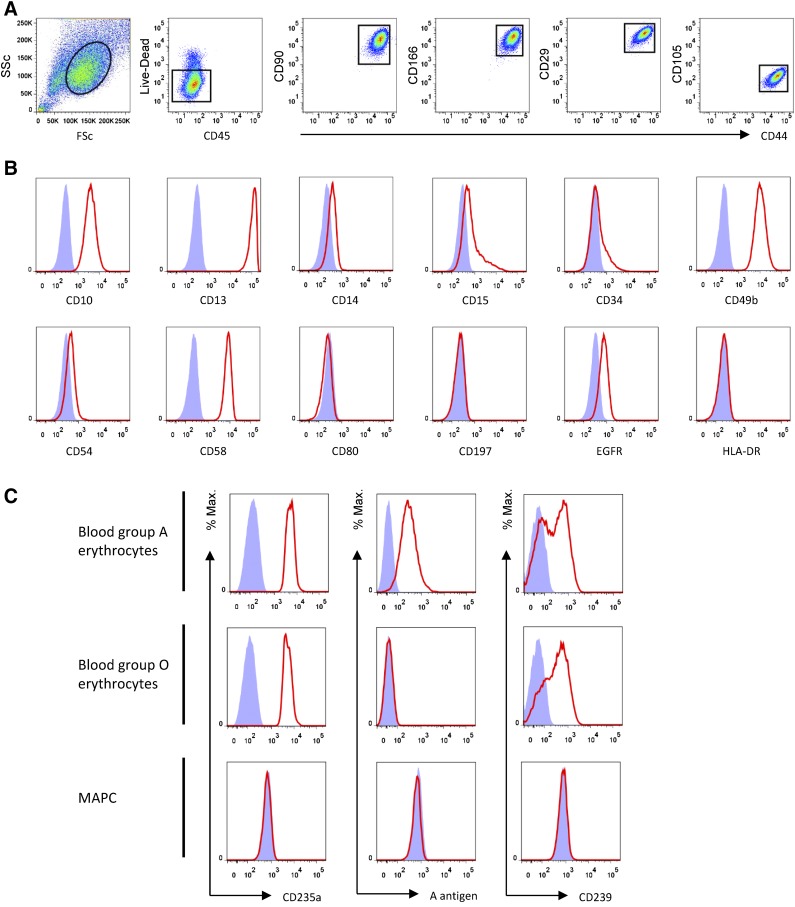
MAPCs are adherent viable adult stem cells ∼15 µm in spherical diameter [10]. MiSOT-I uses a commercial MAPC product, MultiStem (Athersys Inc., Cleveland, OH, http://www.athersys.com). MAPC surface marker analysis showed phenotypic features that are, in part, shared with MSCs (Fig. 1A, 1B). Because of the recipient’s positive anti-Lu(b) status, the MAPCs were typed for basel cell adhesion molecule (BCAM), Lu(b), and glycophorin A expression using flow cytometry. MAPCs expressed neither the ABO blood group antigens nor the Lu(b) antigen (Fig. 1C).
Figure 1.
Characteristics of multipotent adult progenitor cells (MAPCs). (A): Gating strategy for live CD45− CD44+ CD90+ CD166+ CD29+ CD105+ MAPC. (B): Expression of cell surface markers typical of mesenchymal stem cells by MAPCs. (C): Absence of ABO or Lutheran blood group (not shown) antigens. The absence of CD235a (glycophorin A), blood group A antigen, and CD239 (basal cell adhesion molecule) expression means that MAPCs should not be susceptible to complement-mediated lysis on administration to patients with antibodies against these antigens. Abbreviations: EGFR, epidermal growth factor receptor; HLA, human leukocyte antigen; MAPC, multipotent adult progenitor cell.
Case Report
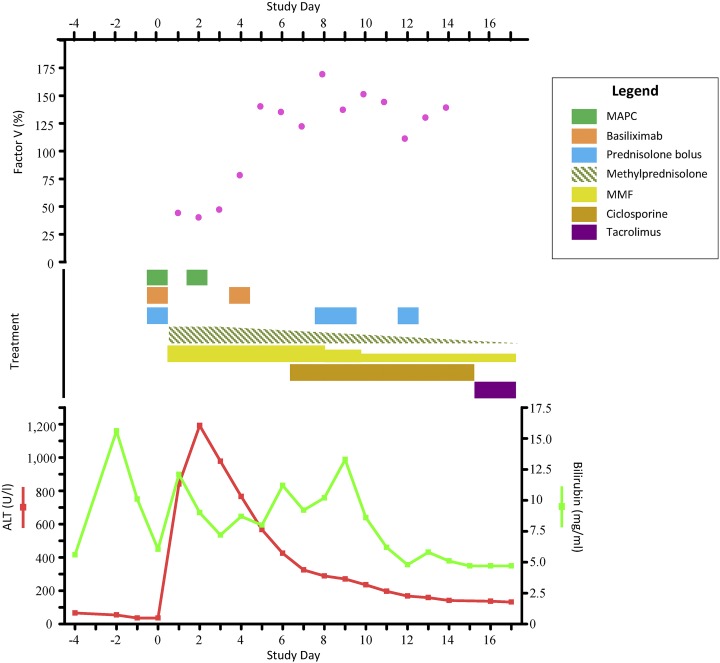
The local ethics committee approved the MiSOT-I protocol, which conforms to the Declaration of Helsinki. The patient provided written informed consent before taking part in the study. Living-related allograft liver transplantation (donor right lobe) was completed according to center practice. In preparation for the intravenous infusion, a single MAPC cryobag containing 1.5 × 108 MAPCs was thawed (37°C) and combined with 18 ml of PlasmaLyte-A infusion solution. After allograft reperfusion, the cell suspension was injected under direct vision into the portal vein approximately 15 mm proximal to the anastomosis using a syringe and needle. Cell infusion (flow rate, 5 ml/min) was without acute cardiocirculatory, renal, pulmonary, or other systemic complications. Doppler ultrasonography of the portal vein before and after MAPC injection showed appropriate postoperative flow patterns and no evidence of thrombosis or stenosis. In addition to the cellular therapy, immunosuppressive treatment followed a “bottom-up” regimen: 2 g of mycophenolate mofetil (MMF) per day; basiliximab (interleukin-2 receptor-blocking antibody) at days 0 and 4; and methylprednisolone, beginning with 1 mg/kg body weight per day, tapered off from day 3. Biliary reconstruction was performed the next day. On day 2, the patient experienced atrial fibrillation that responded to electrical cardioversion. This was reported as a serious adverse event (SAE) unrelated to MAPC use. The alanine aminotransferase (ALT) levels peaked on day 2 (1,191.5 U/l), subsequently normalizing by day 12 (168.0 U/l; Fig. 2).
Figure 2.
Overview of liver function test results and immunosuppressive treatment. Living-related liver transplantation was conducted on day 0. The patient received standard “bottom-up” pharmacological immunosuppression: intraoperative methylprednisolone (500 mg) with subsequent postoperative tapering (starting with 1 mg/kg/day), MMF (2 g/day), and basiliximab (20 mg/kg, days 0 and 4). MAPCs were administered on day 0 (day of transplantation) intraportally and on postoperative day 2. ALT levels peaked on day 2 (1,191.5 U/l); the levels rapidly decreased and had stabilized from day 12 (168.0 U/l). On day 4, the patient became mildly thrombocytopenic and leukopenic; therefore, from day 8, MMF was reduced to 1.5 g/day and subsequently reduced to 1 g/day from day 10 onward. From day 5, the bilirubin and factor V levels increased; a suspected acute rejection was diagnosed on day 6. Methylprednisolone (500 mg) was administered on days 8, 9, and 12. Cyclosporine (300 mg/day) was started on day 7 with initial trough levels of 43 ng/ml (measured directly before the morning dose). Because of the subclinical cyclosporine trough levels, the patient was switched to tacrolimus on day 15; a desired trough level of ∼10 ng/ml was achieved. The liver function test results normalized over the next days. Bilirubin returned to pretransplant levels from day 11, and factor V levels had stabilized from day 9 and showed no additional increase. Abbreviations: ALT, alanine aminotransferase; MAPC, multipotent adult progenitor cell; MMF, mycophenolate mofetil.
A second dose of 1.5 × 108 MAPCs was administered on day 2 by central venous infusion without acute clinical complications. The patient became mildly thrombocytopenic and leukopenic on day 4; therefore, MMF was reduced (1.5 g/day from day 8, 1 g/day from day 10). On day 5, biliary leakage occurred and required surgical intervention, which was reported as an SAE unrelated to MAPC use. From day 5, the bilirubin and factor V levels increased, although the transaminases remained stable (Fig. 2). Consequently, suspected acute rejection was clinically diagnosed on day 6 and reported as an SAE unrelated to MAPC use. Persistent coagulative impairment prevented a confirmatory liver biopsy; therefore, the secondary endpoint of the time to biopsy-proven acute rejection could not be assessed. The clinically diagnosed rejection episode was successfully managed with cyclosporine and methylprednisolone (500 mg on days 8, 9, and 12) treatment. Because of subtherapeutic cyclosporine trough levels (for reasons unknown), immunosuppressive calcineurin inhibitor treatment was switched to tacrolimus. The preoperative creatinine was normal (creatinine, 0.61 mg/dl; urea, 30 mg/dl); however, after the introduction of tacrolimus, the creatinine levels increased (peak creatinine, 1.59 mg/dl; urea, 71 mg/dl). The patient left the intensive care unit on day 8 and was discharged from the unit on day 43. At day 72, the patient’s first routine outpatient visit, he presented with normal liver function (ALT, 51 U/l; bilirubin, 0.9 mg/dl) and normal leukocyte counts (4.93 cells per nl) and reported he was in a positive frame of mind. For reasons unknown, his platelet counts remained relatively low (74 cells per nanoliter). The creatinine (1.40 mg/dl) and urea (48 mg/dl) levels remained moderately elevated. On study day 219, the patient experienced a Banff-rejection activity index (RAI) score 3 biopsy-confirmed acute rejection episode and was treated with a short course of methylprednisolone. This was reported as a SAE not related to the study product. At the latest follow-up visit, the patient was well and had returned to his previous daily routine.
Treatment-Emergent Adverse Events
MiSOT-I uses a toxicity scoring system to quantify treatment-emergent adverse events (TEAEs), which has been previously described [6]. In brief, the toxicity score is calculated from three independent parameters reflecting potentially critical aspects of cellular therapy: intraportal/infusional toxicity (by Doppler ultrasonography), pulmonary toxicity, and systemic toxicity. We detected no severe TEAEs (defined as a score of 3) and 2 intermediate TEAEs (day 3 with a score of 1 and day 29 with a score of 1). Both intermediate TEAEs resulted from a biphasic flow pattern in the hepatic veins that predated surgery and had varied throughout his hospitalization. During the 30-day follow-up observation period, the portal vein (site of injection) showed appropriate flow patterns without signs of stenosis or thrombosis. Additionally, no clinical evidence was found of a lung embolism after intravenous injection.
MAPC Intragraft Biodistribution
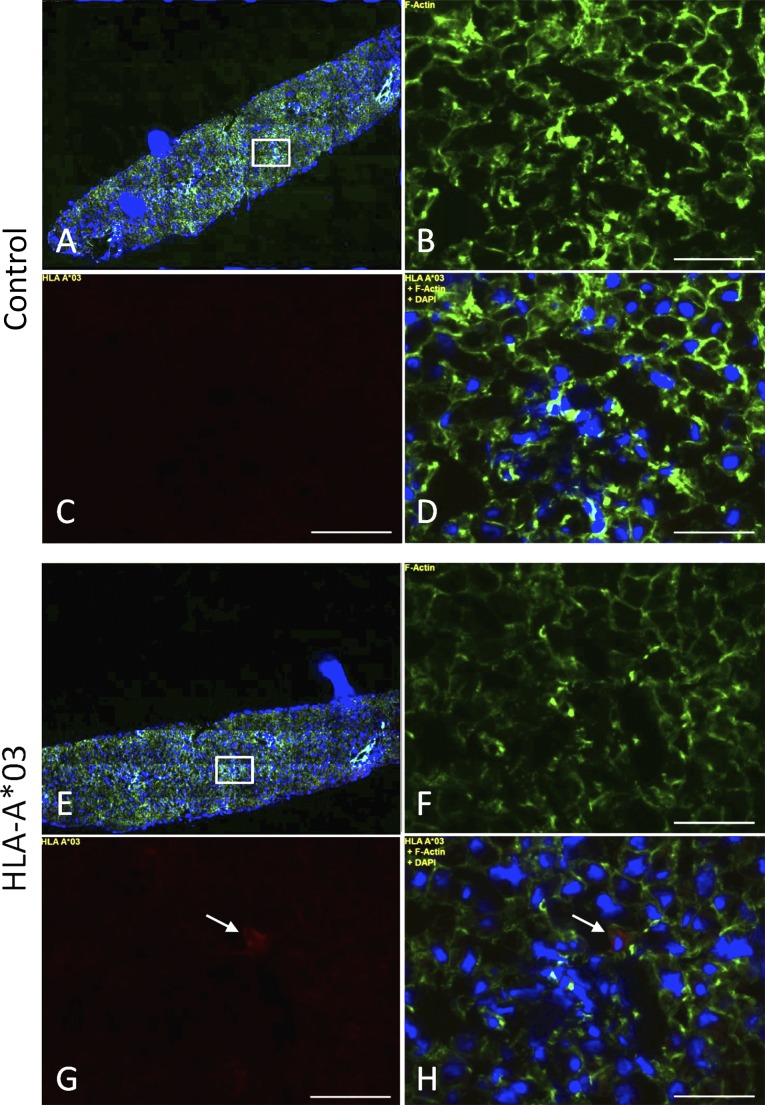
Liver biopsy samples were obtained before reperfusion and shortly after cell infusion to describe the parenchymal biodistribution of the infused cells. MAPCs in the recipient liver tissue were visualized by immunohistochemical staining for HLA-A*03, an HLA class I allele expressed by the infused MAPC product but not by donor and recipient cells. The preinfusion tissue samples were negative for HLA-A*03-specific staining (data not shown). Single HLA-A*03-positive cells of a phenotype and size consistent with MAPCs were detected in the postinfusion samples (Fig. 3), making it likely that the infused cells were present in the liver parenchyma at that point.
Figure 3.
Biodistribution of multipotent adult progenitor cells in liver allograft biopsies. (A–D): Control using second and third antibodies only. (E–H): HLA-A*03. Fluorescent immunostaining of liver biopsy cryosections using anti-actin (green; B, F), anti-HLA-A*03 (red; C, G), and DAPI (blue). (A, E): Wide-field views of anti-actin, anti-HLA-A*03, and DAPI treated samples. (D, H): Superimposed images. Magnified images were obtained using a ×20 objective from the box area indicated (A, E). Scale bars = 50 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; HLA, human leukocyte antigen.
Recipient Leukocyte Profile
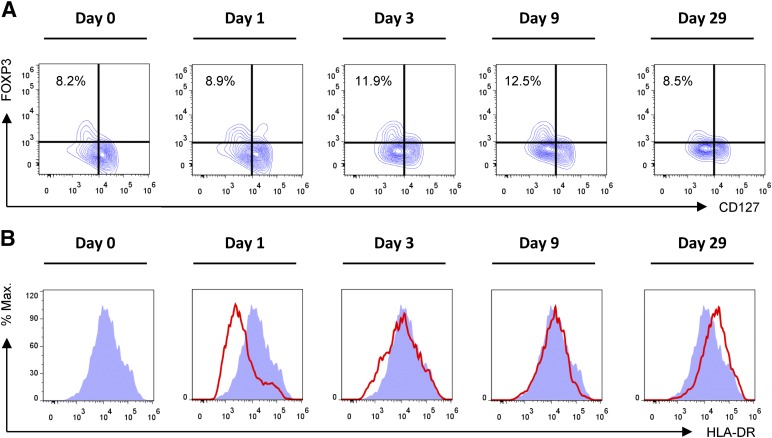
To characterize the recipient’s immunological response to transplantation and MAPC infusion, serial analyses of peripheral blood leukocyte subsets were performed by flow cytometry in the perioperative period (supplemental online Fig. 1). Overall, these data give the impression of early activation of the innate and adaptive immune system. Notably, the frequency of CD4+ FoxP3+ CD127low regulatory T cells was markedly increased on day 3 after the second MAPC infusion (Fig. 4A). In addition, downregulation of HLA-DR expression by CD14+ monocytes relative to pretransplant levels was observed on days 1 and 3, which has previously been associated with diminished immunological reactivity (Fig. 4B). However, these observations should not be interpreted as evidence of clinical efficacy of the cell product at this early stage of investigation.
Figure 4.
Monitoring of peripheral blood leukocyte subsets after MAPC administration and liver transplantation. (A): Frequency of CD4+ FoxP3+ CD127low regulatory T cells (Tregs) after transplantation. Compared with pretransplant frequencies, an increase in Treg frequency within the peripheral blood CD4+ T-cell pool was observed. (B): HLA-DR expression by CD45+ CD14+ peripheral blood monocytes. Compared with pretransplant levels, a relative decrease in HLA-DR expression was observed on days 1 and 3 after transplantation. Abbreviations: % Max., percentage of maximum; HLA, human leukocyte antigen.
Discussion
We have described the first living-related liver recipient to receive MAPCs after transplantation. MAPC administration was feasible in both the operating room and the intensive care unit setting. Immunohistochemistry revealed the presence of MAPCs within the graft after infusion. No severe TEAEs were observed, and no other evidence was seen of acute complications associated with MAPC infusion. To date, the patient has experienced four SAEs (atrial fibrillation, bile leakage, abnormal liver function, and acute rejection) that were judged unrelated to the MAPC infusion. The patient experienced a suspected acute rejection episode that began on day 5 after transplantation. This SAE was not attributed to MAPC treatment, despite its close temporal relationship to the cell infusion, because acute rejection can be expected in this time frame when low-dose induction therapy is used. A Banff-RAI grade 3 biopsy-confirmed acute rejection episode occurred 6 months later and coincided with a common cold infection. We will continue to perform detailed assessments of any future rejection events experienced by the study cohort. Elevated creatinine values correlated with the tacrolimus plasma levels, underlining the general need to reduce calcineurin inhibitor therapy because it is a prime objective of the present approach.
Conclusion
This first-in-human case study has demonstrated that intraportal and intravenous infusion of third-party MAPCs after liver transplantation is clinically feasible. The absence of any acute clinical complications of the cell infusion is reassuring for future administration of MAPCs. Recruitment and follow-up of participants in the MiSOT-I trial continue, and completion of the study is currently projected for autumn 2016.
Supplementary Material
Acknowledgments
We thank I. Kučuk and T. Mark for technical support and S. Melter for collection of clinical data. Athersys Inc. provided financial support and MAPCs (MultiStem) for the present study.
Author Contributions
Y.S.: provision of study materials or patients, data analysis and interpretation, manuscript writing; M.L.: conception and design, provision of study materials or patients; C.L.J.: administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.A.H. and J.H.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; N.A. and R.O.: provision of study materials or patients; R.J.D. and G.V.B.: financial support, provision of study materials or patients; E.K.G.: manuscript writing; H.J.S.: final approval of manuscript; M.H.D.: conception and design, provision of study materials or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
Y.S., C.L.J., and J.H. have uncompensated research funding. R.J.D. has compensated employment in an executive vice president position at Athersys Inc., and has compensated stock options with Athersys Inc. M.H.D. has contracted research with Athersys Inc. G.V.B. has compensated employment and stock options with Athersys Inc. The other authors indicated no potential conflicts of interest.
References
- 1.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231–233; discussion 233–235. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- 3.Eggenhofer E, Popp FC, Mendicino M, et al. Heart grafts tolerized through third-party multipotent adult progenitor cells can be retransplanted to secondary hosts with no immunosuppression. Stem Cells Translational Medicine. 2013;2:595–606. doi: 10.5966/sctm.2012-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 5.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Dillmann J, Popp FC, Fillenberg B, et al. Treatment-emergent adverse events after infusion of adherent stem cells: The MiSOT-I score for solid organ transplantation. Trials. 2012;13:211. doi: 10.1186/1745-6215-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popp FC, Fillenberg B, Eggenhofer E, et al. Safety and feasibility of third-party multipotent adult progenitor cells for immunomodulation therapy after liver transplantation—A phase I study (MISOT-I) J Transl Med. 2011;9:124. doi: 10.1186/1479-5876-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacsovics-Bankowski M, Mauch K, Raber A, et al. Pre-clinical safety testing supporting clinical use of allogeneic multipotent adult progenitor cells. Cytotherapy. 2008;10:730–742. doi: 10.1080/14653240802320245. [DOI] [PubMed] [Google Scholar]
- 9.Streitz M, Miloud T, Kapinsky M, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: Panels and methods from the ONE study. Transp Res. 2013;2:17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boozer S, Lehman N, Lakshmipathy U, et al. Global characterization and genomic stability of human multistem, a multipotent adult progenitor cell. J Stem Cells. 2009;4:17–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.