Abstract
This study compared the timing of appearance of three components of age-related hearing loss that determine the pattern and severity of presbycusis: the functional and structural pathologies of sensory cells and neurons and changes in Gap Detection, the latter as an indicator of auditory temporal processing. Using UM-HET4 mice, genetically heterogeneous mice derived from four inbred strains, we studied the integrity of inner and outer hair cells by position along the cochlear spiral, inner hair cell-auditory nerve connections, spiral ganglion neurons, and determined auditory thresholds, as well as pre-pulse and gap inhibition of the acoustic startle reflex (ASR). Comparisons were made between mice of 5-7, 22-24 and 27-29 months of age. There was individual variability among mice in the onset and extent of age-related auditory pathology.
At 22-24 months of age a moderate to large loss of outer hair cells was restricted to the apical third of the cochlea and threshold shifts in auditory brain stem response were minimal. There was also a large and significant loss of inner hair cell – auditory nerve connections and a significant reduction in Gap Detection. The expression of Ntf3 in the cochlea was significantly reduced. At 27-29 months of age there was no further change in the mean number of synaptic connections per inner hair cell or in gap detection, but a moderate to large loss of outer hair cells was found across all cochlear turns as well as significantly increased ABR threshold shifts at 4, 12, 24 and 48 kHz.
A statistical analysis of correlations on an individual animal basis revealed that neither the hair cell loss nor the ABR threshold shifts correlated with loss of gap detection or with the loss of connections, consistent with independent pathological mechanisms.
Keywords: Aging, Age Related Hearing Loss, Cochlea, NT3, Gap Detection, Auditory
Hearing loss is the most common disability associated with aging, affecting close to a third of people over the age of 65 and half of those over the age of 75 (Gates and Mills, 2005). There is a component to age-related hearing loss (ARHL) or presbyacusis that involves pathologies of sensory cells and neurons. Another component of presbyacusis in both people and animal models is an auditory temporal processing disorder with a reduced ability to detect intervals between sounds, termed “gap detection” (Caspary et al., 2008; Recanzone et al., 2011, Walton 2010 for reviews).
Age-associated “threshold shifts” in which a higher sound intensity is necessary to detect an acoustic signal or to generate an auditory brain stem response (ABR) are generally associated with a loss of hair cells, and outer hair cells in particular. In people, the threshold shift is greatest at higher frequencies of sound with hair cell loss greatest in the base of the cochlea, with some additional low-frequency hearing loss and outer hair cell loss in the apical cochlea (Frisina, 2009; Nelson and Hinojosa, 2006; Gates and Mills, 2005 for reviews). Mid-frequencies of the-cochlea are less affected. A predominant effect on base and apex is also seen in animal models, but animals frequently show more ARHL at lower frequencies and more outer hair cell loss in the apical than basal cochlea (Ohlemiller, 2009; Frisina, 2009 for reviews). However, the extent of apical versus basal outer hair cell loss and lower versus higher frequency hearing loss varies among species and even among strains within species (Ohlemiller, 2009; Frisina, 2009 for reviews).
The underlying causes and mechanisms for the age-related reduction in gap detection have not been clearly identified. The causes could involve both peripheral changes, such as changes in inner hair cells-auditory nerve (IHC-AN) connections and/or aging-associated changes in elements of the central auditory and associated CNS pathways, such as neurotransmitters and receptors (Caspary et al., 2008 Frisina 2009 for reviews).
Another element of ARHL is a modest loss of auditory nerve function with or without overt neuronal pathology (Keithley et al., 1979, 1989; Dazert et al., 1996; see Bao and Ohlemiller, 2010 for review). At least some part of this loss of auditory nerve is secondary to loss of connections between IHC-AN (Kujawa and Liberman, 2006, 2009). The rate and extent of age-related spiral ganglion neuron (SGN) loss in mice is accelerated by exposure of young adults to a mild noise exposure that causes a temporary threshold shift (TTS) (Kujawa and Liberman 2006, 2009). Furthermore, a progressive age-related loss of IHC-AN connections can occur with aging in mice that have not been exposed to noise (Sergeyenko et al., 2013).
Although all these components are integral parts of ARHL, few studies have examined them simultaneously. In order to test possible pathological relationships, the present study examines the numbers of inner and outer hair cells by position along the cochlear spiral (cytocochleograms), IHC-AN connections, spiral ganglion neurons, ABR thresholds, as well as pre-pulse and gap inhibition of the acoustic startle reflex (ASR) in genetically heterogeneous mice at 5-7 months of age, 22-24 months of age and 27-29 months of age. Cochlear expression of three major neurotrophic factor genes, Ntf3, Bdnf and Gdnf, was examined to test for any association between changes in their expression and loss of IHC-AN connections for which they could serve as maintenance factors. The work was conducted using UM-HET4 mice (Schacht et al., 2012), bred through a 4-way cross of grand-parental strains, each of which lacks the sensitive allele at the Ahl locus that predisposes to an early onset ARHL (Johnson et al. 1997; Johnson et al. 2000; Noben-Trauth et al. 2003). UM-HET4 mice comprise a diverse but replicable population in which hearing loss occurs in the last half of the lifespan, resembling in this way the typical age course of hearing loss in humans (Schacht et al., 2012).
2. EXPERIMENTAL PROCEDURES
2.1. Mice
Generation of the UM-HET4 mice has been described previously (Schacht et al., 2012). UM-HET4 mice represent a genetically heterogeneous mouse population that offers two advantages over the inbred stocks more often used for studies of ARHL: genetic heterogeneity, and postponement of hearing loss to the second half of the lifespan. The UM-HET4 mice are generated by a four-way cross between MOLF/EiJ (Jackson Laboratory stock #000550) × 129S1/SvImJ F1 (Jackson Laboratory stock #002448) female mice and C3H/HeJ (Jackson Laboratory stock #000659) × FVB/NJ F1 (Jackson Laboratory stock #001800) male mice. All of the four grandparental strains lack the Ahl allele that typically leads to hearing loss appearing at 2-4 months of age (Johnson et al., 2006 for review), thus facilitating analyses of factors that lead to hearing loss in midlife and old age. Each mouse in the test population inherits 25% of its genome from each of the four distinct inbred grandparental stocks. Each mouse is thus genetically unique, but shares 50% of its alleles with every other mouse in the tested population. At 22-24 months of age the UM-HET4 mice show variability in the extent of their hair cell loss and hearing loss that can be correlated with polymorphisms in specific genetic loci (Schacht et al., 2012).
2.2. Study Design
Three groups of female UM-HET4 mice were assessed. One group was euthanized and evaluated at 5-7 months of age (15 mice), a second group at 22-24 months (26 mice) and a third group at 27-29 months (22 mice). All mice had pre-pulse and gap inhibition of the ASR tested during a four week period prior to euthanasia, and their (ABR) were tested during the week prior to their termination. Following euthanasia, all left cochleae were assessed for hair cell numbers and IHC – AN nerve connections. Right cochleae were used either for qRT-PCR assessments of three neurotrophic factors genes, Bdnf, Ntf3 and Gdnf (right cochleae from 2-3 mice were pooled) or embedded in plastic for quantitative assessment of spiral ganglion neurons. The number of specimens for the qRT-PCR and spiral ganglion neuron assessments in the 22 to 24-months old group was increased by adding the right cochleae of littermates from a previous study (Schacht et al., 2012) which had been similarly treated except for not receiving gap detection assessments.
2.3. Auditory Brain Stem Response
For assessment of ABR, animals were first anesthetized (ketamine 65 mg/kg, xylazine 3.5 mg/kg, and acepromazine 2mg/kg). Body temperature was maintained and ABRs were recorded in an electrically and acoustically shielded chamber (Acoustic Systems, Austin, TX USA). Sub-dermal needle electrodes were placed at vertex (active) and the test ear (reference) and contralateral ear (ground) pinnae. Tucker Davis Technologies (TDT) System III hardware and SigGen/BioSig software (TDT, Alachua, FL USA) were used to present the stimulus and record responses. Tones were delivered through an EC1 driver, with the speculum placed just inside the tragus. Stimulus presentation was 15 ms tone bursts, with 1 ms rise/fall times, presented 10 per second. Up to 1024 responses were averaged for each stimulus level. Responses were collected in 10 dB steps at higher stimulus levels, with additional 5 dB steps near threshold. Threshold was determined by tracking the peak that was repeatable near threshold, typically this was wave IV. Thresholds were interpolated between the lowest stimulus level where a response was observed, and 5 dB lower, where no response was observed.
2.4 Gap Detection
The ability of either a gap in a continuous background sound or a brief pre-pulse to reduce the ASR (Ison, 1982; Ison et al., 1991; Turner et al., 2006) was assessed using equipment and software manufactured by Kinder Scientific (http://www.kinderscientific.com). The mouse was placed inside a test chamber in an acoustically transparent cage set atop a pressure sensor that registers the amplitude and duration of the animal's startle response. The dimensions of the cage were designed to discourage excess movement, but not confine the animal to an abnormal posture. The ASR was assayed by playing a brief (20 ms) broad band noise (2-20 kHz) pulse at 120 dB SPL in order to elicit a “jump,” or startle reflex from the animal. This noise level and duration does not cause any hearing loss. For gap detection, a constant low-level (50 SPL) background noise was played, with a very short (50 ms) gap in the noise presented 100 ms before the startle-inducing sound. In animals with normal hearing, this gap changes their ASR: the amplitude of the response is decreased. For pre-pulse inhibition (PPI), the startle stimulus was presented in quiet and was preceded by a brief, low-level (50dB SPL) noise burst. The values for each condition, presented in pseudo random order, with 5-9 seconds intertrial intervals, are the average of seven trials.
2.5 Cochlear Histo- and Immunostaining
Mice were terminated by an intra-peritoneal (ip) injection of 0.1 ml of sodium pentobarbitrol (FatalPlus) followed by decapitation. The left cochleae were rapidly removed and openings made in the round and oval windows and through the otic capsule in apex. Fixative (4% paraformaldehyde in phosphate buffer) was slowly infused into the scala tympani over 5 minutes followed by immersion of the whole cochlea in fixative for 2 h. Cochleae then received a rinse in phosphate buffered saline (PBS) over 12-16 h. Cochleae were partially decalcified in 5% EDTA over 24 h at 4° C and the otic capsule was then removed. Cochleae were subjected to a blocking step with 3% normal donkey serum and then placed in mouse anti-CTBP2 antibody (BD Transduction Laboratories) diluted 1:200 in PBS plus 0.1% tritonX-100 for 16-20 h at 4°C. Cochleae were then rinsed in PBS three times for 10 minutes each. They next received co-labeling with both donkey anti-mouse immunoglobulin with an Alexafluor 488 fluorescent label (Molecular Probes / Invitrogen) diluted 1:500 in PBS – triton X and with phalloidin (Molecular Probes / Invitrogen) with an Alexafluor 568 fluorescent label, diluted 1:100, incubated for 2 h at room temperature, followed by three rinses in PBS, in the dark. Cochleae were microdissected into three segments, apex, base and hook, and each segment was mounted separately as a surface preparation on a glass slide with Fluoromount (EMS Inc.) and a coverslip placed above. Slides were stored at 4°C until examination.
2.6 Assessment of Inner Hair Cell – Auditory Nerve Connections
Four regions of interest along the cochlear spiral at 1, 2, 3 and 4 mm from the apex, respectively, were examined using a Zeiss laser scanning confocal microscope with a 63x objective. The length of cochlear spiral in each region of interest was approximately 0.2 mm, varying because of differences in the curvature of the cochlear spiral at different positions. A z-series with 1μm slices at 0.3 μm intervals was taken of each region of interest. The digital images were imported into an IMARIS workstation for 3D visualization and into a Metamorph Image Analysis workstation for quantitative analysis. A line was generated in the digital image to separate each inner hair cell, based on the location of nuclei and stereocilia. The number of CTBP2 immunolabeled puncta meeting size and shape criteria and with intensity of labeling at least five times over background was determined for each inner hair cell base in the region of interest and then the mean number of puncta per inner hair cell base in the region of interest was determined.
2.7 Assessment of Hair Cell Loss
Surface preparations of the cochlear spiral were stained with rhodamine labeled phalloidin to label hair cells and the scars replacing missing hair cells (Figure 1). Hair cells were counted under epifluorescence optics on a Leica fluorescent microscope using a 50x objective and a 0.19 mm reticule in the microscope eyepiece. The number of outer hair cells in each row and the number of inner hair cells that were present or absent for each 0.19 mm reticule length was entered into a cytocochleogram program (developed in house e.g. Lemke et al., 2009, Piu et al., 2011) starting at the apex and moving basally until the entire length of the cochlear spiral (5.9 mm average) had been assessed. The program compared hair cell numbers to a normal data base and provided a graph of hair cell loss by position along the cochlear spiral for each cochlea as well as means for the cochleae in each group. Rows of outer hair cells could also be averaged into one combined measure of all outer hair cells at that position, both for individual animals and for means across groups as in Figure 2.
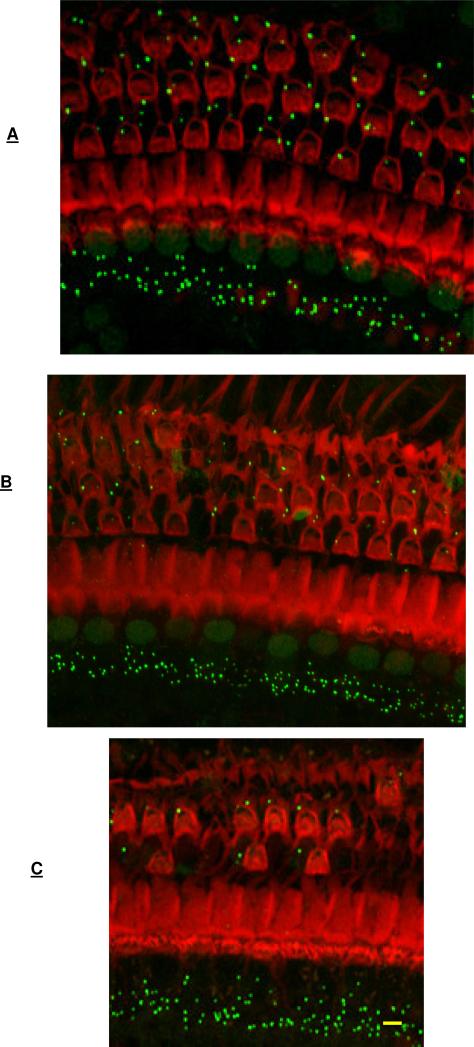
Figure 1.
Representative images of surface preparations from mid-cochlea (3 mm from the apex) of mice at 5-7 months of age (A), 22-24 months of age (B) and 27-29 months of age (C), co-labeled with phalloidin (red label) to image hair cells and scars in regions of missing hair cells and with CTBP2 immunolabeling (green puncta) to mark the ribbons in inner hair cell – auditory nerve synapses. Outer hair cell loss is seen at 22-24 months (B) and at 27-29 months (C) and not at 5-7 months (A). CTBP2 immunolabeling (green puncta) is seen at inner hair cell bases at all ages. Quantitative assessment of hair cell loss is seen in shown in Figure 2. Quantitative assessment of CTBP2 puncta (marker for ribbons and synapses) per inner hair cell is shown in Figure 4. Bar = 10 μm.
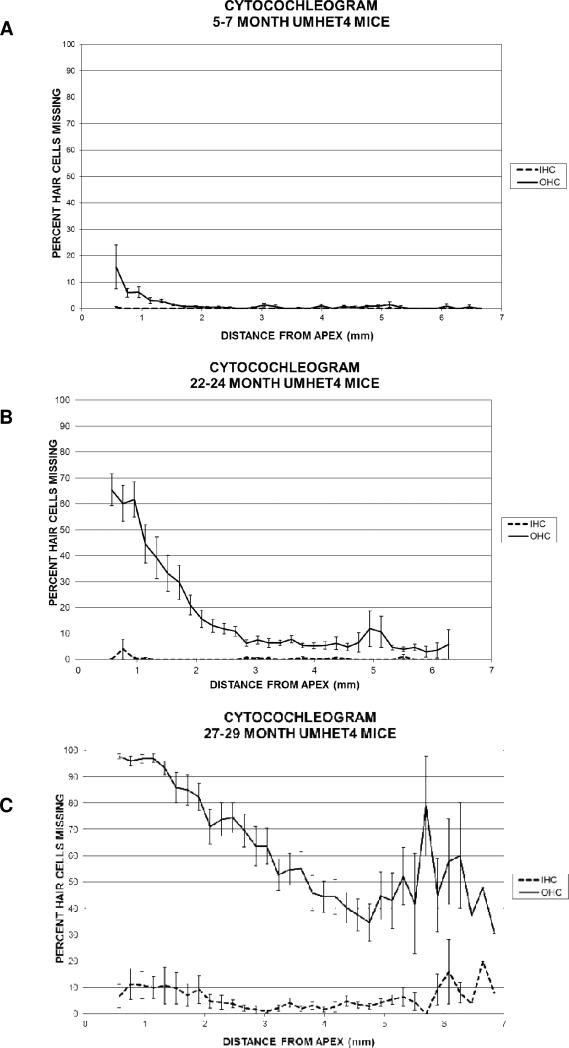
Figure 2.
The mean percentage of inner and outer hair cells that are missing at different locations along the cochlear spiral from apex (left) to base (right) in three groups of UM-HET4 mice terminated at 5-7 months of age (15 mice) (3A); at 22-24 months of age (26 mice) (3B) or at 27-29 months of age (22 mice) (3C). Error bars show standard error of the mean.
2.8 qRT-PCR
Cochleae were rapidly removed following decapitation, the otic capsule dissected away and the remaining cochlear tissues frozen. The right cochleae from 2 mice in each age group were pooled to generate 5 sample pools from 5-7 month old mice, 5 pools from 22-24 month old mice and five pools from 27-29 month old mice. RNA samples from 22-24 month old littermates used in Schacht et al (2012) were available and 8 sample pools were added to the 22-24 month old assessment to make a total of 13 for this age range. Total RNA was isolated from the tissues homogenized in TRIZOL (Invitrogen, Carlsbad, CA) using a modification of the standard TRIZOL protocol. Briefly, chloroform was added to the homogenate, the mixture was vortexed and then centrifuged. The aqueous phase was transferred to a Phase Lock Gel (Heavy) tube (Eppendorf/Brinkmann, Westbury, NY) and extracted with acid phenol:chloroform (1:1). Total RNA was precipitated from the aqueous phase with isopropanol, and re-suspended in RNA Storage Solution (Ambion, Austin, TX). RNA was further purified using the Qiagen RNeasy Micro spin column kit (Qiagen Inc., Valencia, CA) and stored at −80°C. RNA concentration was determined by UV spectrophotometry and quality was assessed on an RNA 6000 Nano LabChip (Agilent, Palo Alto, CA) using an Agilent 2100 Bioanalyzer, to assess the integrity of the 18S and 28S ribosomal RNA bands.
Total RNA was used to generate cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) with Random Primers. The following TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) were used for qRT- PCR reactions: Ntf3= Mm00435413_s1, Bdnf= Mm04230607_s1, Gdnf= Mm00599849_m1, Hprt1= Mm00446966_m1, Gapdh= Mm99999915_g1, Rps16= Mm01617542_g1, Sdha= Mm01352366_m1. Microtiter plates (384-well) were read on an ABI PRISM 7900. The data were analyzed using ABI PRISM 7900 Sequence Detection System software (Applied Biosystems, Foster City, CA). Each gene was assayed in triplicate in every pool of RNA. The 2−Δ1Δ2Ct method was used to calculate fold change in gene expression (Livak and Schmittgen, 2001). To normalize the data, the average of the threshold values for 4 housekeeping genes (hypoxanthine guanine phosphoribosyl transferase - Hprt, glyceraldehyde-3-phosphate dehydrogenase - Gapdh, ribosomal protein S16 - Rps16 and succinate dehydrogenase complex subunit A - Sdha) for any given sample was subtracted from the threshold values for each corresponding sample (Δ1). The normalized threshold values of samples in the same age group were averaged, and the threshold value of the 5-7 month old samples was subtracted from the corresponding threshold values in the 22-24 month old group or the 27-29 month old group (Δ2). The final value was then expressed as logarithm to base 2. This formula (2−(Δ1Δ2Ct)) was used to calculate the relative differences between age groups as fold change.
2.9 Spiral Ganglion Neuron Counts
The right cochleae were removed from randomly selected mice from the 5-7 month group (five mice) and the 22-24 month old group (10 mice) following vascular perfusion of fixative through the heart. Thirteen cochleae from 22-24 month old littermates used in Schacht et al (2012) were added to the 22-24 month old assessment group for a total of 23 animals for this age range (other age groups were not available from that study). Cochleae received intrascalar infusion of fixative and immersion in fixative for 16-20 h at 4°C followed by a rinse in PBS over 12-16 h. They were then decalcified in 5% EDTA for one week followed by dehydration through a graded series of ethanol and embedded in methacrylate resin. Paramodiolar sections were cut at 3 μm, collected on slides and received Paragon stain. Spiral ganglion neuron somata were identified based on size (area greater than 12 μm2) and shape (less than 3:1 aspect ratio) criteria and presence of a nucleus and counts were performed as in Sha et al. (2008). A random section was selected at the beginning of the mid-modiolar sections and every other subsequent section was assessed for a total of six. For each of the six sections, the number of spiral ganglion neurons within three cross-sectional profiles of Rosenthals canal, one in the basal turn, one mid cochlea and one more apical, was counted and divided by the area of each of the Rosenthal's canals to generate a spiral ganglion neuron density for each profile.
2.10. Statistics
Significance for ABR threshold changes and changes in Gap Inhibition or Pre-Pulse Inhibition of the Acoustici Startle Reflex was tested using the unpaired Students T test (Sigmaplot). Significance for changes in CTBP2 counts, Spiral Ganglion Neuron counts and gene expression was determined using ANOVA with a Bonferroni/Dunn Post-hoc test (Statview). For the qRT-PCR data, the Bonferroni/Dunn Post-hoc test compared the Δ1 values (Ct minus the averaged Ct of the housekeeping genes) of the young animals to the Δ1 values of the older animals. Correlation statistics were done as a modeled linear regression/best fit analysis (Sigmaplot).
3. RESULTS
3.1. Auditory Brain Stem Response (ABR) thresholds
Age-related changes in ABR thresholds were observed in the group of 22-24 month old animals compared to the young (5-7 month old) mice. There was a small but significant (p<0.01) age-related increase of 11.5 dB in the mean of auditory ABR thresholds at 4 kHz. Another small 15.0 dB increase in threshold at 48 kHz, the highest frequency tested, did not reach significance. Mid-frequencies (12 and 24 kHz) had changes below 10 dB that were not significant (Figure 3).
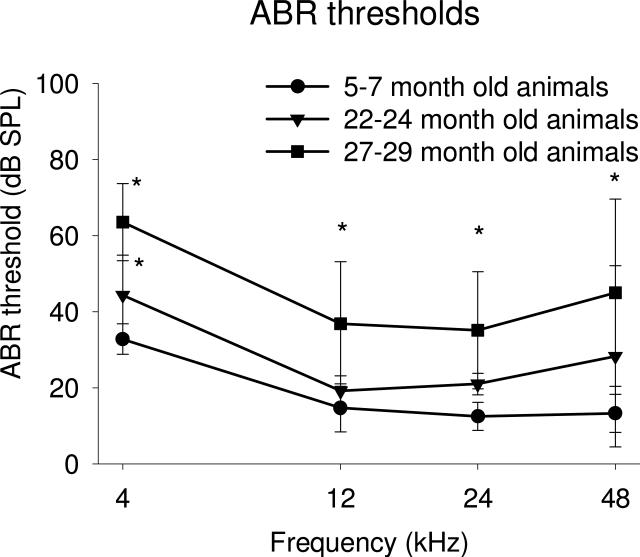
Figure 3.
Comparison of Auditory Brain Stem Response (ABR) measures in separate groups of UM-HET4 mice, assessed at 5-7 months (15 mice), 22-24 months (26 mice) and 27-29 months (22 mice) of age respectively. A small but significant increase (p<0.01) in the mean of auditory ABR thresholds at 4 kHz is seen at 22-24 months compared to 5-7 months, no significant changes were seen at other frequencies. At 27-29 months of age there were significant elevations (p<0.01) in the means of ABR thresholds at all four frequencies tested either when compared to 5-7 months mice or 22-24 months. These mice were also used for the gap detection and morphological assays described in this manuscript. Asterisks indicate significance (p<0.01). Significance was tested using the Student t test (Sigmaplot)
At 27-29 months of age there were significant elevations in the means of ABR thresholds at all four frequencies tested either when compared to young 5-7 month mice or to the 22-24 month old animals, with greatest increases found at 4 kHz and 48 kHz and smaller increases at 12 kHz and 24 kHz. At 4 kHz the mean threshold showed a significant increase of 30.7 dB compared to young animals and of ~20 dB compared to 22-24 months animals. There were also significant increases at 12 kHz with mean threshold increased 22.1 dB compared to young animals and ~15 dB compared to 22-24 months animals. At 24 kHz the mean threshold showed significant increases, 22.6 dB compared to young animals and ~15 dB compared to 22-24 months animals. Significant increases were also found at 48 kHz with the mean threshold increasing 31.7 dB compared to young animals and 16.7 dB compared to 22-24 months animals.
3.2. Hair Cell Loss
The numbers of inner and outer hair cells in 5-7 month old animals (Figure 2A) were normal in all but one mouse, which had moderate loss of outer hair cells limited to the apical third of the cochlea. In the 22 to 24-month old animals (Figure 2B) a moderate (>15%) to large (>60%) loss of outer hair cells was found in the apical quarter of the cochlea, with the loss of outer hair cells lower than 10% for most of the basal two-thirds of the cochlear spiral, consistent with our earlier report from a larger group of over two hundred 22-24 month old UM-HET4 mice (Schacht et al., 2012). In the present study, there was variability in the occurrence of hair cell loss at 22-24 months of age, with some animals showing normal numbers across the cochlear spiral and other animals showing moderate to large loss, also consistent with our earlier report (Schacht et al., 2012). Loss of more than three inner hair cells across the entire cochlea was found in only one of the 26 mice assessed at 22-24 months of age. At 27-29 months of age (Figure 2C), all animals showed outer hair cell loss, also with variability in the amount of loss. There was most often a “cookie cutter” pattern to the outer hair cell loss, with larger losses in basal and apical portions of the cochlea and the least lost in the middle, with the apical quarter showing the greatest loss. Most 27-29 month old mice showed no loss of inner hair cells and only a few animals had a i modest loss in apical and basal regions.
3.3 Inner Hair Cell – Auditory Nerve Connections
C-Terminal Binding Protein 2 (CTBP2) is a component of the ribbon in the IHC-AN synapse that has been used as a marker for IHC-AN connections (e.g. Kujawa and Liberman, 2006, 2009). Sergeyenko et al. (2013) used co-immunolabeling of the pre-synaptic CTBP2 and post-synaptic glutamate receptor (GLUR2) as a marker. They found only a small percentage of “orphan ribbons”, CTBP2 immunolabeling without post-synaptic glutamate receptor immunolabeling adjacent, (<5% at the 12 and 32 kHz regions, and <10% at the 5.6 kHz region), and suggested ribbon counts are an excellent proxy for synaptic counts during the aging process. The current study used CTBP2 immunostaining of ribbons as the sole marker for IHC-AN connections.
The number of CTBP2 immunolabeled ribbons per IHC base (Figure 1A-C & 4A,B) was assessed in ~0.20mm “Regions of Interest” (ROI) at four locations along the cochlear spiral: ~1, 2, 3 and 4 mm from the apex. There was variability in the amount of connections per IHC (Figure 4A) even in the younger (5-7 month old) animals and greater variability in both of the older age groups, with many old animals showing large decrease in number of CTBP2 immunolabeled ribbons and others remaining within the range of the young group. There were significant (p<0.01) decreases in CTBP2 immunolabeled ribbons ranging from 20-34% at both older ages across the four regions of interest (Figure 4A,B). Interestingly, there were no further decreases in the range or mean at 27-29 months of age compared to 22-24 month old animals, despite the increased loss of hair cell loss in the oldest group (Figure 2).
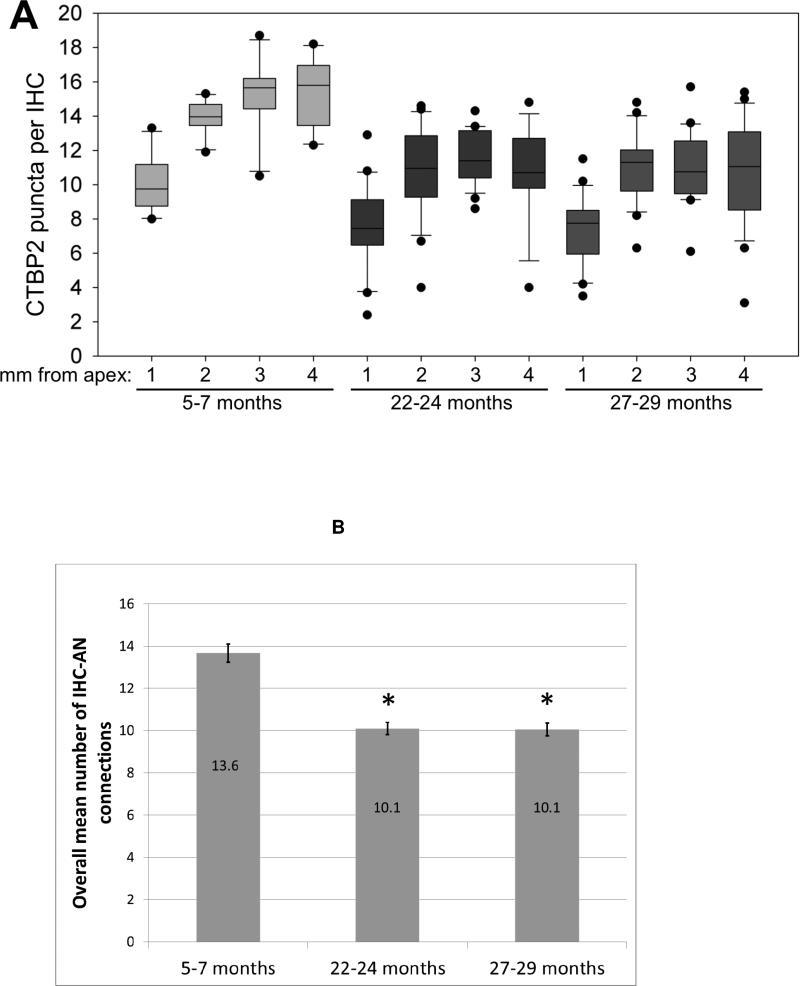
Figure 4.
A) Box plot showing the number of CTBP2 puncta per inner hair cell in four regions of interest (1, 2, 3 and 4 mm from the apex) in three groups of mice, terminated at 5-7 months (15 mice), 22-24 months (26 mice) and 27-29 months (22 mice) of age respectively. The means at 22-24 months are significantly lower than those at 5-7 months in all four regions of interest (p <.01). The means at 27-29 months are not significantly different from those at 22-24 months. Boxes indicate 25th-75th percentile of data; bar indicates median; and whiskers indicate 5th-95th percentile of data. B) When the four regions of interest are combined there is a significant decrease in the overall mean number of connections at 22-24 and 27-29 months of age compared to 5-7 months of age (p <.01) Asterisks indicate significance (p<0.01). Significance was determined using ANOVA with a Bonferroni/Dunn Post-hoc test (Statview).
In the most apical ROI assessed (1 mm from the apex) there was a significant decrease from a mean of 10.1 CTBP2 immunolabeled ribbons per IHC in the 5-7 month old animals to a mean of 7.7 labeled ribbons per IHC in 22-24 month old. There was no significant further decrease in the 27-29 month old animals, in which there was a mean of 7.3 CTBP2 immunolabeled ribbons per IHC. In the region 2 mm from the apex there was a significant decrease in the number of CTBP2 ribbons per IHC from a mean of 13.9 in the 5-7 month old animals to a mean of 11.5 in 22-24 month old animals, which then remained stable in the 27-29 month old animals with a mean of 11.1. In the region 3 mm from the apex the mean number of CTBP2 ribbons per IHC was significantly decreased from 15.2 in young animals to 11.5 in 22-24 month old animals, remaining stable in the 27-29 month old animals with a mean of 11.1. Finally, in the most basal cochlear region assessed (4 mm from the apex) the mean number of CTBP ribbons per IHC had a significant decrease from 15.5 in the young animals to 10.7 labeled ribbons per IHC at 22-24 months, again not decreasing any further in the 27-29 month old animals.
3.4 Spiral Ganglion Neuron Counts
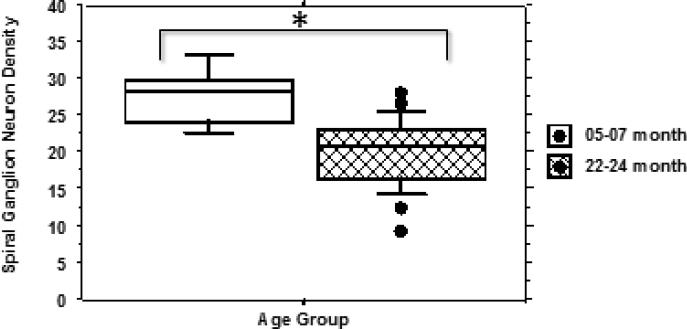
The mean density of spiral ganglion neurons (SGN) within three cross sections (profiles) of Rosenthal's canal (basal, mid-cochlea and more apical) was examined at 5-7 months and 22-24 months of age (Figure 5). There was a significant (<0.01) decrease from a SGN density of 27.8 in the 5-7 month group to 20.1 in the 22-24 month old animals.
Figure 5.
Box plot comparing mean spiral ganglion neuron counts from groups of UM-HET4 mice at 5-7 months of age (5 mice) and 22-24 months of age (23 mice). . The mean number at 22-24 months of age is significantly reduced in comparison with 5-7 months (p <.01). Asterisks indicate significance (p<0.01). Significance was determined using ANOVA with a Bonferroni/Dunn Post-hoc test (Statview).
3.5 Gap Detection
The ASR can be inhibited either by prior presentation of a brief pulse of sound (PPI) or by a short gap in a continuous background sound (Ison, 1982; Ison et al., 1991; Turner et al., 2006). When an animal shows inhibition of the ASR by a pre-pulse of sound but not by a gap in sound, this suggests the animal can hear and process sounds but cannot effectively detect or process small gaps in sound. This is interpreted as a deficit/reduction in Gap Detection, which might indicate a temporal processing disorder. Effective inhibition of the ASR by both a prior sound pulse and a gap would be interpreted as having good Gap Detection ability. A lack of inhibition of the ASR by either sound pre-pulse or a gap would be interpreted by the animal as being a result of a deficit in hearing, and no conclusion can be drawn as to whether the subject had effective or reduced Gap Detection. In some animals, the presence of background sounds at particular frequencies inhibited jump amplitudes; if this inhibition rendered the jump amplitude indistinguishablefrom baseline movement, the animal was not assessed for that frequency.
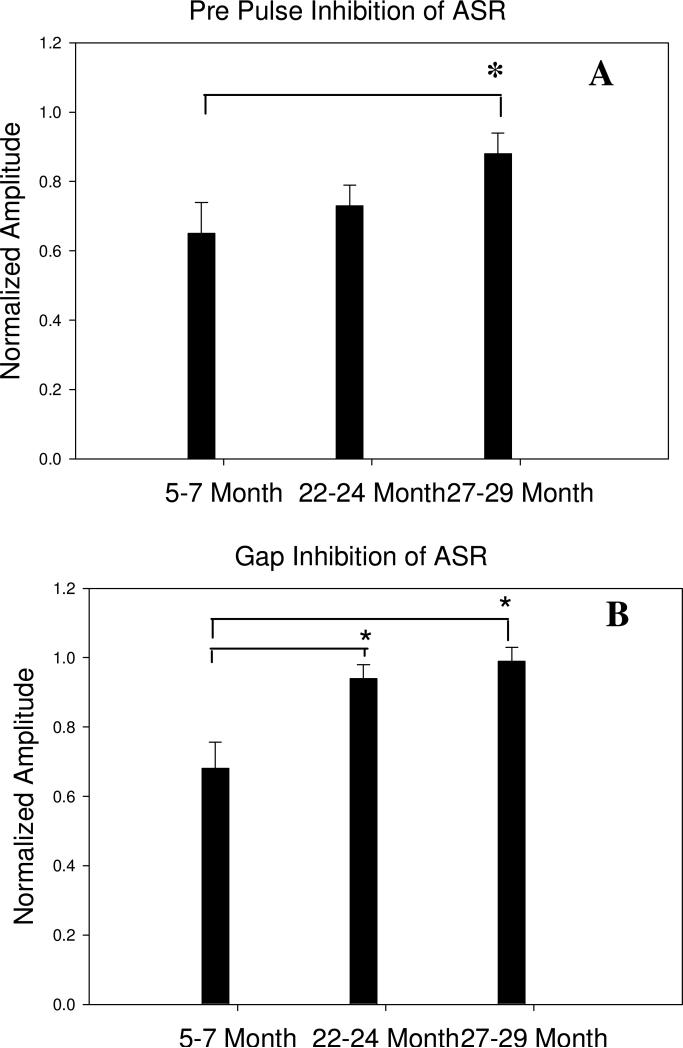
At 5-7 months of age, all but one of the mice showed both PPI of the ASR as well as an effective Gap Inhibition of the ASR, indicating normal auditory processing and Gap Detection (Figure 6). At 22-24 months of age, all 26 mice had retained good PPI. However, many had reduced Gap Inhibition of the ASR, falling along a continuum as seen in Figures 8 and 9. Several animals did not jump during ASR test and so could not be used for the Gap Detection assessment. As a group, the 22-24 month animals showed effective PPI and significantly reduced Gap Inhibition of the ASR (compared to the mean in the 5-7 month old group). In the 27-29 month old animals, 9 of the 22 mice did not jump during the ASR test, and therefore were not used for PPI or Gap Detection assessment. The remaining animals showed a similar pattern to the 22-24 month old group, ranging from good PPI and good Gap Detection to a continuum of reduced Gap Detection with three animals showing both bad PPI and bad Gap Detection. This age-related reduction in Gap Detection in mice is consistent with the literature (e.g. Ison et al., 1998; 2003)
Figure 6.
Comparison of pre-pulse (A) and Gap (B) inhibition of the acoustic startle reflex (ASR) in mice at 5-7 months (15 mice), 22-24 months (based on 18 of the 26 mice, 8 that did not jump are not included) or 27-29 months (based on 13 mice – 9 mice that did not jump are not included) of age. At 5-7 months of age both the pre-pulse and the gap produce a mean inhibition of the ASR of 30-35%. At 22-24 months of age there is no longer a significant gap-inhibition of the ASR while the pre-pulse inhibition remains in the 30-35% range. The difference in Gap Detection between 5-7 months, 22-24, and 27-29 months is significant (p< 0.01), while pre-pulse inhibition has not changed, indicating an age-related decrease in gap detection. At 27-29 months of age there is no longer a significant pre-pulse or gap inhibition of the ASR, indicative of the large hearing loss in many animals. Asterisks indicate significance (p<0.01). Significance was tested using the Student t test (Sigmaplot)
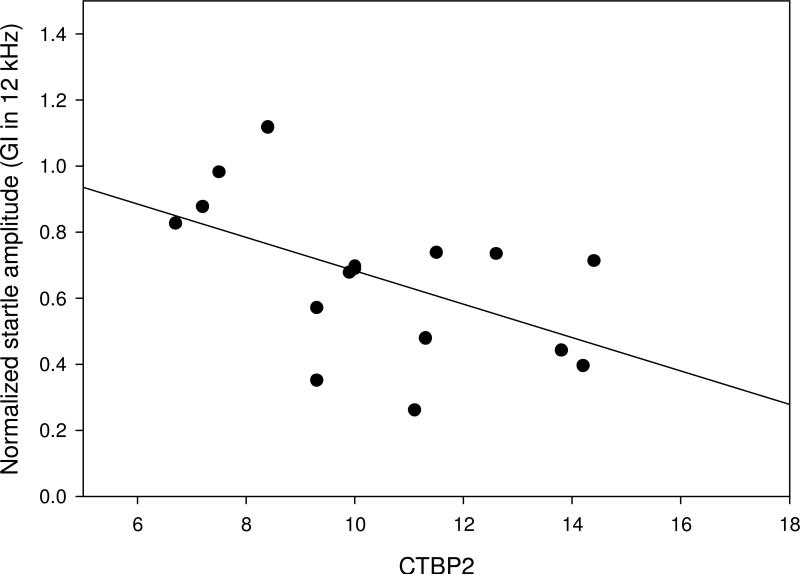
Figure 8.
Scatter plot comparing the number of CTBP2 immunolabeled ribbons per IHC base (as a marker for inner hair cell – auditory nerve connections) to normalized Gap Inhibition. Mice had best Gap Inhibition at 12 kHz, so the region of interest 2 mm from the apex, corresponding to the 12kHz frequency regions (Viberg and Canlon, 2004) was tested for correlation with Normalized Gap Inhibition at 12 kHz, across individual mice at 22-24 months of age. Data are based on 16 of the 26 mice; 10 mice without a robust jump response at 12 kHz are not included. Correlation was weak based on a modeled linear regression/best fit analysis (Sigmaplot).
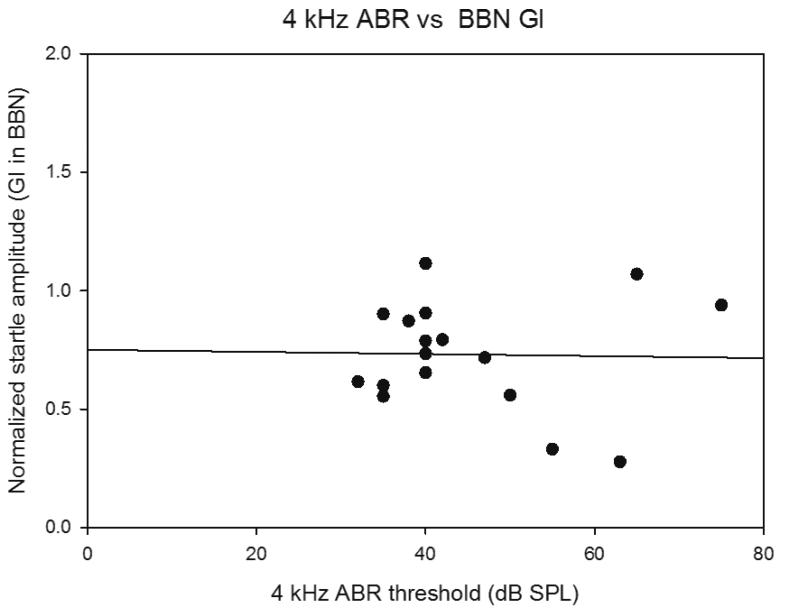
Figure 9.
Scatter plot comparing ABR thresholds changes at 4 kHz (where there was greatest change) and Normalized Gap Inhibition across individual mice at 22-24 months of age. No correlation is observed based on a modeled linear regression/best fit analysis (Sigmaplot).
3.6 Expression of Neurotrophic Factors in the Cochlea
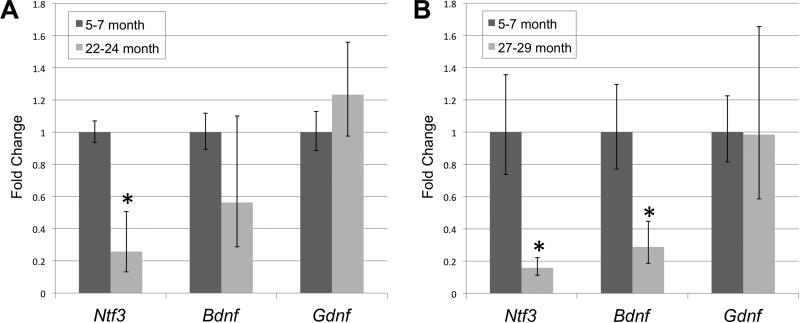
Ntf3 gene expression in the cochleae of 22-24 month old animals significantly (p<0.013) decreased to 26% of the level of its expression in young (5-7 month old) animals (Figure 7). Bdnf expression in 22-24 month old animals showed a downward trend but not a significant decrease. Gdnf expression likewise was not significantly altered. At 27-29 months, Ntf3 expression remained significantly decreased now at 16% of the level in young mice, and the Bdnf expression now showed a significant (<0.01) decrease to 28% of the level of expression in young animals. Gdnf expression at 27-29 months of age remained unchanged from levels in young animals.
Figure 7.
qRT-PCR results comparing gene expression of Ntf3, Bdnf and Gdnf at 5-7 months versus 22-24 months (A) and at 5-7 months versus 27-29 months (B), in whole cochleae from pooled samples (from 2-3 right cochleae) of UM-HET4 mice (5 pools from 5-7 month old mice, 14 pools from 22-24 month old mice; 5 pools from 27-29 month old mice). Ntf3 gene expression in 22-24 month old animals was significantly decreased compared to 5-7 month old mice (p < 0.02). At 27-29 months of age, Ntf3 expression remained significantly decreased from 5-7 month old animals.. Compared to 5-7 months, the decrease in Bdnf expression was not significant at 22-24 months, but became significant in the 27-29 months old animals (p< 0.01). There were no significant changes in Gdnf expression. Significance was determined using an ANOVA with a Bonferroni/Dunn Post-hoc test (Statview) comparing the Δ1 values of the young animals to the the Δ1 values of the older animals. The error was calculated using the standard deviation of the Δ1 as 2−(Δ1Δ2Ct-stdev) and2−(Δ1Δ2Ct+stdev).
3.7 Correlations
One important goal of the study was to compare the appearance of the different components of ARHL and test for correlations. Both a significant loss of IHC-AN connections and a significant decrease in gap detection was found in 22-24 month old mice compared to 5-7 month old animals, and both measures showed no further changes at 27-29 months. There was a continuum of degree of changes for both metrics at 22-24 months of age. Since gap detection and IHC ribbon quantification was performed in the same animals, correlations could be evaluated on an individual animal basis. This was tested in the 22-24 month old mice without hearing loss (less than 10dB threshold shift from 5-7 month measures at any of the frequencies assessed) and good pre-pulse inhibition of the ASR. The increased loss of IHC ribbons in the 12 kHz region (the region of interest 2 mm from the apex) was weakly correlated (p = 0.08; r2 = 0.22) with decreases in gap detection at 12 kHz (Figure 8), suggesting that loss of connections may account for a only small part of the age-related change in gap detection.
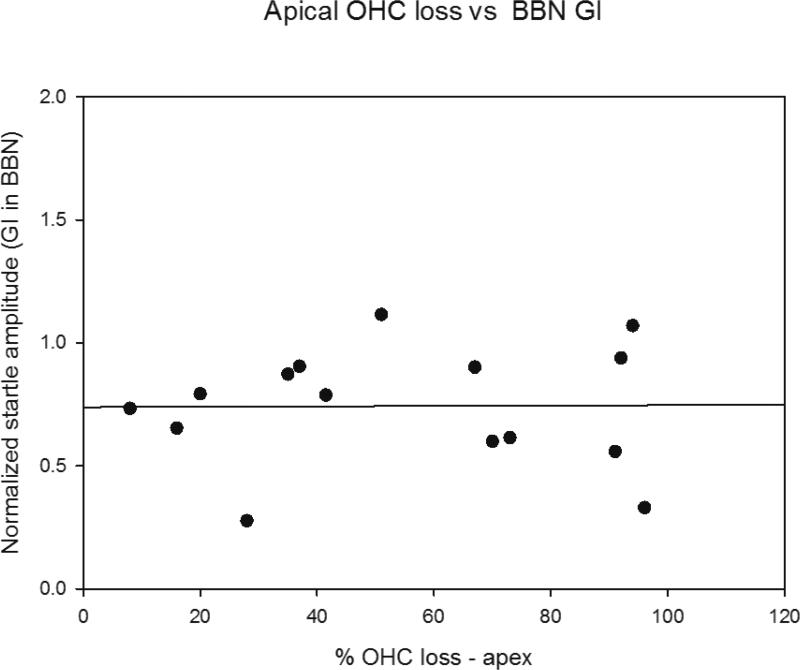
ABR thresholds and hair cell loss were also assessed in the same animals as gap detection and were also examined in scatter plots on an individual animal basis for correlations (Figures 9 and 10). There was no correlation between poor gap detection and elevated ABR thresholds among individual animals in the 22-24 month old mice for any of the frequencies tested (Figure 9 shows scatter plots for 4 kHz and 48 kHz threshold shifts versus gap detection). The combined outer hair cell loss in the apical third, middle third or basal third of the cochlea was determined for each 22-24 month old mouse and compared to gap detection on an individual animal basis. The apical third was the only region where more than minimal hair cell loss was observed at 22-24 months, with variability among animals in the extent. Figure 10 shows a scatter plot for percent outer hair cell loss in the apical third of the cochlea versus gap detection. No correlation between increased outer hair cell loss and poor gap detection was found. No correlation was found for the middle or basal thirds as well (not shown).
Figure 10.
Scatter plot comparing loss of outer hair cells in apical third of the cochlea (where there was greatest loss) versus Normalized Gap Inhibition across individual mice at 22-24 months of age. No correlation is observed based on a modeled linear regression/best fit analysis (Sigmaplot).
4. DISCUSSION
UM-HET4 mice showed individual variability in the timing and extent of different components of ARHL. This allowed assessment of potential relationships and determination whether appearance and severity in one type of pathology could predict appearance and severity of another (in addition to the already known relationship between hair cell loss and ABR threshold shifts). Several pertinent results emerged from this study. An age-related temporal processing deficit was revealed by decreases in gap inhibition of the ASR without loss of PPI, consistent reports in other animal models and people (Anderson et al., 2012; Caspary et al., 2008; Humes et al., 2012; Recanzone et al., 2011, Suta et al., 2011; Walton 2010 for reviews). There was also a significant decrease in IHC-AN connections in 22-24 month old animals, consistent with results in CBA/J mice (Sergeynko et al, 2013). This might suggest a relationship between loss of IHC-AN connections and decreased gap detection and such a correlation has been found following noise exposure in 16-18 week old mice (Hickox and Liberman, 2014). Our results are in agreement with those studies although the observed correlation in the 12 kHz-region (UM-Het4 mice showed best gap detection at 12 kHz) was weak. This analysis suggests that loss of connections may contribute to reduced gap detection but that other factors are likely to dominate, such as the age-related decreases in inhibitory synaptic strength in central auditory pathways that have been reported (Caspary et al., 2008, Frisina 2009 for reviews). There was no correlation between changes in gap detection and ABR threshold shifts (Figure 9) or between reduced gap detection and loss of hair cells at 22-24 months of age (Figure 10).
Both our study and Sergeyenko et al (2013) found that loss of IHC-AN connections occurs at an earlier age than the loss of outer hair cells and elevation of hearing thresholds in most portions of the cochlea. The loss of IHC-AN connections occurs fairly equally across the cochlear spiral while outer hair cell loss and hearing loss is biased towards the most apical and basal cochlea and high and low frequency respectively. The lack of correlation between the timing and location of outer hair cell loss versus loss of IHC-AN connections is also consistent with earlier studies that found that significant age-related loss of auditory neurons is independent of age-related hair cell loss (Keithley et al., 1979, 1989, Dazert et al., 1996, see Bao and Ohlemiller, 2010 for review). This in turn suggests differences in mechanisms underlying age-related hair cell loss and age-related loss of IHC-AN connections.
Recent findings by Wan et al. (2013, 2014) and Wang & Green (2011) provide evidence that NTF3 regulates and maintains IHC-AN connections. Wang and Green (2011) found that lowering NTF3 signaling in inner hair cells decreased the re-innervation of inner hair cells following excitotoxicity induced loss and that increasing NTF3 in organotypic cochlear culture increased reinnervation. Wan et al. al. (2013) found that inducing increased NTF3 expression in supporting cells of young transgenic mice increased the number of IHC-AN connections and that decreasing NTF3 expression decreased the number. NTF3 could therefore play an important role in maintenance of IHC-AN connections across lifespan. The large age-related decrease in Ntf3 gene expression in the mouse cochlea found in the present study by 22-24 months of age could result in a decreased ability to maintain the full complement of connections present in young animals. This could, in turn, explain the 20-34% percent age-related decrease in IHC-AN connections seen in the present study. An important role for BDNF in stabilizing IHC-AN connections has been suggested by studies in transgenic mice (Singer et al, 2014 for review). The decrease in Bdnf expression in the cochlea at 22-24 months in the present study was not significant, but this could be a consequence of variability in the loss of connections among animals. Therefore, a role for BDNF in the age-related loss of connections should not be ruled out.
The present study found no differences between the mean for IHC-AN ribbons per IHC for groups assessed at 22-24 versus 27-29 months of age, with the mean loss across turns approximately 25% at both ages (75% survival). Sergeyenko et al. al. (2013) found a continuous incremental age-related loss of IHC-AN connections in their recent study of CBA/J mice, with ~ 28% loss at 22-24 weeks, ~ 34% loss at 27-29 months continuing up until their last assessment time of 144 weeks (~36 months) of age, where the loss approached 50%. Age-related changes can vary across species and across strains within species due to the wide variety of mechanisms that can influence age-related changes in the cochlea (Frisina, 2009; Ohlemiller, 2009; Someya and Prolla, 2010; Yamasoba et al., 2007 for reviews) and because genetic variation can influence age effects on the auditory system (Schacht et al., 2012). The presence of SGN loss at 22-24 months of age in UM-HET4 mice suggests that some loss of IHC-AN connections is occurring earlier, since there is believed to be a delay between loss of connections and cell death for auditory neurons (Kujawa and Liberman, 2006; 2009).
5. CONCLUSIONS
UM-HE4 mice show individual variability in the timing and extent of age-related changes in ABR thresholds, hair cell loss, IHC-AN connections and gap detection. Two components of ARHL, loss of IHC-AN connections and a decrease in gap detection, occur earlier (at 22-24 months of age) in UM-HET4 mice than a broad loss of sensory cells and threshold shifts (seen across turns at 27-29 months of age) However, the weak correlation between the two parameters at best suggests that loss of IHC-AN connections may account for some but not the majority of influences on decreased gap detection in this aging model. There was no correlation between poor gap detection and elevated ABR thresholds or increased outer hair cell loss and poor gap detection.
An intriguingly large and significant decrease in expression of the neurotrophic factor gene Ntf3, previously associated with IHC-AN connection numbers and stability during postnatal development, was found in the cochlea at 22-24 months. Combined evaluation in future studies of Ntf3 levels and IHC-AN connections in individual UM-HET4 mice will be required to establish a causal relationship between reduced NTF3 and IHC-AN connection loss during aging.
Highlights.
Loss of inner hair cell – auditory nerve connections precedes hair cell loss in most of the cochlea
Loss of gap detection precedes hair cell loss in the basal two-thirds of the cochlea
Loss of connections may contribute to but does not account for all of the age-related reduced gap detection.
Decrease in Ntf3 expression occurs at the same time as loss of inner hair cell – auditory nerve connections
Hair cell loss and ABR thresholds increase significantly from 22-24 months to 27-29 months of age
Acknowledgements
This work was supported by NIH grants P01-AG025164, P30-DC05188 and P30-AG024824. We thank Don Swideriski for his assistance in statistical evaluations and interpretation of correlations.
Abbreviations
- ASR
Acoustic Startle Reflex
- ARHL
Age Related Hearing Loss
- ABR
Auditory Brain Stem Response
- BDNF
Brain Derived Neurotrophic Factors
- GD
Gap Detection
- GDNF
Glial Cell Line Derived Neurotrophic Factor
- IHC-AN
Inner Hair Cell – Auditory Nerve
- i.p.
intraperitoneal
- NTF3
Neurotrophin 3 (previously known as neurotrophic factor 3, or NT3)
- PBS
phosphate buffered saline
- PPI
Pre-pulse Inhibition
- SGN
Spiral Ganglion Neuron
- TTS
Temporary Threshold Shift
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci. 2012 2012 Oct 10;32(41):14156–64. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Ohlemiller KK. Hear Res Age-related loss of spiral ganglion neurons. 2010;264(1-2):93–7. doi: 10.1016/j.heares.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–91. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert S, Feldman ML, Keithley EM. Cochlear spiral ganglion cell degeneration in wild-caught mice as a function of age. Hear Res. 1996;100(1-2):101–6. doi: 10.1016/0378-5955(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Age-related hearing loss: ear and brain mechanisms. Ann N Y Acad Sci. 2009;1170:708–17. doi: 10.1111/j.1749-6632.2009.03931.x. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110(3):577–86. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–20. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23(8):635–66. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: a study of the acoustic startle reflex and its inhibition by brief decrements in noise level. J Acoust Soc Am. 1998;104(3 Pt 1):1696–704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- Ison JR. Temporal acuity in auditory function in the rat: reflex inhibition by brief gaps in noise. J. Comp. Physiol. Psychol. 1982;96:945–054. [PubMed] [Google Scholar]
- Ison JR, Allen P. A diminished rate of “physiological decay” at noise offset contributes to age-related changes in temporal activity in CBA mouse model of presbycusis. J. Acoust. Soc. Amer. 2003;2003;114(1):522–528. doi: 10.1121/1.1577553. [DOI] [PubMed] [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with decortications. Behav. Neurosci. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K. Strain background effects and genetic modifiers of hearing in mice. Brain Res. 2006;1091(1):79–88. doi: 10.1016/j.brainres.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hearing Research. 1997;114(1-2):83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70(2):171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Ryan AF, Woolf NK. Spiral ganglion cell density in young and old gerbils. Hear Res. 1989;38(1-2):125–33. doi: 10.1016/0378-5955(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979;188(3):429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J. Neurosci. 2006;26(7):2115–23. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke LE, McGee DH, Prieskorn DM, Wall GM, Dolan DF, Altschuler RA, Miller JM. Safety of ciprofloxacin and dexamethasone in the guinea pig middle ear. Arch Otolaryngol Head Neck Surg. 2009;135(6):575–80. doi: 10.1001/archoto.2009.30. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2^-DDCT Method. METHODS. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006;116:1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35(1):21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–8. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piu F, Wang X, Fernandez R, Dellamary L, Harrop A, Oinag Y, Sweet J, Tapp R, Dolan DF, Altschuler RA, Lichter J, LeBel C, OTO-104 A sustained release dexamethasone hydrogel for the treatment of otic disorder. Otology and Neurotology. 2011;32:171–9. doi: 10.1097/MAO.0b013e3182009d29. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Engle JR, Juarez-Salinas DL. Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey. Hear Res. 2011;271(1-2):115–22. doi: 10.1016/j.heares.2010.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J, Altschuler RA, Burke DT, Chen S, Dolan D, Galecki AT, Kohrman D, Miller RA. Alleles that modulate late life hearing in genetically heterogeneous mice, Neurobiol Aging. 2012;33(8):1842e15–29. doi: 10.1016/j.neurobiolaging.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear Res. 2008;243(1-2):87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Panford-Walsh R, Knipper M. The function of BDNF in the adult auditory system. Neuropharmacology. 2014;76:719–728. doi: 10.1016/j.neuropharm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Suta D, Rybalko N, Pelánová J, Popelá J, Syka J. Age-related changes in auditory temporal processing in the rat. Exp Gerontol. 2011;46(9):739–46. doi: 10.1016/j.exger.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LR, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav. Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Viberg A, Canlon B. The guide to plotting a cytocochleogram. Hearing Res. 2004;197:1–10. doi: 10.1016/j.heares.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Walton JP. Timing is everything: temporal processing deficits in the aged auditory brainstem. Hear Res. 2010;264(1-2):63–9. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31(21):7938–49. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Corfas G, Stone JS. Inner ear supporting cells: rethinking the silent majority. Semin Cell Dev Biol. 2013;24(5):448–59. doi: 10.1016/j.semcdb.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]