Abstract
Despite current advances in engineering blood vessels over 1 mm in diameter and the existing wealth of knowledge regarding capillary bed formation, studies for the development of microvasculature, the connecting bridge between them, have been extremely limited so far. Here, we evaluate the use of 3-dimensional (3D) microfibers fabricated by hydrogel electrospinning as templates for microvascular structure formation. We hypothesize that 3D microfibers improve extracellular matrix (ECM) deposition from vascular cells, enabling the formation of freestanding luminal multicellular microvasculature. Compared to 2-dimensional cultures, we demonstrate with confocal microscopy and RT-PCR that fibrin microfibers induce an increased ECM protein deposition by vascular cells, specifically endothelial colony-forming cells, pericytes, and vascular smooth muscle cells. These ECM proteins comprise different layers of the vascular wall including collagen types I, III, and IV, as well as elastin, fibronectin, and laminin. We further demonstrate the achievement of multicellular microvascular structures with an organized endothelium and a robust multicellular perivascular tunica media. This, along with the increased ECM deposition, allowed for the creation of self-supporting multilayered microvasculature with a distinct circular lumen following fibrin microfiber core removal. This approach presents an advancement toward the development of human microvasculature for basic and translational studies.—Barreto-Ortiz, S. F., Fradkin, J., Eoh, J., Trivero, J., Davenport, M., Ginn, B., Mao, H.-Q., Gerecht, S. Fabrication of 3-dimensional multicellular microvascular structures.
Keywords: extracellular matrix, fibrin, microfiber, endothelial cell, perivascular cell
The development of artificial microvascular vessels is of critical importance in the fields of cardiovascular diseases, cancer growth, and tissue engineering of vascularized organs. In tissue engineering, there is currently a limit on the size of tissues that can be constructed in vitro due to the diffusion limit of nutrients into developing organs. As such, only thin tissues like skin grafts or avascular tissues like cartilage can be successfully engineered in vitro, with larger constructs requiring the existence of a vascular network within itself to supply the necessary nutrients for cell survival and to avoid necrotic areas postimplantation (1, 2).
Arterioles are the blood vessels found immediately before capillaries, ranging from tens to hundreds of micrometers in diameter. They are composed of an endothelium and its basal lamina, which is comprised mainly of collagen (Col) type IV (Col IV), fibronectin (Fn), laminin (Lmn), and heparin sulfate proteoglycan (3). This is followed by a layer of subendothelial connective tissue and an internal elastic lamina (4–6). These layers are composed mainly of Cols such as type I and III (Col I and Col III, respectively) and elastin (Eln) (5, 7). On top of these 2 layers of extracellular matrix (ECM) proteins lies the tunica media, a layer of perivascular cells increasing in thickness with vessel size (5, 8, 9). In the smallest of arterioles, the perivascular cells are pericytes, which increase in confluency with vessel size and eventually are replaced by vascular smooth muscle cells (vSMCs) (5, 6, 8, 9). The tunica media in arterioles has a primarily circumferential orientation of smooth muscle cells (SMCs), which is necessary for vasoconstriction. Opposite to arterioles, venules collect blood from the capillary beds and are also supported by perivascular cells, though here, the tunica media does not follow a circumferential organization, and the ECM layers are less robust than in arterioles (4, 5, 8–10).
The creation of luminal microvascular conduits with sufficient ECM to be self-standing remains a challenge. Recent novel approaches have used different fabrication techniques in a creative manner to develop more relevant 3-dimensional (3D) conduits (11–14), although none has achieved a fully functional microvascular structure recapitulating both the cellular and ECM protein organization in the multilayer formation present in native vasculature.
Recently, we introduced a modified electrospinning technique that allowed us to fabricate hydrogel microfibers with an internal and external alignment and with tunable diameters between tens and hundreds of micrometers (15). Fibrin microfibers created with this technique support endothelial colony-forming cell (ECFC) growth, alignment, and deposition of basal lamina proteins Col IV, Fn, and Lmn. Furthermore, our studies revealed that the 3D curvature imparted by the scaffold regulates the wrapping organization of the basal lamina proteins and that this process is independent of cytoskeletal organization (both actin and microtubule), as well as of multicellular cell alignment. We also demonstrated that SMCs could be introduced onto a developed endothelial monolayer on the fibrin microfibers to create a multicellular construct and that the SMCs would not only be fully invested on the constructs but would additionally deposit Col I and Eln beneath them, between the SMC and ECFC layers (16).
In the current study, we hypothesize that the fibrin microfiber hydrogel promotes increased ECM protein production by the different vascular cell types, namely ECFCs, pericytes, and SMCs, and that this phenomenon is regulated by the substrate’s spatial dimensionality. We further hypothesize that the fibrin core can be degraded leaving both cellular and ECM conformation intact and that the increased ECM deposition achieved will allow the creation of self-standing multicellular microvascular structures with a distinct circular lumen that recapitulate the multilayer organization found in native blood vessels.
MATERIALS AND METHODS
Cell culture
All cells were cultured in a humidified incubator at 37°C and 5% CO2. Human ECFCs (Lonza, Walkersville, MD, USA) were cultured on Col I (BD Biosciences, Franklin Lakes, NJ, USA)-coated flasks in Endothelial Basal Medium-2 (Lonza) supplemented with EGM-2 BulletKit (Lonza) and 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA) and were used for experiments between passages 7 and 10. Medium was changed every other day, and cells were passaged every 5–7 days with 0.05% trypsin (Life Technologies, Grand Island, NY, USA).
Human vSMCs [American Type Culture Collection (ATCC), Manassas, VA, USA] were used between passages 7 and 10 and cultured in F-12K medium (ATCC) supplemented with 0.01 mg/ml insulin (Akron Biotech, Boca Raton, FL, USA), 10% FBS, 0.05 mg/ml ascorbic acid, 0.01 mg/ml transferrin, 10 ng/ml sodium selenite, 0.03 mg/ml endothelial cell (EC) growth supplement, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 10 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (all from Sigma-Aldrich, St. Louis, MO, USA). Medium was changed every third day, and cells were passaged every 5–7 days with 0.25% trypsin (Life Technologies).
Human placental pericytes (PromoCell, Heidelberg, Germany) were cultured in Pericyte Growth Medium (PromoCell) and used between passages 7 and 10. Medium was changed every other day, and cells were passaged every 5–7 days with 0.05% trypsin.
Preparation of 3D fibrin hydrogel microfiber
Fibrin hydrogel microfibers were generated by the electrospun hydrogel method described previously (16). In brief, 1.5 weight-percent alginate (Sigma-Aldrich) was in-line mixed 2 weight-percent fibrinogen (Sigma-Aldrich) at flow rates of 2 and 1 ml/h, respectively. Both solutions were dissolved in 0.2 weight-percent polyethylene oxide (molecular weight, 4 million; Sigma-Aldrich) before electrospinning. A 4 kV electric potential was applied to the solution before extrusion through a 25-gauge needle. The solution jet was collected in a grounded, rotating bath (30–45 rotations per minute) containing 50 mM CaCl2 solution with 10 U/ml thrombin (Sigma-Aldrich) for 35 minutes. Fibers were left in the collecting solution for 10 minutes and then soaked overnight in 0.25 M sodium citrate to dissolve the alginate. Fibers were then soaked in water for 60 minutes, bundled and stretched to 150% of their initial length, and air-dried for 60 minutes. Microfibers were wrapped around a custom-made plastic frame and sterilized by soaking in 75% ethanol for 2 minutes followed by rinsing twice with sterile water.
Cell seeding and culture on fibrin hydrogel fibers
ECFCs, vSMCs, and pericytes were seeded on fibers as described previously (15, 16). In brief, cells were seeded on microfibers wrapped on a frame at a density of 4 × 105 cells/ml in 5 ml ECFC medium and tumbled overnight at 37°C to facilitate cell attachment. For ECFCs, medium was supplemented with 50 ng/ml VEGF (Pierce, Rockford, IL, USA), whereas for pericytes, medium was supplemented with 30 mM aminocaproic acid (ACA; Sigma-Aldrich). At day 2, frames were transferred to 35 mm Petri dishes and cultured in the same medium in a 5% CO2 humidified incubator at 37°C. Medium was changed every other day thereafter. For cocultures, vSMCs or pericytes were seeded on 5-day ECFC-seeded fibrin fibers at 4 × 105 cells/ml in 5 ml ECFC medium or in ECFC medium with 30 mM ACA, tumbled for 24 hours, and then transferred to 35 mm Petri dishes to continue culture. Medium was changed every other day thereafter.
Preparation of 2-dimensional fibrin-coated surfaces
Cell culture 6-well plates and coverslips were coated with a solution of 0.2 and 0.1% fibrinogen in normal saline, respectively, and then cross-linked with 10 U/ml thrombin in 15 mM CaCl2. The solutions were incubated for 15 minutes before being sterilized with 75% ethanol for 2 minutes and rinsed twice with water.
Cell seeding and culture on 2-dimensional fibrin surfaces
Cells were seeded at 1 × 105 on 6-well plates and at 5 × 104 on coverslips in the same culture medium as their 3D counterpart. Samples were placed in a humidified incubator at 37°C in a 5% CO2 atmosphere, and medium was refreshed every other day up to 5 days of culture.
Plasmin treatment
Fibrin microfibers with or without cells were treated with 15, 1, 0.25, and 0.1 CU/ml plasmin from human plasma (Athens Research and Technology, Athens, GA, USA) in DMEM (Life Technologies for 1, 6, 12, and 24 hours, respectively, in a humidified incubator at 37°C in a 5% CO2 atmosphere. Samples were imaged immediately after treatment.
Live/dead assay
ECFCs cultured in 2-dimensional (2D) substrates and treated with plasmin were incubated with 2 μM calcein acetoxymethyl and 4 μM ethidium homodimer (Life Technologies) in PBS for 30 minutes at 37°C and 5% CO2. Samples were imaged immediately after, and the number of live and dead cells was counted using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining and imaging
Samples were processed as described previously (16). In brief, samples were fixed with 3.7% formaldehyde (Fisher Chemical, Fairlawn, NJ, USA) for 15 minutes, permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in 3.7% formaldehyde for 10 minutes, washed 3 times with PBS, and incubated for 1 hour at room temperature with the indicated primary antibodies (Supplemental Table S1). Samples were double stained with primaries from different hosts (rabbit and mouse). Samples were rinsed 3 times with PBS before being incubated with the appropriate secondary antibodies or conjugated phalloidin at room temperature for 1 hour. Samples were then rinsed with PBS 3 times and counterstained with DAPI for 10 minutes. 2D epifluorescence images were obtained using an Olympus BX60 microscope (Tokyo, Japan). 3D Z-stack and cross-sectional images were obtained and processed using confocal microscopy [LSM 700 (Carl Zeiss Incorporated, Thornwood, NY, USA) and A1-RS1 (Nikon, Melville, NY, USA)]. Because of confocal microscopy limitations when imaging large multicellular structures, only one-half of the structures (the side closest to the objective) could be imaged, resulting in semicircular cross-sections. Samples were therefore flipped over and imaged on both sides to confirm structure uniformity, and images presented are representative for both sides of the structures. Control stains were performed using control IgG primary antibodies and the same corresponding secondary, resulting in minimal to no cross-reactivity or background (Supplemental Fig. S1A).
Spatial-volume rendering
Confocal Z-stack images were processed using standard volume-rendering algorithms in Imaris (Bitplane, Zurich, Switzerland).
RT-PCR
Two-step RT-PCR was performed on 2D and 3D constructs as described previously (17). In brief, total RNA was extracted using a TRIzol protocol (Life Technologies) and quantified using a UV spectrophotometer. RNA was transcribed at 1 μg per sample using reverse-transcriptase Moloney murine leukemia virus (Promega, Madison, WI, USA) and oligo(dT) primers (Promega) according to the manufacturer’s instructions. TaqMan Universal PCR Master Mix and Gene Expression Assay (Applied Biosystems, Foster City, CA, USA) were used to quantitate the expression of COL1A1, COL3A1, COL4A1, FN1, ELN, LAMC1, ACTB (actin, beta), GAPDH (glyceraldehyde 3-phosphate dehydrogenase), and 18S (Life Technologies). The PCR step was performed for 40 cycles of 15 seconds at 95°C and 1 minute at 60°C in an Applied Biosystems StepOne Real-Time PCR System. Relative expressions of the genes were normalized to the amount of ACTB, GAPDH, or 18S in the same cDNA by using the comparative ΔΔCT method provided by the manufacturer. Samples were run in triplicate.
Statistical analyses
All experiments were performed in triplicate for at least 2 biologic replicates. Paired Student's t tests were performed to compare significance between 2D and 3D gene expression (Prism 5.01; GraphPad Software, San Diego, CA, USA), and graphs were plotted with SEM. Significance levels were determined between samples examined and are indicated in the histograms in the figures.
RESULTS
Deposition of ECM proteins by ECFCs in 3D versus 2D substrates
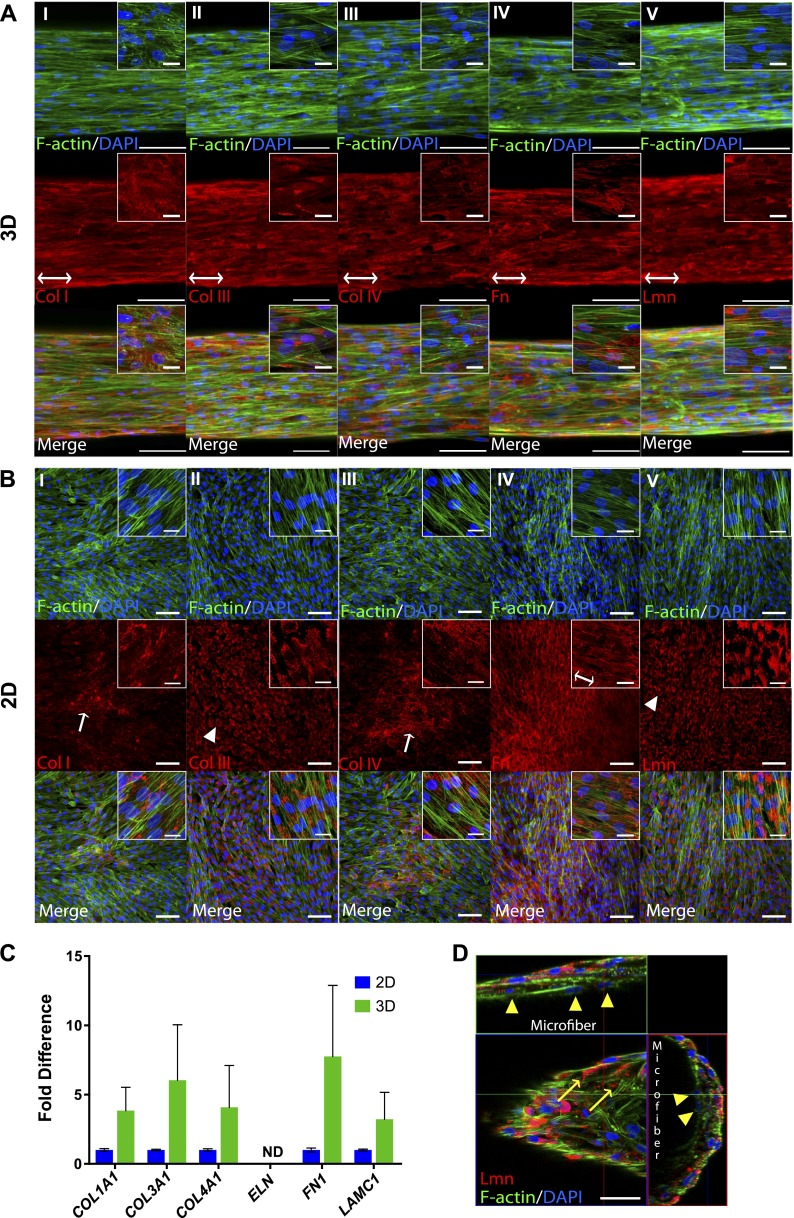
We had originally shown the ability of ECFCs to deposit basal lamina proteins Col IV, Fn, and Lmn when cultured in 2D Petri dishes (18). More recently, we demonstrated the importance of microscale curvature in regulating the organized deposition of these ECM proteins by ECFCs utilizing fibrin microfibers of different sizes (16). Specifically, we showed that microfibers with diameters ranging from 100 to 400 μm guide circumferential ECM deposition by ECFCs. Here, we evaluate whether the 3D fibrin microfibers (average diameter 188.2 ± 13.9 μm) also up-regulate the amount of ECM proteins produced by ECFCs. As shown in Fig. 1, ECFCs deposit wrapping Col IV, Fn, and Lmn after 5 days in culture on 3D fibrin microfibers and also deposit these proteins on 2D fibrin-coated substrates. However, higher amounts of all ECM proteins were observed in 3D versus 2D (Fig. 1A, B). Quantitative RT-PCR performed on these substrates also revealed a higher expression of the genes encoding these ECM proteins in 3D than in 2D (Fig. 1C). Together, these results indicate that the fibrin microfibers promote not only proper ECM organization but also increased ECM deposition compared to 2D cultures.
Figure 1.
Deposition of Col IV, Fn, and Lmn by ECFCs in 3D vs. 2D. Confocal Z-stack image projections are shown of ECM in (A) 3D fibrin microfibers and (B) 2D fibrin-coated surfaces. Red indicates corresponding ECM, blue shows nuclei, and green indicates F-actin. Scale bars, 100 μm. Inset scale bars, 25 μm. C) RT-PCR analysis of expression of ECM genes by ECFCs cultured on 2D vs. 3D. Error bars represent SEM. Significance levels in the distribution are represented by *P < 0.05 and **P < 0.01 (n ≥ 3).
Deposition of ECM proteins by pericytes in 3D versus 2D substrates
To investigate whether the quantity of ECM proteins deposited by perivascular cells was also regulated by substrate spatial dimensionality, we seeded pericytes on fibrin microfibers following the same protocol used for ECFCs. Notably, we observed that pericytes were capable of degrading fibrin microfibers before 5 days, a finding in line with the demonstrated fibrinolytic effects of other mural cells (19) but contradictory to studies that have found pericytes to express fibrinolysis inhibitors (20). To allow us to study ECM deposition after 5 days in culture, we therefore supplemented the medium with 30 mM ACA, which has been used previously to inhibit plasmin activity (21, 22).
As shown in Fig. 2A, pericytes attach and grow on fibrin microfibers. Similarly to ECFCs, pericytes also produce Col IV, Fn, and Lmn both in 2D and 3D. Additionally, they deposit Col I and Col III (Fig. 2A, B). Eln deposition was not observed either in 2D or 3D cultures as confirmed by RT-PCR (Fig. 2C). However, the amount of ECM deposited by pericytes was not distinguishably different in 2D versus 3D based on confocal microscopy. Quantitative RT-PCR analysis revealed that there is an increased expression of COL1A1, COL3A1, COL4A1, FN1, and LAMC1 but that this increase is variable and not statistically significant (Fig. 2C).
Figure 2.
Deposition of Col I, III, and IV, Fn, and Lmn by pericytes in 3D vs. 2D. Confocal Z-stack image projections are shown of ECM in (A) 3D fibrin microfibers and (B) 2D fibrin-coated surfaces. Red indicates corresponding ECM, blue shows nuclei, and green indicates F-actin. Arrows and arrowheads point to randomly deposited and nonpolymerized ECM proteins, respectively. Double-headed arrows show alignment direction of organized ECM deposition. Scale bars, 100 μm. Inset scale bars, 25 μm. C) RT-PCR analysis of expression of ECM genes by SMCs cultured on 2D vs. 3D. ND, not detected. Error bars represent SEM. D) Orthogonal view of pericytes grown on microfibers. Arrowheads point to cells underneath the outer cell layer; arrows point to extracellularly deposited Lmn. Scale bar, 50 μm.
Interestingly, ECM deposition in 2D appears either randomly organized (Fig. 2BI, BIII), nonpolymerized (Fig. 2BII, BV), or following local pericyte orientation (Fig. 2BIV), whereas in 3D, ECM deposition is organized parallel to the cell’s orientation, which follows the longitudinal axis of the microfiber (Fig. 2A). Additionally, Lmn shows a polymerized extracellular deposition in 3D compared to 2D (Fig. 2AV, BV, D). Finally, pericytes can grow in a multilayer organization on the microfibers (Fig. 2D), as opposed to the monolayer formed by ECFCs.
Deposition of ECM proteins by SMCs in 3D versus 2D substrates
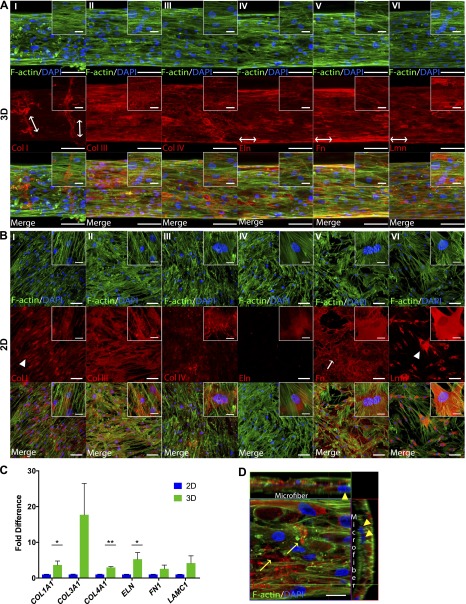
Previously, we had demonstrated that we could introduce SMCs on ECFC-seeded fibrin microfibers and obtain full mural cell investment on the developing microvascular structures. Additionally, we showed deposition of characteristic SMC ECM proteins Eln and Col I between the ECFC and SMC layer (16). To investigate whether the 3-dimensionality of the fibrin microfibers induced a higher ECM deposition by SMCs than 2D cultures, we seeded SMCs directly on fibrin microfibers and on fibrin-coated 2D surfaces. As seen in Fig. 3A, SMCs can attach and grow directly on the microfibers.
Figure 3.
Deposition of Col I, III, and IV, Eln, Fn, and Lmn by SMCs in 3D vs. 2D. Confocal Z-stack image reconstructions are shown of ECM in (A) 3D fibrin fibers and (B) 2D fibrin-coated surfaces. Red indicates corresponding ECM, blue shows nuclei, and green indicates F-actin. Arrows and arrowheads point to randomly deposited and nonpolymerized ECM proteins, respectively. Double-headed arrows show alignment direction of organized ECM deposition. Scale bars, 100 μm. Inset scale bars, 25 μm. C) RT-PCR analysis of expression of ECM genes by pericytes cultured on 2D vs. 3D. Error bars represent SEM (n ≥ 2). Significance levels in the distribution are represented by *P < 0.05 and **P < 0.01. D) Orthogonal view of pericytes grown on microfibers. Arrowheads point to cells underneath the outer cell layer; arrows point to extracellularly deposited Lmn. Scale bar, 20 μm.
Furthermore, SMCs were found to deposit Col I, III, and IV, Eln, Fn, and Lmn both in 2D and 3D cultures (Fig. 3A–C). Remarkably, Col I often presented a wrapping orientation similar to the previously demonstrated deposition of Col IV by ECFCs when cultured in 3D, compared to a sparse mostly intracellular expression in 2D (Fig. 3AI, BI). Additionally, Eln, Fn, and Lmn seem to follow an aligned deposition with cellular orientation along the longitudinal axis of the microfiber in 3D (Fig. 3AIV–VI). In contrast, Fn and Lmn presented a random or intracellular expression in 2D, respectively (Fig. 3BV–VI).
Regarding ECM quantity, Col IV and Eln appeared to be particularly up-regulated in 3D cultures, with Eln deposition being almost null in 2D but uniformly deposited in 3D (Fig. 3AIV, BIV). Indeed, all proteins were found to be expressed in higher quantities in 3D than in 2D via RT-PCR, though COL1A1, COL4A1, and ELN were the only genes significantly up-regulated with average fold differences of 3.7, 3.0, and 4.9 compared to 2D, respectively (Fig. 3C). Similarly to pericytes, SMCs in 3D grow in a multilayer organization and deposit polymerized Lmn extracellularly (Fig. 3D).
Fibrin microfiber degradation
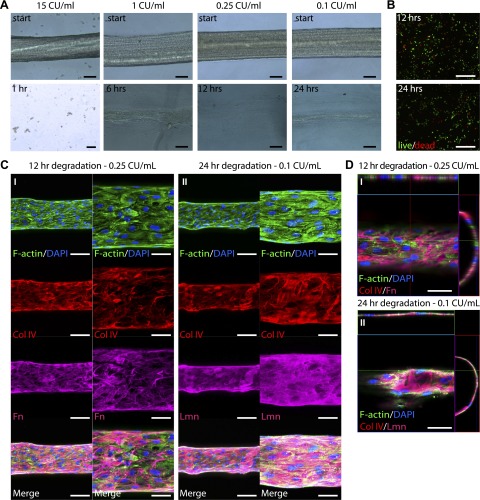
We next sought to investigate whether we could degrade the fibrin microfiber core after EC layer formation while maintaining both cellular viability and intact ECM organization. For this, we first treated fibrin microfibers without cells with different concentrations of plasmin. As expected, plasmin effectively degraded the microfibers in a concentration-dependent manner; we treated microfibers with a range of concentrations for 24 hours and identified treatments that would degrade the microfibers in 1, 6, 12, and 24 hours. Among the concentrations used, we found that 15, 1, 0.25, and 0.1 CU/ml plasmin degraded the microfibers in about 1, 6, 12, and 24 hours, respectively (Fig. 4A). It is important to note that the FBS present in regular culture media was found to interfere with the degradation process (data not shown), and therefore all plasmin treatments were performed in serum-free medium.
Figure 4.
Fiber degradation and viability of cells and ECM after plasmin treatment. A) Light-microscopy images of fibrin microfibers treated with plasmin. Scale bars, 100 μm. B) Immunofluorescence images of viability assays of ECFCs after 12- and 24-hour treatments with plasmin. Red indicates dead cells; green shows live cells. Scale bars, 500 μm. C) Confocal Z-stack image projections of fibrin fibers with ECFCs for 5 days and treated with plasmin for (I) 12 and (II) 24 hours. Scale bars, 100 μm (left) and 50 μm (right). D) Orthogonal view of structures after plasmin treatments. Green indicates F-actin, blue shows nuclei, red indicates Col IV, and magenta shows Fn (I) and Lmn (II) (n ≥ 2). Scale bars, 50 μm.
To investigate whether the degradation treatment would maintain ECFC viability, we treated confluent layers of ECFCs with the identified concentrations for each of the 4 degradation time points chosen: 15 CU/ml for 1 hour, 1 CU/ml for 6 hours, 0.25 CU/ml for 12 hours, and 0.1 CU/ml for 24 hours. Although both 1- and 6-hour treatments resulted in poor cell viability (data not shown), the 12-hour (0.25 CU/ml) and 24-hour (0.1 CU/ml) treatments resulted in 80.6 ± 4.3% and 65.2 ± 1.8% live cells, respectively (Fig. 4B), compared to 90.7 ± 7.4% when cells were cultured in regular medium (data not shown).
Finally, we tested the 12- and 24-hour degradation treatments on ECFC-seeded fibrin microfibers that had been in culture for 5 days. As shown in Fig. 4C, the previously demonstrated ECFC longitudinal alignment as well as the circumferential organization of ECM proteins was maintained after microfiber degradation in both 12- and 24-hour treatment conditions (Fig. 4CI, CII, respectively). Furthermore, the resulting structures had a distinct circular lumen, as shown in Fig. 4D.
Luminal multicellular microvascular structure
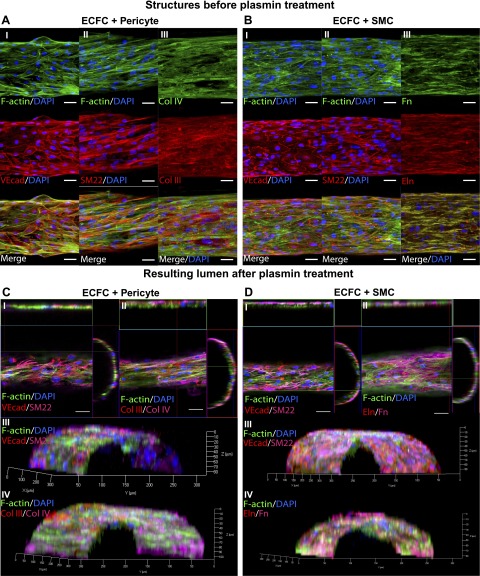
Once we demonstrated that we could degrade the fibrin microfiber using plasmin in conditions that would maintain ECFC viability and ECM protein organization, we proceeded to build multicellular microvascular structures by culturing ECFCs on fibrin microfibers for 5 days, introducing pericytes or SMCs on top of the endothelial monolayer, and continuing culture for 5 more days. As shown in Fig. 5A, B, the resulting structures contain an endothelial monolayer expressing the tight junction protein vascular endothelial cadherin (VEcad) with an ellipsoidal morphology. They also contain a fully invested perivascular multicellular layer expressing SMC and pericyte marker SM22 with an elongated, aligned morphology (shown in more detail in Supplemental Fig. S1B, C). Moreover, abundant ECM protein deposition was observed, including ECM proteins deposited by both ECFCs and mural cells as shown above (Col IV and Fn in Fig. 5AIII, BIII, respectively), mural cells only (Col III in Fig. 5AIII), and SMCs only (Eln in Fig. 5BIII). The resulting structures had a cell-ECM wall thickness of 19.9 ± 3.1 μm and 13.6 ± 0.6 μm for pericyte and SMC cocultures, respectively.
Figure 5.
ECFC and perivascular cell cocultures on fibrin microfibers. Confocal Z-stack image projections are shown of ECFCs grown for 5 days on fibrin microfibers followed by 5 days more of (A) pericyte coculture. Red indicates (I) VEcad, (II) SM22, and (III) Col III, blue shows nuclei, and green indicates F-actin. B) SMC coculture. Red indicates (I) VEcad, (II) SM22, and (III) Eln, blue shows nuclei, and green indicates F-actin. Confocal Z-stack image 3D reconstructions are shown of structures cultured for 5 days with ECFCs followed by 5 more days with perivascular cells and then treated for 12 hours with 0.25 CU/ml plasmin. C) Pericyte coculture. Red indicates (I and III) VEcad and (II and IV) Col III, magenta shows (I and III) SM22 and (II and IV) Col IV, blue indicates nuclei, and green shows F-actin. D) SMC coculture. Red indicates (I and III) VEcad and (II and IV) Eln, magenta shows (I and III) SM22 and (II and IV) Fn, blue indicates nuclei, and green shows F-actin. Scale bars, 50 μm (n ≥ 2).
We then treated constructs with 0.25 CU/ml plasmin for 12 hours and analyzed structures for lumen formation. As shown in the orthogonal views and 3D reconstructions of confocal microscopy images in Fig. 5C, D, both pericyte and SMC cocultures resulted in a distinct circular lumen comprised by ECs and perivascular cells, as evidenced by VEcad and SM22 expression. Furthermore, the structures maintained the ECM protein composition, as shown for Col III and IV (Fig. 5C) and Fn and Eln (Fig. 5D). Orthogonal projections are shown for top Z-stack slices in Fig. 5C, D, and therefore only one plane of the Z-stack image series can be observed. The 3D reconstructions show an angle view of half of the resulting cylindric microvascular structure. To better depict the different cellular and extracellular components of these structures, we processed the confocal images and created 3D spatial-volume renderings of each sample. It is important to note that because these are solid renderings, some aspects of these complex microvascular structures are located underneath the solid surface observed. Nonetheless, as observed in Fig. 6AI, BI, resulting structures contain the same ellipsoidal VEcad (in red) and elongated SM22 (in magenta)-positive cells observed in samples before plasmin treatment. The structures also contain the ECM deposited by each cell type, as shown for Col III and Col IV for ECFC-pericyte cocultures (Fig. 6AII) and for Eln and Fn for ECFC-SMC cocultures (Fig. 6BII).
Figure 6.
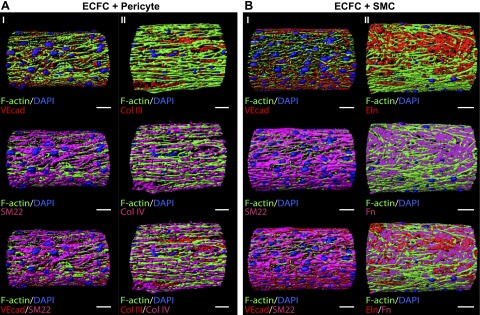
Spatial-volume rendering of multicellular microvascular structures after plasmin treatment. Structures were cultured for 5 days with ECFCs followed by 5 more days with perivascular cells and then treated for 12 hours with 0.25 CU/ml plasmin. A) Pericyte coculture. Red indicates (I) VEcad and (II) Col III, magenta shows (I) SM22 and (II) Col IV, blue indicates nuclei, and green shows F-actin. B) SMC coculture. Red indicates (I) VEcad and (II) Eln, magenta shows (I) SM22 and (II) Fn, blue indicates nuclei, and green shows F-actin. Scale bars, 50 μm.
DISCUSSION
To date, few studies have focused on the development of physiologically relevant microvasculature, which presents a current challenge in the field of tissue engineering due to the difficulty of guiding the organized formation of multicellular constructs with the appropriate cellular and ECM organization. As such, most previous studies in the field of angiogenesis, vasculogenesis, and vascular tissue engineering have either focused on studying the formation of capillaries and vessels with diameters <100 μm (23–31) or have instead aimed to develop large-diameter tissue-engineered vessels, most >3 mm in diameter (32–36).
The challenge of developing physiologically relevant microvascular structures with diameters between 100 μm and 1 mm can be divided into 3 different aspects. First, though it has been established that ECs can create vascular networks in vitro when cultured in 3D matrices such as hydrogels, these networks result in capillary beds with relatively small lumen diameters (23–27). Therefore, to create vessels with a larger diameter, there has to be a system to guide the formation of cylindric vascular structures in the order of hundreds of micrometers. Microfluidic channels have been used extensively to study microvascular development processes, yet most of these studies are done in channels with a square cross section or in nonimplantable devices (37, 38).
Recent studies have developed 3D microfluidic channel arrays with rectangular (12) or circular (11, 39–41) cross sections embedded in hydrogels. However, these present limited success for the second challenge in building microvascular structures, which is constructing a multilayered structure with a continuous endothelium and a robust supporting multicellular mural cell layer. To address the need for mural cell involvement, some of these studies incorporated perivascular cells in the hydrogels encompassing the microfluidic channels and, afterward, seeded ECs in the lumen (11, 12). Although this approach allows the study of cell recruitment, it imposes a barrier for full investment of perivascular cells, which have to migrate through the hydrogels to find the developing microvessels. As such, perivascular cells in these systems were not demonstrated to form a uniform multicellular layer on top of the endothelium. Furthermore, these systems impart a barrier for the detailed study of ECM organization and EC-mural cell interactions due to chemical and physical imaging limitations presented by the hydrogels.
The last challenge in creating microvascular vessels is developing self-supporting structures with the abundant vascular ECM deposition necessary to withstand pulsatile flow as well as vasoconstriction and vasodilatation. As such, ECM proteins such as Cols, Lmn, and Eln have been proven necessary to provide these biomechanical properties. To date, the study of ECM protein deposition by different vascular cells in 3D constructs either alone or in coculture has been extremely limited (42).
We previously established a modified electrospinning technique whereas different biomaterials were spun, collected in an aqueous phase, bundled together, and mechanically stretched to make hydrogel microfibers with an internal and external alignment. We demonstrated that the mild process conditions allowed the encapsulation of cells within the microfibers, as well as seeding of cells postspinning on top of them (15). We then utilized fibrin microfibers fabricated with this technique to guide the organized formation of a confluent and aligned EC monolayer with a cylindric 3D shape and marked expression of EC markers CD31, VEcad, and von Willebrand factor (16). We also discovered that ECFCs deposit ECM proteins with a circumferential organization, perpendicular to the cell’s alignment on the microfibers, a process that is independent of cytoskeletal organization but dependent on fiber diameter. Furthermore, we demonstrated full SMC investment on top of ECs, showing the potential for creating an organized multicellular structure, addressing the first 2 challenges in creating a microvessel.
In the present study, we address the third challenge by analyzing the relative quantities of ECM deposited on our 3D microfibers compared to 2D cultures. For ECFCs, immunofluorescence microscopy revealed increased deposition of ECM proteins Col IV, Fn, and Lmn in 3D. This increased expression was found to be about 2-fold for Fn and Lmn and almost 4-fold for Col IV, as measured by RT-PCR. These results suggest the existence of a geometric or biomechanical-sensing pathway that up-regulates the production of these proteins comprising the basal lamina of native endothelium (43). RT-PCR was used for quantification because it allows us to quantify ECM-insoluble proteins including Col I, III, and IV, Eln, as well as fibrillary Fn (44–46). It also eliminated any cross-reactivity or total protein-loading issues with the microfiber material (fibrin), which is present in exponentially higher quantities than the ECM proteins analyzed.
Similar studies performed on pericytes and SMCs revealed that these cell types produce several different ECM proteins that comprise the subendothelial connective tissue, internal elastic lamina, and ECM of the tunica media of blood vessels (47, 48). In particular, both cell types produced Col types I, III, and IV, as well as Fn and Lmn. Additionally, only SMCs produced Eln, which is in accordance to native vasculature where elastic tissue is found predominantly in arterioles and arteries with a full SMC layer and not in smaller capillaries or venules invested only by pericytes (4, 5, 47). Notably, we saw an increased average expression of all ECM proteins deposited by perivascular cells in 3D compared to 2D, though the up-regulation was only statistically significant for Col I, Col IV, and Eln deposition by SMCs. Furthermore, the morphologic expression of these proteins was found to be more organized in 3D than 2D, and Lmn was found to be deposited extracellularly in 3D only. These results suggest the existence of distinct metabolic pathways that regulate the expression and deposition of different ECM proteins by SMCs and pericytes. Overall, our findings indicate that the fibrin microfibers provide an appropriate microenvironment for abundant, organized ECM deposition.
Up to this point, the microvascular structures still have the fibrin microfiber in their core and are not yet hollow microvascular vessels. However, one of the advantages of fibrin as a biomaterial, besides its natural biocompatibility, bioadhesiveness, and angiogenic-promoting characteristics (49), is its biodegradability in response to plasmin, the enzyme responsible for eliminating fibrin blood clots in our body (50). Varying plasmin concentrations in serum-free medium were shown to degrade fibrin microfibers at different time points. We demonstrate that a 12-hour degradation treatment of 0.25 CU/ml plasmin was able to maintain a cell viability similar to control culture conditions, whereas a 24-hour treatment of 0.1 CU/ml plasmin was more detrimental to cell survival, possibly due to the longer period of serum starvation. Degradation of ECFC-seeded fibrin microfibers after 5 days of culture revealed that both the 12- and 24-hour treatment protocols were able to effectively degrade the fibrin microfiber core while maintaining the intact ECM deposited by ECFCs, resulting in an early microvascular structure comprised of an endothelial layer and its basal lamina with a clear circular lumen.
For luminal multicellular microvascular structure formation, we then introduced perivascular cells on constructs that had been cultured with ECFCs to allow full endothelial layer formation before introducing mural cells. Cocultures were further cultured and shown to be comprised of both ECFCs and mural cells along with their deposited ECM, such as Col III and Col IV for ECFC-pericyte cocultures. Remarkably, ECFC-SMC cocultures presented a bountiful Eln deposition after only 5 days of SMC growth, an elusive achievement in vascular tissue engineering typically shown in SMC cultures of over 4 weeks only (51–54). Finally, the resulting structures were shown to retain both cellular and ECM formation after degradation of the fibrin core, forming a distinct circular lumen. To date, these are the first self-standing, luminal vascular structures with a tunica intima, tunica media, and their corresponding ECM to be fabricated in vitro in only 11 days and with a diameter in the range between 100 and 500 μm.
The next step to continue the microvascular development process will be applying dynamic flow conditions through the microvascular structures since this has been shown to be necessary for proper vessel maturation. Indeed, SMCs have been demonstrated to respond to biomechanical stimuli and to align with the oscillatory strain resulting from pulsatile flow (55–57), resulting in a tunica media with a circumferential alignment of SMCs. Future studies will focus on longer coculture time points to achieve a cell layer of comparable thickness to native vasculature of similar lumen diameter—∼25 μm for a 200 μm arteriole (58)—followed by microfiber core degradation and perfusion of developing structures in a bioreactor device.
CONCLUSIONS
Altogether, we demonstrate that the 3D fibrin hydrogel microfiber system developed leads to an increased ECM protein deposition by vascular cells compared to 2D cultures. In particular, ECFCs presented a significant up-regulation of basal lamina proteins Col IV, Fn, and Lmn, pericytes showed a variable up-regulation of Col I, III, and IV, Fn, and Lmn, and SMCs evidenced a significant up-regulation of Col I and IV and Eln and a variable up-regulation of Col III, Fn, and Lmn. We further demonstrate that this increased ECM deposition, along with a robust perivascular multicellular layer, can support the formation of self-standing microvascular structures with a distinct circular lumen following microfiber core degradation. Overall, the fibrin hydrogel microfibers can be used to guide the stepwise formation of multicellular microvascular structures comprised of an organized EC monolayer, a fully invested multicellular perivascular cell layer, and an abundant amount of the corresponding ECM proteins deposited by each cell type. The system developed can serve as a model for human microvasculature recapitulating its native configuration and can therefore be an excellent tool for translational studies such as drug screening and blood-brain barrier studies, among others.
Supplementary Material
Acknowledgments
The authors thank Andrew Mao and Patrick Peña for assisting with cell culture and sample preparation. This research was partially funded by U.S. National Institutes of Health Grants R01HL107938 (National Heart, Lung, and Blood Institute) and U54CA143868 (National Cancer Institute); and a W.W. Smith Charitable Trust grant (to S.G.).
Glossary
- 2D
2 dimensional
- 3D
3 dimensional
- ACA
aminocaproic acid
- ACTB
actin, beta
- Col
collagen
- EC
endothelial cell
- ECFC
endothelial colony-forming cell
- ECM
extracellular matrix
- Eln
elastin
- FBS
fetal bovine serum
- Fn
fibronectin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Lmn
laminin
- SMC
smooth muscle cell
- VEcad
vascular endothelial cadherin
- vSMC
vascular smooth muscle cell
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Huang G. Y., Zhou L. H., Zhang Q. C., Chen Y. M., Sun W., Xu F., Lu T. J. (2011) Microfluidic hydrogels for tissue engineering. Biofabrication 3, 012001 [DOI] [PubMed] [Google Scholar]
- 2.Auger F. A., Gibot L., Lacroix D. (2013) The pivotal role of vascularization in tissue engineering. Annu. Rev. Biomed. Eng. 15, 177–200 [DOI] [PubMed] [Google Scholar]
- 3.Roy S., Ha J., Trudeau K., Beglova E. (2010) Vascular basement membrane thickening in diabetic retinopathy. Curr. Eye Res. 35, 1045–1056 [DOI] [PubMed] [Google Scholar]
- 4.Hibbs R. G., Burch G. E., Phillips J. H. (1958) The fine structure of the small blood vessels of normal human dermis and subcutis. Am. Heart J. 56, 662–670 [DOI] [PubMed] [Google Scholar]
- 5.Yen A., Braverman I. M. (1976) Ultrastructure of the human dermal microcirculation: the horizontal plexus of the papillary dermis. J. Invest. Dermatol. 66, 131–142 [DOI] [PubMed] [Google Scholar]
- 6.Weber K., Braun-Falco O. (1973) Ultrastructure of blood vessels in human granulation tissue. Arch. Dermatol. Forsch. 248, 29–44 [DOI] [PubMed] [Google Scholar]
- 7.Tsamis A., Krawiec J. T., Vorp D. A. (2013) Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J. R. Soc. Interface 10, 20121004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marieb E. N., Hoehn K. (2007) Human Anatomy & Physiology, 7th ed., Pearson Benjamin Cummings, San Francisco [Google Scholar]
- 9.Standring S. (2008) Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed., Churchill Livingstone/Elsevier, Edinburgh, United Kingdom [Google Scholar]
- 10.Jain R. K. (2003) Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 [DOI] [PubMed] [Google Scholar]
- 11.Miller J. S., Stevens K. R., Yang M. T., Baker B. M., Nguyen D.-H. T., Cohen D. M., Toro E., Chen A. A., Galie P. A., Yu X., Chaturvedi R., Bhatia S. N., Chen C. S. (2012) Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y., Chen J., Craven M., Choi N. W., Totorica S., Diaz-Santana A., Kermani P., Hempstead B., Fischbach-Teschl C., López J. A., Stroock A. D. (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 109, 9342–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann T., Nicholson B. S., Sanders J. E. (2003) Tissue engineering of perfused microvessels. Microvasc. Res. 66, 59–67 [DOI] [PubMed] [Google Scholar]
- 14.Norotte C., Marga F. S., Niklason L. E., Forgacs G. (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30, 5910–5917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S., Liu X., Barreto-Ortiz S. F., Yu Y., Ginn B. P., DeSantis N. A., Hutton D. L., Grayson W. L., Cui F.-Z., Korgel B. A., Gerecht S., Mao H. Q. (2014) Creating polymer hydrogel microfibres with internal alignment via electrical and mechanical stretching. Biomaterials 35, 3243–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreto-Ortiz S. F., Zhang S., Davenport M., Fradkin J., Ginn B., Mao H.-Q., Gerecht S. (2013) A novel in vitro model for microvasculature reveals regulation of circumferential ECM organization by curvature. PLoS One 8, e81061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanjare M., Kusuma S., Gerecht S. (2014) Defining differences among perivascular cells derived from human pluripotent stem cells. Stem Cell Reports 2, 574–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusuma S., Zhao S., Gerecht S. (2012) The extracellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells. FASEBJ. 26, 4925–4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassl E. D., Oegema T. R., Tranquillo R. T. (2002) Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J. Biomed. Mater. Res. 60, 607–612 [DOI] [PubMed] [Google Scholar]
- 20.Kim J. A., Tran N. D., Li Z., Yang F., Zhou W., Fisher M. J. (2006) Brain endothelial hemostasis regulation by pericytes. J. Cereb. Blood Flow Metab. 26, 209–217 [DOI] [PubMed] [Google Scholar]
- 21.Ahmann K. A., Weinbaum J. S., Johnson S. L., Tranquillo R. T. (2010) Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue Eng. Part A 16, 3261–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anonick P. K., Vasudevan J., Gonias S. L. (1992) Antifibrinolytic activities of alpha-N-acetyl-L-lysine methyl ester, epsilon-aminocaproic acid, and tranexamic acid. Importance of kringle interactions and active site inhibition. Arterioscler. Thromb. 12, 708–716 [DOI] [PubMed] [Google Scholar]
- 23.Hanjaya-Putra D., Yee J., Ceci D., Truitt R., Yee D., Gerecht S. (2010) Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J. Cell. Mol. Med. 14, 2436–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanjaya-Putra D., Bose V., Shen Y.-I., Yee J., Khetan S., Fox-Talbot K., Steenbergen C., Burdick J. A., Gerecht S. (2011) Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood 118, 804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis G. E., Koh W., Stratman A. N. (2007) Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res. C Embryo Today 81, 270–285 [DOI] [PubMed] [Google Scholar]
- 26.Montaño I., Schiestl C., Schneider J., Pontiggia L., Luginbühl J., Biedermann T., Böttcher-Haberzeth S., Braziulis E., Meuli M., Reichmann E. (2010) Formation of human capillaries in vitro: the engineering of prevascularized matrices. Tissue Eng. Part A 16, 269–282 [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Aledia A. S., Ghajar C. M., Griffith C. K., Putnam A. J., Hughes C. C., George S. C. (2009) Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng. Part A 15, 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi A., Miyake H., Hattori H., Kuwana R., Hiruma Y., Nakahama K., Ichinose S., Ota M., Nakamura M., Takeda S., Morita I. (2007) In vitro formation of capillary networks using optical lithographic techniques. Biochem. Biophys. Res. Commun. 358, 692–697 [DOI] [PubMed] [Google Scholar]
- 29.Dickinson L. E., Moura M. E., Gerecht S. (2010) Guiding endothelial progenitor cell tube formation using patterned fibronectin surfaces. Soft Matter 6, 5109–5119 [Google Scholar]
- 30.Tsuda Y., Shimizu T., Yamato M., Kikuchi A., Sasagawa T., Sekiya S., Kobayashi J., Chen G., Okano T. (2007) Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials 28, 4939–4946 [DOI] [PubMed] [Google Scholar]
- 31.Baranski J. D., Chaturvedi R. R., Stevens K. R., Eyckmans J., Carvalho B., Solorzano R. D., Yang M. T., Miller J. S., Bhatia S. N., Chen C. S. (2013) Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA 110, 7586–7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaz C. M., van Tuijl S., Bouten C. V. C., Baaijens F. P. T. (2005) Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 1, 575–582 [DOI] [PubMed] [Google Scholar]
- 33.Kelm J. M., Lorber V., Snedeker J. G., Schmidt D., Broggini-Tenzer A., Weisstanner M., Odermatt B., Mol A., Zünd G., Hoerstrup S. P. (2010) A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J. Biotechnol. 148, 46–55 [DOI] [PubMed] [Google Scholar]
- 34.L’Heureux N., Pâquet S., Labbé R., Germain L., Auger F. A. (1998) A completely biological tissue-engineered human blood vessel. FASEB J. 12, 47–56 [DOI] [PubMed] [Google Scholar]
- 35.Dahl S. L., Koh J., Prabhakar V., Niklason L. E. (2003) Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 12, 659–666 [PubMed] [Google Scholar]
- 36.Quint C., Kondo Y., Manson R. J., Lawson J. H., Dardik A., Niklason L. E. (2011) Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 108, 9214–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbridge S. S., Chakrabarti A., DelNero P., Kwee B., Varner J. D., Stroock A. D., Fischbach C. (2013) Physicochemical regulation of endothelial sprouting in a 3D microfluidic angiogenesis model. J. Biomed. Mater. Res. A 101, 2948–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abaci H. E., Shen Y. I., Tan S., Gerecht S. (2014) Recapitulating physiological and pathological shear stress and oxygen to model vasculature in health and disease. Sci. Rep. 4, 4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu W., DeConinck A., Lewis J. A. (2011) Omnidirectional printing of 3D microvascular networks. Adv. Mater. 23, H178–H183 [DOI] [PubMed] [Google Scholar]
- 40.Kolesky D. B., Truby R. L., Gladman A. S., Busbee T. A., Homan K. A., Lewis J. A. (2014) 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26, 3124–3130 [DOI] [PubMed] [Google Scholar]
- 41.He J., Mao M., Liu Y., Shao J., Jin Z., Li D. (2013) Fabrication of nature-inspired microfluidic network for perfusable tissue constructs. Adv. Healthc. Mater. 2, 1108–1113 [DOI] [PubMed] [Google Scholar]
- 42.Davis G. E., Senger D. R. (2005) Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 [DOI] [PubMed] [Google Scholar]
- 43.Laurie G. W., Leblond C. P., Martin G. R. (1982) Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J. Cell Biol. 95, 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lodish H., Berk A., Zipursky S. L., Matsudaira P., Baltimore D., Darnell J. (2000) Molecular Cell Biology, 4th ed, W. H. Freeman, New York [Google Scholar]
- 45.Mecham R. P., Broekelmann T. J., Fliszar C. J., Shapiro S. D., Welgus H. G., Senior R. M. (1997) Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J. Biol. Chem. 272, 18071–18076 [DOI] [PubMed] [Google Scholar]
- 46.Pankov R., Yamada K. M. (2002) Fibronectin at a glance. J. Cell Sci. 115, 3861–3863 [DOI] [PubMed] [Google Scholar]
- 47.Brooke B. S., Karnik S. K., Li D. Y. (2003) Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 13, 51–56 [DOI] [PubMed] [Google Scholar]
- 48.Hungerford J. E., Owens G. K., Argraves W. S., Little C. D. (1996) Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev. Biol. 178, 375–392 [DOI] [PubMed] [Google Scholar]
- 49.Clark R. A. F. (2003) Fibrin is a many splendored thing. J. Invest. Dermatol. 121, xxi–xxii [DOI] [PubMed] [Google Scholar]
- 50.Rijken D. C., Lijnen H. R. (2009) New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 7, 4–13 [DOI] [PubMed] [Google Scholar]
- 51.Kim B. S., Putnam A. J., Kulik T. J., Mooney D. J. (1998) Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol. Bioeng. 57, 46–54 [PubMed] [Google Scholar]
- 52.Gao J., Crapo P., Nerem R., Wang Y. (2008) Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J. Biomed. Mater. Res. A 85, 1120–1128 [DOI] [PubMed] [Google Scholar]
- 53.Patel A., Fine B., Sandig M., Mequanint K. (2006) Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc. Res. 71, 40–49 [DOI] [PubMed] [Google Scholar]
- 54.Long J. L., Tranquillo R. T. (2003) Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 22, 339–350 [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Huang G., Zhang X., Wang L., Du Y., Lu T. J., Xu F. (2014) Engineering cell alignment in vitro. Biotechnol. Adv. 32, 347–365 [DOI] [PubMed] [Google Scholar]
- 56.Liu B., Qu M. J., Qin K. R., Li H., Li Z. K., Shen B. R., Jiang Z. L. (2008) Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys. J. 94, 1497–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanjare M., Kusuma S., Gerecht S. (2013) Perivascular cells in blood vessel regeneration. Biotechnol. J. 8, 434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pocock G., Richards C. D., Richards D. A. (2013) Human Physiology, 4th ed.,Oxford University Press, Oxford, United Kingdom [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.