Abstract
Fibrosis in the old mouse heart arises partly as a result of aberrant mesenchymal fibroblast activation. We have previously shown that endogenous mesenchymal stem cells (MSCs) in the aged heart are markedly resistant to TGF-β signaling. Fibroblasts originating from these MSCs retain their TGF-β unresponsiveness and become inflammatory. In current studies, we found that these inflammatory fibroblasts secreted higher levels of IL-6 (3-fold increase, P < 0.05) when compared with fibroblasts derived from the young hearts. Elevated IL-6 levels in fibroblasts derived from old hearts arose from up-regulated expression of Ras protein-specific guanine nucleotide releasing factor 1 (RasGrf1), a Ras activator (5-fold, P < 0.01). Knockdown of RasGrf1 by gene silencing or pharmacologic inhibition of farnesyltransferase (FTase) or ERK caused reduction of IL-6 mRNA (more than 65%, P < 0.01) and decreased levels of secreted IL-6 (by 44%, P < 0.01). In vitro, IL-6 markedly increased monocyte chemoattractant protein-1-driven monocyte-to-myeloid fibroblast formation after transendothelial migration (TEM; 3-fold, P < 0.01). In conclusion, abnormal expression of RasGrf1 promoted production of IL-6 by mesenchymal fibroblasts in the old heart. Secreted IL-6 supported conversion of monocyte into myeloid fibroblasts. This process promotes fibrosis and contributes to the diastolic dysfunction in the aging heart.—Cieslik, K. A., Trial, J., Entman, M. L. Mesenchymal stem cell-derived inflammatory fibroblasts promote monocyte transition into myeloid fibroblasts via an IL-6-dependent mechanism in the aging mouse heart.
Keywords: fibrosis, RasGrf1, transendothelial migration
In previous work from our group, we have provided evidence for an important role of inflammatory signaling in the development of cardiac interstitial fibrosis (1–3). Extensive studies with models of daily noninfarctive fibrosis and angiotensin infusion in young animals revealed the up-regulated inflammatory cascade resulting in development of interstitial fibrosis in the absence of significant cardiomyocyte loss. Fibrosis was closely tied to the induction of monocyte chemoattractant protein-1 (MCP-1) (2, 3) followed by the uptake of mononuclear leukocytes. Migrated monocytes polarized initially into M1 macrophages followed by those with an M2 phenotype, a highly orchestrated sequence of events that was influenced by the presence of specific cytokine and lymphokine expression patterns (4). The myeloid cell influx was reduced after 2 weeks following the suppression of MCP-1 by TGF-β (3), which is a well-described modulator of mononuclear inflammatory processes (5–7). The hallmark of this fibrotic process is the presence of the myeloid fibroblast, which arises from M2 macrophages (4) and has also been described by others in renal and lung fibrosis (8, 9).
Simultaneous studies in our laboratory demonstrated that interstitial fibrosis occurring in an aged mouse heart was likewise associated with signaling cascades inducing the myeloid fibroblast (10). In contrast to our previous models that employ young animals, fibrosis in the aging heart continued and was associated with persistence of MCP-1 (10) as well as other inflammatory mediators that we had seen in the more acute studies. Studies in the aging mouse demonstrated that the source of persistent MCP-1 driving the myeloid fibroblast formation was fibroblasts derived from dysregulated mesenchymal stem cells (MSCs). Studies of both the MSCs and the MSC-derived fibroblasts demonstrated that they poorly responded to TGF-β signaling (11), and therefore, their production of MCP-1 was not efficiently controlled by the TGF-β pathway.
In the current communication, we wish to focus on the observation that the mesenchymal fibroblast acquires a phenotype that appears to prolong chronic inflammatory reactions within the uninjured aged myocardium. Our earlier studies focused on MCP-1 because of its association with the generation of the myeloid fibroblasts (10, 12). The current study sought to examine more closely the signaling abnormalities associated with the MSC-derived inflammatory fibroblast. It depended on the critical role of a markedly up-regulated Ras pathway and confirmed the involvement of farnesyltransferase (FTase) and ERK1/2 signaling (as previously reported) (13). The most striking cytokine abnormality associated with this augmented signaling was an increase in IL-6 expression that is controlled by Ras protein-specific guanine nucleotide releasing factor 1 (RasGrf1). The pathophysiologic consequence of increased IL-6 expression was demonstrated in an in vitro transendothelial migration (TEM) assay, where we have established that although MCP-1 is a major driving force of increased myeloid cell uptake, the addition of IL-6 significantly facilitates the generation of the myeloid fibroblasts. The studies are presented in the context of a potential mechanism of chronic cardiac inflammation associated with aging.
MATERIALS AND METHODS
Reagents
PD 0325901 was purchased from Cayman Chemical (Ann Arbor, MI, USA), Jak inhibitor was purchased from EMD Millipore (Billerica, MA, USA), FTI-277 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), brefeldin A was obtained from Sigma-Aldrich (St. Louis, MO, USA), mouse recombinant IL-6 was acquired from Biolegend (San Diego, CA, USA), and human recombinant MCP-1, human IL-6, soluble human IL-6R, soluble human gp130, and sc-144 hydrochloride were purchased from R&D Systems (Minneapolis, MN, USA).
Animals
Male C57BL/6J mice aged 3, 14, 30 mo were obtained from the Center for Comparative Medicine, Baylor College of Medicine or the U.S. National Institutes of Health (NIH) National Institute of Aging (Bethesda, MD, USA). All animals were used in agreement with the Baylor College of Medicine Animal Care and Research Advisory Committee guidelines.
Cell isolation and culture
The hearts were cut into 1 mm3 pieces, digested with Liberase TH (Roche Diagnostics, Indianapolis, IN, USA) and incubated in a 37°C shaking water bath with regular trituration by pipette to obtain a single cell suspension. Afterward, cells were centrifuged at 250 g for 5 min. The cell pellet was washed and then suspended in fibroblast growth medium (DMEM/F12, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Rockford, IL, USA) and 1% antibiotic-antimycotic (Life Technologies). This procedure results in a cell mix that comprises 1% stem cells (14). These cultures can be sustained past the Hayflick limit; therefore, we refer to them as “fibroblasts derived from young or old MSCs” (which originated from young or old hearts, respectively). Cells used for these studies were cultured up to passage number 9. It has been demonstrated by others (15, 16) and is in agreement with our data that the fibroblast imprinted phenotype is stable after prolonged in vitro culture.
For studies that require quiescence, cells were incubated in low glucose (1 g/L) DMEM (Life Technologies) supplemented with 1% antibiotic-antimycotic (Life Technologies). The cell cycle was synchronized within 24 h, the medium then was changed again, and the cell extract/medium was collected after an additional 24 h.
For all cell culture data, each experiment was done in triplicate. Biologic replicates (number of donors) are indicated in the figure legend.
Immunofluorescence
Hearts were perfused with ZnCl2/acetate-Tris fixative for 15 min and left in fixative for a total of 4 h before dehydration and embedding in paraffin. Cultured cells were fixed in 2% paraformaldehyde. Heart sections or cells were permeabilized (if applicable) with 0.5% Triton X-100. Primary antibody incubation lasted overnight for heart sections or 1 h for cultured cells. After washes, cells were incubated with an appropriate secondary antibody for 45 min (if applicable). Following secondary antibody incubation, cells were washed in PBS, and nuclei were counterstained with DAPI containing mounting medium (Life Technologies).
Because IL-6 is a secreted protein, accumulation of IL-6 in the cytoplasm was achieved by treating cells for 6 h with a protein transport inhibitor (Brefeldin A, 10 ng/ml).
Anti-IL-6 and anti-RasGrf1 antibodies were obtained from Abcam (Cambridge, MA, USA), and anti-procollagen type I and DDR2 antibodies were purchased from Santa Cruz Biotechnology. Anti-CD45 antibody was purchased from BD Biosciences (San Diego, CA, USA).
Quantitative morphometry
Five to 10 sections per heart were stained with appropriate antibodies. For each section, at least 4 pictures were taken. Sections from 3 young and 3 old (30 mo of age) animals were used for this experiment.
Quantitative PCR
Total RNA was isolated from whole hearts with TRIzol reagent (Life Technologies). RNA was transcribed to cDNA by using a Verso kit (Thermo Fisher Scientific). Quantitative PCR was performed on a CFX96 Real Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using SYBR Green Super mix (Bio-Rad) and specific primers. Gene expression was measured by the comparative CT method to calculate the amount of target mRNA normalized to an endogenous reference (Hprt or 18S). Data were expressed as a fold of mRNA level relative to mRNA expression detected in control samples. Each sample was tested in triplicate to assure reproducibility. Primers were evaluated using MIQE guidelines (17).
Primer sequences
The following primer sequences were used: IL-6, sense 5′-AGTTGCCTTCTTGGGACTGA-3′ and antisense 5′-ACAGGTCTGTTGGGAGTGGT-3′; RasGrf1, sense 5′-GCCAACACAGGCTTTTCCTCT-3′ and antisense 5′-GGAGCACATTCAGCACACGAT-3′; CLCF-1, sense 5′-ACCTACCTGAACTACCTGGGG-3′ and antisense 5′-ATTGAGGCTTCGCCACACTT-3′; CT-1, sense 5′-ACCGAATATATATGGAAGACCACC-3′ and antisense 5′-TGTTGCTGCACGTATTCCTC-3′; IL-11, sense 5′-TGCTGACAAGGCTTCGAGTAG-3′ and antisense 5′-ACATCAAGAGCTGTAAACGGC-3′; LIF, sense 5′-AACCAGATCAAGAATCAACTGGC-3′ and antisense 5′-TGTTAGGCGCACATAGCTTTT-3′; Hprt, sense 5′-GCCCCAAAATGGTTAAGGTT-3′ and antisense 5′-TTGCGCTCATCTTAGGCTTT-3′; and 18S, sense 5′-CGGACAGGATTGACAGATTG-3′ and antisense 5′-CAAATCGCTCCACCAACTAA-3′.
ELISA (IL-6 and soluble IL-6R)
Cells were seeded (105 cells/flask) then synchronized for 24 h in serum-free medium before medium was changed again. Treated or untreated cells were incubated for an additional 24 h, and then medium was collected and samples were analyzed using an IL-6 ELISA and IL-6R ELISA kits (R&D Systems), as per the manufacturer's instructions. For transfection experiments, cells were seeded at 3 × 103 cell/cm2 density.
Transfection
Transfection was carried out according to a protocol supplied by the manufacturer (Origene, Rockville, MD, USA). In brief, 24 h before transfection cells were seeded (3 × 103 cell/cm2), small interference RNA (dicer substrate 27-mer duplexes) at 1 nM concentration were transfected into cells using siTran 1.0 (Origene) and OPTI-MEM medium (Life Technologies). Twenty-four hours after transfection, medium was changed to DMEM/F12 (Life Technologies) containing 1% FBS. Medium, total RNA, or cells were processed 48 h after transfection.
TEM
Human cardiac microvascular endothelial cells obtained from Lonza (Walkersville, MD, USA) were grown on collagen type IV-coated inserts that allow cell migration through 8 μm pores. When endothelial cells reached 100% confluence, peripheral blood mononuclear cells (PBMCs) were isolated and added to the inserts. MCP-1 was used as a chemoattractant and other mediators were added as indicated. After 4 d, migrated cells were fixed at the bottom of the wells and visualized by Giemsa stain. Myeloid fibroblasts are elongated and noticeably different from other cells (18). The number of fibroblasts in the bottom of the wells was counted. Normal donor blood was obtained from human volunteers under a protocol approved by the Institutional Review Board of Baylor College of Medicine. The investigation conformed to the principles outlined in the Declaration of Helsinki. Each donor’s cells were measured in triplicate. Because of differences in absolute numbers of fibroblasts among donors, the number of cells in the experimental well was divided by the number in the corresponding control well (diluent) to normalize the data. Human recombinant proteins were purchased from R&D Systems.
Protein array
Fibroblasts derived from 3- and 30-mo-old hearts were used for this experiment. The cell cycle was synchronized by changing growth medium (see cell isolation and culture) to serum free medium for 24 h. Next, cells were incubated in fresh serum free medium for the following 24 h and then medium was collected. Secreted proteins were concentrated using a spin concentrator with 5 kDa cutoff (Sartorius, Bohemia, NY, USA). To minimize protein degradation collected medium was supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Protein (75 µg) was loaded onto mouse cytokine antibody array membranes (RayBiotech, Inc., Norcross, GA, USA). Membranes were processed according to manufacturer’s instructions, images that were developed using X-ray film developer were scanned, and densitometry was assessed by NIH ImageJ software. Data are expressed as the means ± se of the signal compared with background chemiluminescence.
Ras activation assay
Quiescent fibroblasts derived from 3- and 30-mo-old hearts were harvested in ice-cold lysis buffer per manufacturer’s instructions. Lysates (50 μl) were loaded into wells of 96 well plate precoated with Raf-1 (Raf1 binds activated Ras) (Cell Biolabs, San Diego, CA, USA). Next, the plate was washed and incubated with anti-pan-Ras antibody followed by washing and incubation with secondary horseradish peroxidase-conjugated antibody. After washing, plate was incubated with substrate solution for 20 min. Stop solution was added subsequently and the absorbance of each well was read at 450 nm.
Statistical analysis
Results are presented as means ± se. Comparison between 2 groups was made by an unpaired Student’s t test. For multiple group comparison, a 1-way ANOVA test was applied. Differences were considered statistically significant if P < 0.05.
RESULTS
Mesenchymal fibroblasts derived from the old MSCs express higher levels of IL-6
Initial analysis of fibroblasts originating from old and young cardiac MSCs revealed differential expression of various cytokines. For this experiment, quiescent fibroblasts were cultured in low glucose medium for 24 h then medium was collected and secreted proteins were analyzed via protein array. Analysis of cytokine panel (22 cytokines) revealed that fibroblasts derived from 30-mo-old MSCs secreted 3- to 7-fold higher levels of IL-6, MCP-1, Rantes (regulated on activation, normal T cell expressed and secreted) and VEGF (Table 1). As we recently reported that mesenchymal fibroblasts derived from aged hearts express higher levels of MCP-1 than younger controls (12), and we proposed that this elevated level of MCP-1 contributed to progression of cardiac fibrosis as observed in the mouse heart (10), we decided to examine if other cytokines released by old fibroblasts may contribute to this process as well. Because IL-6 has been implicated in fibrosis (19) and heart failure (20, 21), we elected to investigate the role of IL-6 in the infiltration of myeloid cells into the myocardium.
TABLE 1.
Increased secretion of inflammatory mediators by mesenchymal fibroblasts derived from 30-mo-old mouse hearts
| Protein | Aged-to-young (<3-mo-old) ratio | sem |
|---|---|---|
| IL-2 | 1.98 | 0.71 |
| IL-3 | 0.58 | 0.09 |
| IL-4 | 0.95 | 0.15 |
| IL-5 | — a | |
| IL-6 | 4.65 | 2.09 |
| IL-9 | 1.89* | 0.22 |
| IL-10 | 0.92 | 0.15 |
| IL-12p40p70 | 0.86 | 0.18 |
| IL-12p70 | 1.36 | 0.20 |
| IL-13 | 1.57 | 0.34 |
| IL-17 | ND | |
| IFN-γ | 1.41 | 0.12 |
| GCSF | 1.46 | 0.22 |
| GMCSF | 1.04 | 0.14 |
| MCP-1 | 4.16b,* | 0.17 |
| MCP-5 | 1.35 | 0.24 |
| Rantes | 3.42 | 1.07 |
| SCF | 2.75 | 1.01 |
| Thrombopoietin | 0.79 | 0.06 |
| sTNFRI | 1.49 | 0.18 |
| TNFα | 1.44 | 0.27 |
| VEGF | 6.90 | 3.02 |
Very low levels secreted by aged fibroblasts only. bPreviously reported values (12). *P < 0.05.
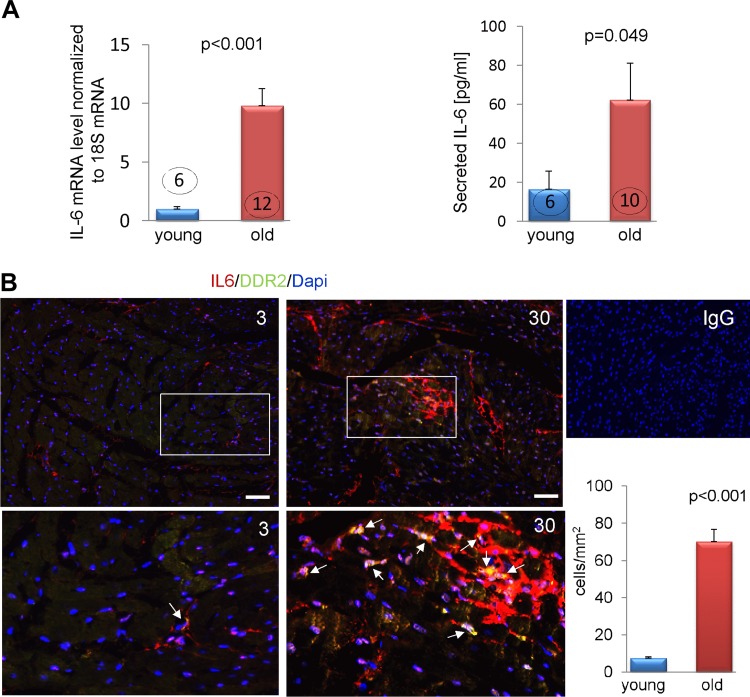
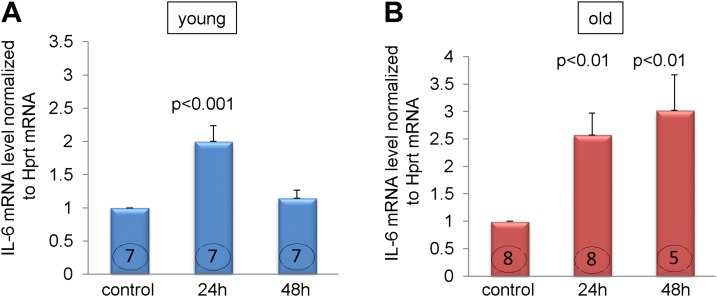
Quiescent mesenchymal fibroblasts originated from young and old MSC were analyzed for expression of IL-6 using more sensitive (than protein array) methods. We found that fibroblasts derived from old MSCs maintain elevated transcription levels of IL-6 by 9-fold (Fig. 1A, left panel) and secreted a 3-fold higher level of IL-6 protein (determined by ELISA) (Fig. 1A, right panel) within 24 h compared with controls derived from young MSCs. Next, we verified in heart sections the presence of cells expressing discoid domain receptor 2 (DDR2, a marker of cardiac fibroblasts) that simultaneously express IL-6. We found that these fibroblasts were mostly absent in young hearts (Fig. 1B). Morphometric analysis indicated that the number of DDR2+IL-6+ fibroblasts in the old hearts increases by 8-fold (Fig. 1B, bottom panel). Concomitantly, the number of IL-6+ procollagen type I+ fibroblasts in the old heart was also increased. Interestingly, we also observed increased number of IL-6+ procollagen type I+ fibroblasts in the middle-aged mice (14 mo old), indicating that the adverse changes leading to a switch of phenotype happen relatively early in life (Supplemental Fig. 1).
Figure 1.
The expression of IL-6 in cardiac mesenchymal fibroblasts is affected by aging. A) Expression of IL-6 in quiescent fibroblasts derived from young and old MSCs were analyzed by quantitative PCR and by ELISA, 24 h after the cell cycle was synchronized. B) Double immunofluorescence labeling of IL-6 (red) and DDR2 (green) in 3- and 30-mo-old mouse hearts. Double positive cells are visualized in yellow/orange color. Middle panel shows magnified image of the section marked by white squares. Bottom panel shows morphometric analysis of IL-6+DDR2+ cells in heart sections. Nuclei were stained with DAPI. Scale bar, 20 μm. P < 0.05 denotes statistical significance. The number in each column corresponds to the number of donor animals from which cells were derived for these experiments except for immunofluorescence staining where sections from 3 animals per age-group were analyzed. Young or 3 signifies cells or hearts derived from 3-mo-old mice and old or 30 denotes cells or hearts obtained from 30-month old mice. Arrows point to double positive IL6+DDR2+ fibroblasts.
Because IL-6 can be also expressed by myeloid cells, old hearts were analyzed for the presence of IL-6+CD45+ cells. We found very few double positive cells; the majority of cells expressing IL-6 were CD45neg (Supplemental Fig. 1B).
Increased IL-6 expression in fibroblasts derived from old MSCs depends on the Ras-ERK pathway
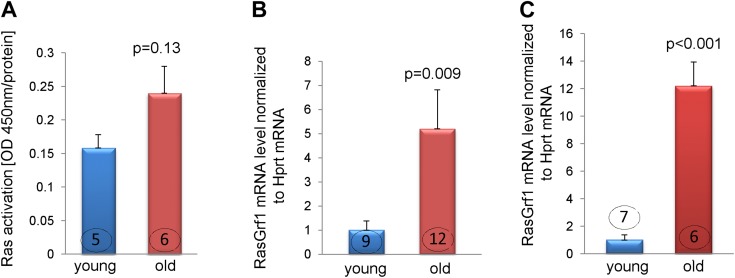
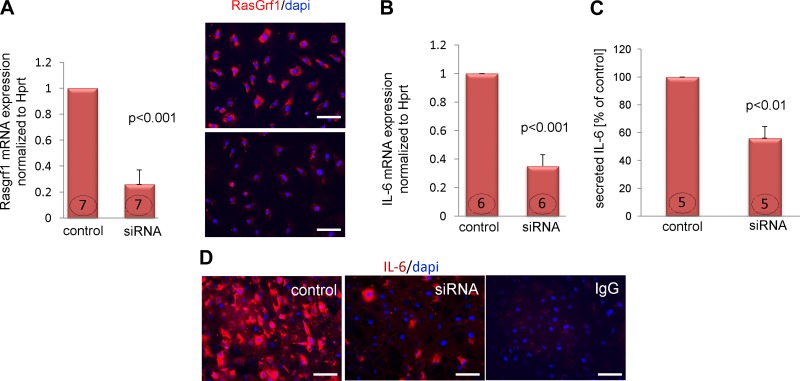
The aging fibroblast has been associated with a secretory phenotype (22). The mechanism by which the expression of soluble factors is up-regulated is not fully understood; however, our previously published data showed that at least some of these effects are mediated via the FTase-ERK pathway (13). Participation of FTase in these processes suggests that Ras proteins may be involved as well, because the farnesyl chain (transferred by FTase) attaches Ras to the cell membrane and allows proper Ras function. Fibroblasts derived from young and old MSCs were subjected to a Ras activation assay. We found that there was a 60% increase in Ras activation (measured as GTP-bound Ras) in fibroblasts that originated from old MSCs (Fig. 2A) and although that difference was not statistically significant (P = 0.13), there is a consistent trend based on our current and previous data (13) suggesting that Ras pathway is involved in activation of the secretory fibroblast phenotype. Next, we analyzed the expression of RasGrf1, a protein that catalyzes the exchange of GDP to GTP and effectively changes inactive Ras to its active form. The expression of RasGrf1 was elevated by 5-fold in fibroblasts derived from the old MSCs (Fig. 2B), and by 12-fold in old whole-heart lysates isolated from 30-mo-old animals (Fig. 2C). Next, we questioned if RasGrf1 expression controls IL-6 transcriptional activation. To answer that question, we demonstrated that transient RasGrf1 knockdown (Fig. 3A; left and right panels) resulted in an ~75% reduction in RasGrf1 mRNA level, and noticeable reduction of protein expression. RasGrf1 knockdown also affected IL-6 mRNA and protein levels, which were down-regulated by about 65 and 44%, respectively (Fig. 3B, C), suggesting that the Ras pathway is involved in IL-6 transcriptional control. Fig. 3D shows representative images illustrating the effect of RasGrf1 silencing on cytoplasmic IL-6 expression. These images support results shown on Fig. 3B, C.
Figure 2.
RasGrf1 expression is elevated in fibroblasts derived from old MSCs. A). Ras activation as measured in quiescent fibroblasts derived from young and old MSCs. Results were normalized to protein content of cell lysates. B and C) Increased expression of RasGrf1 mRNA in fibroblasts derived from old MSCs (B) or old hearts (as the whole-heart lysate) (C) as measured by quantitative PCR. Young and old signifies cells derived from 3- and 30-mo-old mice, respectively. P < 0.05 denotes statistical significance; the number in each column corresponds to the number of donor animals from which cells were derived.
Figure 3.
RasGrf1 expression controls IL-6 transcription in fibroblasts derived from the old MSCs. A). Silencing RasGrf1 by siRNA in fibroblasts derived from old MSCs reduced RasGrf1 mRNA (left panel) and protein (right panel) levels. Fibroblasts were transfected with scrambled siRNA (labeled: control) or siRNA targeting RasGrf1 (labeled: siRNA). RNA and protein analysis was carried 48 h posttransfection. B and C) RasGrf1 silencing caused a decrease of IL-6 transcription (B) and reduced levels of secreted IL-6 (C). D) Cells transfected with scrambled siRNA or RasGrf1 siRNA were treated with 10 ng/ml brefeldin A for 6 h (starting 42 h posttransfection). Cytoplasmic IL-6 was visualized by immunofluorescence labeling. Scale bar, 50 μm. P < 0.05 denotes statistical significance; the number in each column corresponds to the number of donor animals from which cells were derived for these experiments except for immunofluorescence staining where transfected cells from 3 independent experiments were analyzed.
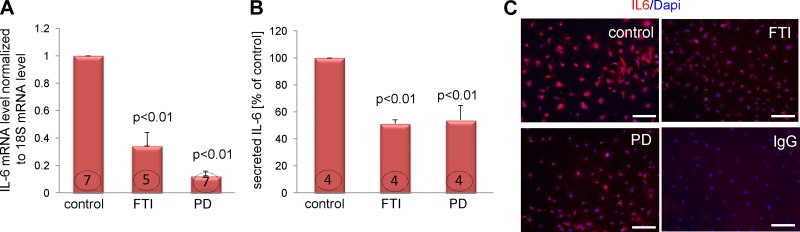
To further examine the downstream effectors of this pathway, we treated quiescent fibroblasts derived from old MSCs with an FTase inhibitor (100 nM FTI-277) or an inhibitor of ERK signaling (2 μM PD0325901) 24 h before measuring IL-6 levels. We found that both inhibitors reduced IL-6 messenger RNA level by at least 70% (Fig. 4A) as well as the amount of secreted IL-6 by 50% (Fig. 4B). Likewise, inhibition of FTase or ERK reduced cytoplasmic IL-6 levels as well (Fig. 4C).
Figure 4.
The FTase-ERK pathway contributes to IL-6 expression in cardiac fibroblasts. Quiescent fibroblasts derived from the old MSCs were treated with 100 nM FTI-277 (FTI) or 2μM PD 0325901 (PD) for 24 h. A–C) IL-6 mRNA level (A), IL-6 secretion (B), and cytoplasmic IL-6 (C) are shown. To visualize cytoplasmic IL-6 cells were treated with 10 ng/ml brefeldin A for 6 h. Scale bar, 50 μm. P < 0.05 denotes statistical significance; the number in each column corresponds to the number of donor animals from which cells were derived for these experiments. For immunofluorescence staining treated cells from 3 independent experiments were analyzed.
As the regulation of IL-6 gene expression has been studied by others extensively (23, 24), it has been suggested that in some cells IL-6 expression can be modulated via its positive feedback loop (25). Therefore, we treated fibroblasts derived from young and old MSCs with recombinant IL-6 (50 ng/ml) (19) for 24 and 48 h. We found that although fibroblasts from both age-groups responded similarly to the treatment with recombinant IL-6 within the first 24 h by up-regulating its own expression by at least 2-fold (Fig. 5, left and right panel), the clear difference was when cells were stimulated for 48 h. Cells derived from young MSCs reduced elevated IL-6 expression almost to the level observed in the untreated controls, whereas cells derived from the old MSCs not only maintained but even slightly increased their expression of IL-6 mRNA after 48 h, suggesting that there is a control mechanism present in fibroblasts derived from young but not old MSCs.
Figure 5.
Differential response to recombinant IL-6 in fibroblasts derived from young and old MSCs. Quiescent fibroblasts were treated with one dose of recombinant IL-6 (50 ng/ml) and then cells were harvested 24 and 48 h later. A) Response of fibroblast derived from the young MSCs. B) Responses from fibroblasts derived from the old MSCs. P < 0.05 denotes statistical significance; the number in each column corresponds to the number of donor animals from which cells were derived for these experiments. Young signifies cells derived from 3-mo-old mice, and old denotes cells derived from 30-mo-old mice.
IL-6 enhances MCP-1-driven leukocyte-to-fibroblast transition
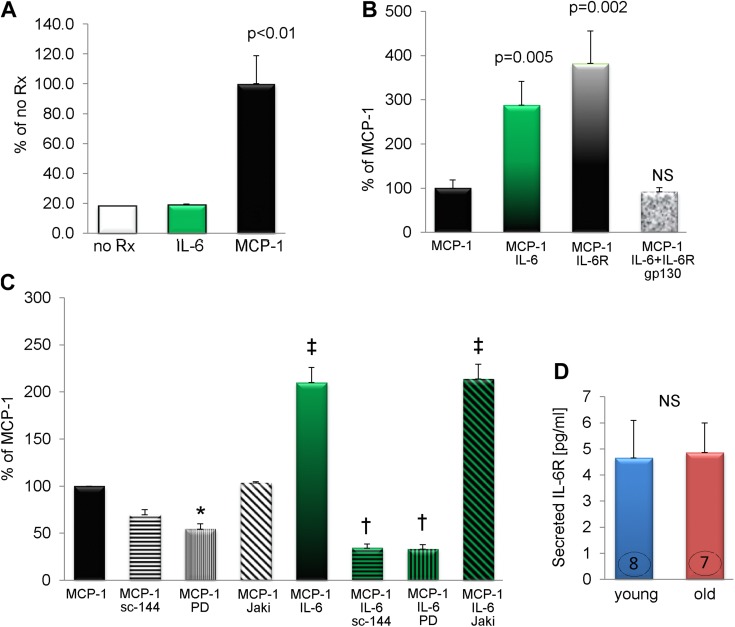
In the in vitro TEM assay, endothelial cells were grown on inserts (that allow cell migration via 8 μm pores) to form a monolayer (see schema on Supplemental Fig. 2). We have used this in vitro method in studying myeloid fibroblast formation and found it predicts and informs our previous studies (2, 4, 10). Once cells reached 100% confluence, PBMCs were placed on top of the endothelial cell layer. Medium below the insert was supplemented with or without chemoattractant and 4 d later cells that migrated through the pores and became M2 macrophage-derived myeloid fibroblasts (that can be identified by a change of morphology) were counted. In this experiment, we tested several hypotheses. First, we asked the question if IL-6 attracts PBMCs through the endothelial barrier. We found that the number of cells that migrated in response to IL-6 was equal to the number of cells that migrated in control wells (in which medium below the insert was not supplemented with any chemoattractant) (Fig. 6A). When we counted cells that migrated toward MCP-1, we found (as we have shown before) (2, 4) robust cell migration and maturation into M2 macrophages-myeloid fibroblasts. This MCP-1-dependent monocyte-to-fibroblast transition was considered a positive control. Next, we asked if IL-6 alters MCP-1-dependent responses: medium below the insert was supplemented with IL-6 and MCP-1 together (Fig. 6B). The number of cells that responded to this treatment nearly tripled suggesting that IL-6 facilitates this transition.
Figure 6.
IL-6 facilitates MCP-1-driven monocyte to myeloid fibroblast maturation. For experiments (A–C) PBMCs were added on top of a confluent endothelial cell monolayer seeded on an insert. Medium below the insert was supplemented as indicated. The following reagents were used: IL-6 (10 ng/ml), MCP-1 (650 ng/ml), IL-6R (200 ng/ml), soluble gp130 (300 ng/ml), sc-144 hydrochloride (5 μM), PD 0325901 (1 μM), Jak inhibitor (2 μM). noRX, no supplementation; sc-144, sc-144 hydrochloride; PD, PD 0325901; Jaki, Jak inhibitor. D) Fibroblasts derived from young and old MSCs secrete the same level of soluble IL-6R. P < 0.05 denotes statistical significance. n = 3, 5, 4 for (A), (B), and (C), respectively. (D) Number in each column corresponds to the number of donor animals from which cells were used for these experiments. Young signifies cells derived from 3-mo-old MSCs, and old denotes cells derived from 30-mo-old MSCs. *P < 0.05 (vs. MCP-1), †P < 0.001 (vs. MCP-1+IL-6), ‡P < 0.001 (vs. MCP-1). NS, no statistical significance.
It has been demonstrated that IL-6 requires 2 types of receptors for signaling, IL-6R and gp130 (26). Some cells generate a soluble IL-6R that binds to gp130 on other cells. This allows IL-6 signaling on cells that do not express IL-6R (or expression is low), such as endothelial cells. We found that the soluble IL-6R supplementation also enhanced MCP-1-driven leukocyte to M2 macrophage/myeloid fibroblast maturation. On the other hand, soluble gp130 reduced IL-6-mediated signaling via IL-6/IL-6R sequestration (Fig. 6B), as described by others (27). The fact that addition of the soluble IL-6 receptor increased monocyte-to-fibroblast maturation indicated that there may be a supply of endogenous IL-6 generated during the TEM. This possibility was validated by the fact that the inhibitors of IL-6 signaling pathway such as inhibitor of gp130 signaling (sc-144 hydrochloride) decreased the MCP-1-dependent myeloid fibroblast numbers by 30% (Fig. 6C).
Activation of gp130 receptor results in an initiation of 2 signaling cascades: Janus kinase-signal transducer and activator of transcription (Jak-Stat) and Ras-ERK pathways (28). IL-6+MCP-1-mediated monocyte-to-myeloid fibroblast transition was reduced in the presence of gp130 receptor inhibitor (sc-144 hydrochloride) and ERK pathway inhibitor (PD 0325901) (Fig. 6C). The degree of inhibition indicated that IL-6 became the dominant effector under conditions of exogenous excess. In contrast, IL-6-mediated transition of monocyte into myeloid fibroblasts was not dependent on the Jak pathway because addition of Jak inhibitor did not alter the number of myeloid fibroblasts. An ERK inhibitor also reduced number of myeloid fibroblasts in wells treated only with MCP-1. ERK-dependent transition of monocyte to myeloid fibroblast has also been demonstrated by others (29).
Finally, we examined whether fibroblasts derived from old MSC might secrete IL-6R as a mechanism for enhanced IL-6 signaling. We demonstrated that fibroblasts from both age-groups (young and old) secreted the same level of IL-6R, suggesting that the mechanism behind enhanced monocyte-to-fibroblast transition in the aged mouse heart depends on elevated IL-6 levels rather than an increased donation of soluble IL-6R by mesenchymal fibroblasts derived from old MSC (Fig. 6D).
Expression of other than IL-6 gp130 family members by mesenchymal fibroblasts
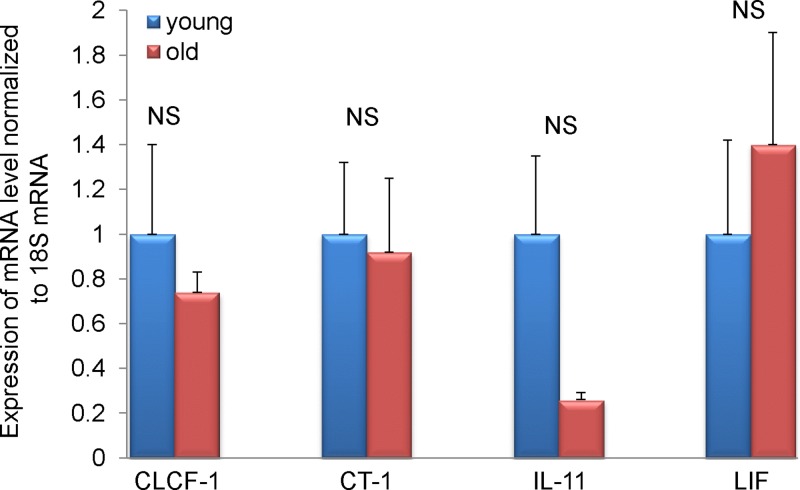
There are several other cytokines that signal via gp130 receptor such as cardiotrophin-like cytokine factor 1 (CLCF1), cardiotrophin-1 (CT-1), leukemia inhibitory factor (LIF), oncostatin M (OSM), IL-11, IL-27, and ciliary neurotrophic factor (CNTF). We have chosen to test the levels of mRNA expression of CLCF-1, CT-1, LIF, IL-11, and OSM based on their role in heart physiology/pathophysiology. Similar levels of CLCF-1, CT-1, and LIF mRNA were detected in fibroblasts derived from 3- and 30-mo-old MSCs (Fig. 7). There was a decrease of IL-11 expression in old fibroblasts, but the difference did not reach statistical significance (P = 0.1). Interestingly it has been demonstrated that IL-11 ameliorates postmyocardial infarction fibrosis (30), corroborating the notion that the aging heart displays greater postinfarct remodeling (31). Additionally, OSM mRNA could not be detected.
Figure 7.
Expression of gp130 family cytokines in fibroblasts derived from young and old MSCs. Expression of CLCF-1, CT-1, IL-11, and LIF measured by quantitative PCR. NS, no statistical significance. Young and old signify cells derived from 3- and 30-mo-old MSCs, respectively. n = 5, 8 for fibroblast derived from young and old MSCs, respectively.
DISCUSSION
Inflammatory fibroblasts that secrete IL-6 and other cytokines have also been described in other pathophysiologic conditions such as rheumatoid arthritis (32), cancer (33), pulmonary hypertension (34), inflammatory dilated cardiomyopathy (35), chronic hepatitis (36), and systemic sclerosis (37). Inflammatory fibroblasts can promote tissue damage during injury. They can also switch the acute inflammatory phase into chronic inflammation (38). It has been reported that fibroblasts originating from an inflamed environment acquire an inflammatory phenotype characterized by release of MCP-1, IL-6, and IL-8 (39). It has been recognized that proinflammatory fibroblasts can drive the homing of circulating leukocytes (40) by affecting the endothelium via cytokine-induced expression of adhesion molecules (41). On the other hand, once leukocytes transmigrate into the tissue they can influence fibroblasts; cytokines secreted by M1 (classically activated macrophages) induce fibroblasts to release proinflammatory cytokines and enzymes degrading extracellular matrix, whereas M2 factors increase fibroblast proliferation and collagen production (42). Because we have reported both proinflammatory cytokine production (12) and increased collagen production in fibroblasts derived from old MSCs (13), each cell type (MSC-derived fibroblasts and myeloid cells) may augment each other’s pathophysiologic role in fibrosis.
Our previous data suggested that fibroblasts derived from old MSCs demonstrated marked activation of FTase (13). This let to our investigation of the Ras signaling cascade. The difference in basal Ras activity between young and old fibroblasts that we reported in Fig. 2A was not statistically significant. As provided by Cell Biolabs product manual, Ras activity in stimulated HeLa cells was 45–70% higher than basal level, suggesting that the observed 60% difference between young and old fibroblasts may be of biologic relevance. The elevated Ras activity in fibroblasts derived from old MSCs was accompanied by a 5-fold increase in RasGrf1 expression. Knockdown of RasGrf1 markedly reduced IL-6 transcription and secretion. In agreement with our data, increased expression of RasGrf1 has been associated with another inflammatory condition, rheumatoid arthritis (43). Moreover, knockdown of RasGrf1 in fibroblast like synoviocytes resulted in a 50% reduction of IL-6 secretion (43), similar to our findings. Furthermore, down-regulation of Ras signaling resulted in suppression of ERK activation and IL-6 production (44, 45).
RasGrf1 in aging
RasGrf1 is an imprinted gene that is expressed only after birth (46). Genetic deletion of RasGrf1 leads to changes in the metabolism of aged mice to resemble calorie-restricted models (47), which are known to promote longevity and have reduced circulating insulin levels (48). This correlates well with our own observation that aged mice have high circulating insulin levels and the demonstration that insulin activated the Ras-ERK pathway in fibroblasts derived from old MSCs (13). We also reported that insulin negatively influences pluripotent stem cells by causing loss of stemness (indicated by a reduced expression of Nanog) (13) and premature differentiation. Others have shown that pluripotent stem cells, when isolated from aged animals, had elevated expression of RasGrf1 compared with young controls (49). Thus overactive Ras signaling may contribute to premature aging and loss of effective repair mechanism via depletion of the existing stem cell pool.
MSCs as an inflammatory mediator in the aged heart
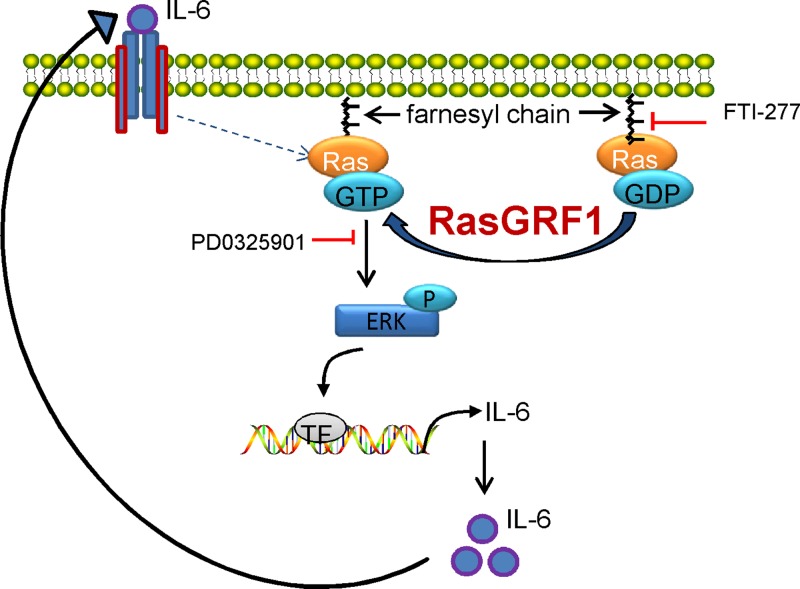
Our data, when considered with our previous experiments, allow us to propose the following construct to explain the inflammatory fibroblast and its role in fibrosis. MSCs from the aged heart and fibroblasts derived from them were markedly unresponsive to TGF-β. Because TGF-β ordinarily suppresses chemokine synthesis (7), we have postulated that this abnormality accounts for the persistent expression of MCP-1. MCP-1 attracts mononuclear cells to the heart, allowing their transition into M2 macrophage/myeloid fibroblasts (2, 4). In the current report, we demonstrate that the fibroblasts derived from old MSCs had a marked increase in RasGrf1, which is responsible for the augmented Ras pathway and increase in IL-6 synthesis dependent upon it (see schema on Fig. 8). Our data demonstrate that MCP-1 and IL-6 interact to markedly augment production of myeloid fibroblasts from M2 macrophages. These fibroblasts have been associated with pathologic fibrotic states in the kidney, lung, and heart (8–10).
Figure 8.
Role of elevated RasGrf1 expression in amplified IL-6 levels in fibroblasts derived from the old MSCs. Increased expression of RasGrf1 promotes exchange of GDP into GTP on Ras and renders its activation. Ras activation is also controlled by FTase activity since use of the FTase inhibitor (FTI-277) reduces IL-6 expression. Downstream of Ras and FTase, ERK activation controls IL-6 expression; use of PD 0325901 (ERK inhibitor) down-regulates IL-6 levels. Finally, IL-6 that is secreted activates its own transcription in a feed-forward loop.
As a result of defective TGF-β signaling, we also found that MSCs from aged heart do not respond to the signaling through TGF-β ligands that maintains stemness and prevents differentiation (11–13). There is, thus, also an increase in MSC-derived fibroblasts that are actively secreting collagen in response to the FTase-ERK pathway (13).
Altered phenotype is stable in fibroblasts derived from old MSCs
In this study, we have used cultured fibroblasts that originated from young and old MSCs. We have shown before that MSCs derived from aged hearts expressed higher levels of MCP-1 than the young controls and that the defect was passed onto their progeny cells; fibroblasts derived from these MSC expressed the same inflammatory phenotype (12). Importantly, fibroblasts consistently maintained their inflammatory phenotype passage after passage. Others have shown that fibroblasts derived from an inflammatory environment express higher levels of cytokines, when compared with fibroblasts derived from normal tissue (50). Moreover, it has been demonstrated that once the change in expression occurred, the imprinted phenotype was stable after prolonged in vitro culture (15, 16), which is in agreement with our own data.
CONCLUSIONS
This report provides a novel insight into the mechanism of inflammation and TEM supported by stromal cells. To our knowledge, we are the first to point out the RasGrf1-IL-6 axis in the aging mouse heart. Collectively, data presented here and before (10, 12) suggest that chronic, low-level inflammation derived from fibroblasts mediates an influx of mononuclear leukocytes in the aging heart that may promote fibrosis and lead to heart failure. A link between IL-6 and M2 polarized macrophages (51) and a correlation between elevated IL-6 signaling and heart failure in patients has been established (20). Learning about the molecular mechanisms behind these processes may result in development of novel strategies for maintenance of the healthy aging heart wherein dysregulated Ras signaling or more specifically RasGrf1 becomes a new target for chronic inflammatory diseases.
Supplementary Material
Acknowledgments
The authors thank Dr. Jeffrey Crawford for conducting protein array experiment. They also thank Christina Sam and Dorellyn Lee for excellent technical assistance. This work was supported by U.S. National Institutes of Health National Heart, Lung, and Blood Institute Grant R01HL089792 (to M.L.E.), a Medallion Foundation grant (to K.A.C.), and the Hankamer Foundation.
Glossary
- CLCF1
cardiotrophin-like cytokine factor 1
- CNTF
ciliary neurotrophic factor
- CT-1
cardiotrophin-1
- FBS
fetal bovine serum
- FTase
farnesyltransferase
- LIF
leukemia inhibitory factor
- MCP-1
monocyte chemoattractant protein-1
- MSC
mesenchymal stem cell
- OSM
oncostatin M
- PBMC
peripheral blood mononuclear cell
- TEM
transendothelial migration
- RasGrf1
Ras protein-specific guanine nucleotide-releasing factor 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Haudek S. B., Xia Y., Huebener P., Lee J. M., Carlson S., Crawford J. R., Pilling D., Gomer R. H., Trial J., Frangogiannis N. G., Entman M. L. (2006) Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl. Acad. Sci. USA 103, 18284–18289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haudek S. B., Trial J., Xia Y., Gupta D., Pilling D., Entman M. L. (2008) Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc. Natl. Acad. Sci. USA 105, 10179–10184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haudek S. B., Cheng J., Du J., Wang Y., Hermosillo-Rodriguez J., Trial J., Taffet G. E., Entman M. L. (2010) Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 49, 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trial J., Cieslik K. A., Haudek S. B., Duerrschmid C., Entman M. L. (2013) Th1/M1 conversion to th2/m2 responses in models of inflammation lacking cell death stimulates maturation of monocyte precursors to fibroblasts. Front. Immunol. 4, 287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 90, 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D., et al. (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg M. W., Shimizu K., Lebedeva M., Haspel R., Takayama K., Chen Z., Frederick J. P., Wang X. F., Simon D. I., Libby P., Mitchell R. N., Jain M. K. (2004) Essential role for Smad3 in regulating MCP-1 expression and vascular inflammation. Circ. Res. 94, 601–608 [DOI] [PubMed] [Google Scholar]
- 8.Xia Y., Entman M. L., Wang Y. (2013) CCR2 regulates the uptake of bone marrow-derived fibroblasts in renal fibrosis. PLoS ONE 8, e77493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L., Louie M. C., Vannella K. M., Wilke C. A., LeVine A. M., Moore B. B., Shanley T. P. (2011) New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L341–L353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cieslik K. A., Taffet G. E., Carlson S., Hermosillo J., Trial J., Entman M. L. (2011) Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J. Mol. Cell. Cardiol. 50, 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cieslik K. A., Trial J., Entman M. L. (2011) Defective myofibroblast formation from mesenchymal stem cells in the aging murine heart rescue by activation of the AMPK pathway. Am. J. Pathol. 179, 1792–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cieslik K. A., Trial J., Crawford J. R., Taffet G. E., Entman M. L. (2014) Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. J. Mol. Cell. Cardiol. 70, 56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cieslik K. A., Trial J., Carlson S., Taffet G. E., Entman M. L. (2013) Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: role of elevated circulating insulin levels. FASEB J. 27, 1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson S., Trial J., Soeller C., Entman M. L. (2011) Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc. Res. 91, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsey M. L., Goshorn D. K., Squires C. E., Escobar G. P., Hendrick J. W., Mingoia J. T., Sweterlitsch S. E., Spinale F. G. (2005) Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc. Res. 66, 410–419 [DOI] [PubMed] [Google Scholar]
- 16.Squires C. E., Escobar G. P., Payne J. F., Leonardi R. A., Goshorn D. K., Sheats N. J., Mains I. M., Mingoia J. T., Flack E. C., Lindsey M. L. (2005) Altered fibroblast function following myocardial infarction. J. Mol. Cell. Cardiol. 39, 699–707 [DOI] [PubMed] [Google Scholar]
- 17.Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L., Vandesompele J., Wittwer C. T. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 [DOI] [PubMed] [Google Scholar]
- 18.Haudek S. B., Gupta D., Dewald O., Schwartz R. J., Wei L., Trial J., Entman M. L. (2009) Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc. Res. 83, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meléndez G. C., McLarty J. L., Levick S. P., Du Y., Janicki J. S., Brower G. L. (2010) Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56, 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plenz G., Song Z. F., Tjan T. D., Koenig C., Baba H. A., Erren M., Flesch M., Wichter T., Scheld H. H., Deng M. C. (2001) Activation of the cardiac interleukin-6 system in advanced heart failure. Eur. J. Heart Fail. 3, 415–421 [DOI] [PubMed] [Google Scholar]
- 21.Tsutamoto T., Hisanaga T., Wada A., Maeda K., Ohnishi M., Fukai D., Mabuchi N., Sawaki M., Kinoshita M. (1998) Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J. Am. Coll. Cardiol. 31, 391–398 [DOI] [PubMed] [Google Scholar]
- 22.Acosta J. C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J. P., Athineos D., Kang T. W., Lasitschka F., Andrulis M., Pascual G., Morris K. J., Khan S., Jin H., Dharmalingam G., Snijders A. P., Carroll T., Capper D., Pritchard C., Inman G. J., Longerich T., Sansom O. J., Benitah S. A., Zender L., Gil J. (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libermann T. A., Baltimore D. (1990) Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 10, 2327–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T., Akira S. (1993) Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 90, 10193–10197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigert C., Düfer M., Simon P., Debre E., Runge H., Brodbeck K., Häring H. U., Schleicher E. D. (2007) Upregulation of IL-6 mRNA by IL-6 in skeletal muscle cells: role of IL-6 mRNA stabilization and Ca2+-dependent mechanisms. Am. J. Physiol. Cell Physiol. 293, C1139–C1147 [DOI] [PubMed] [Google Scholar]
- 26.Jones S. A., Horiuchi S., Topley N., Yamamoto N., Fuller G. M. (2001) The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 15, 43–58 [DOI] [PubMed] [Google Scholar]
- 27.Jones S. A., Rose-John S. (2002) The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim. Biophys. Acta 1592, 251–263 [DOI] [PubMed] [Google Scholar]
- 28.Ogata A., Chauhan D., Teoh G., Treon S. P., Urashima M., Schlossman R. L., Anderson K. C. (1997) IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J. Immunol. 159, 2212–2221 [PubMed] [Google Scholar]
- 29.Williams S. M., Golden-Mason L., Ferguson B. S., Schuetze K. B., Cavasin M. A., Demos-Davies K., Yeager M. E., Stenmark K. R., McKinsey T. A. (2014) Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J. Mol. Cell. Cardiol. 67, 112–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obana M., Maeda M., Takeda K., Hayama A., Mohri T., Yamashita T., Nakaoka Y., Komuro I., Takeda K., Matsumiya G., Azuma J., Fujio Y. (2010) Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation 121, 684–691 [DOI] [PubMed] [Google Scholar]
- 31.Bujak M., Kweon H. J., Chatila K., Li N., Taffet G., Frangogiannis N. G. (2008) Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J. Am. Coll. Cardiol. 51, 1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georganas C., Liu H., Perlman H., Hoffmann A., Thimmapaya B., Pope R. M. (2000) Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-kappa B but not C/EBP beta or c-Jun. J. Immunol. 165, 7199–7206 [DOI] [PubMed] [Google Scholar]
- 33.Studebaker A. W., Storci G., Werbeck J. L., Sansone P., Sasser A. K., Tavolari S., Huang T., Chan M. W., Marini F. C., Rosol T. J., Bonafé M., Hall B. M. (2008) Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 68, 9087–9095 [DOI] [PubMed] [Google Scholar]
- 34.El Kasmi K. C., Pugliese S. C., Riddle S. R., Poth J. M., Anderson A. L., Frid M. G., Li M., Pullamsetti S. S., Savai R., Nagel M. A., Fini M. A., Graham B. B., Tuder R. M., Friedman J. E., Eltzschig H. K., Sokol R. J., Stenmark K. R. (2014) Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J. Immunol. 193, 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L., Ong S., Talor M. V., Barin J. G., Baldeviano G. C., Kass D. A., Bedja D., Zhang H., Sheikh A., Margolick J. B., Iwakura Y., Rose N. R., Ciháková D. (2014) Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 211, 1449–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt A. P., Haughton E. L., Lalor P. F., Filer A., Buckley C. D., Adams D. H. (2009) Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology 136, 705–714 [DOI] [PubMed] [Google Scholar]
- 37.Sato S., Hasegawa M., Takehara K. (2001) Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J. Dermatol. Sci. 27, 140–146 [DOI] [PubMed] [Google Scholar]
- 38.Burman A., Haworth O., Bradfield P., Parsonage G., Filer A., Thomas A. M., Amft N., Salmon M., Buckley C. D. (2005) The role of leukocyte-stromal interactions in chronic inflammatory joint disease. Joint Bone Spine 72, 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsonage G., Falciani F., Burman A., Filer A., Ross E., Bofill M., Martin S., Salmon M., Buckley C. D. (2003) Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb. Haemost. 90, 688–697 [DOI] [PubMed] [Google Scholar]
- 40.Tieu B. C., Lee C., Sun H., Lejeune W., Recinos A. III, Ju X., Spratt H., Guo D. C., Milewicz D., Tilton R. G., Brasier A. R. (2009) An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 119, 3637–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGettrick H. M., Smith E., Filer A., Kissane S., Salmon M., Buckley C. D., Rainger G. E., Nash G. B. (2009) Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 39, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ploeger D. T., Hosper N. A., Schipper M., Koerts J. A., de Rond S., Bank R. A. (2013) Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 11, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abreu J. R., de Launay D., Sanders M. E., Grabiec A. M., van de Sande M. G., Tak P. P., Reedquist K. A. (2009) The Ras guanine nucleotide exchange factor RasGRF1 promotes matrix metalloproteinase-3 production in rheumatoid arthritis synovial tissue. Arthritis Res. Ther. 11, R121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto A., Fukuda A., Seto H., Miyazaki T., Kadono Y., Sawada Y., Nakamura I., Katagiri H., Asano T., Tanaka Y., Oda H., Nakamura K., Tanaka S. (2003) Suppression of arthritic bone destruction by adenovirus-mediated dominant-negative Ras gene transfer to synoviocytes and osteoclasts. Arthritis Rheum. 48, 2682–2692 [DOI] [PubMed] [Google Scholar]
- 45.de Launay D., Vreijling J., Hartkamp L. M., Karpus O. N., Abreu J. R., van Maanen M. A., Sanders M. E., Grabiec A. M., Hamann J., Ørum H., Vervoordeldonk M. J., Fluiter K., Tak P. P., Reedquist K. A. (2010) Silencing the expression of Ras family GTPase homologues decreases inflammation and joint destruction in experimental arthritis. Am. J. Pathol. 177, 3010–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itier J. M., Tremp G. L., Léonard J. F., Multon M. C., Ret G., Schweighoffer F., Tocqué B., Bluet-Pajot M. T., Cormier V., Dautry F. (1998) Imprinted gene in postnatal growth role. Nature 393, 125–126 [DOI] [PubMed] [Google Scholar]
- 47.Borrás C., Monleón D., López-Grueso R., Gambini J., Orlando L., Pallardó F. V., Santos E., Viña J., Font de Mora J. (2011) RasGrf1 deficiency delays aging in mice. Aging (Albany, N.Y. Online) 3, 262–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Font de Mora J., Esteban L. M., Burks D. J., Núñez A., Garcés C., García-Barrado M. J., Iglesias-Osma M. C., Moratinos J., Ward J. M., Santos E. (2003) Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J. 22, 3039–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratajczak M. Z., Kucia M., Liu R., Shin D. M., Bryndza E., Masternak M. M., Tarnowski M., Ratajczak J., Bartke A. (2011) RasGrf1: genomic imprinting, VSELs, and aging. Aging (Albany, N.Y. Online) 3, 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogaboam C. M., Steinhauser M. L., Chensue S. W., Kunkel S. L. (1998) Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int. 54, 2152–2159 [DOI] [PubMed] [Google Scholar]
- 51.Fernando M. R., Reyes J. L., Iannuzzi J., Leung G., McKay D. M. (2014) The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS ONE 9, e94188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.