Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) containing the α5 subunit modulate nicotine consumption, and the human CHRNA5 rs16969968 polymorphism, causing the replacement of the aspartic acid residue at position 398 with an asparagine (α5DN), has recently been associated with increased use of tobacco and higher incidence of lung cancer. We show that in ventral midbrain neurons, the α5 subunit is essential for heteromeric nAChR-induced intracellular-free Ca2+ concentration elevations and that in α5−/− mice, a class of large-amplitude nicotine-evoked currents is lost. Furthermore, the expression of the α5DN subunit is not able to restore nicotinic responses, indicating a loss of function by this subunit in native neurons. To understand how α5DN impairs heteromeric nAChR functions, we coexpressed α4, α5, or α5DN subunits with a dimeric concatemer (β2α4) in a heterologous system, to obtain nAChRs with fixed stoichiometry. Both α5(β2α4)2 and α5DN(β2α4)2 nAChRs yielded similar levels of functional expression and Ca2+ permeability, measured as fractional Ca2+ currents (8.2 ± 0.7% and 8.0 ± 1.9%, respectively), 2-fold higher than α4(β2α4)2. Our results indicate that the loss of function of nicotinic responses observed in α5DN-expressing ventral midbrain neurons is neither due to an intrinsic inability of this subunit to form functional nAChRs nor to an altered Ca2+ permeability but likely to intracellular modulation.—Sciaccaluga, M., Moriconi, C., Martinello, K., Catalano, M., Bermudez, I., Stitzel, J. A., Maskos, U., Fucile, S. Crucial role of nicotinic α5 subunit variants for Ca2+ fluxes in ventral midbrain neurons.
Keywords: nicotine dependence, nicotinic acetylcholine receptors, Ca2+ permeability, fractional Ca2+ current
Neuronal nicotinic acetylcholine receptors (nAChRs) are homo- or heteropentameric ion channels selective for cations (1). α5 Subunit participates in functional heteromeric nAChRs only in the presence of other α and β subunits [α5* nAChRs; (2–4)], and it confers specific activation, desensitization, and ion selectivity to these receptors (2, 4–8). Recently, the α5 subunit containing the nonsynonymous variant rs16969968, a single nucleotide polymorphism in which the aspartic acid residue at position 398 is replaced with an asparagine (α5DN), has been associated with increased use of tobacco (9, 10) and with a higher incidence of lung cancer (11, 12). The α5 subunit is important for nicotine aversion (13, 14), and in its absence (i.e., α5−/− mice), self-administration of nicotine increases (13, 15), which suggests that α5DN-induced increase in nicotine consumption in humans may be due to partial loss of α5* nAChR function (15, 16). This subunit has been shown to increase Ca2+ permeability of heteromeric nAChRs (4, 6). The ability of nAChRs to allow significant Ca2+ entry in presynaptic terminals represents a key step in cholinergic modulation of neurotransmitter release (17), and the expression of α5 subunit in neurons of the reward-related mesocorticolimbic pathways (18, 19) and in structures linked to aversion and withdrawal response, such as medial habenula and interpeduncular nucleus (20–22), strongly suggests that Ca2+ fluxes mediated by α5* nAChRs are highly relevant for drug-dependence mechanisms. To clarify the role of α5 and α5DN subunits in modulating neuronal intracellular free Ca2+ concentration ([Ca2+]i), we studied the nicotine-evoked Ca2+ mobilization in ventral midbrain neurons from wild-type (WT), α5−/−, and homozygous α5DN mice. Furthermore, to resolve discrepancies in the effects of the DN polymorphism on Ca2+ permeability of α5* nAChRs (8, 23), we measured, for the first time, the fractional Ca2+ current [Pf; i.e., the percentage of total current carried by Ca2+ ions; (24, 25)] through human or mouse α5* nAChRs. To quantify the contribution of the α5 isoforms to the Ca2+ permeability of midbrain nAChRs, WT and α5DN subunits were heterologously expressed along with α4 and β2 subunits, abundant in developing and adult ventral tegmental area (VTA) dopaminergic neurons (26) and forming most α5* nAChRs in these cells (27). To avoid data ambiguity produced by assembly of mixed receptor populations (6, 28, 29), α4 and β2 subunits were expressed as concatenated β2α4 dimers, giving rise to fixed stoichiometry nAChRs. We show that in native ventral midbrain neurons, the α5 subunit is crucial for nicotine-evoked responses and that the function of this subunit is only partially substituted by α5DN, even though in vitro experiments demonstrate that both isoforms give rise to functional nAChRs that produce comparable Pf values.
MATERIALS AND METHODS
Animals
α5−/− mice were originally obtained from Mariella De Biasi (Baylor University, Waco, TX, USA) (30). Pan-genomic analysis indicated a small contribution of C57BL6/N to the genetic background. Mice were therefore backcrossed for another 7 generations to a C57BL6/J mice strain of WT animals used in this study. The α5−/− mice were housed at Charles River Laboratories (Chatillon-sur-Chalaronne, France) under EOPS conditions and were sent to collaborators from there. Mice engineered to possess the α5DN variation were generated via homologous recombination. The α5DN mice were generated by Ingenious Targeting Laboratory (Ronkonkoma, NY, USA) using C57BL/6 embryonic stem cells and genomic constructs derived from a C57BL/6 bacterial artificial chromosome library. The targeting construct was mutated to convert an aspartic acid (D) codon (GAT) to an asparagine (N) codon (AAT) at amino acid position 397 of the mouse α5 subunit gene (Chrna5). This position is equivalent to position 398 in the human α5 gene (CHRNA5). The neoselection cassette of the targeting construct was removed by breeding the α5DN mice to a C57BL/6 strain expressing a ZP3-Cre transgene [C57BL/6-TgN(Zp3-Cre)93Knw; The Jackson Laboratory, Bar Harbor, ME, USA). Removal of the neocassette was verified by PCR. Experiments described in the present work were conducted in accordance with the guidelines on the ethical use of animals from the European Community Council Directive of November 24, 1986 (86/609/EEC).
Primary ventral midbrain cultures
Primary ventral midbrain cultures were prepared as described previously (31), with some modifications. Ventral mesencephalon tissues were microdissected from embryonic day 14 mouse embryos, kept in ice-cold HBSS (Gibco, Life Technologies, Carlsbad, CA, USA) containing 20 mM d-glucose, 1% penicillin-streptomycin, and 100 μM ascorbic acid, and then incubated in a solution containing 0.05% (w/v) trypsin/EDTA and 0.05% (w/v) DNase dissolved in PBS. After 15 minutes at 37°C, tissues were mechanically dissociated. Cells were resuspended in Neurobasal medium (Life Technologies) supplemented with 2 mM l-glutamine and B27 supplement, seeded on poly-l-ornithine/laminin (Becton Dickinson, San Diego, CA, USA)-coated dishes (2 × 105 cells/ml), and grown at 37°C in a 5% CO2 humidified atmosphere. No fetal bovine serum (FBS) was added to the cultures; 50% of the medium was changed every 2 days. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated.
Whole-cell currents in VTA slices
VTA slices were prepared from 3-week-old male mice. Mice were anesthetized with 2-bromo-2-chloro-1,1,1-trifluoroethane and then decapitated. Brains were quickly removed, and horizontal slices (250 μm) were cut in ice-cold glycerol-based artificial cerebrospinal fluid (ACSF; 2 mM KCl, 2.4 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 11 mM glucose, and 250 mM glycerol) with a DSK Microslicer DTK-1000 (Ted Pella, Inc., Redding, CA, USA). Single slices were kept in a slice incubation chamber at room temperature with oxygenated ACSF (125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1.25 mM NaH2PO4, 25 mM NaHCO3, and 10 mM glucose) and transferred into a recording chamber, continually superfused with oxygenated ACSF, within 1–8 hours after preparation. Whole-cell patch-clamp recordings were performed on VTA neurons at room temperature, at a holding potential of −70 mV. Only neurons from parabrachial-pigmented nucleus and paranigral nucleus exhibiting the electrophysiologic features described by Klink et al. (26) for dopaminergic VTA neurons were included in the analysis. In particular, the functional parameters considered were fast after hyperpolarization (>17 mV), hyperpolarization-dependent (Ih) current activation with proper time-dependent relaxation (Ih Sag >30%), and spike width (>2 ms). Membrane currents were recorded using glass electrodes (3–4 MΩ) filled with 135 mM KCl, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 5 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and 2 mM Mg-ATP [pH 7.3, with potassium hydroxide (KOH)] or 135 mM KCl, 10 mM HEPES, 0.5 mM Fura-2, and 2 mM Mg-ATP (pH 7.3, with KOH) when [Ca2+]i was simultaneously monitored (see below). Nicotine was delivered to cells by pressure applications (10–20 psi for 1 second; Picospritzer II; General Valve, Fairfield, NJ, USA) from glass micropipettes positioned above whole-cell voltage-clamped neurons. All measures on VTA neurons were performed in the presence of 10 nM methyllycaconitine (MLA), 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 20 μM AP5, and 10 μM bicuculline, and in these conditions, nicotine-induced currents were blocked by 50 μM dihydro-β-erythroidine.
Whole-cell currents in transfected GH4C1 cells
Rat pituitary GH4C1 cells were grown in Ham’s F10 medium supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin, at 37°C in a 5% CO2 humidified atmosphere. Cells were plated on poly-L-lysine-coated cover slides (1 × 105 cells/ml) and transiently transfected 24 hours later using Magnetofection (NeuroMag; OZ Biosciences, Marseille, France) according to the manufacturer’s protocol, adding 0.5 μg human α4, α5, or α5D398N and (α4β2)2 cDNA subtype per well. In distinct experiments, mouse α5 or α5D397N and mouse (α4β2)2 cDNA were used. Human and mouse β2 and α4 subunits were engineered as previously described (28). The C terminus of the β2 subunit was linked to the N terminus of the α4 subunit through an AGS linker [(AGS)6]. The signal sequence of the second subunit and the end codon of the first subunit were deleted. Recordings were carried out 48–72 hours following transfection. Whole-cell currents were recorded at room temperature using borosilicate glass patch pipettes having a tip resistance of 3–5 MΩ filled with the following internal solutions: for Pf measurements (see below), 140 mM N-methyl-d-glucamine, 10 mM HEPES-HCl, and 0.5 mM Fura-2 (pH 7.3); otherwise, 140 mM CsCl, 10 mM HEPES-HCl, 2 mM Mg-ATP, and 0.5 or 5 mM BAPTA (as indicated) (pH 7.3). The patch series resistance was compensated by 80–95%, and measurements were performed at a holding potential of −70 mV, unless otherwise indicated. Cell capacitance was routinely compensated using the amplifier function and the value used to estimate cell surface. Membrane currents were filtered at 2 kHz upon acquisition with an Axopatch 200A amplifier (Axon Instruments, Union City, CA, USA) and analyzed off-line. During recordings, cells were continuously superfused using a gravity-driven perfusion system consisting of independent tubes for normal and nicotine-containing external solutions: 140 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose, and 10 mM HEPES/NaOH (pH 7.3). The terminals of the tubes were positioned 50–100 μm away from the patched cell and connected to a fast exchanger system (RSC-100; Bio-Logic, Claix, France). Data sampling and analysis were performed using pClamp10 software (Molecular Devices, Sunnyvale, CA, USA). Fast desensitization exponential constants τ were determined by best fitting the first 500 ms of whole-cell current decay, in the presence of 100 μM nicotine, to the following function: f(t) = A exp(−t/τ), where A is the current amplitude.
[Ca2+]i measurements
Measures of [Ca2+]i were obtained by time-resolved digital fluorescence microscopy using the Ca2+ indicator Fura-2 (excitation 340 and 380 nm; emission 510 nm) in different cell systems: primary ventral midbrain neurons, single neurons in VTA slices, and transfected GH4C1 cells. Single VTA neurons were loaded with cell-impermeant Fura-2 (500 μM) through the patch pipette (see above), whereas other cell types were incubated with the cell-permeant Fura-2 acetoxymethylester (2 μM; Molecular Probes, Life Technologies) for 45 minutes at 37°C in culture medium. Ca2+ transients were elicited by applying 100 μM nicotine for 2 seconds. Measures on primary ventral midbrain cultures and on VTA neurons were performed in the presence of 10 nM MLA, 20 μM CNQX, 20 μM AP5, and 10 μM bicuculline. Data were recorded and analyzed by a conventional system driven by the MetaFluor software (Molecular Devices).
Pf
The procedure used for Pf determination follows the method originally proposed by Zhou and Neher (24), which has the advantage to be independent of any assumption on ion-permeation properties (25, 32). All measurements were performed in GH4C1 cells, which are small (membrane capacitance ranges between 8 and 20 pF) and round and allow almost perfect voltage clamp, thus being an ideal preparation for the determination of Pf, which requires that current flows uniquely through the channel under study. Cells were loaded with cell-impermeant Fura-2 through the patch pipette used to measure nicotine-evoked currents. Recordings of fluorescence signals and whole-cell membrane currents were synchronized, and images were acquired and stored on a personal computer and analyzed offline. All optical parameters and digital camera settings were maintained throughout this study to avoid nonhomogeneous data. The changes of [Ca2+]i were expressed as ΔF/F (i.e., the ratio of time-resolved fluorescence variation over the basal fluorescence), using only 1 excitation wavelength, 380 nm, to increase the temporal resolution. Determinations were carried out after the basal fluorescence had reached a stable value. Cells displaying high-basal F340:F380 ratio values (>1 in our conditions) and/or low-basal F380 values (<100 arbitrary units) were discarded. In order to evaluate Pf, the F:Q ratio between the fluorescence increase (F) and total charge that had entered the cell at each fluorescence acquisition time (Q) was defined as F/Q = (ΔF/F)/Q. For each cell, we used the F/Q points that, immediately after the onset of the nicotine-induced response, exhibited a linear relationship, indicating that the Ca2+-buffering capability of Fura-2 was not saturated. The F:Q ratio value was then measured as the slope of the linear regression best fitting the F-Q plot. Finally, Pf was determined by normalizing the ratio obtained in standard medium (F/Q) to the calibration ratio, measured when Ca2+ ions were the only permeant ionic species (F/QCa): Pf = (F/Q)/(F/QCa). Calibrations were performed on different days throughout the whole experimental period, using nicotine-evoked pure Ca2+ currents. Cell-by-cell calibration was not feasible because only the first nicotine application to each cell was used for analysis, in order to avoid data variation often observed with repetitive applications.
Statistical analysis
Statistical significance of differences observed in cell distributions was assessed by the χ2 test (P < 0.05). Mean differences between groups were assessed by the ANOVA test (P < 0.05). All data are shown as means ± sem.
RESULTS
Role of α5 subunit in embryonic ventral midbrain neurons in culture
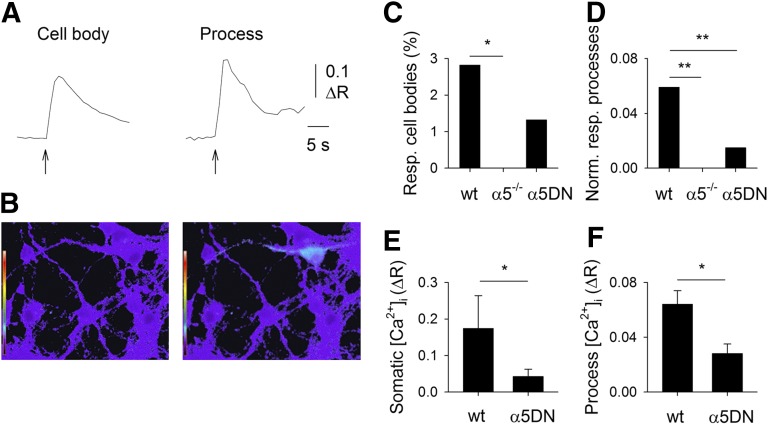
To analyze the role of the α5 subunit in the cholinergic modulation of [Ca2+]i in VTA neurons, we measured Ca2+ transients elicited by the application of 100 μM nicotine to primary cultures of embryonic ventral midbrain neurons from WT, α5−/−, and α5DN mice (Fig. 1A). To isolate the contribution of heteromeric nAChRs, these experiments were conducted in the presence of 10 nM MLA, a selective blocker of α7* nAChR, 20 μM CNQX, 20 μM AP5, and 10 μM bicuculline, to prevent Ca2+ signals due to nicotine-evoked glutamate or GABA release. Nicotine-induced Ca2+ transients were detected in 10 out of 355 cell bodies in WT cultures and in 21 cell processes in the same 35 optical fields, whereas in cells from α5−/− animals, Ca2+ signals were never observed (219 cell bodies in 29 optical fields; P = 0.029; Fig. 1A–C), indicating that the α5 subunit is necessary to trigger detectable Ca2+ entry in ventral midbrain neurons in culture, directly through nAChRs and/or through voltage-operated Ca2+ channels activated by nAChR-mediated depolarization. To further confirm this finding, nicotine-induced whole-cell currents were recorded from ventral midbrain neurons in culture from WT or α5−/− mice: slowly desensitizing heteromeric-like responses were observed in 4 out of 9 WT neurons, whereas never in 43 α5−/− neurons (data not shown). In cultures from α5DN mice, nicotine elicited [Ca2+]i elevations in 8 out of 606 cell bodies and in 9 cell processes in the same 85 optical fields (Fig. 1C, D). The proportion of responsive processes was significantly lower in α5DN neurons than in WT (P < 0.001, Fig. 1D), showing a partial loss of function of the polymorphic subunit. This finding is in agreement with the observation that the mean Ca2+ transient amplitudes of responsive cell bodies and processes were significantly higher in WT cultures than in α5DN (P = 0.034, Fig. 1E; P = 0.010, Fig. 1F).
Figure 1.
α5 Subunit is essential to observe nicotine-evoked [Ca2+]i elevations mediated by heteromeric nAChRs in mouse ventral midbrain neurons in culture, with α5DN less efficient than WT subunit. A) Typical [Ca2+]i transients elicited by 100 μM nicotine (2-second application, in the presence of 10 nM MLA, 20 μM CNQX, 20 μM AP5, and 10 μM bicuculline) in a cell body (left) and in a process (right) of 2 different ventral midbrain neurons in primary culture. B) Typical digital images of Fura-2-loaded ventral midbrain neurons before (left) and after (right) nicotine application. The images display in pseudocolors the ratio between the images obtained with excitation wavelengths of 340 and 380 nm (emission wavelength 510 nm). Note the color change associated with [Ca2+]i increase in soma and processes of a single neuron. C) Percentage of neurons exhibiting somatic nicotine-evoked [Ca2+]i elevations, in cultures from WT, α5−/−, and α5DN mice, as indicated. Note the absence of responsive (Resp.) α5−/− cells. *P = 0.029. D) Number of nicotine-evoked [Ca2+]i elevations observed in neuronal processes normalized to the number of the analyzed optical fields, in cultures from WT, α5−/−, and α5DN mice, as indicated. Note the absence of responsive processes in α5−/− cells and the significant reduction of responsive α5DN processes. Norm. resp., normal responsive. **P < 0.001. E) Mean amplitude of nicotine-evoked [Ca2+]i elevations in the cell bodies of responsive neurons in cultures from WT and α5DN mice, as indicated. *P = 0.034. F) Mean amplitude of nicotine-evoked [Ca2+]i elevations in neuronal processes in cultures from WT and α5DN mice, as indicated. *P = 0.010.
Role of α5 subunit in VTA neurons in slices
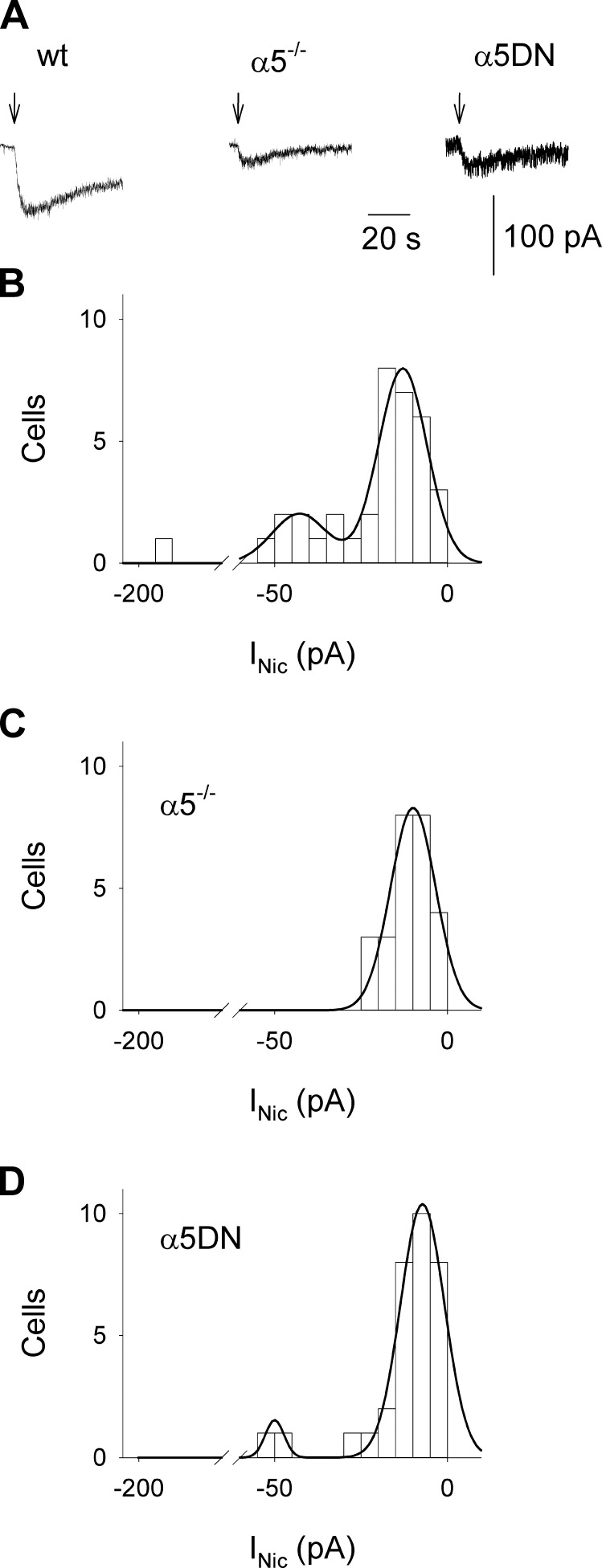
To confirm in a more physiologic system our observations on the role of the α5 subunit in nicotine-evoked Ca2+ fluxes, we studied nicotine-induced Ca2+ signals in a selected population of VTA neurons in slices, identified by location and homogeneous electrophysiologic properties as described by Klink et al. [(26); see Materials and Methods]. As for neurons in culture, all measurements were done in the presence of 10 nM MLA, 20 μM CNQX, 20 μM AP5, and 10 μM bicuculline. Individual neurons were dialyzed via a patch pipette with Fura-2 to simultaneously record nicotine-evoked inward currents and Ca2+ transients. Although nAChR-mediated inward currents were recorded from most neurons (Fig. 2A), it was never possible to detect current-associated Ca2+ signals, probably because of the low-current amplitudes and/or the possible extrasomatic location of nAChRs. In VTA slices from WT animals, the distribution of current amplitudes elicited by the application of 100 μM nicotine was best fitted by the sum of 2 Gaussian functions, indicating the presence of at least 2 different populations of heteromeric nAChRs (Fig. 2B). By contrast, in α5−/− mice, only 1 low-amplitude current population was observed (Fig. 2C), suggesting that the high-amplitude subset of WT neurons may be due to the activation of α5* nAChRs. In slices from α5DN mice, it was possible to observe not only the low-amplitude currents but also the high-amplitude ones, although at a significantly reduced frequency in comparison to WT (P < 0.001; Fig. 2D), confirming that in VTA neurons, the expression of α5DN subunit is only partially able to functionally substitute the α5 subunit.
Figure 2.
α5 Subunit is essential for the presence of a high-amplitude subpopulation of inward currents mediated by heteromeric nAChRs in neurons in mouse VTA slices, with α5DN less efficient than WT subunit. A) Typical nicotine-evoked inward currents (INic) recorded in the presence of 10 nM MLA, 20 μM CNQX, 20 μM AP5, and 10 μM bicuculline in VTA neurons from WT, α5−/−, and α5DN mice, as indicated. Arrows indicate nicotine pressure applications (100 μM for 2 seconds). B) Histogram representing the current amplitude distribution for WT neurons (n = 36). Data were best fitted to the sum of 2 Gaussian functions, with mean ± SD values of −13 ± 7 and −43 ± 8 pA, respectively. C) Histogram representing the current amplitude distribution for α5−/− neurons (n = 26). Data were best fitted to a single Gaussian function. Mean ± sd values were −10 ± 7 pA. D) Histogram representing the current amplitude distribution for α5DN neurons (n = 32). Data were best fitted to the sum of 2 Gaussian functions, with mean ± sd values of −7 ± 6 and −50 ± 3 pA, respectively.
Functional expression of α5* and α5DN* nAChRs in GH4C1 cells
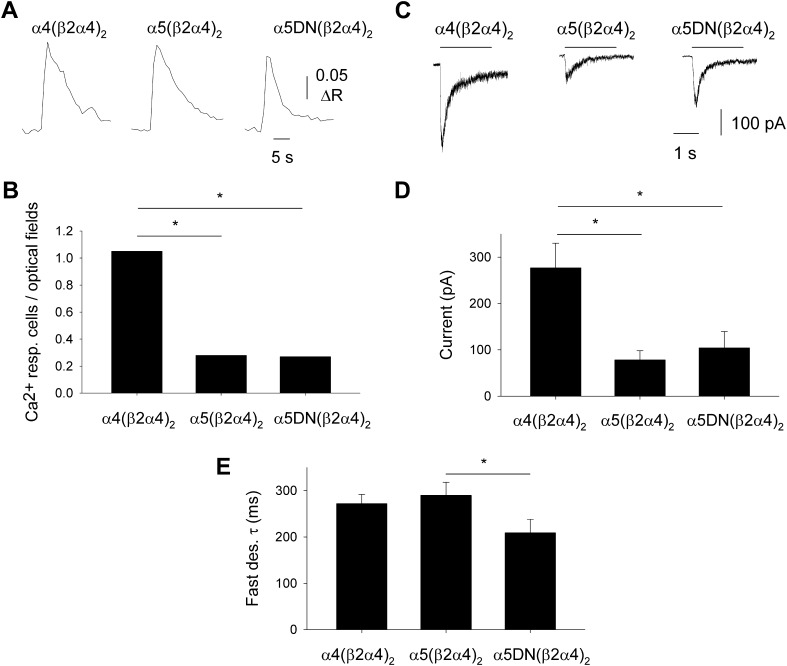
Taken together, our data indicate that the α5 subunit isoforms differ in their ability to allow detectable cellular responses in VTA neurons upon nicotinic stimulation. To illuminate the mechanisms underlying this difference, we studied the functional expression of both α5 isoforms along with α4 and β2 subunits, in a heterologous expression system, GH4C1 cells. The coexpression of these 3 subunits may lead to the assembly of several different heteromeric nAChRs in the same cells (e.g., α4β2 and α5α4β2), which may confound the analysis of functional data. To avoid multiple nAChR subtypes, a single α subunit was coexpressed with a dimeric concatemer formed by β2 and α4 subunits (26, 27), which did not form functional nAChRs when expressed alone (data not shown). In this way, we were able to study in parallel nAChRs with fixed stoichiometry: α4(β2α4)2, α5(β2α4)2, and α5DN(β2α4)2 nAChRs. All these receptors were functionally expressed in GH4C1 cells, in distinct experiments for human and mouse subtypes, and able to exert Ca2+ transients upon application of 100 μM nicotine (Fig. 3A). To quantify nAChR expression, we analyzed the percentage of nicotine-responding cells and whole-cell current amplitudes, given that in nonclamped GH4C1 cells, the nicotine-induced Ca2+ signals are due not only to Ca2+ entry through nAChRs but also to activation of voltage-dependent Ca2+ channels. When human α4 was present along with the human dimeric concatemer, the percentage of responsive cells was significantly higher than in the presence of α5 subunits (P < 0.001; Fig. 3B). This finding was confirmed by the mean amplitudes of the inward currents elicited by 100 μM nicotine in GH4C1 cells expressing the same human nAChRs (Fig. 3C), significantly higher for α4(β2α4)2, and not different for α5(β2α4)2 and α5DN(β2α4)2 nAChRs (P < 0.001; Fig. 3D). Similarly, when the corresponding mouse nAChRs were studied, no significant difference was observed between nicotine-evoked currents recorded from cells expressing α5(β2α4)2 or α5DN(β2α4)2 (data not shown). The analysis of fast desensitization induced by 100 μM nicotine revealed a significant functional difference between α5(β2α4)2 and α5DN(β2α4)2 nAChRs, with the latter decaying faster (Fig. 3E; P = 0.042).
Figure 3.
The α5 and α5DN subunits are equally able to form functional Ca2+-permeable nAChRs when heterologously coexpressed with dimeric concatemer β2α4 in GH4C1 cells. A) Typical [Ca2+]i transients elicited by 100 μM nicotine (2-second application) in single GH4C1 cells transiently transfected with human cDNAs encoding for dimeric concatemer β2α4 and for α4 (left), α5 (middle), or α5DN (right) subunits. B) Number of GH4C1 cells exhibiting nicotine-evoked [Ca2+]i transients, normalized to the number of analyzed optical fields (n = 59, 218, and 111 for α4, α5, and α5DN, respectively). In single optical fields, the number of cells was comprised between 30 and 60. Both α5 subunits yielded significantly less-functional nAChRs than α4 subunit (*P < 0.001), whereas no differences were observed between the 2 α5 isoforms. C) Typical nicotine-evoked inward currents recorded in single GH4C1 cells transfected as in (A) as indicated. D) Mean amplitude of nicotine-evoked currents. The number of recorded cells was 23, 28, and 15 for α4, α5, and α5DN, respectively. These data confirmed that both α5 subunits formed significantly less-functional nAChRs than α4 subunit (*P < 0.001), with no differences between α5 isoforms. E) Mean fast desensitization (des.) exponential constant values (*P = 0.042).
Pf of α5(β2α4)2 and α5DN(β2α4)2 nAChRs
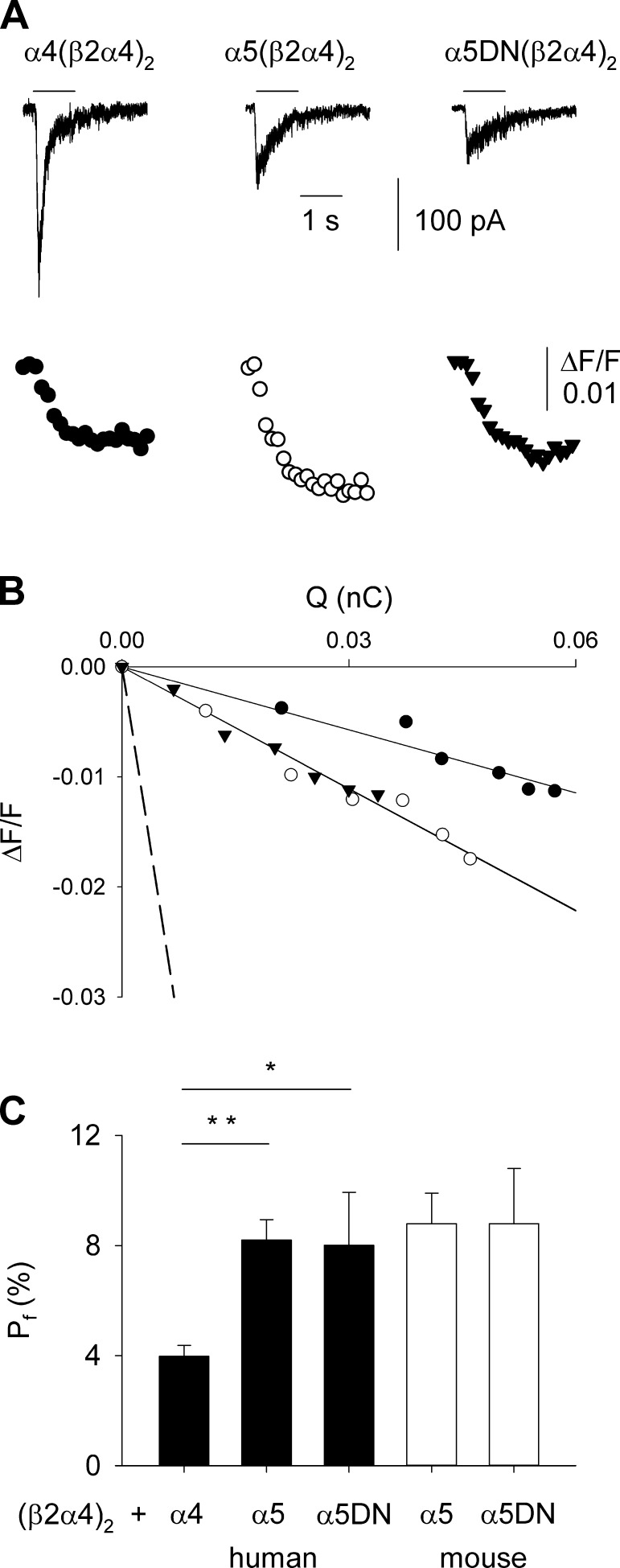
Given the known role of α5 subunit in modulating Ca2+-related properties of nAChRs (4, 6, 23), we measured the ability of Ca2+ ions to permeate these receptor channels in GH4C1 cells, in terms of Pf (see Materials and Methods), simultaneously recording nicotine-evoked currents and variations of [Ca2+]i (Fig. 4A). The Pf value of human α4(β2α4)2 was 4.0 ± 0.4%, whereas with both human α5 isoforms, the Pf values were doubled: 8.2 ± 0.7 and 8.0 ± 1.9% for α5(β2α4)2 and α5DN(β2α4)2, respectively (Fig. 4B, C). The Pf values for mouse α5(β2α4)2 and α5DN(β2α4)2 were not significantly different from human ones (8.8 ± 1.1 and 8.8 ± 2.0%, respectively; Fig. 4C). These data indicate that α5 and α5DN subunits confer similar Ca2+ permeability to heteromeric α5* nAChRs.
Figure 4.
The α5 and α5DN subunits confer similar high Pf-to-α4β2* nAChRs. A) Simultaneous recordings of whole-cell current (top) and Ca2+ transient (bottom), induced by nicotine (100 μM; horizontal bar), in GH4C1 cells transiently transfected with cDNAs encoding for dimeric concatemer β2α4 and for human α4, α5, or α5DN subunits, as indicated. Holding potential is −70 mV. At the excitation wavelength of 380 nm, [Ca2+]i increase corresponds to a downward deflection of fluorescence intensity. Traces are aligned and share the same temporal scale. B) Linear relationships between ΔF/F and Q obtained from the same cells as in (A). The dashed line represents the mean slope obtained from calibration experiments (4.37 nC−1; n = 9). C) Bar chart of Pf values measured for human (black bars) α4(β2α4)2 (n = 15), α5(β2α4)2 (n = 7), and α5DN(β2α4)2 (n = 6) or mouse (white bars) α5(β2α4)2 (n = 7) and α5DN(β2α4)2 (n = 8). Error bars represent sem. *P = 0.003; **P < 0.001.
Ca2+-dependent modulation of α5(β2α4)2 and α5DN(β2α4)2 nAChRs
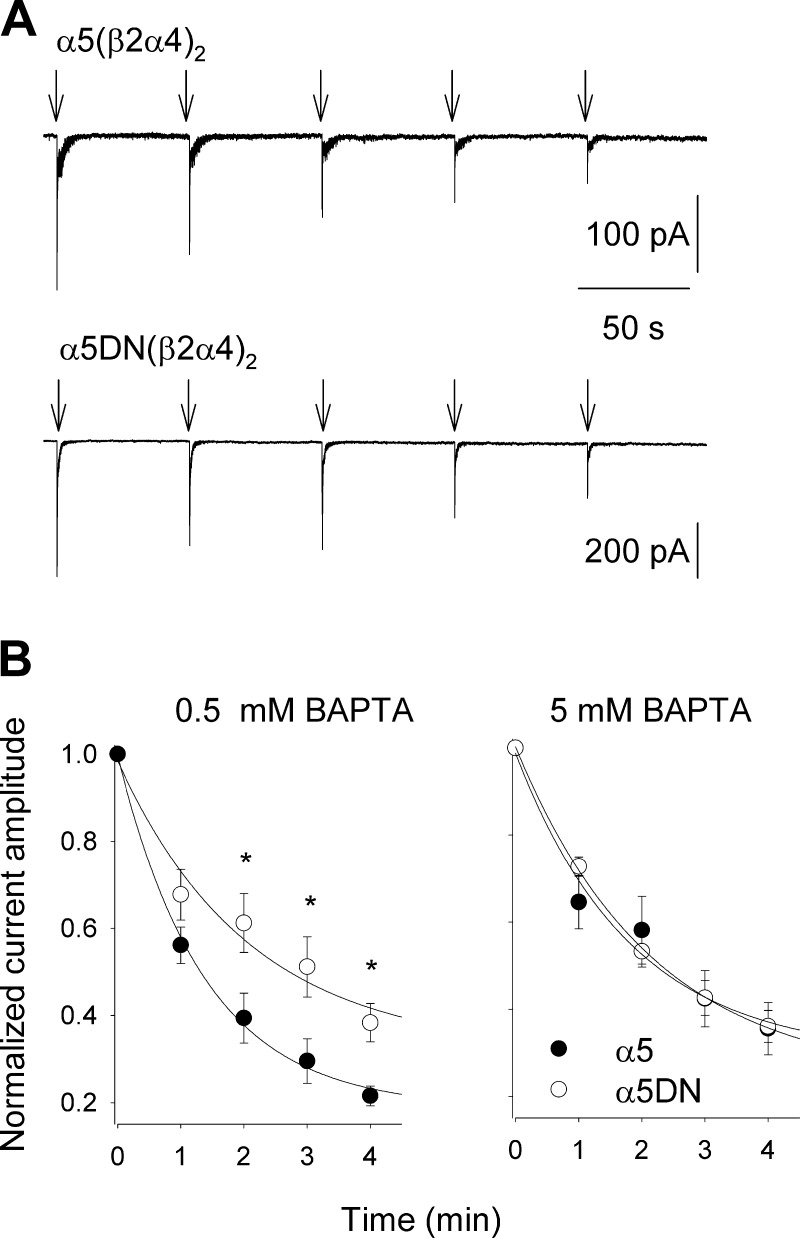
Ca2+ flux through activated nAChRs determines a local increase of [Ca2+]i, which may directly or indirectly affect the functional properties of these receptors. Considering that α5 and α5DN subunits differ only in respect of a negatively charged residue in the intracellular M3-M4 loop, which may house sites for Ca2+-receptor interaction, we studied the effects of [Ca2+]i modulation on the whole-cell currents elicited by 100 μM nicotine in GH4C1 cells expressing human α5(β2α4)2 or α5DN(β2α4)2 nAChRs. Both current amplitude and fast desensitization were unaffected by the intracellular concentration of the Ca2+ chelator BAPTA (from 0.5 to 5 mM; data not shown). However, a significant functional difference emerged upon repetitive stimulation (100 μM nicotine for 1 second every minute): in the presence of 0.5 mM BAPTA, the peak amplitudes of currents mediated by α5(β2α4)2 decayed significantly more than those mediated by α5DN-containing receptors (Fig. 5), whereas no significant difference was observed when the BAPTA concentration was 10-fold greater (Fig. 5B). This result indicates that the aspartate residue in position 398 confers a functional Ca2+ dependence to the α5 subunit.
Figure 5.
Intracellular Ca2+ differently modulates the stability of whole-cell currents mediated by α5(β2α4)2 or α5DN(β2α4)2 nAChRs expressed in GH4C1 cells. A) Typical traces of whole-cell currents mediated by α5(β2α4)2 (top) or α5DN(β2α4)2 (bottom) in transiently transfected GH4C1 cells upon repetitive application of 100 μM nicotine (1 second every minute; 0.5 mM BAPTA in intracellular solution). Holding potential is −70 mV. B) Time course of normalized current amplitudes evoked by repetitive nicotinic stimulations, in the presence of 0.5 mM BAPTA in the intracellular solution (left) or 5 mM BAPTA (right). Note the significant difference between time courses of α5(β2α4)2 (solid circles; left, n = 6; right, n = 7) and α5DN(β2α4)2 (open circles; left, n = 6; right, n = 9) observed with low Ca2+ buffer. *P = 0.035, *P = 0.030, and *P = 0.009, from left to right.
DISCUSSION
In this study, we have described 1) a pivotal role of the α5 subunit in shaping nicotine-evoked Ca2+ transients and whole-cell currents mediated by heteromeric nAChRs; 2) the inability of the α5DN isoform, containing a nonsynonymous variant equivalent to human rs16969968 associated with increased tobacco consumption and lung cancer (9–12), to fully substitute for WT α5 subunit at a functional level; 3) similar functional expression of both human (or mouse) α5 isoforms in heteromeric nAChRs of known stoichiometry; 4) similar Ca2+ permeability, measured in terms of Pf, for α5(β2α4)2 and α5DN(β2α4)2 nAChRs, which is 2-fold higher than for α4(β2α4)2 nAChR; and 5) an intracellular Ca2+-dependent modulation of α5(β2α4)2 nAChRs absent in α5DN(β2α4)2 nAChRs. The α5 subunit has recently been shown to be necessary to maintain high α4 subunit expression in VTA neurons, consequently leading to higher nicotine-evoked current amplitudes (19), and to allow in the same cells a nicotine-induced firing increase (15). Our results confirm and strengthen these findings, showing that in α5−/− mice, a class of large amplitude currents mediated by MLA-insensitive nAChRs is lost, as well as detectable [Ca2+]i changes evoked by nicotine. The loss of nicotinic response observed in α5−/− mice appears to be larger in cultured neurons (embryonic) than in neurons in slices (adult), likely due to the different patterns of nicotinic subunit expression in different developmental phases: in VTA, during the prenatal period, α5 subunit is highly expressed, whereas α6 subunit, which assembles with α4 and β2 subunits in the adult and may compensate for the α5 absence, is scarcely expressed (27). The functional properties conferred by α5 to heteromeric nAChRs expressed in VTA neurons are extremely relevant in modulating nicotine consumption because animals lacking this subunit self-administer higher doses of nicotine than their WT counterpart, which avoids high nicotine levels (15). Furthermore, we have shown a clear loss of function of α5DN isoform in native neurons of knockin mice, where responses mediated by heteromeric nAChR are significantly smaller than in WT animals. A similar loss of function has been described in α5−/− mice in which a5DN expression was obtained by selective lentiviral infection and associated with increased nicotine self-administration (15). Overall, these findings indicate that a key role in high nicotine self-administration is played by the decreased ability of α5DN-containing nAChRs to properly translate cholinergic signaling in cell depolarization, firing increase, and Ca2+ entry in VTA neurons.
Thus, the question is how a single intracellular residue change may lead to the observed loss of function. There are 2 main possibilities: 1) the DN substitution impairs some functional property of α5* nAChRs, such as single-channel conductance, ion selectivity, and open probability; or 2) the DN substitution lowers functional expression of α5DN subunit and hence reduces the surface density of α5* nAChRs in VTA neurons. To discriminate between these 2 hypotheses, it is mandatory to analyze the functional properties of α5* and α5DN* nAChRs in heterologous systems, but this approach is complicated by the assembly and expression of multiple forms of heteromeric nAChRs (3, 33). For instance, when α4, β2, and α5 cDNAs are cotransfected in mammalian cells or injected in Xenopus oocytes, there could be the functional expression of the following nAChRs: (α4)3(β2)2, (α4)2(β2)3, (α4)2(β2)2α5, α4(β2)2(α5)2, etc. Of course, the presence of α5 subunit may change the probability of the formation of each nAChR subtype, but this mechanism is not a priori known, and moreover, this subunit may substitute either an acetylcholine-binding or -nonbinding subunit in α4β2* nAChR (34). Indeed, several studies have described the functional properties of α5DN* nAChRs in heterologous systems in the presence of mixed nAChR populations (10, 14, 23), and their results must be discussed keeping in mind this experimental approach. To avoid the problem of multiple nAChR populations with different subunit stoichiometry, we expressed human (or mouse, with overlapping results) α4, α5, or α5DN subunits with a dimeric concatemer (β2α4), so that only 1 nAChR subtype could be formed in each condition, analogously to what was previously done by others (6, 8, 28, 29). Studying these receptor channels, we found the same level of current amplitudes mediated by both α5(β2α4)2 and α5DN(β2α4)2 nAChRs in GH4C1 cells, at difference with observations in ventral midbrain neurons. Considering that α5* and α5DN* nAChRs exhibit the same single-channel conductance (35), our results suggest that in GH4C1, the expression levels of α5(β2α4)2 and α5DN(β2α4)2 nAChRs are similar and that the loss of function observed in α5DN neurons is not due to an intrinsic low probability of α5DN to assemble into heteromeric nAChRs.
Furthermore, we have shown that Ca2+ permeability of α5(β2α4)2 and α5DN(β2α4)2 nAChRs is not different, and 2-fold higher than that of α4(β2α4)2 nAChR. The high Pf values are likely due to the presence of a couple of glutamate residues at the extracellular mouth of the α5 M2 transmembrane region, where other α subunits house only 1 glutamate residue (36). Ca2+ permeability of nAChRs is a physiologically relevant parameter (36) because Ca2+ influx through these receptor channels has been shown to presynaptically modulate neurotransmitter release (17). The expression of α5 subunit has been associated with increased Ca2+ permeability of heteromeric nAChRs (4), but results concerning α5DN isoform appear controversial. The polymorphic subunit lowered pure Ca2+ currents flowing through α5(α4β2)2 nAChRs (8), a phenomenon related to decreased Ca2+ permeability by those authors but that may be due to Ca2+-induced changes in open probability (37–39). By contrast, no significant differences were observed between PCa:PNa ratio values of α3β4, α3β4α5, and α3β4α5DN nAChRs, measured by means of reversal potential shift (23), suggesting from one side that α5 isoforms do not change ion selectivity but from the other that α5 subunit is not able to increase Ca2+ permeability in comparison to α3 subunit, in contrast with previous results (4). The approach used in the present study, to measure Ca2+ permeability in terms of Pf, has been shown to yield more reliable results than shift of reversal potential, based on constant field assumptions (32), whereas the “pure Ca2+ current” method (6–8) does not separate the effects due to ion selectivity from Ca2+-induced modulation of open probability. Thus, our data provide the first quantification of the Ca2+ permeability of α5* and α5DN* nAChRs. Furthermore, showing that the ratio between Ca2+ and Na+ flowing through α5* and α5DN* nAChRs is the same, our results clearly indicate that the observed decrease in Ca2+ influx in α5DN-expressing ventral midbrain neurons is not caused by an altered Ca2+ selectivity.
On the basis of careful comparison of experimental conditions, we propose that our findings are in agreement with previously published data. The α5DN subunit does not reduce Ca2+ permeability of α5(β2α4)2 nAChRs, but it lowers the pure Ca2+ current (8), likely because the increase of external Ca2+ produces a parallel increase of [Ca2+]i upon nicotinic stimulation (23), and in turn, internal Ca2+ can modulate open probability of α5* nAChRs (39). It is highly probable that the α5 and α5DN polymorphs interact differently with Ca2+ ions in the intracellular M3-M4 loop because the aspartate-asparagine substitution occurs there. This hypothesis is strongly supported by our observation that α5(β2α4)2- and α5DN(β2α4)2-mediated current peak amplitudes evoked by repetitive stimulation decrease to different levels when intracellular Ca2+ is weakly buffered. Our data are in agreement with the [Ca2+]i-modulated recovery from desensitization observed in habenular nAChRs (40) and indicate that intracellular Ca2+ directly or indirectly interacts with aspartate 398, leading to a functional modification of α5* nAChRs. In our experimental conditions, this modulation is restricted to current stability and does not affect first current amplitude or fast desensitization kinetics. However, the same basic mechanism could explain also the different responses observed between α3β4α5 and α3β4α5DN nAChRs only in the presence of high external Ca2+ concentrations (23): increased Ca2+ influx leads to high [Ca2+]i, which in turn modulates differently α5* and α5DN* nAChRs. The reported nonsignificant difference between PCa/PNa values of α3β4 and α3β4α5 nAChRs [2.9 and 3.7, respectively; (23)] may be due to the presence of a nonnegligible α3β4 nAChR population in α3β4α5-transfected cells.
Our results indicate that the reduction of nAChR-mediated Ca2+ signals observed in α5DN-expressing ventral midbrain neurons by us and others (15, 19) is not due to an intrinsic inability of this subunit to form functional nAChRs or to an altered Ca2+ permeability but to a significant α5DN-related decrease of nAChR-mediated currents. Future studies will be necessary to clarify how the partial loss of function of α5DN* nAChRs in neurons is related to changes in their intracellular modulation, and its impact on neuronal signaling and hence on the cholinergic control of reward/aversion pathways.
Acknowledgments
The authors thank Ms. Giuseppina Chece for animal genotyping. This study has been supported by the European Research Area Net (ERA-NET) Neuron NicoGene project (to S.F. and U.M.). J.A.S. is supported by U.S. National Institutes of Health (NIH) National Cancer Institute Grant CA089392 and NIH National Institute on Drug Abuse Grant DA015663.
Glossary
- ACSF
artificial cerebrospinal fluid
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- [Ca2+]i
intracellular free Ca2+ concentration
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- FBS
fetal bovine serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- Ih
hyperpolarization dependent current
- KOH
potassium hydroxide
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- Pf
fractional Ca2+ current
- VTA
ventral tegmental area
- WT
wild-type
REFERENCES
- 1.Gotti C., Clementi F. (2004) Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 74, 363–396 [DOI] [PubMed] [Google Scholar]
- 2.Ramirez-Latorre J., Yu C. R., Qu X., Perin F., Karlin A., Role L. (1996) Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 380, 347–351 [DOI] [PubMed] [Google Scholar]
- 3.Fucile S., Barabino B., Palma E., Grassi F., Limatola C., Mileo A. M., Alemà S., Ballivet M., Eusebi F. (1997) Alpha 5 subunit forms functional alpha 3 beta 4 alpha 5 nAChRs in transfected human cells. Neuroreport 8, 2433–2436 [DOI] [PubMed] [Google Scholar]
- 4.Gerzanich V., Wang F., Kuryatov A., Lindstrom J. (1998) alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J. Pharmacol. Exp. Ther. 286, 311–320 [PubMed] [Google Scholar]
- 5.Groot-Kormelink P. J., Boorman J. P., Sivilotti L. G. (2001) Formation of functional alpha3beta4alpha5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br. J. Pharmacol. 134, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapia L., Kuryatov A., Lindstrom J. (2007) Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol. Pharmacol. 71, 769–776 [DOI] [PubMed] [Google Scholar]
- 7.Kuryatov A., Onksen J., Lindstrom J. (2008) Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol. Pharmacol. 74, 132–143 [DOI] [PubMed] [Google Scholar]
- 8.Kuryatov A., Berrettini W., Lindstrom J. (2011) Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)₂α5 AChR function. Mol. Pharmacol. 79, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saccone S. F., Hinrichs A. L., Saccone N. L., Chase G. A., Konvicka K., Madden P. A., Breslau N., Johnson E. O., Hatsukami D., Pomerleau O., Swan G. E., Goate A. M., Rutter J., Bertelsen S., Fox L., Fugman D., Martin N. G., Montgomery G. W., Wang J. C., Ballinger D. G., Rice J. P., Bierut L. J. (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 16, 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierut L. J., Stitzel J. A., Wang J. C., Hinrichs A. L., Grucza R. A., Xuei X., Saccone N. L., Saccone S. F., Bertelsen S., Fox L., Horton W. J., Breslau N., Budde J., Cloninger C. R., Dick D. M., Foroud T., Hatsukami D., Hesselbrock V., Johnson E. O., Kramer J., Kuperman S., Madden P. A., Mayo K., Nurnberger J. Jr, Pomerleau O., Porjesz B., Reyes O., Schuckit M., Swan G., Tischfield J. A., Edenberg H. J., Rice J. P., Goate A. M. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165, 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorgeirsson T. E., Geller F., Sulem P., Rafnar T., Wiste A., Magnusson K. P., Manolescu A., Thorleifsson G., Stefansson H., Ingason A., Stacey S. N., Bergthorsson J. T., Thorlacius S., Gudmundsson J., Jonsson T., Jakobsdottir M., Saemundsdottir J., Olafsdottir O., Gudmundsson L. J., Bjornsdottir G., Kristjansson K., Skuladottir H., Isaksson H. J., Gudbjartsson T., Jones G. T., Mueller T., Gottsäter A., Flex A., Aben K. K., de Vegt F., Mulders P. F., Isla D., Vidal M. J., Asin L., Saez B., Murillo L., Blondal T., Kolbeinsson H., Stefansson J. G., Hansdottir I., Runarsdottir V., Pola R., Lindblad B., van Rij A. M., Dieplinger B., Haltmayer M., Mayordomo J. I., Kiemeney L. A., Matthiasson S. E., Oskarsson H., Tyrfingsson T., Gudbjartsson D. F., Gulcher J. R., Jonsson S., Thorsteinsdottir U., Kong A., Stefansson K. (2008) A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung R. J., McKay J. D., Gaborieau V., Boffetta P., Hashibe M., Zaridze D., Mukeria A., Szeszenia-Dabrowska N., Lissowska J., Rudnai P., Fabianova E., Mates D., Bencko V., Foretova L., Janout V., Chen C., Goodman G., Field J. K., Liloglou T., Xinarianos G., Cassidy A., McLaughlin J., Liu G., Narod S., Krokan H. E., Skorpen F., Elvestad M. B., Hveem K., Vatten L., Linseisen J., Clavel-Chapelon F., Vineis P., Bueno-de-Mesquita H. B., Lund E., Martinez C., Bingham S., Rasmuson T., Hainaut P., Riboli E., Ahrens W., Benhamou S., Lagiou P., Trichopoulos D., Holcátová I., Merletti F., Kjaerheim K., Agudo A., Macfarlane G., Talamini R., Simonato L., Lowry R., Conway D. I., Znaor A., Healy C., Zelenika D., Boland A., Delepine M., Foglio M., Lechner D., Matsuda F., Blanche H., Gut I., Heath S., Lathrop M., Brennan P. (2008) A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452, 633–637 [DOI] [PubMed] [Google Scholar]
- 13.Fowler C. D., Lu Q., Johnson P. M., Marks M. J., Kenny P. J. (2011) Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frahm S., Slimak M. A., Ferrarese L., Santos-Torres J., Antolin-Fontes B., Auer S., Filkin S., Pons S., Fontaine J. F., Tsetlin V., Maskos U., Ibañez-Tallon I. (2011) Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70, 522–535 [DOI] [PubMed] [Google Scholar]
- 15.Morel C., Fattore L., Pons S., Hay Y. A., Marti F., Lambolez B., De Biasi M., Lathrop M., Fratta W., Maskos U., Faure P. (2014) Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry 19, 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler C. D., Kenny P. J. (2014) Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76(Pt B), 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wonnacott S. (1997) Presynaptic nicotinic ACh receptors. Trends Neurosci. 20, 92–98 [DOI] [PubMed] [Google Scholar]
- 18.Charpantier E., Barnéoud P., Moser P., Besnard F., Sgard F. (1998) Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport 9, 3097–3101 [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S., Santos N., Holgate J., Haass-Koffler C. L., Hopf F. W., Kharazia V., Lester H., Bonci A., Bartlett S. E. (2013) The α5 subunit regulates the expression and function of α4*-containing neuronal nicotinic acetylcholine receptors in the ventral-tegmental area. PLoS One 8, e68300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks M. J., Pauly J. R., Gross S. D., Deneris E. S., Hermans-Borgmeyer I., Heinemann S. F., Collins A. C. (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 12, 2765–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grady S. R., Moretti M., Zoli M., Marks M. J., Zanardi A., Pucci L., Clementi F., Gotti C. (2009) Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 29, 2272–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salas R., Sturm R., Boulter J., De Biasi M. (2009) Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29, 3014–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tammimäki A., Herder P., Li P., Esch C., Laughlin J. R., Akk G., Stitzel J. A. (2012) Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by α3β4α5 nicotinic acetylcholine receptors. Neuropharmacology 63, 1002–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z., Neher E. (1993) Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 425, 511–517 [DOI] [PubMed] [Google Scholar]
- 25.Neher E. (1995) The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology 34, 1423–1442 [DOI] [PubMed] [Google Scholar]
- 26.Klink R., de Kerchove d’Exaerde A., Zoli M., Changeux J. P. (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 21, 1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azam L., Chen Y., Leslie F. M. (2007) Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience 144, 1347–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Nelson M. E., Kuryatov A., Choi C., Cooper J., Lindstrom J. (2003) Human alpha4beta2 acetylcholine receptors formed from linked subunits. J. Neurosci. 23, 9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groot-Kormelink P. J., Broadbent S. D., Boorman J. P., Sivilotti L. G. (2004) Incomplete incorporation of tandem subunits in recombinant neuronal nicotinic receptors. J. Gen. Physiol. 123, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salas R., Orr-Urtreger A., Broide R. S., Beaudet A., Paylor R., De Biasi M. (2003) The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol. 63, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 31.Pruszak, J., Just, L., Isacson, O., Nikkhah, G. (2009) Isolation and culture of ventral mesencephalic precursor cells and dopaminergic neurons from rodent brains. Curr. Protoc. Stem Cell Biol. Chapter 2: Unit 2D.5 [DOI] [PubMed] [Google Scholar]
- 32.Burnashev N., Zhou Z., Neher E., Sakmann B. (1995) Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol. 485, 403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwart R., Vijverberg H. P. (1998) Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 54, 1124–1131 [PubMed] [Google Scholar]
- 34.Jin X., Bermudez I., Steinbach J. H. (2014) The nicotinic α5 subunit can replace either an acetylcholine-binding or nonbinding subunit in the α4β2* neuronal nicotinic receptor. Mol. Pharmacol. 85, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li P., McCollum M., Bracamontes J., Steinbach J. H., Akk G. (2011) Functional characterization of the α5(Asn398) variant associated with risk for nicotine dependence in the α3β4α5 nicotinic receptor. Mol. Pharmacol. 80, 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fucile S. (2004) Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium 35, 1–8 [DOI] [PubMed] [Google Scholar]
- 37.Adams D. J., Nutter T. J. (1992) Calcium permeability and modulation of nicotinic acetylcholine receptor-channels in rat parasympathetic neurons. J. Physiol. Paris 86, 67–76 [DOI] [PubMed] [Google Scholar]
- 38.Mulle C., Léna C., Changeux J. P. (1992) Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron 8, 937–945 [DOI] [PubMed] [Google Scholar]
- 39.Girod R., Crabtree G., Ernstrom G., Ramirez-Latorre J., McGehee D., Turner J., Role L. (1999) Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann. N. Y. Acad. Sci. 868, 578–590 [DOI] [PubMed] [Google Scholar]
- 40.Guo X., Lester R. A. (2007) Regulation of nicotinic acetylcholine receptor desensitization by Ca2+. J. Neurophysiol. 97, 93–101 [DOI] [PubMed] [Google Scholar]