Abstract
Alternative activation of alveolar macrophages is linked to fibrosis following exposure to asbestos. The scavenger receptor, macrophage receptor with collagenous structure (MARCO), provides innate immune defense against inhaled particles and pathogens; however, a receptor for asbestos has not been identified. We hypothesized that MARCO acts as an initial signaling receptor for asbestos, polarizes macrophages to a profibrotic M2 phenotype, and is required for the development of asbestos-induced fibrosis. Compared with normal subjects, alveolar macrophages isolated from patients with asbestosis express higher amounts of MARCO and have greater profibrotic polarization. Arginase 1 (40-fold) and IL-10 (265-fold) were higher in patients. In vivo, the genetic deletion of MARCO attenuated the profibrotic environment and pulmonary fibrosis in mice exposed to chrysotile. Moreover, alveolar macrophages from MARCO−/− mice polarize to an M1 phenotype, whereas wild-type mice have higher Ym1 (>3.0-fold) and nearly 7-fold more active TGF-β1 in bronchoalveolar lavage (BAL) fluid (BALF). Arg432 and Arg434 in domain V of MARCO are required for the polarization of macrophages to a profibrotic phenotype as mutation of these residues reduced FIZZ1 expression (17-fold) compared with cells expressing MARCO. These observations demonstrate that a macrophage membrane protein regulates the fibrotic response to lung injury and suggest a novel target for therapeutic intervention.—Murthy, S., Larson-Casey, J. L., Ryan, A. J., He, C., Kobzik, L., Carter, A. B. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure.
Keywords: MARCO, mitochondria, asbestos
Lung macrophages are critical in regulating host responses to lung injury because they are the first line of defense against inhaled pathogens and environmental toxins. Macrophages are important not only in mounting an immune response to foreign agents but also in repair of the injury (1). These divergent functions are dependent on their ability to switch between phenotypically distinct subpopulations, the classically activated macrophages (CAMs; M1) and the alternatively activated macrophages (AAMs; M2), depending on stimulation by T-helper (Th)1 or Th2 cytokines, respectively (2, 3). It is known that macrophage polarization is tightly regulated in certain conditions, as especially seen in differences in Th1- and Th2-induced innate immunity (4, 5). CAMs are responsible for the generation of proinflammatory cytokines, whereas AAMs are anti-inflammatory and often associated with fibrotic conditions, including pulmonary fibrosis (2, 5, 6). Although Th2-induced AAMs are protective for parasite infection, allergic responses, and asthma (4, 7, 8), the profibrotic AAMs express characteristic marker proteins involved in profibrotic changes, such as 1) anti-inflammatory and profibrotic immune effectors, 2) up-regulated l-arginine metabolism by arginase 1 to generate polyamines and proline, and 3) alteration in extracellular matrix dynamics.
Reactive oxygen species (ROS) generated in macrophages are required for the development of pulmonary fibrosis following chrysotile exposure (9–11). Our prior observations demonstrate that the primary source of ROS in macrophages is the mitochondria and that mitochondrial H2O2 generation, rather than Th2 cytokines, plays a significant role in the alternative activation of macrophages to the profibrotic phenotype in the setting of pulmonary fibrosis (6, 9–11). Thus, understanding the regulation of mitochondrial ROS generation in alveolar macrophages is critical for the development of therapeutic approaches in the treatment of pulmonary fibrosis. Currently, little is known regarding upstream signaling events following inhalation of environmental particles.
SRs are cell surface pattern recognition receptors expressed by macrophages and serve as part of the innate immune system (12, 13). They bind and take up bacteria, oxidized lipids, and macrophage receptor with collagenous structure (MARCO), a member of class A family of SR, also binds unopsonized environmental particles (12, 14–19). The structure of MARCO is organized into 5 domains consisting of a short N-terminal intracellular region, a transmembrane domain, and a long extracellular region composed of a spacer domain, a collagenous domain, and a C-terminal scavenger receptor (SR) cysteine-rich (SRCR) domain (domain V) (12, 13). The secreted domains of SR-As have been shown to bind crocidolite (20); however, an intact macrophage receptor for asbestos has not been demonstrated. We hypothesized that MARCO is a cell surface receptor for asbestos and is required for chrysotile-induced pulmonary fibrosis by mediating the generation of mitochondrial ROS and polarizing macrophages to the profibrotic phenotype. Our results demonstrate that the macrophage cell surface receptor, MARCO, plays a significant role in modulating macrophage polarization following lung injury and therefore may constitute a novel therapeutic target to halt the development of pulmonary fibrosis.
MATERIALS AND METHODS
Materials
Chrysotile asbestos was provided Dr. Peter S. Thorne (College of Public Health, University of Iowa, Iowa City, IA, USA). TiO2, p-hydroxyphenylacetic acid (pHPA), horseradish peroxidase (HRP), α-ketoglutarate, NADPH anti-β-actin antibody, anti-mouse antibody, and anti-rabbit antibody were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Antibodies directed to MARCO and SR-A were from Santa Cruz Biotechnology (Dallas, TX, USA). The antibody against Rieske was from Abcam (Cambridge, MA, USA), and the antibody directed to gp91phox was from BD Biosciences (San Jose, CA, USA).
Human subjects
The Human Subjects Review Board of the University of Iowa Carver College of Medicine approved the protocol of obtaining alveolar macrophages from normal volunteers and patients with asbestosis. Criteria for selecting normal volunteers and patients with asbestosis are described elsewhere (11). Fiberoptic bronchoscopy with BAL was performed after subjects received intramuscular atropine (0.6 mg) and local anesthesia. Three subsegments of the lung were lavaged with five 20-ml aliquots of normal saline, and the first aliquot in each was discarded. The percentage of alveolar macrophages obtained from BAL was determined by Wright-Giemsa stain and varied from 90–99%.
Cell culture
Human monocyte (THP-1) and mouse alveolar macrophage (MH-S) cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum and penicillin/streptomycin. All experiments were performed with RPMI supplemented with 0.5% serum. In vitro, chrysotile was used at a final dose of 10 μg/cm2.
Mice
Wild-type (WT) C57Bl/6 mice were from Jackson Laboratories (Bar Habor, ME, USA). MARCO−/− mice have been described elsewhere (21). All protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. Pulmonary fibrosis determined as previously described (6, 10). Mice were exposed intratracheally to (100 μg/50 μl normal saline) titanium hydroxide (TiO2) or chrysotile asbestos. Alveolar macrophages from mice were obtained by BAL, and cell differential was determined by Wright-Giemsa stain.
Plasmids, small interfering RNA, and transfections
Full-length MARCO and truncation mutants 442 and 420 have been previously described (15). Using full-length MARCO as a template for PCR amplification, the 431 truncation mutant was cloned into the HindIII/EcoR1 sites of pcDNA3.1 after shuttling through PCR4-TOPO (Invitrogen, Carlsbad, CA, USA). Site-directed mutants of MARCO were generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) using full-length MARCO as the template. DNA sequences of all plasmid constructs were verified by DNA sequencing (University of Iowa DNA facility, Iowa City, IA, USA). Plasmid vectors were transfected into cells using X-treme gene 9 transfection reagent (Roche, Indianapolis, IN, USA), according to the manufacturer’s instructions (11). Scrambled universal negative control small interfering RNA (siRNA), Rieske siRNA, and MARCO siRNA were from Integrated DNA Technologies (Coralville, IA, USA). Cells were transfected with 100 nM of siRNA complexed to Dharmafect 2 (GE Dharmacon, Lafayette, CO, USA) for 48–72 h.
Isolation of mitochondria and membranes
Mitochondria and membranes were isolated as previously described (6, 9).
Determination of H2O2 generation
Extracellular H2O2 production was determined fluorometrically, as previously described (9). Mitochondrial H2O2 was determined in cells incubated for 30 min with or without chrysotile. Mitochondria were resuspended in phenol-red free HBSS supplemented with 6.5 mM glucose, 1 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 6 mM sodium bicarbonate, 1.6 mM pHPA, 95 µg/ml HRP, and 5 mM α-ketoglutarate. Fluorescence of the pHPA-dimer was measured using a spectrofluorometer at excitation of 323 nm and emission of 400 nm.
Quantitative real-time PCR
Isolation of total RNA, reverse transcription of RNA, and quantitative real-time PCR were performed as described previously (6). Mouse FIZZ-1, mouse β-actin, human TGF-β1, and human hypoxanthine-guanine phosphoribosyltransferase (HPRT) primers are previously described (6, 9). The following human primer sets were also used: arginase-1, 5′-TTC TCA AAG GGA CAG CCA CG-3′ and 5′-TAG GGA TGT CAG CAA AGG GC-3′ and IL-10, 5′-GAT CCA GTT TTA CCT GGA GGA G-3′ and 5′-CCT GAG GGT CTT CAG GTT CTC-3′. Data were calculated by the ΔΔCT method, and after normalizing to β-actin or HPRT, the results were expressed as arbitrary units.
Hydroxyproline determination
Lung tissue was dried to stable weight and acid hydrolyzed with 6 N HCl for 24 h at 120°C. Hydroxyproline concentration was normalized to dry weight of the lung, and the concentration was determined as described previously (10).
HPLC/mass spectrometry analysis
A Sciex (Toronto, ON, Canada) API 4000 triple-quadrupole mass spectrometer was used for this study. The Turbo V source was operated under positive electrospray at 600°C. All gases were nitrogen and the declustering potential, collision exit potential, and collision energy were optimized for glutathione (GSH) and glutathione disulfide (GSSG). Liquid chromatography was performed on a Shimadzu (Kyoto, Japan) Prominence HPLC system. GSH and GSSG separations were performed on a SeQuant ZIC-HILIC (The Nest Group, Southboro, MA, USA) column, 20 × 2.1 mm with a particle size of 3.5 µ. The guard column was a SeQuant ZIC-HILIC 20 × 2.1 mm, particle size 5 µ. Unknown samples were quantified using Analyst 1.6.2 software. The mass transitions used were 433/201 for NEM derivatized GSH and 613/355 for GSSG. The injection volume was 5 µl and the flow rate was 0.4 ml/min.
ELISA
Ym-1, IL-1b and TNF-α in BAL fluid was measured by ELISA (R&D, Minneapolis, MN, USA), according to manufacturer’s instructions.
Arginase assay
Arginase activity in cell lysates was determined using the QuantiChrom Arginase Assay kit (BioAssay Systems, Hayward, CA, USA) according to manufacturer’s instructions.
Statistical analysis
Statistical comparisons were performed with Student’s t test or 1-way ANOVA followed by Tukey’s multiple comparison test. Values in figures are expressed as means with standard errors and P < 0.05 was considered to be significant.
RESULTS
Alveolar macrophages from patients with asbestosis express high levels of MARCO, have increased mitochondrial oxidative stress, and have a profibrotic phenotype
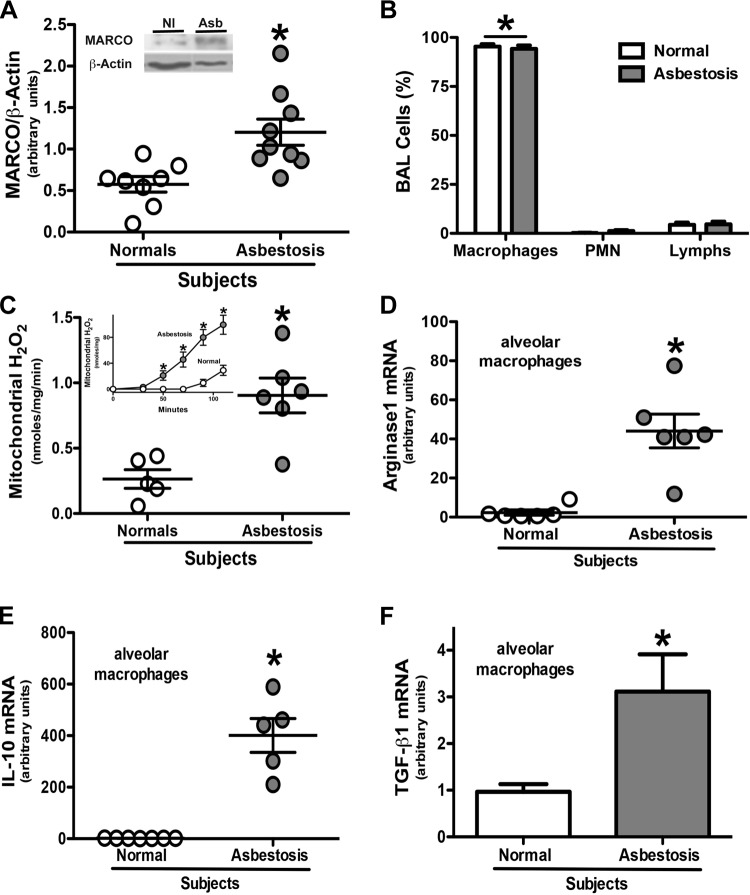
Alveolar macrophages use MARCO to bind and phagocytize bacteria, oxidized lipids, and environmental particles (15, 16, 19, 21). We hypothesized that MARCO binds chrysotile asbestos, promotes a profibrotic environment in the lung, and is linked to pulmonary fibrosis. We first investigated its significance in patients with asbestosis. Compared with normal subjects, alveolar macrophages from patients with asbestosis expressed significantly more MARCO (Fig. 1A). To validate the importance of this difference in MARCO expression, we investigated if alveolar macrophages were the predominant cell type in the BALF obtained from the subjects. Alveolar macrophages comprised 90–99% of the cells in both patients with asbestosis and normal subjects (Fig. 1B).
Figure 1.
Alveolar macrophages from patients with asbestosis have higher MARCO expression, greater mitochondrial oxidative stress, and a predominant M2 phenotype. A) MARCO and β-actin expression in alveolar macrophages obtained by BAL from normal subjects (Nl, n = 8) and patients with asbestosis (Asb, n = 9) were determined by immunoblotting. Densitometric analysis and a representative blot (inset) are shown. Results are expressed as arbitrary units. *P < 0.05. B) Cells were obtained by BAL from normal subjects (n = 5) and patients with asbestosis (n = 5). Cell differential was determined by Wright-Giemsa stain. *P < 0.0001 macrophages vs. all other cell types. Mitochondria were isolated from alveolar macrophages from normal subjects and patients with asbestosis. C) H2O2 production rate and (inset) H2O2 production measured by pHPA assay. Results are expressed as nanomoles per milligram per minute. *P < 0.05. Nl (n = 5) and Asb (n = 6). Total RNA was isolated from alveolar macrophages obtained from normal subjects and patients with asbestosis by BAL. D) Arginase 1, *P < 0.0008 (n = 6); (E) IL-10, *P < 0.0001 (Nl, n = 7; Asb, n = 5); and (F) TGF-β1 mRNA, * P < 0.03 (n = 5) were measured and are expressed in arbitrary units.
We have shown that alveolar macrophages from patients with asbestosis generate greater H2O2 compared with normal subjects and that the primary source of chrysotile-induced H2O2 is the mitochondria (9, 11, 22). We determined the relative contribution of ROS from the mitochondria in patients with asbestosis. Alveolar macrophages obtained from patients produced hydrogen peroxide (H2O2) at a significantly higher rate compared with alveolar macrophages from normal subjects (Fig. 1C). This is consistent with a significantly greater levels of mitochondrial H2O2 generated from patients with asbestosis compared with normal subjects (Fig. 1C, inset).
Because increased mitochondrial oxidative stress is linked to polarization of macrophages to an profibrotic phenotype, we questioned if alveolar macrophages from patients with asbestosis had a predominant AAM phenotype (6). The expression of the markers, Arginase 1 (Fig. 1D) and IL-10 (Fig. 1E), were found to be greater than 40-fold and 265-fold higher, respectively, in alveolar macrophages isolated from patients with asbestosis compared with normal subjects. Moreover, the expression of TGF-β1 was significantly higher in the alveolar macrophages from patients with asbestosis (Fig. 1F). These data suggest that the expression of the macrophage cell surface receptor, MARCO, and profibrotic macrophage polarization is present in humans with asbestos-induced pulmonary fibrosis.
Chrysotile induces MARCO expression in vivo and in vitro
Because patients with asbestosis had an increase in MARCO expression compared with normal subjects, we asked if this increased expression of MARCO in alveolar macrophages was secondary to exposure to asbestos. WT mice exposed to chrysotile were killed at 3, 7, 14, or 21 d later. MARCO was not detected in alveolar macrophages from mice unexposed to chrysotile. In contrast, it was present at all times following chrysotile exposure (Fig. 2A).
Figure 2.
Asbestos induces MARCO expression in vivo and in vitro. A) WT mice were intratracheally administered 100 μg/mouse chrysotile asbestos and, at the indicated times, mice were killed and alveolar macrophages were obtained by BAL. Cells were lysed, and MARCO and β-actin expression determined by immunoblotting. Alveolar macrophages were exposed to (B) chrysotile or (C) TiO2 for the designated time, and MARCO, SR-A, and β-actin expression were determined by immunoblot analysis. D) Macrophages were exposed to chrysotile for 180 min. Membrane and cytosol fractions were isolated and MARCO, gp91phox, and β-actin expression were determined by immunoblot analysis.
To corroborate our in vivo findings and to establish the earliest time at which chrysotile induces MARCO expression, we exposed alveolar macrophages to chrysotile over 180 min. MARCO was minimally present in unexposed cells, but it increased in a time-dependent manner in cells exposed to chrysotile (Fig. 2B). In contrast, another member of the class A family of SRs, SR-A1, was found to be constitutively present in alveolar macrophages and was not altered by chrysotile exposure. In addition, induction in MARCO expression was specific for chrysotile as TiO2 had minimal, if any, effect (Fig. 2C).
The increase in MARCO expression following chrysotile exposure was largely accounted for by expression in cell membranes, whereas little to no MARCO was detected in the cytoplasm (Fig. 2D). In aggregate, these data demonstrate that chrysotile induces MARCO expression and membrane localization in alveolar macrophages and suggest MARCO has an important role in initiating signaling in alveolar macrophages.
MARCO is required for the development of chrysotile-induced pulmonary fibrosis
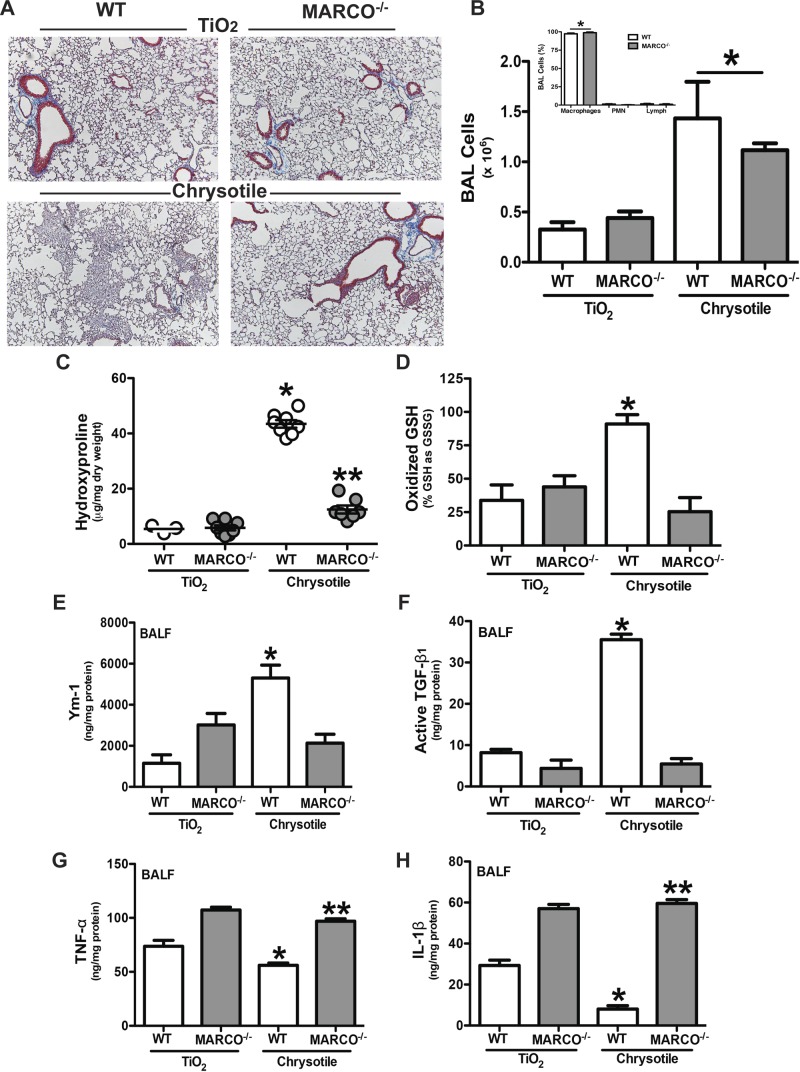
Based on the ex vivo data and the fact that chrysotile induces MARCO expression, we hypothesized that MARCO is required for the fibrotic response to lung injury. To investigate its role in pulmonary fibrosis following lung injury, we exposed WT and MARCO−/− mice to either the inert particle, TiO2, as a negative control, or to chrysotile asbestos. The histologic staining of lungs for collagen revealed that both strains of mice exposed to TiO2 had normal lungs, whereas the lungs of WT mice exposed to chrysotile showed dense, aberrant collagen deposition and destruction of the normal architecture. In contrast, the lungs of the MARCO−/− mice exposed to chrysotile were essentially normal (Fig. 3A). There was no difference between the strains in the number of inflammatory cells in the BALF. The number of cells increased with chrysotile exposure (Fig. 3B), and the predominant cell type (95–100%) in both strains was alveolar macrophages (Fig. 3B, inset). These histologic findings were confirmed biochemically by demonstrating that the lungs of MARCO−/− mice had significantly less hydroxyproline than WT mice exposed to chrysotile (Fig. 3C).
Figure 3.
MARCO is required for the development of pulmonary fibrosis. WT and MARCO−/− mice were intratracheally administered 100 μg/mouse of TiO2 or chrysotile asbestos. Twenty-one days later, mice were killed, BAL was performed, and lungs were removed. A) Lungs were stained for collagen with Masson’s trichrome stain. Representative sections from WT (n = 4) and MARCO−/− (n = 4) mice are shown. B) Cells obtained by BAL from WT (n = 5, TiO2; n = 6, chrysotile) and MARCO−/− (n = 5, TiO2; n = 7, chrysotile) mice. *P < 0.0001 vs. TiO2-exposed mice. (Inset) Cell differential from chrysotile-exposed mice was determined by Wright-Giemsa stain. *P < 0.0001 (n = 4) macrophages vs. all other cell types. C) Hydroxyproline content in lungs is shown as microgram hydroxyproline per milligram lung dry weight. *P < 0.0001 vs. all other groups, and **P < 0.0014 vs. TiO2-exposed mice. WT (n = 3, TiO2; n = 8, chrysotile) and MARCO−/− (n = 8, TiO2; n = 7, chrysotile) mice. D) GSH and GSSG were measured by HPLC and mass spectrometry analysis and are expressed as oxidized glutathione (GSSG) relative to its reduced form (GSH). *P < 0.05. WT (n = 6) and MARCO−/− (n = 7, TiO2; n = 6, chrysotile) mice. BALF was obtained by BAL. ELISA was performed for (E) Ym-1, *P < 0.0002 vs. all other groups, WT (n = 5, TiO2; n = 8, chrysotile) and MARCO−/− (n = 7, TiO2; n = 8, chrysotile); (F) active TGF-β1, *P < 0.0001 vs. all other groups; (G) TNF-α, *P < 0.0001 vs. all other groups and **P < 0.0009 vs. WT groups; and (H) IL-1β, * P < 0.0001 vs. all other groups and **P < 0.0001 vs. WT groups. WT (n = 4, TiO2; n = 8, chrysotile) and MARCO−/− (n = 5, TiO2; n = 8, chrysotile) (F–H).
Because the development of pulmonary fibrosis is associated with lung oxidative stress, we determined if there was a difference in the redox environment of lungs in WT and MARCO−/− mice. The lungs from WT mice had a significantly higher percentage of glutathione in its oxidized disulfide form (GSSG) than lungs from MARCO−/− mice, suggesting that MARCO is required, at least in part, for oxidative changes in the lung following chrysotile exposure in vivo (Fig. 3D).
The polarization of alveolar macrophages to an M2 phenotype is often associated with fibrotic repair of injured tissues, including the lung (23–25). Because the deletion of MARCO in vivo is protective from fibrotic development, we hypothesized that MARCO induced the polarization of macrophages to a profibrotic M2 phenotype. Genetic deletion of MARCO attenuated the profibrotic and enhanced a proinflammatory microenvironment. Compared with WT mice, BALF from MARCO−/− mice exposed to chrysotile had significantly lower levels of the AAM marker, Ym1, which was near the concentration of Ym1 in the BALF from TiO2-exposed WT mice (Fig. 3E). Moreover, active TGF-β1 in the WT mice was nearly 7-fold higher that in the BALF from chrysotile-exposed MARCO−/− mice (Fig. 3F). In contrast, MARCO−/− mice had significantly higher levels of the M1 markers, TNF-α (Fig. 3G) and IL-1β (Fig. 3H), that was constitutive as they did not change with chrysotile exposure. Chrysotile exposure reduced the M1 markers in WT BALF. These results, for the first time, demonstrate that MARCO is linked to the development of a profibrotic microenvironment by modulation of macrophage polarization and is required for fibrotic response to lung injury.
MARCO is required for mitochondrial oxidative stress
Based on our prior observations that mitochondrial ROS is linked to macrophage phenotype and pulmonary fibrosis (6, 9, 11, 26), we investigated if MARCO modulated mitochondrial H2O2 production. We first inhibited MARCO activity by incubating alveolar macrophages with fucoidan, an inhibitor of SR activity. Exposure to chrysotile dramatically increased the rate of mitochondrial H2O2 production in cells exposed to vehicle, and H2O2 generation was significantly reduced in cells exposed to fucoidan (Fig. 4A). To specifically link this effect to MARCO, we silenced the expression of MARCO in macrophages. Chrysotile significantly increased the rate of H2O2 generation in isolated mitochondria from cells transfected with the scrambled siRNA, whereas knockdown of MARCO was less than in control cells expressing the scrambled siRNA (Fig. 4B).
Figure 4.
MARCO is required for mitochondrial H2O2 production. A) Macrophages were incubated for 1 h with 500 μg/ml fucoidan before being exposed to chrysotile. Mitochondria were isolated and the production of H2O2 was determined. Results are expressed as nanomoles per milligram per minute. *P < 0.0001 control + chrysotile vs. fucoidan + chrysotile. n = 3. B) Macrophages were transfected with either scrambled or MARCO siRNA, and 48 h later cells were cultured in the presence or absence of chrysotile. Mitochondria were isolated and H2O2 production was measured. Results are expressed as nanomoles per milligram per minute. *P < 0.0001 vs. all other groups. n = 3. (Inset) Knockdown of MARCO by immunoblot analysis. C) Macrophages were transfected as in B. After 48 h, cells were exposed to chrysotile. Cell binding to chrysotile was counted in fifteen fields by microscopy. n = 15. * P < 0.0004. (Inset) Knockdown of MARCO by immunoblot analysis. D) Cells were transfected with scrambled or Rieske siRNA and 48 h later transfected with empty pcDNA3.1 or vector expressing MARCOWT. Twenty-four hours later, cells were exposed to chrysotile and H2O2 production was determined in whole cells. Results are expressed as nanomoles per milligram per minute. n = 3. *P < 0.05 –chrysotile vs. +chrysotile; **P < 0.05 vs. scrambled siRNA + empty + chrysotile; #P < 0.05 vs. scrambled siRNA + empty + chrysotile; ##P < 0.05 vs. scrambled siRNA + MARCOWT + chrysotile. (Inset) Rieske knockdown was determined by immunoblotting.
To determine if the interaction of MARCO with asbestos is required for the effects of chrysotile exposure, we investigated if MARCO-deficient macrophages had a defect in binding chrysotile. The interaction of chrysotile in macrophages with knockdown of MARCO was less than one-half of that observed in cells expressing the scrambled siRNA (Fig. 4C). These data suggest that MARCO is required, in part, for the downstream signal transduction in chrysotile-exposed macrophages.
To validate that MARCO-mediated H2O2 production is derived from the mitochondria, we silenced the iron-sulfur protein, Rieske, a component of cytochrome bc1 complex (complex III) in the mitochondria. Complex III is the major source of ROS in the mitochondria, and Rieske is one of four redox centers in complex III and is necessary for mitochondrial H2O2 generation (9, 27). Chrysotile exposure increased the rate of H2O2 production in cells transfected with empty vector, and overexpression of MARCOWT increased the rate further. In contrast, silencing Rieske prevented chrysotile-induced H2O2 production in cells transfected with the empty vector, and it significantly abrogated H2O2 production in cells overexpressing MARCOWT (Fig. 4D). These results strongly suggest that MARCO is an upstream regulator of mitochondrial ROS production.
Arg432 and Arg434 in the extracellular domain V of MARCO are required for macrophage polarization
To determine if a specific region of MARCO is required for the generation of mitochondrial ROS, we transfected alveolar macrophages with an empty vector, a vector expressing the full-length MARCO (MARCOWT), or MARCO truncation mutants within domain V, which is the region important for particle and pathogen recognition (12, 15, 28) (Fig. 5A). Chrysotile increased the rate of H2O2 release from cells expressing the empty vector, and overexpression of MARCOWT significantly enhanced this effect (Fig. 5B). The rate of H2O2 production in macrophages transfected with MARCO442 also increased significantly with chrysotile; however, chrysotile was unable to induce H2O2 in cells expressing the MARCO431 and MARCO420 constructs. These observations suggest that MARCO directly modulates chrysotile-induced mitochondrial oxidative stress within the region spanning 432–442 in domain V.
Figure 5.
Arginine residues in domain V of MARCO mediates mitochondrial ROS and promotes M2 polarization. A) Schematic diagram of truncation mutants of MARCO. B) Macrophages were transfected with empty or vectors expressing MARCOWT or truncation mutants. After 24 h cells were exposed to chrysotile and H2O2 production was measured. C) Schematic diagram of site-directed MARCO mutants. D) Macrophages were transfected with empty or vectors expressing MARCOWT or site-directed mutants. After 24 h cells were exposed to chrysotile, mitochondria were isolated, and H2O2 production was measured. B, D) Results show mean ± sem, n = 3, *P < 0.05 –chrysotile vs. +chrysotile; **P < 0.05 vs. MARCOWT + chrysotile. Macrophages were transfected with empty vector or vectors expressing WT or R432A,R434A mutant and 24 h later were exposed to chrysotile for (E) 4 or (F) 24 h. E) Fizz-1 mRNA and F) arginase activity were determined. Results show mean ± sem, n = 3, *P < 0.05 –chrysotile vs. +chrysotile; **P < 0.05 vs. MARCOWT + chrysotile; ***P < 0.05 vs. empty + chrysotile and MARCOWT + chrysotile.
To determine if specific amino acid residues within this region are required to modulate mitochondrial ROS production, we utilized prior evidence showing that chrysotile attains a negative surface charge at physiologic or acidic pH by dissolution of Mg(OH)2 on the surface (29–31). The negative surface charge suggests that positively charged amino acids within this region are potential binding residues for chrysotile. The positively charged residues between 432 and 442 are Arg432 and Arg434. To determine if these residues are required for chrysotile-induced mitochondrial ROS production, we transfected cells with MARCO constructs in which the arginine residues were mutated to alanine: R432A, R434A, and R432A,R434A (Fig. 5C). Chrysotile significantly increased H2O2 levels in cells expressing the MARCOWT. In contrast, mutation of residues 432 or 434 significantly attenuated this increase and mutation of both residues completely abrogated chrysotile-induced H2O2 production (Fig. 5D). Taken together, these observations show that MARCO, via 2 arginine residues in its extracellular domain V, modulates chrysotile-mediated mitochondrial oxidative stress.
The previously mentioned residues form an R-X-R motif that has been demonstrated to be important in binding to gram-negative bacteria (32). Because these residues are required for mitochondrial ROS generation, we determined if the R-X-R motif is also necessary for polarization of macrophages to the profibrotic phenotype. Chrysotile increased gene expression of the marker FIZZ-1 in cells expressing the empty vector, and this effect was significantly enhanced in cells expressing MARCOWT (Fig. 5E). In contrast, FIZZ-1 expression was decreased in cells expressing MARCOR432A,R434A to levels that were significantly below control expression. MARCOR432A,R434A also inhibited the activity of another marker, Arginase 1, compared with MARCOWT (Fig. 5F). These data strongly suggest that the R-X-R motif in the extracellular domain V of MARCO binds chrysotile in alveolar macrophages and indicate that these residues are required for mitochondrial oxidative stress and macrophage polarization to the profibrotic M2 phenotype. In aggregate, these observations demonstrate that MARCO is required, at least in part, for pulmonary fibrosis following exposure to an environmental toxicant.
DISCUSSION
The development of pulmonary fibrosis, including asbestosis, is a complex process that involves input from multiple cell types. Alveolar macrophages play an important role in this process as they are among the first set of cells to mount an immune response to inhaled pathogens and environmental insults. Macrophages are also key regulators of the redox state of the lung and participate in the repair following injury. However, in certain situations repair is deregulated resulting in an exaggerated fibrotic response. This is prevalent in the fibrotic phenotype that is associated with exposure to environmental irritants, such as chrysotile asbestos (33, 34). Although the response alveolar macrophages utilize to combat chrysotile exposure involves phagocytosis (35, 36), the mechanisms that allow macrophages to identify the fibers are poorly understood as no receptor for chrysotile asbestos is known. In this study, our observations demonstrate that MARCO recognizes chrysotile and mediates its effects on mitochondrial oxidative stress, macrophage polarization, and the development of a profibrotic phenotype.
MARCO is a class A SR that binds bacteria, oxidized lipids, and environmental particles (12, 15, 19, 21). The end result of binding environmental particles and pathogens is a contained inflammatory response that is exacerbated in the absence of MARCO (17, 37, 38). Little is known about downstream events triggered by MARCO, especially in the context of pulmonary fibrosis. Increased MARCO expression coupled with higher mitochondrial H2O2 production in alveolar macrophages from patients with asbestosis and decreased lung oxidative stress in MARCO−/− mice exposed to chrysotile supports the notion that MARCO is linked to oxidative stress. Although one study has shown that MARCO increases ROS levels in mast cells, neither the source of ROS nor the signaling events leading to ROS generation were determined (39). In the current study, we demonstrate that MARCO is required, at least in part, for mediating mitochondrial oxidative stress induced by chrysotile exposure. Our previous observations demonstrate the importance of mitochondrial H2O2 in the development and progression of pulmonary fibrosis (6, 9, 11). Results from the current study extend these observations and uncover a novel upstream modulator of pulmonary oxidative stress.
Domain V in the extracellular region of MARCO has been shown to bind environmental particles and bacteria (12, 15, 16, 32, 40). TiO2 binding to MARCO can be blocked with a specific antibody. The binding of Escherichia coli to MARCO occurs within the cysteine-rich domain V (32). The generation of a S423R mutant increases bacterial binding compared with the WT MARCO, suggesting that a positively charged amino acid enhanced the binding of negatively charged bacteria. Chrysotile becomes negatively charged in physiologic or acidic pH environments (29–31). In the current study, we found that positively charged residues, Arg432 and Arg434, are required to initiate signaling after chrysotile exposure. Moreover, MARCO-deficient macrophages have significantly less binding to chrysotile. These novel findings suggest that chrysotile binds MARCO at a conserved R-X-R motif in domain V and that this motif is likely essential for mediating its effects on the development and progression of pulmonary fibrosis.
Although other particles, such as TiO2 (15, 16) and silica (18), also bind domain V of MARCO, they do not elicit the same response as chrysotile. A likely explanation for these differences could be that the particles bind to different sites in domain V. Indeed, TiO2 is reported to bind between residues 420 and 431 (15) and silica binds between 443 and 520 (18). Our observations taken together with these previous reports suggest that the cysteine-rich domain V of MARCO is capable of binding various particles and pathogens that, by engaging different regions of the protein, elicit specific downstream effects. Another intriguing possibility is that MARCO may serve as a docking site for chrysotile and other particles and, depending on the region bound by these foreign agents, MARCO may then interact with specific downstream effectors to transduce unique signaling events. To our knowledge, no interacting partners for MARCO have been identified.
Mitochondrial H2O2 generation has been shown to be critical for the alternate activation of macrophages to the profibrotic M2 phenotype (6). SRs are an important component of the innate immune system and are known to modulate the inflammatory state of the lung. Previous observations demonstrate that MARCO binds LPS, and the response is induced in classically activated macrophages indicating a role in host pathogen clearance (41, 42). However, MARCO is not activated nor does it promote the secretion of proinflammatory cytokines in all situations, suggesting that it has a more complex role. In fact, studies have demonstrated that MARCO and other SRs decrease the proinflammatory environment in bacterial infections, ozone, or silica exposure (17, 21, 38). These studies show that MARCO−/− mice had an increase in pulmonary inflammation characterized by increased BAL cell numbers and proinflammatory cytokines, which is appropriate for the Th1, granulomatous response following silica exposure; however, this study found no significant difference in fibrosis in WT and MARCO−/− mice following silica exposure (17). Although not investigated, these prior data suggest that MARCO polarizes the macrophage to an alternatively activated phenotype. The polarization to this phenotype is often beneficial to decrease inflammation and enhance repair of injured tissue, an event that clearly occurs following exposure to asbestos. Another factor that may promote AAM polarization is asbestos-induced lung disease has a long latency period due to the slow clearance of fibers from the lung. An imbalance to a predominant AAM phenotype, however, can result in a fibrotic response (2, 6). Although these previous studies appear contradictory, they suggest that tissue macrophages develop mixed M1/M2 phenotypes in complex pathologic conditions, such as lung injury, and polarize to a predominant phenotype depending on the duration and stage of injury and/or repair (43). Results presented in the current study demonstrate that MARCO promotes polarization to a profibrotic M2 phenotype and precipitates an exaggerated fibrotic response to lung injury. These observations suggest that MARCO is a novel therapeutic target to halt the progression of a devastating disease for which no effective treatment is currently available.
Acknowledgments
This work was supported, in whole or in part, by U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences Grant 2R01ES015981-07 (to A.B.C.) and the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biological Laboratory Research and Development Merit Review Grant 1BX001135-03 (to A.B.C.). For the GSH and GSSG analysis, the authors thank the Targeted Metabolomics and Proteonomics Laboratory supported by NIH Grant P30 DK079337 [University of Alabama at Birmingham (UAB) O'Brien Acute Kidney Injury Center], the UAB Lung Health Center, and the UAB Center for Free Radical Biology.
Glossary
- AAM
alternatively activated macrophage (M2)
- BAL
bronchoalveolar lavage
- BALF
BAL fluid
- CAM
classically activated macrophage (M1)
- GSH
glutathione
- GSSG
glutathione disulfide
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- HRP
horseradish peroxidase
- MARCO
macrophage receptor with collagenous structure
- M1
CAM
- M2
AAM
- pHPA
p-hydroxyphenylacetic acid
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- SR
scavenger receptor
- SRCR
SR cysteine-rich
- Th
T-helper
- WT
wild-type
REFERENCES
- 1.Harraz M. M., Marden J. J., Zhou W., Zhang Y., Williams A., Sharov V. S., Nelson K., Luo M., Paulson H., Schöneich C., Engelhardt J. F. (2008) SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Invest. 118, 659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 3.Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 4.Herbert D. R., Hölscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M., Leeto M., Kirsch R., Hall P., Mossmann H., Claussen B., Förster I., Brombacher F. (2004) Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 [DOI] [PubMed] [Google Scholar]
- 5.Nair M. G., Du Y., Perrigoue J. G., Zaph C., Taylor J. J., Goldschmidt M., Swain G. P., Yancopoulos G. D., Valenzuela D. M., Murphy A., Karow M., Stevens S., Pearce E. J., Artis D. (2009) Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206, 937–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C., Ryan A. J., Murthy S., Carter A. B. (2013) Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J. Biol. Chem. 288, 20745–20757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnan A., van Pee D., Bongrand P., Vervloet D. (1998) Alveolar macrophage interleukin (IL)-10 and IL-12 production in atopic asthma. Allergy 53, 1092–1095 [DOI] [PubMed] [Google Scholar]
- 8.Moreira A. P., Hogaboam C. M. (2011) Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J. Interferon Cytokine Res. 31, 485–491 [DOI] [PubMed] [Google Scholar]
- 9.He C., Murthy S., McCormick M. L., Spitz D. R., Ryan A. J., Carter A. B. (2011) Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J. Biol. Chem. 286, 15597–15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murthy S., Adamcakova-Dodd A., Perry S. S., Tephly L. A., Keller R. M., Metwali N., Meyerholz D. K., Wang Y., Glogauer M., Thorne P. S., Carter A. B. (2009) Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L846–L855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn-Heaford H. L., Ryan A. J., Murthy S., Racila A. M., He C., Sieren J. C., Spitz D. R., Carter A. B. (2012) Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J. Biol. Chem. 287, 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elomaa O., Kangas M., Sahlberg C., Tuukkanen J., Sormunen R., Liakka A., Thesleff I., Kraal G., Tryggvason K. (1995) Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80, 603–609 [DOI] [PubMed] [Google Scholar]
- 13.Gough P. J., Gordon S. (2000) The role of scavenger receptors in the innate immune system. Microbes Infect. 2, 305–311 [DOI] [PubMed] [Google Scholar]
- 14.Arredouani M. S., Kobzik L. (2004) The structure and function of marco, a macrophage class a scavenger receptor. Cell. Mol. Biol. (Noisy-le-grand) 50 Online Pub, OL657–OL665 [PubMed] [Google Scholar]
- 15.Arredouani M. S., Palecanda A., Koziel H., Huang Y. C., Imrich A., Sulahian T. H., Ning Y. Y., Yang Z., Pikkarainen T., Sankala M., Vargas S. O., Takeya M., Tryggvason K., Kobzik L. (2005) MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J. Immunol. 175, 6058–6064 [DOI] [PubMed] [Google Scholar]
- 16.Palecanda A., Paulauskis J., Al-Mutairi E., Imrich A., Qin G., Suzuki H., Kodama T., Tryggvason K., Koziel H., Kobzik L. (1999) Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 189, 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakur S. A., Beamer C. A., Migliaccio C. T., Holian A. (2009) Critical role of MARCO in crystalline silica-induced pulmonary inflammation. Toxicol. Sci. 108, 462– 471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakur S. A., Hamilton R. Jr., Pikkarainen T., Holian A. (2009) Differential binding of inorganic particles to MARCO. Toxicol. Sci. 107, 238– 246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arredouani M. S., Yang Z., Imrich A., Ning Y., Qin G., Kobzik L. (2006) The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell Mol. Biol. 35, 474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resnick D., Freedman N. J., Xu S., Krieger M. (1993) Secreted extracellular domains of macrophage scavenger receptors form elongated trimers which specifically bind crocidolite asbestos. J. Biol. Chem. 268, 3538–3545 [PubMed] [Google Scholar]
- 21.Dahl M., Bauer A. K., Arredouani M., Soininen R., Tryggvason K., Kleeberger S. R., Kobzik L. (2007) Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J. Clin. Invest. 117, 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy S., Ryan A., He C., Mallampalli R. K., Carter A. B. (2010) Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 285, 25062–25073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karo-Atar D., Moshkovits I., Eickelberg O., Königshoff M., Munitz A. (2013) Paired immunoglobulin-like receptor-B inhibits pulmonary fibrosis by suppressing profibrogenic properties of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 48, 456–464 [DOI] [PubMed] [Google Scholar]
- 24.Tao B., Jin W., Xu J., Liang Z., Yao J., Zhang Y., Wang K., Cheng H., Zhang X., Ke Y. (2014) Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J. Immunol. 193, 2801–2811 [DOI] [PubMed] [Google Scholar]
- 25.Gibbons M. A., MacKinnon A. C., Ramachandran P., Dhaliwal K., Duffin R., Phythian-Adams A. T., van Rooijen N., Haslett C., Howie S. E., Simpson A. J., Hirani N., Gauldie J., Iredale J. P., Sethi T., Forbes S. J. (2011) Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 184, 569–581 [DOI] [PubMed] [Google Scholar]
- 26.Jain M., Rivera S., Monclus E. A., Synenki L., Zirk A., Eisenbart J., Feghali-Bostwick C., Mutlu G. M., Budinger G. R., Chandel N. S. (2013) Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J. Biol. Chem. 288, 770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turrens J. F., Alexandre A., Lehninger A. L. (1985) Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 237, 408–414 [DOI] [PubMed] [Google Scholar]
- 28.Ojala J. R., Pikkarainen T., Tuuttila A., Sandalova T., Tryggvason K. (2007) Crystal structure of the cysteine-rich domain of scavenger receptor MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J. Biol. Chem. 282, 16654–16666 [DOI] [PubMed] [Google Scholar]
- 29.Bales R. C., Morgan J. J. (1985) Surface charge and adsorption properties of chrysotile asbestos in natural waters. Environ. Sci. Technol. 19, 1213–1219 [DOI] [PubMed] [Google Scholar]
- 30.Light W. G., Wei E. T. (1977) Surface charge and asbestos toxicity. Nature 265, 537–539 [DOI] [PubMed] [Google Scholar]
- 31.Seshan K. (1983) How are the physical and chemical properties of chrysotile asbestos altered by a 10-year residence in water and up to 5 days in simulated stomach acid? Environ. Health Perspect. 53, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brännström A., Sankala M., Tryggvason K., Pikkarainen T. (2002) Arginine residues in domain V have a central role for bacteria-binding activity of macrophage scavenger receptor MARCO. Biochem. Biophys. Res. Commun. 290, 1462–1469 [DOI] [PubMed] [Google Scholar]
- 33.Guidotti T. L., Miller A., Christiani D., Wagner G., Balmes J., Harber P., Brodkin C. A., Rom W., Hillerdal G., Harbut M., Green F. H. Y.; American Thoracic Society (2004) Diagnosis and initial management of nonmalignant diseases related to asbestos. Am. J. Respir. Crit. Care Med. 170, 691–715 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz D. A., Galvin J. R., Frees K. L., Dayton C. S., Burmeister L. F., Merchant J. A., Hunninghake G. W. (1993) Clinical relevance of cellular mediators of inflammation in workers exposed to asbestos. Am. Rev. Respir. Dis. 148, 68–74 [DOI] [PubMed] [Google Scholar]
- 35.Scheule R. K., Holian A. (1989) IgG specifically enhances chrysotile asbestos-stimulated superoxide anion production by the alveolar macrophage. Am. J. Respir. Cell Mol. Biol. 1, 313–318 [DOI] [PubMed] [Google Scholar]
- 36.Wright A., Donaldson K., Davis J. M. (1983) Cytotoxic effect of asbestos on macrophages in different activation states. Environ. Health Perspect. 51, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh S., Gregory D., Smith A., Kobzik L. (2011) MARCO regulates early inflammatory responses against influenza: a useful macrophage function with adverse outcome. Am. J. Respir. Cell Mol. Biol. 45, 1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton R. F. Jr., Thakur S. A., Mayfair J. K., Holian A. (2006) MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J. Biol. Chem. 281, 34218–34226 [DOI] [PubMed] [Google Scholar]
- 39.Brown J. M., Swindle E. J., Kushnir-Sukhov N. M., Holian A., Metcalfe D. D. (2007) Silica-directed mast cell activation is enhanced by scavenger receptors. Am. J. Respir. Cell Mol. Biol. 36, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Sankala M., Ojala J. R., Sun Y., Tuuttila A., Isenman D. E., Tryggvason K., Pikkarainen T. (2006) A phage display screen and binding studies with acetylated low density lipoprotein provide evidence for the importance of the scavenger receptor cysteine-rich (SRCR) domain in the ligand-binding function of MARCO. J. Biol. Chem. 281, 12767–12775 [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay S., Chen Y., Sankala M., Peiser L., Pikkarainen T., Kraal G., Tryggvason K., Gordon S. (2006) MARCO, an innate activation marker of macrophages, is a class A scavenger receptor for Neisseria meningitidis. Eur. J. Immunol. 36, 940–949 [DOI] [PubMed] [Google Scholar]
- 42.van der Laan L. J., Döpp E. A., Haworth R., Pikkarainen T., Kangas M., Elomaa O., Dijkstra C. D., Gordon S., Tryggvason K., Kraal G. (1999) Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J. Immunol. 162, 939–947 [PubMed] [Google Scholar]
- 43.Martinez F. O., Gordon S. (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]