Abstract
Regeneration of human cartilage is inherently inefficient; an abundant autologous source, such as human induced pluripotent stem cells (hiPSCs), is therefore attractive for engineering cartilage. We report a growth factor-based protocol for differentiating hiPSCs into articular-like chondrocytes (hiChondrocytes) within 2 weeks, with an overall efficiency >90%. The hiChondrocytes are stable and comparable to adult articular chondrocytes in global gene expression, extracellular matrix production, and ability to generate cartilage tissue in vitro and in immune-deficient mice. Molecular characterization identified an early SRY (sex-determining region Y) box (Sox)9low cluster of differentiation (CD)44lowCD140low prechondrogenic population during hiPSC differentiation. In addition, 2 distinct Sox9-regulated gene networks were identified in the Sox9low and Sox9high populations providing novel molecular insights into chondrogenic fate commitment and differentiation. Our findings present a favorable method for generating hiPSC-derived articular-like chondrocytes. The hiChondrocytes are an attractive cell source for cartilage engineering because of their abundance, autologous nature, and potential to generate articular-like cartilage rather than fibrocartilage. In addition, hiChondrocytes can be excellent tools for modeling human musculoskeletal diseases in a dish and for rapid drug screening.—Lee, J., Taylor, S. E. B., Smeriglio, P., Lai, J., Maloney, W. J., Yang, F., Bhutani, N. Early induction of a prechondrogenic population allows efficient generation of stable chondrocytes from human induced pluripotent stem cells.
Keywords: hiPSC-derived chondrocytes, prechondrogenic population, cartilage regeneration
Articular cartilage in joints has a very limited intrinsic capacity for repair, presumably due to its avascular nature and a lack of stem or progenitor cell access (1). Therefore, no current therapies are effective for focal cartilage defects caused by injury or trauma, even in young adults. Such defects frequently lead to degenerative conditions such as osteoarthritis (OA). Therapies for age-associated OA are limited to pain management or total joint arthroplasty (2, 3). The cells currently used in clinics for cartilage repair—autologous chondrocytes and mesenchymal stem cells (MSCs)—have limited availability, and the additional surgery for harvesting autologous chondrocytes is associated with morbidity. Most important, the cells generate a functionally inferior fibrocartilage rather than hyaline articular cartilage. Alternative stem cell sources, including adipose-derived stem cells (ADSCs) (4), clonal blood marrow precursors (5), and, more recently, nasal cartilage-derived progenitor cells (6), have been explored for generating articular cartilage. It has also been reported that juvenile or neonatal chondrocytes have a regenerative potential for cartilage generation superior to that of adult chondrocytes (7); however, their widespread use is limited by donor availability.
The breakthrough discovery that somatic cells can be reprogrammed to embryonic stem cell (ESC)-like induced pluripotent stem cells (iPSCs) has provided a potentially limitless patient-autologous cell source (8–10). The reprogramming approach has now been extended to convert one somatic cell type to another, as well—for example, skin fibroblasts to neurons or cardiac cells, bypassing the iPSC state (11, 12). Patient-derived iPSCs or skin fibroblasts can be ideal autologous cells for chondrocyte generation, as these cells avoid immune rejection and can be readily expanded before chondrocyte generation. Another potential advantage could be the generation of developmentally younger chondrocytes, akin to the neonatal chondrocytes. Indeed, a recent report demonstrated that mouse iPSC-derived chondrocytes have the potential to generate hyaline cartilage and engraft efficiently into an ex vitro cartilage defect (13). We therefore sought to develop methodologies to direct human (h)iPSC differentiation toward chondrocytes (or chondroprogenitor cells) with a higher regenerative potential. Toward this end, we sought to optimize a growth factor-based protocol to improve differentiation of hiPSC toward articular chondrocytes for the ease of future clinical applications. In addition, our goal was to characterize the molecular identity of the hiPSC-derived chondrocytes and to define the molecular events associated with stepwise chondrogenic differentiation.
MATERIALS AND METHODS
Viral preparation and infection for hiPSC generation
HEK293FT cells were plated at a density of 6 × 106 cells per T225 flask and incubated overnight. The cells were transfected with 10 μg VSV-G (envelope protein), 15 μg pUMVC (packaging plasmid), and 10 μg of the gene of interest [SRY (sex-determining region) box (Sox)2, octamer-binding transcription factor (Oct)4, oncogene of the avian myelocytomatosis virus (c-Myc), or Kruppellike factor (Klf)4] with Lipofectamine (Life Technologies, Carlsbad, CA, USA). The supernatant was collected 48 h after transfection and filtered through a 0.45 μm filter. After it was spun at 17,100 rpm for 2 h 20 min, the viral pellet was resuspended to make 100× stock solutions. To generate hiPSCs, retrovirally transduced human fibroblasts were seeded at 5 × 104 cells per well of a 6-well dish 1 d before transduction. The medium was replaced with virus-containing supernatant supplemented with 8 μg/ml polybrene and incubated for 24 h. The transduced fibroblasts were then cultured in iPSC medium [DMEM/F12 (Life Technologies-Gibco, Grand Island, NY, USA), 20% Knockout Serum Replacement (Life Technologies), nonessential amino acids, penicillin, streptomycin, β-mercaptoethanol, and 10 ng/ml fibroblast growth factor (FGF)-2] on mitomycin-treated MEFs. The lentivirally transduced hiPSC line was a gift from Dr. Vittorio Sebastiano (Stanford University).
Chondrocyte culture
Articular chondrocytes were harvested from grossly normal cartilage pieces discarded during notchplasty or debridement from patients (with no prior history of OA) undergoing anterior cruciate ligament (ACL) reconstruction (a 27 yr-old woman and a 35 year-old man), under protocols approved by the Institutional Review Board of Stanford University for studies involving human subjects. Cartilage was dissected, and the chondrocytes were dissociated from the matrix (14). Chondrocytes were cultured in a monolayer in Dulbecco’s modified Eagle’s medium (ThermoScientific, Inc., Waltham, MA, USA) supplemented with 25 mg/ml ascorbate, 2 mM l-glutamine, 1% penicillin/streptomycin antibiotics, and 10% fetal bovine serum (FBS; Invitrogen-Life Technologies, Carlsbad, CA, USA) at 37°C.
Chondrogenic differentiation of hiPSCs
In brief, hiPSCs were cultured on growth factor-reduced Matrigel (BD Biosciences, San Jose, CA, USA) in mTeSR-1 (Stem Cell Technologies, Vancouver, BC, Canada) medium before differentiation. hiPSCs were dissociated by incubation with 1 mg/ml Dispase (Stem Cell Technologies) for 10 min at 37°C in 5% CO2. To form embryoid bodies (EBs), small clumps of hiPSCs were resuspended in aggregation medium containing STEMPRO34 (Life Technologies), 30 mg/ml transferrin, 5 mg/ml ascorbic acid, and 13 μg/ml monothioglycerol (15) with the addition of 10 μg/ml Y27632 (Stemgent, Inc., Detroit, MI, USA), 25 ng/ml Wnt3a [wingless-type MMTV (mouse mammary tumor virus) integration site family; BD Biosciences], and 50 ng/ml Activin A (BD Biosciences). After 4 d, EBs were plated on fibronectin-coated tissue culture plates in chondrogenic differentiation medium (DMEM/F12, 10% FBS, 1% insulin-transferrin-selenium, 1% non-essential amino acids, 2% B27, and β-mercaptoethanol) and supplemented serially with growth factors for 14 d. Concentrations and timing of growth factor supplementation are described in Supplemental Fig. S2. After 14 d of differentiation, the cells were passaged, and the hiChondrocytes were maintained in chondrogenic growth medium (Lonza, Walkersville, MD, USA) on gelatin-coated tissue culture plates and passaged before confluence.
Western blot analysis
Proteins were extracted from cells by solubilizing the cells in RIPA buffer containing 1× protease inhibitor cocktail. Total protein (25 μg) was loaded and resolved on SDS-polyacrylamide gels, transferred to PVDF membranes, and probed with the following primary antibodies: anti-Oct4 and anti-Nanog (Cell Signaling Technology, Danvers, MA, USA) and β-actin (Sigma-Aldrich, St. Louis, MO, USA).
Quantitative real-time PCR
RNA was isolated with the RNeasy kit (Qiagen, Valencia, CA, USA). First-strand cDNA was primed with oligo (dT) primers, and qPCR was performed with TaqMan primer sets (Life Technologies-Applied Biosystems, Foster City, CA, USA). Relative expression levels were normalized to GAPDH and 18s and calculated by the 2−ΔCt method.
In vitro pellet cultures
Chondrocytes were dissociated and suspended in a chondrogenic growth medium (Lonza). Four 2 × 105 chondrocytes in 0.4 ml cell suspension were transferred into each 15 ml tube. Chondrocyte pellets were formed by centrifugation at 500 g for 10 min. The pellets were cultured at 37°C under 5% CO2 with chondrogenic growth medium. The medium was changed every second day for the rest of the 4 wk culture period.
Cell encapsulation in 3D hydrogels/in vivo transplantation in mice
Chondroitin sulfate methacrylate was synthesized by modifying a reported method. Briefly, CS sodium salt from bovine trachea (Sigma-Aldrich) was reacted with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide in a 2-(N-morpholino) ethanesulfonic acid buffer for 5 min, followed by the addition of 2-aminoethyl methacrylate (AEMA). For hiChondrocyte encapsulation, 15 × 106 cells/ml cells were suspended in a hydrogel solution consisting of 5% weight/volume (w/v) poly(ethyleneglycol diacrylate) (MW = 5000 g/mol), 3% w/v chondroitin sulfate methacrylate, and 0.05% w/v photoinitiator (Irgacure D 2959; Ciba Specialty Chemicals, Basel, Switzerland) in medium. Cell-laden hydrogels were cultured in chondrocyte growth medium for 24 h and either cultured in vitro or engrafted subcutaneously in NCr-Foxn1nu-immunodeficient mice (Taconic, Oxnard, CA, USA). All samples were cultured in vitro or in mice for 4 wk before they were harvested for further analyses. All animal procedures were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Flow cytometry
Cells were dissociated into a single-cell suspension by using TrypLE (Invitrogen-Life Technologies) and fixed in BD Cytofix buffer (BD Biosciences) for 20 min at room temperature. The cells were permeabilized by washing and incubating them with BD Permeabilization/Wash (BD Biosciences) buffer at 1 × 107 cells per 1 ml for 10 min. The cells were stained by incubating them with antibodies (mouse anti-human Sox9, anti-human CD44-PE/Cy7, and anti-human CD140-Alexa647; BD Biosciences) for 30 min. Primary antibodies were diluted according to the manufacturer’s instructions, and secondary antibodies (donkey anti-mouse IgG Alexa 488; BD Biosciences) were diluted by 1:250. The cells were scanned with an LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Ashland, OR, USA).
Biochemical analyses
Cell-hydrogel constructs were weighed wet, lyophilized, weighed dry, and digested in papainase solution (Worthington Biochemical, Lakewood Township, NJ, USA) at 60°C for 16 h, as described previously (16). DNA content was measured using the PicoGreen assay (Molecular Probes-Life Technologies, Eugene, OR, USA) with lambda phage DNA as the standard. Sulfated glycosaminoglycan (GAG) content was quantified with the 1,9-dimethylmethylene blue dye-binding assay with shark CS (Sigma-Aldrich) as the standard. GAG content of the acellular hydrogels was determined as a negative control and subtracted from the amount of GAG released by the encapsulated cells during the 4 wk of culture.
Microarray analysis
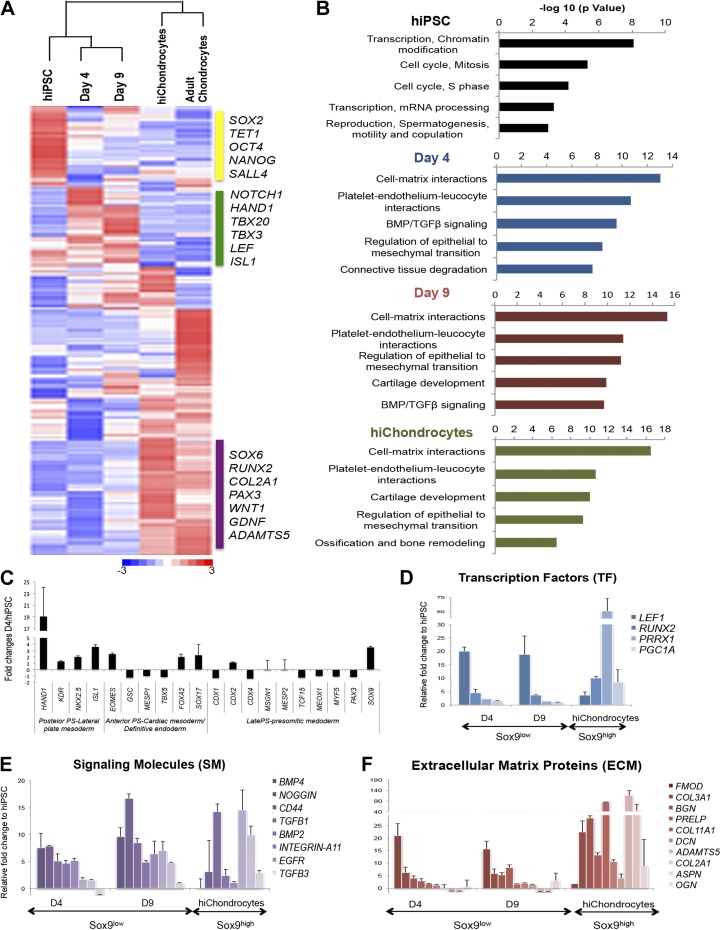
For the microarray analyses, to analyze the changes in gene expression over the differentiation process, Human Gene 1.0 ST Arrays (Affymetrix, Santa Clara, CA, USA) were performed on 2 biologic replicates at each time point. The hiPSC replicates were set as the baseline arrays and the differentiating chondrocyte replicates as the experimental arrays. Normalization, comparison of gene expression values, filtering of significant expression probes, and clustering analysis of expression values were performed within dChip (Harvard School of Public Health, Boston, MA, USA) as described in the manual (17). To examine the changes in lineage-specific genes over the course of differentiation, gene lists for ESC) regulation and cartilage development were obtained from MetaCore (Thomson Reuters, New York, NY, USA). Clustering analysis performed on these combined gene lists enabled the generation of the heat map in Fig. 4. Network analysis of the genes that had a 1.5-fold or greater upregulation in expression at each stage of differentiation when compared to the hiPSCs (the hiPSCs were compared to adult chondrocytes) was performed using MetaCore. Raw microarray data was uploaded to the Gene Expression Omnibus [accession number: GSE62914; National Center for Biotechnology Information, Bethesda, MD, USA].
Figure 4.
Genome-wide gene expression analyses during chondrogenic differentiation of hiPSC. A) Hierarchical clustering and representative heatmap of genome-wide gene expression at different stages during hiPSC differentiation to hiChondrocytes and comparison with undifferentiated hiPSC and adult chondrocytes. B) Top gene ontology terms significantly enriched at different stages during chondrogenic differentiation of hiPSCs. C) Gene expression for characteristic markers of mesodermal subtypes and posterior, mid-, anterior, and late PS observed at an early stage (d 4) during chondrogenic differentiation of hiPSCs. D–F) Expression of known Sox9-regulated genes including transcription factors, signaling molecules, and ECM proteins in the Sox9low and Sox9high populations induced during chondrogenic differentiation of hiPSCs. Data presented as means ± se for gene expression vs. hiPSCs (n = 2 biologic replicates).
Statistical analyses
Statistical significance of data was determined by applying a 2-tailed Student’s t test to values obtained from independent experiments.
RESULTS
hiPSC differentiation into articular chondrocytes
We sought to optimize a growth factor-based protocol for quick and efficient differentiation of hiPSCs toward chondrocytes. Toward this goal, we used hiPSCs established by the original Yamanaka approach (9) of introducing 4 pluripotency factors—octamer-binding transcription factor (Oct)-4; SRY (sex-determining region Y) box, (Sox)-2; Krüppellike factor (Klf)-4; and v-myc avian myelocytomatosis viral oncogene homolog (c-Myc) (OSKM)—in human foreskin fibroblasts (BJ fibroblasts; ATCC, Manassas, VA, USA) by retroviral transduction (18). In addition, we independently established hiPSCs from human foreskin fibroblasts using the 4 Yamanaka factors in a single lentiviral cassette, thereby using a different method for hiPSC generation. The hiPSCs were characterized for pluripotency in vitro (Supplemental Fig. S1A, B) and in vivo by their ability to form teratomas (18).
To differentiate the cells into chondrocytes, we initially tried the stepwise protocol, using growth factors previously established by Oldershaw et al. (19) for differentiation of human (h)ESC cultures into chondrocytes. However, multiple experiments in which this protocol was used for inducing chondrogenic differentiation in hiPSC cultures were unsuccessful, mainly because of a decline in iPSC viability. The results were not surprising, considering that variable gene expression and DNA methylation patterns have been reported within established hESC lines (20) as well as between ESCs and iPSCs (21, 22). After a few trials, we discovered that dissociation and aggregation of hiPSCs in suspension cultures to form EBs for a short period was an optimal initial step (Fig. 1A and Supplemental Fig. S2). Another major modification was the use of the p160-Rho-associated coil kinase (ROCK) inhibitor Y27632, which has been shown to prevent cell dissociation-induced apoptosis in both ESC and iPSC cell cultures (23). Besides promoting pluripotent stem cell survival, Y27632 has been reported to induce an increase in SOX9 gene expression in chondroprogenitor cells (24). We therefore reasoned that the addition of Y27632 would also be beneficial for promoting differentiation of iPSCs toward a chondrogenic fate. To elucidate the precise effect of ROCK inhibitor, we performed hiPSC differentiation in EBs for the first 4 d in the presence and absence of the inhibitor and then studied the relative number of EBs and the percentage of Sox9-positive cells by fluorescence-activated cell sorting (FACS). As shown in Supplemental Fig. S3, we confirmed the effect of the ROCK inhibitor, both in supporting EB differentiation and in increasing the percentage of Sox9-positive cells among the live cells. This analysis definitively established the positive effect of ROCK inhibitor in enhancing chondrogenic differentiation in hiPSCs.
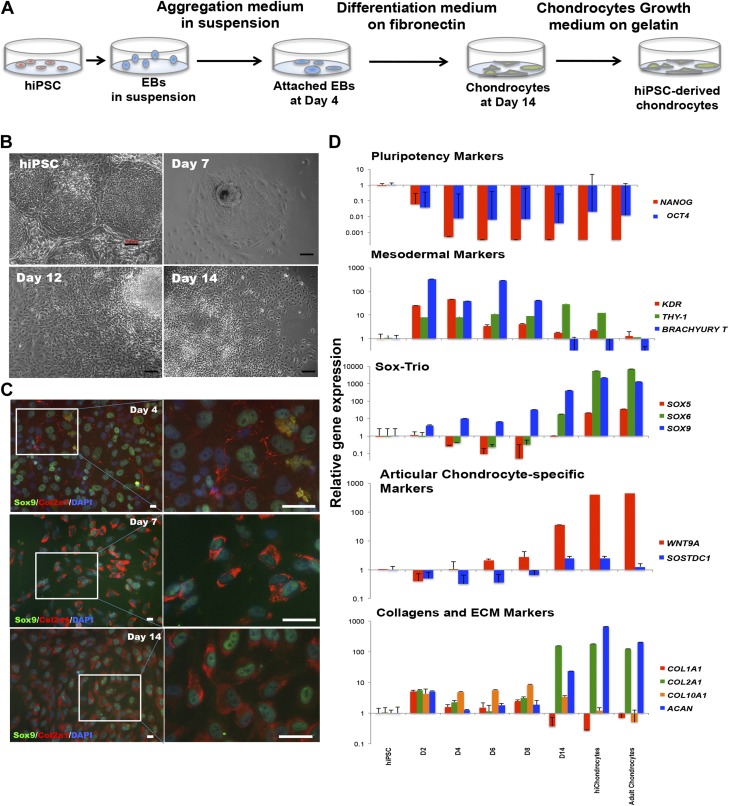
Figure 1.
Efficient differentiation of hiPSCs into stable chondrocytes. A) Chondrogenic differentiation of hiPSCs. B) Representative morphologic changes in hiPSCs during chondrogenic differentiation at different stages (undifferentiated hiPSCs, d 7, 12, and 14 after differentiation). Scale bar, 100 μm. C) Representative immunofluorescence staining of chondrocyte markers, Sox9 and Col2a1 during hiPSC differentiation at d 4, 7, and 14. Scale bar, 100 μm. D) Gene expression analyses using quantitative real-time PCR for pluripotency markers (OCT4 and NANOG), mesodermal markers (KDR, Brachyury T, and THY-1), Sox trio chondrogenic markers (SOX9, SOX6, and SOX5), joint interzone-specific markers (WNT9A and SOSTDC1) and collagens and ECM markers (COL1A1, COL2A1, COL10A1, and ACAN). Data are expressed as means ± sd for gene expression vs. hiPSCs (logarithmic scale) and represent 3 independent experiments.
hiPSCs were cultured as EBs in the first stage in aggregation medium containing Y27632 with a stepwise treatment of Wnt3a, Activin A, FGF2, and bone morphogenetic protein (BMP)-4 in the first 4 d. The rationale was to skew the EB differentiation toward a mesodermal fate while maintaining cellular viability. In the second stage spanning d 4–9, the EBs were cultured in differentiation medium with continued treatment with FGF2 and BMP4, along with follistatin to reduce endodermal gene expression. Neurotrophin (NT)-4 was added to increase cell survival, as had been reported (19). The differentiating cells were adherent while continuing their differentiation at this stage (Fig. 1B). In the final stages of differentiation, growth differentiation factor 5 was added to induce chondrogenic differentiation and maturation. FGF2 and NT4 were maintained while BMP4 was gradually removed. After d 4, the cells were continually differentiated and passaged only after d 14. During differentiation, the EBs first generated from pluripotent hiPSC colonies consisted of a heterogeneous mix of differentiated mesodermal cells. A chondrocytelike morphology was evident in the cells by d 12 and became more homogenous upon passaging after d 14. Upon immunostaining for Sox9, a low expression representing mesodermal cells was observed as early as d 4 after the adherence of EBs and was more pronounced in a small fraction of cells at d 7. Collagen (Col) II, a characteristic extracellular matrix (ECM) marker for chondrocytes was not yet detected by immunostaining for Col2a1 at d 4, but its accumulation was observed by d 7 (Fig. 1C). Cells coexpressing Sox9 and Col II increased during the differentiation process demonstrating efficient differentiation of hiPSCs into chondrocytes. By d 14, a high expression of both Sox9 and Col II was observed in the differentiated chondrocytes and was maintained after cell passaging, therefore showing a stable chondrocyte fate. At the early stage of differentiation (d 2), we observed a rapid loss of the pluripotency markers [Nanog homeobox (NANOG) and OCT4] (Fig. 1D). In contrast to the down-regulation of pluripotency-associated markers, genes indicative of mesodermal expression such as KDR, THY-1 (known as CD90), and Brachyury T, were expressed at higher levels as early as d 2–4, indicating that the differentiation process mediates an earlier mesodermal transition than previously observed during human ESC differentiation (19). By d 8, the mesodermal markers were reduced, and the chondrogenic transcription factor Sox9 was significantly increased in accordance with the immunostaining results. Gene expression of other Sox family members, SOX6 and SOX5, continually increased during chondrogenic differentiation. By d 14, chondrocyte-specific collagen (COL2A1) as well as Aggrecan (ACAN) showed higher gene expression levels, but not the fibrocartilage marker, COL1A1 or the hypertrophy marker COL10A1. More important, markers associated with joint interzone development, WNT9A (25, 26) and the BMP inhibitor sclerostin domain-containing protein 1 (SOSTDC1) (25) were found to be highly expressed, indicating that the differentiation was directed toward an articular chondrocyte fate rather than a growth plate chondrocyte. Moreover, hiChondrocytes maintained the coexpression of Sox9 and Col II upon multiple rounds of cell passaging after 14 d of differentiation as well as postcryopreservation (Supplemental Fig. S4A). Taken together, these findings demonstrate that the reported differentiation method is efficient and effective.
Efficient generation of hyaline-like cartilage in vitro by hiChondrocytes
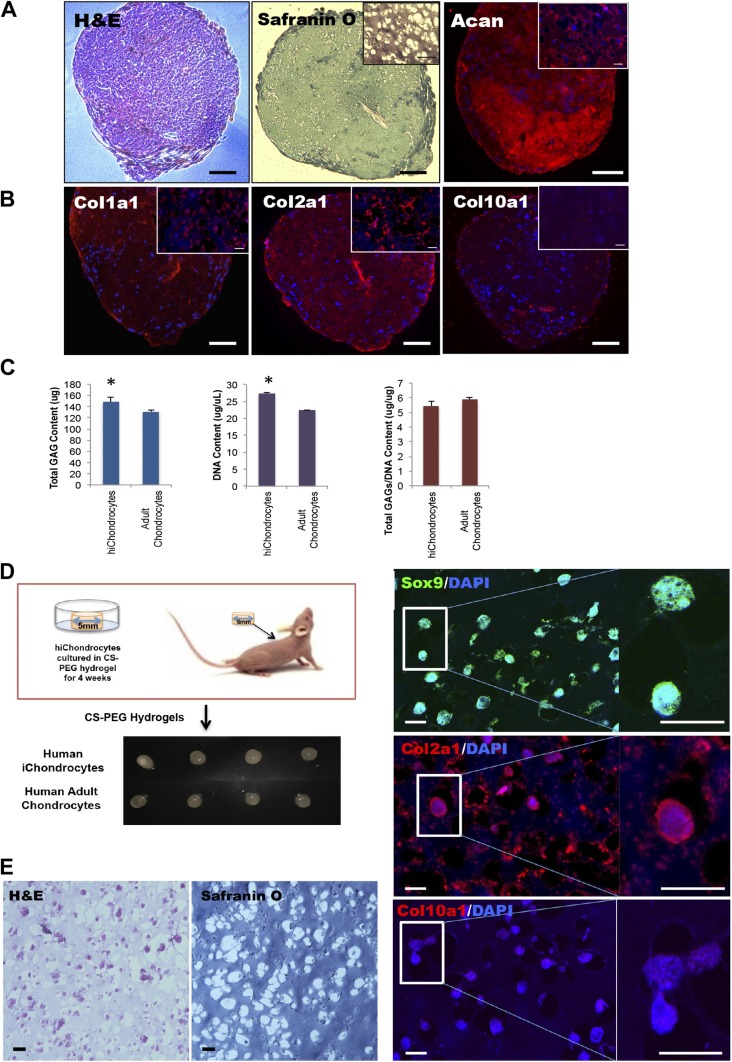
To assess the chondrogenic potential of hiChondrocytes, we centrifuged 2 × 105 cells to form 3-dimensional pellets and subsequently cultured them for 28 d in vitro in the presence of chondrocyte growth medium containing insulin-like growth factor, FGF2, insulin, and transferrin. Safranin-O staining of sectioned pellets showed the production of GAGs in pellets formed from hiChondrocytes (Fig. 2A). Next, we examined the extracellular matrix components produced by hiChondrocytes in pellet culture, using immunohistochemical staining for Acan, and different types of collagens (Col1a1, Col2a1, and Col10a1). Both Acan and cartilage-specific type II collagen (Col2a1) were abundantly present throughout the cartilage pellets formed by hiChondrocytes (Fig. 2B). In contrast, these cartilage pellets showed little staining for type I collagen (Col1a1) or type X collagen (Col10a1), showing a lack of both characteristic fibrocartilage and hypertrophic markers. Quantification of the fluorescence intensity using Image J (National Institutes of Health, Bethesda, MD, USA) determined the staining intensity for type II collagen to be 20-fold higher than that of type I collagen (Supplemental Fig. S4B).
Figure 2.
A–C) In vitro and (D–F) in vivo cartilage generation by hiChondrocytes. A) Representative hematoxylin and eosin (H&E), Safranin O, and (Acan) staining. B) Representative immunofluorescence staining for Col1a1, Col2a1, and Col10a1 in hiChondrocyte-derived cell pellets. C) Quantification of GAG production in hiChondrocyte-derived cell pellets in vitro. Data are presented as means ± sd of 3 independent experiments. *P < 0.05. D) hiChondrocytes encapsulated in CS-PEG hydrogels and engrafted subcutaneously in immunodeficient mice. E) Representative H&E and Safranin O staining of hydrogel-capsulated hiChondrocytes cultured for 4 wk in vivo. F) Confocal microscopy for representative immunofluorescence staining for chondrocyte markers, Sox9, Col2a1, and Col10a1 in hydrogel capsulated hiChondrocytes cultured for 4 wk in vivo. Scale bars, 100 μm; (B) inset 20 μm.
Quantification of GAG production from the cartilage pellets generated by hiChondrocytes demonstrated higher amounts of GAG production compared to the 3D pellets generated by adult chondrocytes (Fig. 2C). The primary adult chondrocytes used were those isolated from the knees of non-OA patients undergoing ACL reconstructive surgery using institutional review board-approved protocols (14). The higher GAG production was due to the increased proliferation of hiChondrocytes, as compared to the adult chondrocytes resulting in a higher number of cells, as indicated by the DNA content (Fig. 2C and Supplemental Fig. S5A). Upon normalization for the DNA content (i.e., number of cells), the amount of GAG production per cell by hiChondrocytes was similar to that of the adult chondrocytes. Therefore, the hiChondrocytes generated in the described protocol were stable and comparable in function to adult chondrocytes.
Upon directly assessing cell growth for 2 distinct hiChondrocyte populations compared to 2 primary human adult chondrocytes (a 27-year-old woman and a 35-year-old man), we observed that the hiChondrocytes grew faster than the adult chondrocytes. The hiChondrocytes cell growth showed a doubling time of approximately 35 ± 0.6 h, faster than the adult human chondrocytes, which showed a doubling time of 45.7 ± 1.3 h (Supplemental Fig. S5A).
In vivo cartilage generation by hiChondrocytes in the absence of tumor
We next investigated the in vivo potential of hiChondrocytes for cartilage tissue formation and tested whether any tumorigenic cells were present in these differentiated cell populations. The hiChondrocytes were encapsulated in chondroitin sulfate containing polyethylene glycol (CS-PEG)-based hydrogels (16, 27), cultured for 24 h in vitro and engrafted subcutaneously in NCr-Foxn1nu-immunodeficient mice (Fig. 2D–F). These CS-PEG hydrogels have been used by many groups, including ours, as biomimetic scaffolds for supporting cartilage generation by adult chondrocytes and stem cells, including MSCs and ADSCs (16, 28). The hiChondrocyte-engrafted hydrogels were harvested from the mice after 28 d and demonstrated formation of cartilaginous tissue similar to that formed by the control adult chondrocyte-engrafted hydrogels (Fig. 2D). No tumors were detected in the mice with hiChondrocyte-seeded hydrogel transplants, either in the scaffold or surrounding tissues, by visual inspection or upon harvesting. Further histologic analyses showed characteristic cartilaginous tissues and GAG staining with Safranin-O (Fig. 2E). Immunostaining for Sox9 and Col2a1 showed high expression, confirming the long-term stability of the chondrocyte phenotype in hiChondrocytes (Fig. 2F). Likewise, immunostaining of the hydrogel sections showed that Col2a1 was highly expressed, whereas the expression of Col10a1 was negligible. Quantification of GAG production from the in vivo cartilage tissue generated in biomimetic hydrogels demonstrated similar amounts of GAG production (normalized to DNA content) by hiChondrocytes compared with that of adult chondrocytes, in agreement with the in vitro observations (Supplemental Fig. S5B). A comparison of the chondrogenic gene expression between hiChondrocytes and adult chondrocytes after harvesting from the engrafted hydrogels showed that the expression levels of SOX9, SOX6, and SOX5 in hiChondrocytes were comparable to that in adult human chondrocytes, as was the expression of the ECM genes [COL1A1, COL10A1, and ACAN] was similar in both the populations (Supplemental Fig. S5C), confirming the long-term stability of hiChondrocytes. In addition, hiChondrocytes were capable of generating cartilage without tumorigenesis after in vivo transplantation for 4 wk. Altogether, the in vivo studies demonstrated both the safety and efficacy of our optimized differentiation protocol.
Chondrogenic differentiation of hiPSC involves early induction of a Sox9lowCD44lowCD140low population
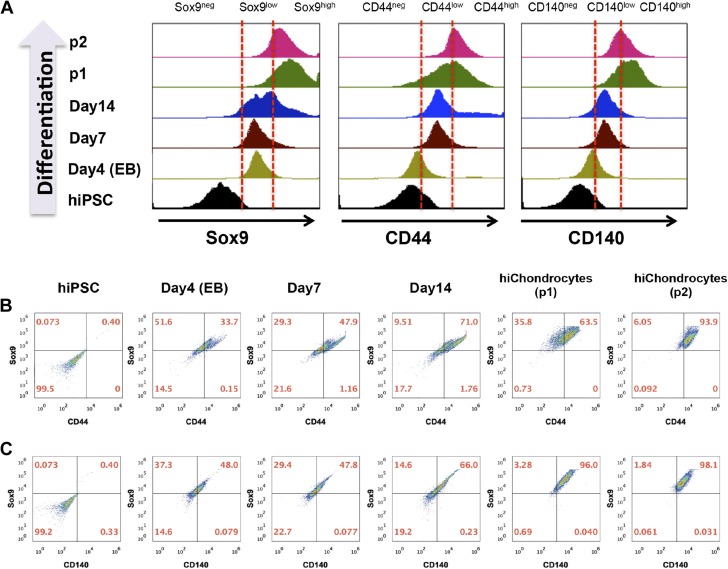
To further define the nature and frequency of the intermediate cell populations during the differentiation of hiPSC to hiChondrocytes, we initially used flow cytometric analyses to follow the expression of the key transcriptional regulator of chondrocyte fate Sox9 during differentiation. Although hiPSCs did not express Sox9 as expected, 64–85% of the differentiating cells expressed it as early as d 4 after the initial aggregation stage (Supplemental Fig. S6). By d 14, 76–89% of the cells were Sox9-positive and that portion increased to a homogenous 92–99% Sox9-positive cells upon passaging. We noted particularly that the intensity of the Sox9 expression gradually increased during chondrogenic differentiation, revealing 2 distinct chondrogenic populations, with Sox9low and Sox9high expression, respectively (Fig. 3A). Since Sox9 has been observed to be expressed in early mesodermal populations as well (29, 30), we analyzed the expression of the mature chondrocyte cell-surface markers, cluster of differentiation (CD)44 and CD140, respectively, in the Sox9-expressing cells to map the emergence of the precise chondrogenic fate during hiPSC differentiation. Both CD44 and CD140 showed 2 distinct low- and high-expression populations, similar to Sox9. The early chondrogenic populations at d 4 and 7 were Sox9lowCD44lowCD140low, which eventually matured into a homogenous Sox9highCD44highCD140high hiChondrocyte population after differentiation (Fig. 3B, C). These single-cell analyses demonstrate that the differentiation protocol leads to a highly efficient chondrogenic differentiation of hiPSCs.
Figure 3.
Single-cell flow cytometric analyses during chondrogenic differentiation of hiPSCs. A) Representative analyses of Sox9-, CD44-, and CD140-expressing cells during chondrogenic differentiation of hiPSC. Representative flow cytometric analyses of (B) CD44 and (C) CD140 coexpression in Sox9-expressing cells at d 0, 4, 7, 14 after induction of hiPSC differentiation and in hiChondrocytes (passages 1, 2).
To test the reproducibility of chondrocyte differentiation, we performed single-cell FACS analysis for Sox9 at different times in the differentiation protocol during the stepwise growth-factor treatment for 14 d and quantified the Sox9low and Sox9high populations (Fig. 3A and Supplemental Fig. S6B). The cells are exclusively Sox9low at d 4 and 7 and were exclusively Sox9high after passage 1. The representative Supplemental Fig. S6B demonstrates 3 batches (1, 3, and 4) from lentivirally generated hiPSCs and 2 batches (7 and 10) from retrovirally generated hiPSCs. Differentiation in all the batches is consistent with a 5–9% variation in the overall Sox9-expressing population at d 4, 9, and 14 that eventually gave rise to a very homogenous Sox9high population after the first passage of cells. After passage 1, the yield of the hiChondrocytes was determined to be 97 ± 2% Sox9high, demonstrating the robustness and reproducibility of the differentiation protocol. Chondrocyte differentiation experiments were repeated at least 15 times (15 independent biologic replicates), on both the independently established hiPSC lines, and >90% differentiation efficiency into hiChondrocytes was consistently observed showing the reliability and reproducibility of the differentiation protocol (Supplemental Fig. S6B).
We further tried to enrich for the Sox9highCD44highCD140high chondrocytes at d 14 after differentiation by FACS for CD44high cells (Supplemental Fig. S7A). The CD44high hiChondrocytes were expanded in monolayer culture and characterized. Whereas gene expression for SOX9 and WNT9A (enriched in articular chondrocytes) was comparable in the CD44high-sorted, unsorted, and adult chondrocytes, the CD44high-sorted hiChondrocytes showed a higher expression for COL2A1 than the unsorted hiChondrocytes (Supplemental Fig. S7B). However, qualitative analyses demonstrated an efficient generation of cartilage and ECM by unsorted and CD44high sorted hiChondrocytes, comparable to adult chondrocytes upon culture into 3-dimensional pellets (Supplemental Fig. S7C), suggesting that the unsorted hiChondrocyte populations were functionally similar to the CD44high-sorted hiChondrocytes.
Genome-wide gene expression analyses reveal 2 distinct gene networks regulated by Sox9 during hiPSC differentiation into hiChondrocytes
To understand the molecular mechanisms underlying hiPSC differentiation into hiChondrocytes, we analyzed genome-wide gene expression changes during the course of hiPSC differentiation, using exon microarrays. We performed the global gene expression analyses at the 3 distinct stages of hiChondrocyte specification: 1) mesodermal induction stage (differentiation d 0–4); 2) prechondrogenic stage (differentiation d 4–9); and 3) chondrocyte maturation stage (differentiation d 9–14), comparing these stages to the originating hiPSC and adult chondrocytes. Two independent biologic replicates were used for hiPSCs (d 0), each differentiation time point (d 4 and 9), and hiChondrocytes generated and compared to 2 primary adult human chondrocytes. An unsupervised clustering and Pearson correlation analyses of the different datasets clearly showed that the differentiating populations at d 4 and 9 clustered together, whereas the hiPSCs were distinct from these populations (Supplemental Fig. S8A, B). Similarly, hiChondrocytes were similar to adult chondrocytes, but distinct from the intermediate differentiating populations and hiPSCs (Supplemental Fig. S8C). For the initial analyses, we identified a subset of 270 genes, including the known critical markers for pluripotency, cartilage differentiation, and chondrocytes, as well as early mesodermal genes (Fig. 4A). Upon differentiation, gene expression of pluripotency markers, including SOX2, TET1, OCT4, NANOG, and SALL4, was immediately down-regulated, as observed clearly by d 4, concurrent with an increase in expression of mesodermal genes, including NOTCH1, HAND1, TBX20, TBX3, LEF1, NKX2.5, and ISL1, in the intermediate stages of differentiation at d 4 and 9. Gene expression patterns of hiChondrocytes showed similarity to adult chondrocytes in the spectrum of genes essential for cartilage development and chondrogenesis (SOX6, RUNX2, COL2A1, PAX3, WNT1, GDNF, and ADAMTS5). These mature chondrogenic genes, however, were gained later in differentiation, as the early stages (d 4 and 9) showed lower expression levels for these genes. The array data were validated by the independent measurement of the key chondrogenic factors by real-time PCR (Fig. 1D, Supplemental Fig. S8C) including the Sox trio, ECM genes, and other transcription factors.
Pathway enrichment analyses showed chromatin modifiers and cell cycle regulators to be enriched in hiPSCs, whereas cell-matrix interactors, cartilage development, and bone ossification and remodeling pathways were enriched in adult chondrocytes. For the intermediate stages, regulation of epithelial-to-mesenchymal transition, cell-matrix interactions, and BMP/TGFβ signaling were the significant pathways identified (Fig. 4B). It has been an unresolved question whether the primitive streak (PS) mesodermal induction consists of a common mesenchymal progenitor or distinct progenitor cells destined for the downstream lineages. In a recent elegant study, investigators addressed this complex question and suggested the existence of multiple distinct progenitors, further identifying the spatial and temporal patterns of genes that particularly characterize a prechondrogenic progenitor (31).
To test whether our differentiation protocol leads to an enrichment of the newly described prechondrogenic mesodermal progenitors, we sought to compare the gene expression of different mesodermal developmental genes (Fig. 4C). Indeed, we observed that, when compared to hiPSCs, the early mesodermal populations at d 4 of differentiation showed an enrichment for the genes associated with posterior PS lateral plate mesoderm that later matures into the prechondrogenic mesoderm, whereas the genes associated with the anterior PS that develops into cardiac mesoderm or definitive endoderm showed a lower expression. These results suggest that hiPSC differentiation is specifically guided toward the prechondrogenic mesoderm in our experimental design, thereby resulting in a highly efficient generation of chondrocytes.
The transcription factor Sox9 is expressed in mesenchymal cells, chondroprogenitors, and mature chondrocytes (29, 32), but a molecular understanding of the differences in the Sox9 targets between these different stages is lacking. We examined genes that were identified as containing Sox9 interaction sites in a chondrogenic rat sarcoma cell line by chromatin immunoprecipitation, followed by global array (ChIP-chip) (33) in the Sox9low mesodermal population and the Sox9high chondrocytes. We divided the Sox9-regulated genes into 3 groups: transcription factors, signaling molecules, and ECM proteins (Fig. 4D–F). In the group of transcription factors (Fig. 4D), expression of LEF1, which interacts with β-catenin under Wnt stimulation (32), was high in the Sox9low population (d 4 and 9), whereas RUNX2, PRRX1 (34), and the transcriptional coactivator PGC-1α (35) expression was high in Sox9high hiChondrocytes. In the signaling molecules group, BMP2, BMP4, and TGFb1 were highly expressed in Sox9low but were down-regulated in the Sox9high hiChondrocytes, whereas CD44, integrin, and epidermal growth factor receptor were highly expressed in those cells (Fig. 4E). We noted especially that the ECM proteins in the third group (Fig. 4F) exhibited the most significant increase in Sox9-regulated ECM genes, including COL3A1, BGN, COL11A1, DCN, ADAMTS5, COL2A1, ASPN, and OGN in Sox9high hiChondrocytes. The ECM proteins encoded by these genes are critical for human articular cartilage function.
DISCUSSION
We report an efficient and relatively quick method of generating articularlike chondrocytes from hiPSCs. We capitalized on previously reported developmental pathways to optimize directed stage-specific cues for guiding hiPSC differentiation toward chondrocytes (19, 25). hiPSCs represent a novel and potentially unlimited resource for generating chondrocytes and engineered cartilage tissue for therapeutic applications, as well as for modeling acquired and genetic musculoskeletal diseases in vitro for initial drug discovery and development.
Previous studies have demonstrated generation of chondrocytes from human ESCs through stage-specific modulation of multiple signaling pathways (19). In brief, we optimized and applied these protocols to hiPSCs with some critical changes that include a short-term differentiation of the hiPSCs in EBs, with the addition of Wnt3a and Activin A, to induce preferential differentiation toward the mesoderm. Another key modification was the addition of the p160-ROCK inhibitor Y27632. We observed that generation of EBs by hiPSCs was greatly reduced in the absence of Y27632. Inhibition of the RhoA/ROCK pathway by Y27632 early in the differentiation time course increased both hiPSC differentiation in EBs and the percentage of Sox9-expressing cells, thereby enhancing the overall chondrogenic differentiation of hiPSCs (Supplemental Fig. S3).
Following the differentiation protocol with combined growth factor treatment, we demonstrated a highly efficient generation of hiChondrocytes, with almost 98% cells exhibiting the mature chondrogenic phenotype of Sox9highCD140highCD44high gene expression. It is notable that the hiChondrocytes expressed markers associated with joint interzone development that are found only in articular chondrocytes (WNT9A and SOSTDC1) (25, 26), as well as the chondrogenic markers Col2a1 and Acan, but neither Col10a1, which is associated with hypertrophy, nor Col1a1, which is a fibrocartilage marker.
Functionally, the hiChondrocytes demonstrated a potential comparable to adult chondrocytes in generating cartilage tissue in vitro as well as in vivo, as shown by histology, immunostaining, and quantitative estimation of accumulated GAGs and ECM deposition. Furthermore, FACS for CD44high cells at d 14 after differentiation to enrich for the Sox9highCD44highCD140high chondrocytes did not lead to any significant improvement in cartilage generation in 3-dimensional pellets. We therefore conclude that such a sorting method, which can lead to loss of cells, is not beneficial. However, the CD44high-sorted hiChondrocytes did show differential gene expression compared with the unsorted and adult chondrocytes in having a higher expression of COL2A1; hence, further studies on the detailed characterization of the CD44high population and its subsets could be beneficial.
The hiChondrocytes generated are also “safe” in vivo, as they do not form any teratomas; however, it must be mentioned that having an appropriate scaffold such as the CS-PEG hydrogels used in this study would contribute toward maintaining a stable cell fate by providing the right niche cues to the transplanted cells. Such a lack of teratoma formation after differentiation of mouse iPSCs into chondrocytes has been demonstrated by the Guilak (13) and Keller (25) groups as well, whereas investigators in a study demonstrating transdifferentiation of fibroblasts into chondrocytes using defined factors reported generation of teratomas in the transdifferentiated chondrocytes that had a persistent high expression of Sox9 (36). The hiChondrocytes generated are stable and do not dedifferentiate upon cryopreservation, as in up to 3 passages of monolayer culture of hiChondrocytes in vitro, the process of freezing and thawing did not diminish the expression of the chondrogenic markers, Sox9 and Col2a1 (Supplemental Fig. S4A). In addition, the hiChondrocytes had a proliferative advantage over adult chondrocytes that could be beneficial for their expansion for research or therapeutic applications (Supplemental Fig. S5A).
Chondrogenic differentiation in early development is stringently controlled by distinct signaling networks (26); therefore, chondrogenic differentiation of hiPSCs toward chondrocytes provides an in vitro platform to identify and dissect the stepwise molecular events during this process. Comparison with normal adult chondrocytes via global gene expression analyses showed a close similarity between hiChondrocytes and adult chondrocytes, with a clear distinction from parental hiPSC. Our findings suggest that a combination of inhibition of the RhoA/ROCK pathway, Wnt, and Activin A signaling initially increased the hiPSC differentiation in the EBs toward the mesoderm. Using our differentiation protocol, we have identified that the posterior PS markers, as was recently described to characterize a prechondrogenic mesodermal population (19), were markedly induced in the early populations during hiPSC differentiation (d 4), whereas the anterior PS/cardiac mesodermal markers were reduced (Fig. 4C). These results highlight that the combination of Wnt3A, Activin A, and the ROCK inhibitor Y27632 led to a preferential enrichment of prechondrogenic mesoderm at the onset of hiPSC differentiation in our method.
Another interesting mechanistic insight from our study has been that Sox9 regulates distinct networks in the Sox9low mesodermal population and the late-emerging Sox9high chondrocyte population. Although Sox9 has been known as a key regulator in mesodermal populations and for chondrogenic differentiation, the binding and regulation of its targets has not been characterized in detail in these populations (by chromatin immunoprecipitation and global sequencing, for example). Also, the observations that the mesodermal/prechondrogenic population is predominantly Sox9low at the single-cell level, maturing to a Sox9high population, rather than some cells gaining high levels of Sox9 with others having no Sox9, informs us that the cellular differentiation process is homogenous and that the maturation of Sox9low to Sox9high expression is a distinct step in chondrogenic differentiation. In addition, our study shows that Sox9 has a distinct set of binding targets when its levels are low and that it can especially activate these target genes (like LEF and BMP4, as in Fig. 4D, E) only at low levels. As expected, a different set of genes appeared to be activated when the levels were high (Fig. 4F), thereby providing new and interesting insights into the gene networks regulated by Sox9. In future studies, it will be interesting to illuminate the differences in the cofactors and binding targets when Sox9 levels are low or high, to get detailed mechanistic information about Sox9 function.
In conclusion, our study findings enable us to define an optimal protocol for efficient chondrocyte differentiation of hiPSCs as a potentially attractive source for cartilage generation for therapeutic purposes, as well as for disease modeling and drug screening. In addition, this study provides comprehensive analyses of the underlying molecular events during hiPSC differentiation toward chondrocytes, including identification of 2 distinct Sox9low and Sox9high regulatory networks that operate in the early mesodermal and mature chondrocyte fates. The findings therefore provide the groundwork for the future use of patient-specific hiPSCs to understand how these gene networks are impaired or modulated during inherited or acquired cartilage diseases.
Supplementary Material
Acknowledgments
The authors thank Prof. John P. Cooke (Methodist Hospital Research Institute), Profs. Renee Reijo Pera (Montana State University), and Drs. Vittorio Sebastiano (Stanford University) and Arwen Hunter (Stem Cell Technologies) for help with hiPSC generation and Natalia Kosovilka (Stanford University School of Medicine Protein and Nucleic Acid Facility) for running the microarrays. FACS data were performed on an instrument in the shared FACS facility obtained using U.S. National Institutes of Health S10 Shared Instrument Grant S10RR027431-01. No financial support or other benefits have been obtained from any commercial sources for this study. The authors declare no conflicts of interest.
Glossary
- ACAN
aggrecan
- ACL
anterior cruciate ligament
- ADSC
adipose-derived stem cell
- BMP
bone morphogenic protein
- CD
cluster of differentiation
- COL
chondrocyte-specific collagen
- CS-PEG
chondroitin sulfate containing polyethylene glycol
- EB
embryoid body
- ECM
extracellular matrix
- ESC
embryonic stem cell
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- GAG
glycosaminoglycan
- hESC
human embryonic stem cell
- hiChondrocyte
hiPSC-derived chondrocyte, hiPSC, human induced pluripotent stem cell
- MSC
mesenchymal stem cell
- NANOG
Nanog homeobox
- NT
neurotrophin
- OA
osteoarthritis
- OCT
octamer-binding transcription factor
- PS
primitive streak
- ROCK
Rho-associated coil kinase
- SOSTDC
sclerostin domain-containing
- SOX
SRY (sex-determining region Y) box
- WNT
wingless-type MMTV (mouse mammary tumor virus) integration site family
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Huey D. J., Hu J. C., Athanasiou K. A. (2012) Unlike bone, cartilage regeneration remains elusive. Science 338, 917-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makris E. A., Gomoll A. H., Malizos K. N., Hu J. C., Athanasiou K. A. (2015) Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 11, 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmander L. S., Roos E. M. (2007) Clinical update: treating osteoarthritis. Lancet 370, 2082–2084 [DOI] [PubMed] [Google Scholar]
- 4.Stoddart M. J., Grad S., Eglin D., Alini M. (2009) Cells and biomaterials in cartilage tissue engineering. Regen. Med. 4, 81–98 [DOI] [PubMed] [Google Scholar]
- 5.Chan C. K., Lindau P., Jiang W., Chen J. Y., Zhang L. F., Chen C. C., Seita J., Sahoo D., Kim J. B., Lee A., Park S., Nag D., Gong Y., Kulkarni S., Luppen C. A., Theologis A. A., Wan D. C., DeBoer A., Seo E. Y., Vincent-Tompkins J. D., Loh K., Walmsley G. G., Kraft D. L., Wu J. C., Longaker M. T., Weissman I. L. (2013) Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc. Natl. Acad. Sci. USA 110, 12643–12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelttari K., Pippenger B., Mumme M., Feliciano S., Scotti C., Mainil-Varlet P., Procino A., von Rechenberg B., Schwamborn T., Jakob M., Cillo C., Barbero A., Martin I. (2014) Adult human neural crest-derived cells for articular cartilage repair. Sci. Transl. Med. 6, 251ra119. [DOI] [PubMed] [Google Scholar]
- 7.Adkisson H. D. IV, Martin J. A., Amendola R. L., Milliman C., Mauch K. A., Katwal A. B., Seyedin M., Amendola A., Streeter P. R., Buckwalter J. A. (2010) The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am. J. Sports Med. 38, 1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 10.Wernig M., Meissner A., Foreman R., Brambrink T., Ku M., Hochedlinger K., Bernstein B. E., Jaenisch R. (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 [DOI] [PubMed] [Google Scholar]
- 11.Vierbuchen T., Ostermeier A., Pang Z. P., Kokubu Y., Südhof T. C., Wernig M. (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ieda M., Fu J. D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B. G., Srivastava D. (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekman B. O., Christoforou N., Willard V. P., Sun H., Sanchez-Adams J., Leong K. W., Guilak F. (2012) Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 109, 19172–19177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor S. E., Smeriglio P., Dhulipala L., Rath M., Bhutani N. (2014) A global increase in 5-hydroxymethylcytosine levels marks osteoarthritic chondrocytes. Arthritis Rheumatol. 66, 90–100 [DOI] [PubMed] [Google Scholar]
- 15.Sturgeon C. M., Ditadi A., Awong G., Kennedy M., Keller G. (2014) Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 32, 554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smeriglio P., Lai J. H., Dhulipala L., Behn A. W., Goodman S. B., Smith R. L., Maloney W. J., Yang F., Bhutani N. (2015) Comparative potential of juvenile and adult human articular chondrocytes for cartilage tissue formation in three-dimensional biomimetic hydrogels. Tissue Eng. Part A 21, 147–155 [DOI] [PubMed] [Google Scholar]
- 17.Li C., Wong W. H. (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 98, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Sayed N., Hunter A., Au K. F., Wong W. H., Mocarski E. S., Pera R. R., Yakubov E., Cooke J. P. (2012) Activation of innate immunity is required for efficient nuclear reprogramming. Cell 151, 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldershaw R. A., Baxter M. A., Lowe E. T., Bates N., Grady L. M., Soncin F., Brison D. R., Hardingham T. E., Kimber S. J. (2010) Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 28, 1187–1194 [DOI] [PubMed] [Google Scholar]
- 20.Allegrucci C., Young L. E. (2007) Differences between human embryonic stem cell lines. Hum. Reprod. Update 13, 103–120 [DOI] [PubMed] [Google Scholar]
- 21.Laurent L. C., Ulitsky I., Slavin I., Tran H., Schork A., Morey R., Lynch C., Harness J. V., Lee S., Barrero M. J., Ku S., Martynova M., Semechkin R., Galat V., Gottesfeld J., Izpisua Belmonte J. C., Murry C., Keirstead H. S., Park H. S., Schmidt U., Laslett A. L., Muller F. J., Nievergelt C. M., Shamir R., Loring J. F. (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenther M. G., Frampton G. M., Soldner F., Hockemeyer D., Mitalipova M., Jaenisch R., Young R. A. (2010) Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell 7, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J. B., Nishikawa S., Nishikawa S., Muguruma K., Sasai Y. (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 [DOI] [PubMed] [Google Scholar]
- 24.Woods A., Wang G., Beier F. (2005) RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 280, 11626–11634 [DOI] [PubMed] [Google Scholar]
- 25.Craft A. M., Ahmed N., Rockel J. S., Baht G. S., Alman B. A., Kandel R. A., Grigoriadis A. E., Keller G. M. (2013) Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 140, 2597–2610 [DOI] [PubMed] [Google Scholar]
- 26.Pacifici M., Koyama E., Shibukawa Y., Wu C., Tamamura Y., Enomoto-Iwamoto M., Iwamoto M. (2006) Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann. N. Y. Acad. Sci. 1068, 74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varghese S., Hwang N. S., Canver A. C., Theprungsirikul P., Lin D. W., Elisseeff J. (2008) Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 27, 12-21 [DOI] [PubMed] [Google Scholar]
- 28.Lai J. H., Kajiyama G., Smith R. L., Maloney W., Yang F. (2013) Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci. Rep. 3, 3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiyama H., Kim J. E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T., de Crombrugghe B. (2005) Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665–14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dy P., Wang W., Bhattaram P., Wang Q., Wang L., Ballock R. T., Lefebvre V. (2012) Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 22, 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendjan S., Mascetti V. L., Ortmann D., Ortiz M., Karjosukarso D. W., Ng Y., Moreau T., Pedersen R. A. (2014) NANOG and CDX2 pattern distinct subtypes of human mesoderm during exit from pluripotency. Cell Stem Cell 15, 310–325 [DOI] [PubMed] [Google Scholar]
- 32.Akiyama H., Lyons J. P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., Deng J. M., Taketo M. M., Nakamura T., Behringer R. R., McCrea P. D., de Crombrugghe B. (2004) Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh C. D., Maity S. N., Lu J. F., Zhang J., Liang S., Coustry F., de Crombrugghe B., Yasuda H. (2010) Identification of SOX9 interaction sites in the genome of chondrocytes. PLoS ONE 5, e10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ten Berge D., Brouwer A., Korving J., Martin J. F., Meijlink F. (1998) Prx1 and Prx2 in skeletogenesis: roles in the craniofacial region, inner ear and limbs. Development 125, 3831–3842 [DOI] [PubMed] [Google Scholar]
- 35.Kawakami Y., Tsuda M., Takahashi S., Taniguchi N., Esteban C. R., Zemmyo M., Furumatsu T., Lotz M., Izpisúa Belmonte J. C., Asahara H. (2005) Transcriptional coactivator PGC-1alpha regulates chondrogenesis via association with Sox9. Proc. Natl. Acad. Sci. USA 102, 2414–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiramatsu K., Sasagawa S., Outani H., Nakagawa K., Yoshikawa H., Tsumaki N. (2011) Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J. Clin. Invest. 121, 640–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.