This pooled analysis of case–control studies found an increased risk of pancreatic cancer with higher levels of dietary vitamin D intake. Additional studies are required to determine whether or not our finding has a causal basis.
Keywords: vitamin D, case–control studies, pancreatic cancer, pooled analysis
Abstract
Background
The potential role of vitamin D in the aetiology of pancreatic cancer is unclear, with recent studies suggesting both positive and negative associations.
Patients and methods
We used data from nine case–control studies from the International Pancreatic Cancer Case–Control Consortium (PanC4) to examine associations between pancreatic cancer risk and dietary vitamin D intake. Study-specific odds ratios (ORs) were estimated using multivariable logistic regression, and ORs were then pooled using a random-effects model. From a subset of four studies, we also calculated pooled estimates of association for supplementary and total vitamin D intake.
Results
Risk of pancreatic cancer increased with dietary intake of vitamin D [per 100 international units (IU)/day: OR = 1.13, 95% confidence interval (CI) 1.07–1.19, P = 7.4 × 10−6, P-heterogeneity = 0.52; ≥230 versus <110 IU/day: OR = 1.31, 95% CI 1.10–1.55, P = 2.4 × 10−3, P-heterogeneity = 0.81], with the association possibly stronger in people with low retinol/vitamin A intake.
Conclusion
Increased risk of pancreatic cancer was observed with higher levels of dietary vitamin D intake. Additional studies are required to determine whether or not our finding has a causal basis.
introduction
Pancreatic cancer is a highly lethal malignancy for which the only potential cure is surgical resection [1]. Screening tests for early detection are limited and it is largely asymptomatic until advanced stages [2]. Consequently, when most cases are diagnosed surgery is no longer feasible [2]. Identifying modifiable risk factors is therefore important. Smoking is a confirmed risk factor [3]. Increased body mass index (BMI) [4], diabetes [5], non-O blood group [6] and heavy alcohol intake [7] have also been associated with increased risk.
Ecological studies have shown an inverse relationship between ultraviolet radiation, the primary source of vitamin D, and pancreatic cancer risk [8], mortality [9, 10] and incidence [11]. Circulating 25-hydroxyvitamin D (25(OH)D) concentrations arise from cutaneous production following sun exposure and vitamin D intake from diet and supplements. Two pooled analyses of the association between pre-diagnosis 25(OH)D levels and pancreatic cancer risk have produced contradictory results [12, 13]; one found that people with high 25(OH)D levels (≥100 nmol/l) were at significantly higher risk compared with people with lower levels (50 to <75 nmol/l) [12], while the other found an inverse association between 25(OH)D levels and risk [13]. Similarly, studies of dietary and supplementary intake have produced inconsistent results. A recent pooled analysis of cohort studies [14] failed to confirm that higher total vitamin D intake is associated with reduced risk [15], while results from a case–control study suggested that, among men, higher dietary intake was associated with increased risk [16].
Given the interest in increasing food fortification or in recommending routine supplementation, further investigation of an association between vitamin D and pancreatic cancer is warranted. We conducted a pooled analysis of vitamin D intake (dietary, supplementary and total) and pancreatic cancer risk using data from case–control studies participating in the International Pancreatic Cancer Case–Control Consortium (PanC4).
methods
studies
Nine PanC4 case–control studies included dietary assessments which enabled the calculation of dietary vitamin D intake [8, 16–23]. Six studies were conducted in North America, one each in Italy and Australia and one was a multi-national study. The publically available archive of the United States Third National Health and Nutrition Examination Survey (NHANES III) was used to derive the NHANES case–control study. Characteristics of these studies are summarised in supplementary Table S1, available at Annals of Oncology online.
We obtained original datasets from each participating study. After exclusions, data from 2963 patients with pancreatic adenocarcinoma and 8527 control subjects were available for the current analysis. For 6 cases in the Queensland Pancreatic Cancer Study (QPCS) study, 13 cases in the Ontario study and 156 cases and 137 controls in the Surveillance of Environmental Aspects Related to Cancer in Humans (SEARCH) study, a proxy respondent was interviewed. Each study obtained written informed consent.
exclusions
Study subject data were excluded for 1175 cases and 389 controls. Subjects were excluded if they had not completed food frequency questionnaires (FFQs) or if data were missing for any variables included in minimally adjusted models. For all studies except Ontario, where a restricted set of FFQ entries led to an under-estimation of total energy, we also excluded men with calculated energy intakes <800 or >5000 kcal/day, and women with energy intakes <700 or >4000 kcal/day.
exposure variables
Seven studies used a comprehensive FFQ to assess the usual dietary habits of participants. The Ontario and NHANES studies used the Brief Block FFQ and a 24-h dietary recall, respectively. Dietary intake of vitamin D was calculated by each study using country-specific nutrient-density databases. Additional details on FFQs and calculation of dietary intake have either been published elsewhere [16, 21–25] or are given in the supplementary Methods, available at Annals of Oncology online. For each study, except Ontario, dietary vitamin D intake was energy-adjusted using the residuals method [26].
For four studies [Mayo, University of Minnesota (UM), QPCS and University of California, San Francisco (UCSF)], we also considered supplementary (supplementary Methods, available at Annals of Oncology online) and total vitamin D intake. Total intake was calculated as the sum of supplementary and energy-adjusted dietary intakes.
statistical analysis
We used a two-stage modelling approach to estimate the association between vitamin D intake and pancreatic cancer risk [27]. In the first stage, unconditional logistic regression was used to estimate study-specific odds ratios (ORs) and associated standard errors for the exposure of interest. Minimally adjusted estimates were produced using models that included age, sex, study centre (multicentre studies only) and extent of proxy use (SEARCH only). Fully adjusted ORs were estimated using models that also included smoking status, total daily energy intake and other study-specific confounders (supplementary Table S2, available at Annals of Oncology online) that were selected using the process outlined in the supplementary Methods, available at Annals of Oncology online. Because of its small number of cases, the NHANES study did not allow for the calculation of fully adjusted estimates. In the second stage, study-specific ORs were pooled using a random-effects model [28]. Between-studies heterogeneity was assessed using the Q-statistic.
Our primary analysis included dietary vitamin D intake as a continuous exposure. In secondary analyses, dietary vitamin D intake was categorised into four levels based on approximate quartiles of intake of controls. In one approach, quartiles were calculated using controls from all studies, and the same thresholds [<110, 110–159, 160–229 and ≥230 international units (IU)/day] were applied across all studies. In the other approach, we used study-specific quartiles. Supplementary intake was categorised into three levels: no intake from supplements; 1–399 IU/day; and ≥400 IU/day. Total vitamin D intake was categorised into four levels using study-specific thresholds based on quartiles of total intakes of controls. We also examined linear trends for the categorical exposures by modelling the median value of each level as a continuous variable in the multivariable logistic regression models. To evaluate the influence of individual studies, we carried out sensitivity analyses by excluding one study at a time from the analysis and recalculating the pooled OR.
We generated estimates within strata of sex, smoking status (never, ever) and retinol/vitamin A intake. Retinol and vitamin A have antagonistic effects of on vitamin D [29, 30], and retinol/vitamin A intake was classified as low or high based on approximate study-specific medians using either dietary or total intake, depending on availability. We used the Wald statistic to assess the significance of the pooled cross-product terms between the exposure and the binary stratification variable. In these analyses, the exposure and interaction terms were the continuous variables of exposure. Using data from four studies (Mayo, QPCS, SEARCH, Yale), we also repeated our primary analysis, with cases subdivided on the basis of cancer stage (‘early’, ‘late’). A tumour was defined to be early stage if it was: resectable (Mayo); TNM stage IA or IB (QPCS); isolated and limited to primary organ with no invasion of adjacent lymph nodes, and no liver metastasis (SEARCH); and in situ or local (Yale). ‘Late’ stage was defined as: locally advanced or metastatic (Mayo); TNM stage IIA, IIB, III or IV (QPCS); invasion of adjacent lymph nodes or liver metastasis (SEARCH) and regional, distant or metastatic (Yale).
Analyses were carried out in SAS version 9.2 (SAS Institute, Inc., Cary, NC). Figures were produced using the Rmeta package in R [31]. All P values are two-sided and we used a nominal significance level of P < 0.05 to denote statistical significance.
results
Participant characteristics are shown in Table 1. For both cases and controls, the median attained age was 66 years. The proportion of men was slightly higher among cases (56%) than controls (53%). Compared with controls, cases reported higher BMI, and greater proportions of cases than controls had histories of smoking, diabetes or pancreatitis. In six of the studies, cases were less educated than controls, but the opposite was observed in the Italian study (data not shown).
Table 1.
Characteristics of 2963 cases of pancreatic cancer and 8527 controls included in the pooled analysis of vitamin D intake and pancreatic cancer risk
| Characteristics | Cases (N = 2963) |
Controls (N = 8527) |
||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Study | ||||
| Italy | 319 | (10.8) | 647 | (7.6) |
| Mayo | 582 | (19.6) | 1238 | (14.5) |
| NHANES | 34 | (1.1) | 970 | (11.4) |
| Ontario | 263 | (8.9) | 1281 | (15.0) |
| QPCS | 352 | (11.9) | 581 | (6.8) |
| SEARCH | 362 | (12.2) | 912 | (10.7) |
| UCSF | 510 | (17.2) | 1679 | (19.7) |
| UM | 179 | (6.0) | 539 | (6.3) |
| Yale | 362 | (12.2) | 680 | (8.0) |
| Sex | ||||
| Men | 1656 | (55.9) | 4509 | (52.9) |
| Women | 1307 | (44.1) | 4018 | (47.1) |
| Age (years) | ||||
| <40 | 32 | (1.1) | 299 | (3.5) |
| 40–49 | 182 | (6.1) | 664 | (7.8) |
| 50–59 | 616 | (20.8) | 1745 | (20.5) |
| 60–69 | 1036 | (35.0) | 2639 | (30.9) |
| 70–79 | 882 | (29.8) | 2504 | (29.4) |
| ≥80 | 215 | (7.3) | 676 | (7.9) |
| Racea | ||||
| White | 2510 | (94.5) | 6649 | (92.3) |
| Black | 79 | (3.0) | 329 | (4.6) |
| Other | 66 | (2.5) | 227 | (3.2) |
| Missing | 308 | 1324 | ||
| Body mass index (kg/m2)b | ||||
| <25 | 1098 | (40.2) | 3392 | (42.9) |
| 25 to <30 | 1059 | (38.7) | 3089 | (39.0) |
| 30 to <35 | 410 | (15.0) | 1012 | (12.8) |
| ≥35 | 167 | (6.1) | 418 | (5.3) |
| Missing | 229 | 616 | ||
| Ever smoker | ||||
| No | 1086 | (36.8) | 3862 | (45.3) |
| Yes | 1869 | (63.2) | 4656 | (54.7) |
| Missing | 8 | 9 | ||
| History of diabetes | ||||
| No | 2302 | (79.7) | 7599 | (89.9) |
| Yes | 587 | (20.3) | 853 | (10.1) |
| Missing | 74 | 75 | ||
| History of pancreatitisc | ||||
| No | 2215 | (90.3) | 6784 | (99.0) |
| Yes | 238 | (9.7) | 69 | (1.0) |
| Missing | 510 | 1674 | ||
International Pancreatic Cancer Case–Control Consortium.
aNo information available from Ontario study.
bNo information available from UM study.
cNo information available from NHANES or Italy studies.
NHANES, National Health and Nutrition Examination Survey; QPCS, Queensland Pancreatic Cancer Study; SEARCH, Surveillance of Environmental Aspects Related to Cancer in Humans; UCSF, University of California, San Francisco; UM, University of Minnesota.
Compared with included cases, excluded cases reported higher BMI (27.7 versus 26.8 kg/m2), and a greater proportion of excluded cases had histories of diabetes (24% versus 20%) and pancreatitis (14% versus 10%). A smaller proportion of excluded controls were Caucasian (82% versus 92%) compared with included controls.
dietary vitamin D intake
An increase in dietary intake of vitamin D of 100 IU/day was associated with a 13% increase in pancreatic cancer risk [OR = 1.13, 95% confidence interval (CI) 1.07–1.19, P = 7.4 × 10−6] (Table 2, Figure 1). No evidence of between-studies heterogeneity was found (P = 0.52). Results were essentially unchanged when we adjusted for pack-years of cigarette smoking instead of smoking status, and when we excluded data from proxy respondents. Further, no single study unduly influenced the magnitude of the effect or its statistical significance (supplementary Table S3, available at Annals of Oncology online). The association remained when we restricted the analysis to the four studies with supplement data and excluded people with any supplementary vitamin D intake (OR = 1.18, 95% CI 1.08–1.30, P = 3.0 × 10−4, P-heterogeneity = 0.56).
Table 2.
Study-specifica and pooled odds ratios (ORs)b for pancreatic cancer per 100 IU/day increase in dietary vitamin D intakec
| Study | Ca:Cod | Dietary vitamin D intake (IU/day)c |
|||||
|---|---|---|---|---|---|---|---|
| Median (IQR) |
Minimally adjusted |
Fully adjusted |
|||||
| Cases | Controls | OR (95% CI) | P value | OR (95% CI) | P value | ||
| All participants | |||||||
| Italy | 319:647 | 119 (94, 154) | 111 (85, 140) | 1.33 (1.04, 1.70) | 0.02 | 1.29 (0.98, 1.69) | 0.07 |
| Mayo | 582:1238 | 152 (105, 219) | 158 (107, 225) | 0.94 (0.84, 1.05) | 0.28 | 1.18 (1.02, 1.36) | 0.03 |
| NHANES | 34:970 | 200 (100, 261) | 162 (84, 259) | 1.03 (0.91, 1.16) | 0.66 | NA | NA |
| Ontario | 263:1281 | 131 (80, 194) | 113 (76, 172) | 1.14 (0.98, 1.33) | 0.09 | 1.06 (0.90, 1.26) | 0.47 |
| QPCS | 352:581 | 138 (102, 179) | 136 (101, 175) | 0.93 (0.79, 1.11) | 0.44 | 0.97 (0.81, 1.16) | 0.73 |
| SEARCH | 362:912 | 142 (106, 178) | 144 (107, 181) | 1.08 (0.88, 1.33) | 0.44 | 1.09 (0.88, 1.36) | 0.41 |
| UCSF | 510:1679 | 249 (181, 340) | 230 (168, 321) | 1.06 (0.99, 1.15) | 0.11 | 1.17 (1.07, 1.28) | 4.6 × 10−4 |
| UM | 179:539 | 226 (160, 323) | 228 (148, 322) | 1.00 (0.87, 1.16) | 0.95 | 1.21 (0.99, 1.49) | 0.07 |
| Yale | 362:680 | 172 (118, 239) | 168 (118, 234) | 1.00 (0.89, 1.13) | 0.94 | 1.08 (0.94, 1.25) | 0.29 |
| Pooled | 2963:8527 | 1.03 (0.98, 1.09) | 0.22 | 1.13 (1.07, 1.19) | 7.4 × 10−6 | ||
| Between-studies heterogeneity | Q = 10.8, P = 0.21 | Q = 6.1, P = 0.52 | |||||
| Men | |||||||
| Italy | 172:345 | 118 (95, 150) | 108 (82, 133) | 1.58 (1.09, 2.28) | 0.01 | 1.58 (1.03, 2.42) | 0.04 |
| Mayo | 320:592 | 151 (111, 211) | 159 (109, 218) | 0.96 (0.82, 1.13) | 0.64 | 1.23 (0.99, 1.53) | 0.06 |
| NHANES | 21:534 | 197 (101, 222) | 160 (82, 258) | 0.93 (0.70, 1.23) | 0.61 | NA | NA |
| Ontario | 134:678 | 133 (88, 202) | 116 (79, 170) | 1.23 (1.01, 1.51) | 0.04 | 1.11 (0.88, 1.39) | 0.37 |
| QPCS | 216:344 | 141 (102, 183) | 132 (100, 167) | 1.01 (0.82, 1.24) | 0.91 | 1.05 (0.85, 1.31) | 0.63 |
| SEARCH | 201:452 | 147 (113, 175) | 144 (106, 180) | 1.08 (0.84, 1.37) | 0.55 | 1.12 (0.87, 1.44) | 0.38 |
| UCSF | 282:873 | 243 (181, 321) | 222 (164, 310) | 1.07 (0.97, 1.19) | 0.19 | 1.15 (1.02, 1.31) | 0.02 |
| UM | 104:305 | 226 (170, 314) | 230 (142, 319) | 1.05 (0.86, 1.28) | 0.62 | 1.42 (1.03, 1.95) | 0.03 |
| Yale | 206:386 | 167 (118, 237) | 164 (120, 224) | 1.01 (0.86, 1.19) | 0.91 | 1.01 (0.83, 1.23) | 0.91 |
| Pooled | 1656:4509 | 1.06 (0.99, 1.13) | 0.10 | 1.15 (1.06, 1.23) | 2.9 × 10−4 | ||
| Between-studies heterogeneity | Q = 9.6, P = 0.29 | Q = 6.6, P = 0.48 | |||||
| Women | |||||||
| Italy | 147:302 | 119 (94, 155) | 115 (87, 146) | 1.15 (0.82, 1.61) | 0.42 | 1.11 (0.77, 1.60) | 0.57 |
| Mayo | 262:646 | 155 (99, 239) | 157 (104, 232) | 0.91 (0.78, 1.07) | 0.27 | 1.20 (0.99, 1.46) | 0.06 |
| NHANES | 13:436 | 220 (76, 351) | 164 (86, 263) | 1.24 (0.99, 1.56) | 0.06 | NA | NA |
| Ontario | 129:603 | 123 (66, 181) | 109 (72, 174) | 1.03 (0.82, 1.30) | 0.78 | 1.00 (0.78, 1.30) | 0.98 |
| QPCS | 136:237 | 135 (101, 174) | 143 (103, 195) | 0.81 (0.60, 1.08) | 0.15 | 0.84 (0.61, 1.15) | 0.28 |
| SEARCH | 161:460 | 139 (94, 178) | 145 (109, 182) | 1.11 (0.77, 1.61) | 0.58 | 1.06 (0.72, 1.58) | 0.76 |
| UCSF | 228:806 | 263 (183, 355) | 241 (175, 330) | 1.06 (0.94, 1.18) | 0.34 | 1.18 (1.04, 1.34) | 0.01 |
| UM | 75:234 | 229 (153, 333) | 226 (153, 324) | 0.94 (0.76, 1.17) | 0.60 | 1.09 (0.84, 1.43) | 0.51 |
| Yale | 156:294 | 178 (115, 241) | 179 (118, 245) | 1.00 (0.84, 1.19) | 0.99 | 1.12 (0.89, 1.41) | 0.34 |
| Pooled | 1307:4018 | 1.02 (0.95, 1.09) | 0.67 | 1.12 (1.03, 1.21) | 0.01 | ||
| Between-studies heterogeneity | Q = 8.7, P = 0.36 | Q = 5.0, P = 0.65 | |||||
| P-interaction | 0.27 | 0.17 | |||||
| Never smokers | |||||||
| Italy | 131:326 | 119 (95, 154) | 113 (89, 140) | 1.37 (0.91, 2.07) | 0.14 | 1.32 (0.85, 2.07) | 0.22 |
| Mayo | 240:669 | 156 (108, 219) | 160 (107, 231) | 0.91 (0.77, 1.08) | 0.27 | 1.16 (0.94, 1.43) | 0.16 |
| NHANES | 11:425 | 206 (181, 313) | 171 (90, 280) | 0.97 (0.73, 1.30) | 0.85 | NA | NA |
| Ontario | 108:578 | 146 (84, 199) | 116 (78, 183) | 1.15 (0.91, 1.46) | 0.24 | 1.11 (0.86, 1.45) | 0.42 |
| QPCS | 140:285 | 138 (102, 177) | 133 (100, 173) | 1.01 (0.76, 1.33) | 0.96 | 1.07 (0.80, 1.44) | 0.65 |
| SEARCH | 123:394 | 130 (83, 174) | 138 (101, 175) | 1.12 (0.84, 1.50) | 0.45 | 1.10 (0.82, 1.49) | 0.52 |
| UCSF | 160:638 | 258 (195, 346) | 233 (167, 321) | 1.09 (0.96, 1.24) | 0.19 | 1.23 (1.07, 1.42) | 3.8 × 10−3 |
| UM | 65:259 | 240 (174, 321) | 228 (155, 323) | 1.01 (0.80, 1.26) | 0.95 | 1.07 (0.80, 1.44) | 0.64 |
| Yale | 108:288 | 173 (116, 243) | 176 (122, 239) | 0.93 (0.75, 1.15) | 0.51 | 0.94 (0.72, 1.23) | 0.67 |
| Pooled | 1086:3862 | 1.03 (0.96, 1.11) | 0.40 | 1.14 (1.05, 1.24) | 2.0 × 10−3 | ||
| Between-studies heterogeneity | Q = 6.9, P = 0.55 | Q = 3.9, P = 0.79 | |||||
| Ever smokers | |||||||
| Italy | 185:316 | 118 (93, 151) | 108 (80, 138) | 1.20 (0.87, 1.65) | 0.27 | 1.27 (0.90, 1.79) | 0.18 |
| Mayo | 340:566 | 151 (102, 219) | 157 (106, 217) | 0.98 (0.85, 1.15) | 0.84 | 1.32 (1.07, 1.63) | 0.01 |
| NHANES | 23:545 | 197 (89, 261) | 153 (80, 240) | 1.13 (0.92, 1.40) | 0.25 | NA | NA |
| Ontario | 152:700 | 122 (79, 190) | 110 (73, 165) | 1.14 (0.93, 1.39) | 0.20 | 1.02 (0.82, 1.28) | 0.85 |
| QPCS | 212:296 | 138 (101, 182) | 137 (103, 178) | 0.88 (0.71, 1.10) | 0.27 | 0.94 (0.74, 1.18) | 0.57 |
| SEARCH | 239:517 | 148 (114, 183) | 149 (111, 183) | 1.09 (0.80, 1.49) | 0.59 | 1.09 (0.79, 1.52) | 0.60 |
| UCSF | 350:1041 | 246 (180, 335) | 230 (168, 321) | 1.06 (0.96, 1.16) | 0.26 | 1.14 (1.02, 1.28) | 0.02 |
| UM | 113:280 | 220 (159, 315) | 229 (135, 320) | 1.01 (0.84, 1.22) | 0.89 | 1.35 (1.01, 1.81) | 0.05 |
| Yale | 254:392 | 171 (118, 236) | 164 (113, 227) | 1.06 (0.92, 1.22) | 0.45 | 1.16 (0.97, 1.38) | 0.12 |
| Pooled | 1868:4653 | 1.05 (0.99, 1.11) | 0.11 | 1.14 (1.06, 1.23) | 4.9 × 10−4 | ||
| Between-studies heterogeneity | Q = 5.0, P = 0.75 | Q = 7.5, P = 0.38 | |||||
| P-interaction | 0.75 | 0.79 | |||||
| Low retinol/vitamin Ae | |||||||
| Italy | 176:308 | 117 (94, 146) | 107 (79, 137) | 1.36 (0.97, 1.89) | 0.07 | 1.26 (0.88, 1.80) | 0.21 |
| Mayo | 310:598 | 153 (101, 227) | 159 (109, 227) | 0.88 (0.75, 1.03) | 0.11 | 1.18 (0.96, 1.44) | 0.12 |
| NHANES | 17:484 | 122 (76, 203) | 124 (65, 196) | 1.01 (0.88, 1.16) | 0.88 | NA | NA |
| QPCS | 187:275 | 130 (99, 164) | 125 (92, 166) | 0.97 (0.78, 1.21) | 0.81 | 1.00 (0.80, 1.25) | 0.99 |
| SEARCH | 179:459 | 136 (103, 163) | 133 (93, 171) | 1.17 (0.80, 1.71) | 0.42 | 1.14 (0.77, 1.70) | 0.51 |
| UCSF | 274:821 | 231 (162, 314) | 209 (152, 302) | 1.08 (0.96, 1.20) | 0.19 | 1.20 (1.05, 1.38) | 0.01 |
| UM | 86:272 | 216 (154, 295) | 199 (131, 284) | 1.12 (0.89, 1.39) | 0.33 | 1.42 (1.02, 1.97) | 0.04 |
| Yale | 177:342 | 173 (110, 252) | 162 (112, 233) | 1.08 (0.91, 1.29) | 0.37 | 1.34 (1.07, 1.68) | 0.01 |
| Pooled | 1406:3559 | 1.04 (0.97, 1.12) | 0.31 | 1.20 (1.10, 1.30) | 2.5 × 10−5 | ||
| Between-studies heterogeneity | Q = 8.7, P = 0.27 | Q = 4.7, P = 0.58 | |||||
| High retinol/vitamin Ae | |||||||
| Italy | 143:339 | 124 (94, 158) | 115 (90, 144) | 1.34 (0.91, 1.96) | 0.14 | 1.27 (0.84, 1.93) | 0.25 |
| Mayo | 272:640 | 151 (107, 207) | 158 (105, 224) | 1.00 (0.85, 1.18) | 0.97 | 1.09 (0.90, 1.32) | 0.36 |
| NHANES | 17:486 | 222 (190, 313) | 206 (120, 318) | 1.02 (0.77, 1.36) | 0.90 | NA | NA |
| QPCS | 165:306 | 148 (113, 204) | 148 (113, 200) | 0.92 (0.70, 1.21) | 0.55 | 0.98 (0.73, 1.30) | 0.86 |
| SEARCH | 183:453 | 149 (113, 189) | 155 (121, 192) | 1.03 (0.79, 1.34) | 0.81 | 1.04 (0.78, 1.39) | 0.80 |
| UCSF | 236:858 | 280 (204, 355) | 253 (188, 337) | 1.08 (0.97, 1.19) | 0.18 | 1.15 (1.03, 1.30) | 0.02 |
| UM | 93:267 | 240 (176, 331) | 260 (177, 342) | 0.92 (0.75, 1.12) | 0.39 | 1.16 (0.89, 1.52) | 0.27 |
| Yale | 185:338 | 172 (125, 233) | 174 (125, 235) | 0.94 (0.79, 1.11) | 0.44 | 0.88 (0.70, 1.10) | 0.25 |
| Pooled | 1294:3687 | 1.02 (0.95, 1.08) | 0.65 | 1.09 (1.01, 1.18) | 0.03 | ||
| Between-studies heterogeneity | Q = 5.6, P = 0.59 | Q = 6.0, P = 0.42 | |||||
| P-interaction | 0.40 | 0.08 | |||||
| Cases restricted to ‘early’-stage cancerf | |||||||
| Mayo | 173:1238 | 149 (106, 235) | 158 (107, 225) | 0.97 (0.82, 1.16) | 0.78 | 0.99 (0.63, 1.56) | 0.98 |
| QPCS | 26:581 | 139 (101, 166) | 136 (101, 175) | 0.99 (0.63, 1.58) | 0.98 | 1.14 (0.90, 1.44) | 0.27 |
| SEARCH | 82:912 | 131 (107, 176) | 144 (107, 181) | 1.33 (0.99, 1.79) | 0.06 | 0.98 (0.68, 1.42) | 0.92 |
| Yale | 49:680 | 175 (118, 223) | 168 (118, 234) | 0.88 (0.65, 1.19) | 0.42 | 1.34 (0.95, 1.87) | 0.09 |
| Pooled | 330:3411 | 1.03 (0.87, 1.21) | 0.77 | 1.13 (0.96, 1.32) | 0.14 | ||
| Between-studies heterogeneity | Q = 4.2, P = 0.24 | Q = 1.8, P = 0.61 | |||||
| Cases restricted to ‘late’-stage cancerf | |||||||
| Mayo | 371:1238 | 157 (106, 218) | 158 (107, 225) | 0.94 (0.82, 1.07) | 0.36 | 1.00 (0.82, 1.21) | 0.96 |
| QPCS | 261:581 | 139 (102, 183) | 136 (101, 175) | 0.94 (0.78, 1.14) | 0.53 | 1.19 (1.01, 1.42) | 0.04 |
| SEARCH | 110:912 | 127 (85, 173) | 144 (107, 181) | 1.20 (0.90, 1.60) | 0.22 | 1.10 (0.95, 1.28) | 0.20 |
| Yale | 313:680 | 172 (118, 243) | 168 (118, 234) | 1.02 (0.91, 1.16) | 0.71 | 1.21 (0.86, 1.70) | 0.26 |
| Pooled | 1055:3411 | 0.99 (0.92, 1.07) | 0.82 | 1.11 (1.01, 1.22) | 0.03 | ||
| Between-studies heterogeneity | Q = 2.8, P = 0.42 | Q = 2.2, P = 0.53 | |||||
Results presented for all participants, stratified by sex, smoking behaviour and retinol/vitamin A intake, and for analyses where cases were subdivided on the basis of cancer stage.
aStudy-specific ORs calculated using unconditional logistic regression. Minimally adjusted estimates produced using models that included age (continuous), sex, study centre (multicentre studies only) and extent of proxy use (SEARCH only). Fully adjusted estimates produced using models that included the adjustment variables shown in supplementary Table S2, available at Annals of Oncology online. Fully adjusted estimates were not calculated for the NHANES study due to the small number of cases.
bPooled ORs calculated using random-effects models.
cDietary vitamin D intake was energy-adjusted by the residuals method for all studies except Ontario.
dNumbers used to calculate fully adjusted estimates may differ due to missing confounder data.
eThresholds used to categorise retinol/vitamin A intake: Italy (dietary retinol equivalent), 1050 µg/day (Milan) and 1110 µg/day (Pordenone); Mayo (total vitamin A), 1790 µg/day; QPCS (total retinol), 370 µg/day; SEARCH (dietary retinol), 410 µg/day (Adelaide), 560 µg/day (Utrecht), 110 µg/day (Warsaw); UCSF (total vitamin A), 14 000 IU/day; UM (total retinol), 2870 IU/day; Yale (dietary retinol), 950 µg/day. Estimates not calculated for Ontario because retinol/vitamin A intake not available.
fEarly stage defined as: Mayo, resectable; QPCS, TNM stage IA or IB; SEARCH, isolated pancreatic mass limited to primary organ, no invasion of adjacent lymph nodes and no liver metastasis; Yale, in situ or local. Late stage defined as: Mayo, locally advanced or metastatic; QPCS, TNM stage IIA, IIB, III or IV; SEARCH, invasion of adjacent lymph nodes or liver metastasis; Yale, regional, distant or metastatic.
Ca, cases; CI, confidence interval; Co: controls; IU, international units; NHANES, National Health and Nutrition Examination Survey; QPCS, Queensland Pancreatic Cancer Study; SEARCH, Surveillance of Environmental Aspects Related to Cancer in Humans; UCSF, University of California, San Francisco; UM, University of Minnesota.
Figure 1.
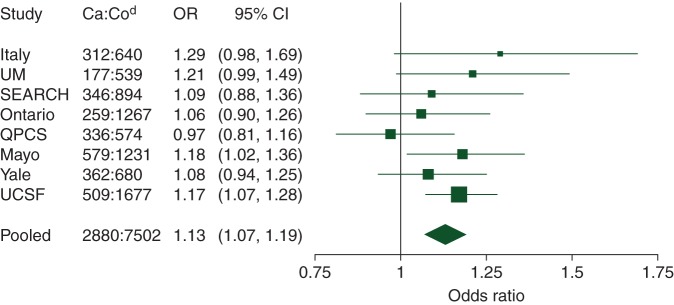
Study-specifica and pooled odds ratios (ORs)b for pancreatic cancer for an increase in dietary vitamin D intakec of 100 IU/day: fully adjusted estimates. aStudy-specific ORs calculated using unconditional logistic regression adjusted for the confounders in supplementary Table S2, available at Annals of Oncology online. Dietary vitamin D intake included in models as a continuous exposure. bPooled OR calculated using a random-effects model. cDietary vitamin D intake was energy-adjusted by the residuals method for all studies except Ontario. dNumber of cases (Ca) and controls (Co) used to produce fully adjusted estimates. Differences with numbers given in Table 2 are due to missing confounder data. CI, confidence interval; QPCS, Queensland Pancreatic Cancer Study; UM, University of Minnesota; SEARCH, Surveillance of Environmental Aspects Related to Cancer in Humans; UCSF, University of California, San Francisco.
Supplementary Figure S1, available at Annals of Oncology online, shows a forest plot of study-specific and pooled ORs for a dietary vitamin D intake of ≥230 IU/day compared with <110 IU/day. People with an intake ≥230 IU/day had a 1.3-fold increased risk (OR = 1.31, 95% CI 1.10–1.55, P = 2.4 × 10−3, P-heterogeneity = 0.81) (supplementary Table S4, available at Annals of Oncology online). The association was even stronger when intake was categorised using study-specific quartiles (supplementary Figure S2 and Table S5, available at Annals of Oncology online).
Stratified analyses suggested that the association might be stronger in people with low retinol/vitamin A intake (Table 2) (P-interaction = 0.08). The association did not differ by sex or smoking behaviour, and estimates were not meaningfully different when cases were subdivided by early- or late-stage cancer (Table 2).
supplementary and total vitamin D intake
These results were based on four studies only. We found no association between pancreatic cancer risk and supplementary vitamin D intake, and a suggestion of a positive association with total vitamin D intake (supplementary Tables S6 and S7, available at Annals of Oncology online, respectively). Significant between-studies heterogeneity was present for comparisons of the lowest and highest levels of supplementary and total intakes (P = 0.01 and P = 0.003, respectively). When QPCS estimates were excluded, the pooled OR for supplementary intake (≥400 IU/day versus none) increased from 1.03 to 1.20 (95% CI 0.95–1.50, P-heterogeneity = 0.35), and the pooled OR for total intake became significant (OR = 2.01, 95% CI 1.50–2.69, P-heterogeneity = 0.66).
discussion
In this pooled analysis of case–control studies, we found a modest positive association between pancreatic cancer risk and dietary vitamin D intake. This association appeared to be stronger in people with low retinol/vitamin A intakes. Significant between-studies heterogeneity was seen when comparing highest and lowest levels of supplementary and total vitamin D intakes. A positive association with total intake was suggested, but there was no association with supplementary intake.
When dietary vitamin D intake was treated as a continuous exposure, seven of the eight study-specific ORs exceeded unity, with significance or borderline significance achieved in four of them. Our result confirms the previously published finding from the UCSF study [16], although we did not observe that the association differed between men and women. When UCSF participants were removed, the association here was not altered. Our findings are not consistent with a pooled analysis of data from the Nurses' Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). That analysis found that total vitamin D intake ≥600 IU/day was associated with 41% lower risk compared with intake <150 IU/day and, although not statistically significant, risk also appeared to decrease with dietary intake [15]. The largest pooled analysis of cohort studies, however, found no associations with either dietary or total vitamin D intake [14]; the NHS, HPFS and Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) study were among the 11 cohorts from the United States and Europe used in the analysis of dietary vitamin D intake.
A pooled analysis of five cohort studies, including the NHS and HPFS, found a significant inverse association between pancreatic cancer risk and plasma 25(OH)D levels [13]. In contrast, a positive association between serum 25(OH)D levels and risk (>65.5 versus <32.0 nmol/l: OR = 2.92) was observed in the ATBC study of male, Finnish smokers [32]. A subsequent pooled analysis of eight cohort studies, including the ATBC cohort, found pancreatic cancer risk doubled with high serum 25(OH)D levels (≥100 versus 50 to <75 nmol/l: OR = 2.12) [12]. Unlike that analysis, we found evidence of a trend, as opposed to threshold, effect.
It has been speculated that tumour growth might be related to vitamin D's influence on growth factors, including insulin [12, 32]. It is unclear what, if any, role vitamin D might play in tumour initiation. The stronger association that we observed in people with low retinol/vitamin A intake might be explained by antagonistic effects of retinol and vitamin A on vitamin D [29, 30].
Given the limitations of case–control studies of pancreatic cancer, non-causal explanations for our findings need to be considered, especially since dietary vitamin D intake (even in the highest category) was relatively modest. A recent cancer diagnosis may influence dietary recall, potentially biasing our results. Furthermore, since cases are usually diagnosed at an advanced stage, it is possible that their diets may have changed before diagnosis. For example, if cases increased their intake of easily digestible carbohydrates, including vitamin D-fortified products, before diagnosis, then our result may be due to reverse causality. However, estimated effects were not substantially different when cases were subdivided by early- or late-stage cancer. Another explanation is that the observed relationship is due to either residual confounding or an unmeasured confounder. Residual confounding due to smoking is unlikely given the almost identical effects among never and ever smokers. On the other hand, despite our careful adjustment for known or putative risk factors, a spurious relationship due to an unknown confounder is still possible. Exposure to ultraviolet radiation makes a significant contribution to vitamin D status. We did not take sun exposure into account and thus cannot exclude effect modification or confounding by this variable. In addition, since pancreatic cancer is highly fatal, it is possible that survival bias occurred.
We had reduced power to detect associations with supplementary or total intake. The suggestion of a positive association with total intake is presumably driven by the association with dietary intake. The QPCS study was responsible for the between-studies heterogeneity for comparisons of highest and lowest levels of intake; pooled estimates that take into account the QPCS study should be interpreted with caution. People in the QPCS study with a supplementary intake of ≥400 IU/day were at significantly lower risk compared with those with no such intake. In contrast, the other study-specific ORs were all above unity. The QPCS study, which was conducted in Australia, asked specific questions regarding intake of vitamin D-containing supplements and information was collected regarding brands and doses. The other three studies were from the United States and used a somewhat less structured approach to capturing information about supplementary vitamin D intake.
Our study has several strengths. It was adequately powered to detect a reasonable magnitude of association with dietary intake. The large sample size also permitted stratified analyses. The two-stage methodology allowed us to develop parsimonious models while still carefully adjusting for study-specific confounders. We therefore avoided problems associated with ‘overfitted’ models, such as inflated standard errors and numerically unstable estimates. Finally, we used data from diverse geographical locations, although we caution that our results might not generalise to non-white populations.
In conclusion, we observed a significant positive association between dietary vitamin D intake and risk of pancreatic cancer. This suggests that a favourable effect of vitamin D intake on pancreatic cancer is unlikely, but does not exclude the possibility that vitamin D obtained through ultraviolet exposure has a beneficial effect. The possibility of a harmful effect suggests that caution should be exercised in recommending large dietary intake of vitamin D. Further investigations are warranted to determine whether or not this association has a causal basis.
funding
The QPCS study was supported by a project grant (#442302) from the National Health and Medical Research Council (NHMRC) (Australia). REN is supported by a fellowship from the NHMRC. MW is supported by the Centre for Research Excellence in Sun and Health. The Italy study was supported by the Italian Association for Cancer Research (AIRC). The Mayo study was supported by the National Institutes of Health grant P50 CA102701 (Mayo Clinic SPORE in Pancreatic Cancer). The Ontario study was supported by the Canadian Institutes of Health Research (grant MOP-106631 to MC) and the National Institutes of Health (RO1 CA97075, as part of PACGENE consortium). The Netherlands investigation in the Surveillance of Environmental Aspects Related to Cancer in Humans (SEARCH) study was supported by the Dutch Ministry of Public Health, Welfare and Sports (formerly Welfare, Health and Culture). The University of California, San Francisco (UCSF) study was supported in part by National Cancer Institute grants (CA59706, CA108370, CA109767, CA89726 and CA098889), and by the Rombauer Pancreatic Cancer Research Fund. Cancer incidence data collection in the UCSF study was supported by the California Department of Public Health, the National Cancer Institute's Surveillance, Epidemiology and End Results Program (contract N01-PC-35136) awarded to the Northern California Cancer Center. The University of Minnesota (UM) study was supported by a National Cancer Institute grant (RO1-CA-58697 to KA and PI). The Yale Connecticut Pancreatic Cancer Study was supported by the National Cancer Institute at the U.S. National Institutes of Health (grant 5R01CA098870). The funding sources for this study did not participate in the design or conduct of the study, nor in collection, management, analysis or interpretation of study data, nor in preparation, review or approval of this manuscript. The Yale Connecticut Pancreatic Cancer Study gratefully acknowledges the cooperation of the 30 Connecticut hospitals, including Stamford Hospital, in allowing patient access. The Connecticut study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in that study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Annaka Schulte and Ayelet Borgida for assistance with data preparation.
references
- 1.Hartwig W, Werner J, Jager D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013; 14: e476–e485. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008; 393: 535–545. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer 2007; 120: 1993–1998. [DOI] [PubMed] [Google Scholar]
- 5.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92: 2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risch HA, Lu L, Wang J et al. ABO blood group and risk of pancreatic cancer: a study in Shanghai and meta-analysis. Am J Epidemiol 2013; 177: 1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tramacere I, Scotti L, Jenab M et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer 2010; 126: 1474–1486. [DOI] [PubMed] [Google Scholar]
- 8.Tran B, Whiteman DC, Webb PM et al. Association between ultraviolet radiation, skin sun sensitivity and risk of pancreatic cancer. Cancer Epidemiol 2013; 37: 886–892. [DOI] [PubMed] [Google Scholar]
- 9.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002; 94: 1867–1875. [DOI] [PubMed] [Google Scholar]
- 10.Neale RE, Youlden DR, Krnjacki L, Kimlin MG, van der Pols JC. Latitude variation in pancreatic cancer mortality in Australia. Pancreas 2009; 38: 387–390. [DOI] [PubMed] [Google Scholar]
- 11.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. Pancreas 2010; 39: 669–674. [DOI] [PubMed] [Google Scholar]
- 12.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolpin BM, Ng K, Bao Y et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2012; 21: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genkinger JM, Wang M, Li R et al. Dairy products and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Ann Oncol 2014; 25: 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner HG, Michaud DS, Giovannucci E et al. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev 2006; 15: 1688–1695. [DOI] [PubMed] [Google Scholar]
- 16.Zablotska LB, Gong Z, Wang F, Holly EA, Bracci PM. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control 2011; 22: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McWilliams RR, Bamlet WR, de Andrade M et al. Nucleotide excision repair pathway polymorphisms and pancreatic cancer risk: evidence for role of MMS19L. Cancer Epidemiol Biomarkers Prev 2009; 18: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services (DHHS). National Center for Health Statistics Third National Health and Nutrition Examination Survey, 1988–1994, NHANES III Second Laboratory Data File (CD-ROM, Series 11, No. 2A). Hyattsville, MD: Centers for Disease Control and Prevention, 1998. [Google Scholar]
- 19.Cotterchio M, Lowcock E, Hudson TJ, Greenwood C, Gallinger S. Association between allergies and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2014; 23: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risch HA, Yu H, Lu L, Kidd MS. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst 2010; 102: 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravi F, Polesel J, Bosetti C et al. Dietary intake of selected micronutrients and the risk of pancreatic cancer: an Italian case-control study. Ann Oncol 2011; 22: 202–206. [DOI] [PubMed] [Google Scholar]
- 22.Howe GR, Ghadirian P, Bueno de Mesquita HB et al. A collaborative case-control study of nutrient intake and pancreatic cancer within the search programme. Int J Cancer 1992; 51: 365–372. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Dhakal IB, Gross MD et al. Physical activity, diet, and pancreatic cancer: a population-based, case-control study in Minnesota. Nutr Cancer 2009; 61: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control 2011; 22: 1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Third National Health and Nutrition Examination survey (NHANES III), 1988–94. NHANES III Total Nutrient Intakes File Documentation. Series 11, No. 2A. In. 1998.
- 26.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986; 124: 17–27. [DOI] [PubMed] [Google Scholar]
- 27.Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med 2001; 20: 2115–2130. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 29.Rohde CM, DeLuca HF. All-trans retinoic acid antagonizes the action of calciferol and its active metabolite, 1,25-dihydroxycholecalciferol, in rats. J Nutr 2005; 135: 1647–1652. [DOI] [PubMed] [Google Scholar]
- 30.Rohde CM, Manatt M, Clagett-Dame M, DeLuca HF. Vitamin A antagonizes the action of vitamin D in rats. J Nutr 1999; 129: 2246–2250. [DOI] [PubMed] [Google Scholar]
- 31.Lumley T. rmeta: Meta-analysis. R package version 2.16.2012. http://CRAN.R-project.org/package=rmeta (14 March 2014, date last accessed).
- 32.Stolzenberg-Solomon RZ, Vieth R, Azad A et al. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res 2006; 66: 10213–10219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


