In this paper, the predictive role of tumor-infiltrating lymphocyte (TIL) was evaluated in more than 800 breast cancer patients enrolled in two phase III randomized clinical trials comparing adjuvant anthracyclines versus no adjuvant chemotherapy. Basing on these data, TIL could not serve as a parameter to select patients for anthracycline treatment.
Keywords: breast cancer, adjuvant chemotherapy, tumor-infiltrating lymphocytes, anthracyclines
Abstract
Background
Tumor-infiltrating lymphocytes (TILs) are emerging as strong prognostic factor for early breast cancer patients, especially in the triple-negative subtype. Here, we aim to validate previous findings on the prognostic role of TIL in the context of two randomized adjuvant trials and to investigate whether lymphocyte infiltrates can predict benefit from adjuvant anthracyclines.
Patients and methods
A total of 816 patients enrolled and treated at the Gustave Roussy in the context of two multicentric randomized trials comparing adjuvant anthracyclines versus no chemotherapy were included in the present analysis. Primary end point was overall survival (OS). Hematoxilin and eosin slides of primary tumors were retrieved and evaluated for the percentage of intratumoral (It) and stromal (Str) TIL. Each case was also defined as high-TIL or low-TIL breast cancer adopting previously validated cutoffs.
Results
TIL were assessable for 781 of 816 cases. High-TIL cases were more likely grade 3 and estrogen receptor (ER)-negative (P < 0.001). In multivariate analysis, both continuous It-TIL and Str-TIL were strong prognostic factors for OS [hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.77–0.95 P = 0.003; HR 0.89, 95% CI 0.81–0.96, P = 0.005 for It-TIL and Str-TIL, respectively]. The prognostic effect of continuous TIL was limited to triple-negative and HER2-positive patients. Ten-year OS rates were: 89% and 68% for triple-negative high-TIL and low-TIL, respectively (HR 0.44, 95% CI 0.18–1.10, P = 0.07) and 78% and 57% for HER2-positive high-TIL versus low-TIL, respectively (HR 0.46, 95% CI 0.20–1.11, P = 0.08). Either continuous or binary TIL variables did not predict for the efficacy of anthracyclines. Test for interaction P value was not significant in the whole study population and in subgroups (ER+/HER2−, HER2+, ER−/HER2−).
Conclusions
We confirmed the prognostic role of TIL in triple-negative early breast cancer and suggested a prognostic impact in HER2+ patients as well. Basing on our data, TIL should not be used as a parameter to select patients for anthracyclines chemotherapy.
introduction
Immune system is a key player in cancer progression. Preclinical data suggest that chemotherapy can to trigger an antitumor immune response, by causing an immunogenic cell death that allows antigen cross-presentation, activation of dendritic cells and tumor-specific cytotoxic T cells [1]. A few hypothesis-generating studies on small breast cancer series indicated that exposure to cytotoxic drugs such as anthracyclines and taxanes could lead to the attraction of lymphocytes to the tumor bed [2, 3].
The presence of a host antitumor immunity has been shown to influence the response to cytotoxic treatments. Denkert et al. demonstrated that a high tumor infiltration by lymphocytes at diagnosis is associated with a higher likelihood of pathological complete response after neoadjuvant chemotherapy [4]. The prognostic role of tumor-infiltrating lymphocytes (TIL) in breast cancer has been evaluated in the context of randomized adjuvant trials. In summary, TIL at baseline are associated with high-proliferative, high-grade and estrogen receptor (ER)-negative tumors and represent a strong prognostic factor for certain breast cancer subtypes, mainly for triple-negative breast cancer (TNBC) [5, 6]. However, in all these trials, patients were treated with adjuvant chemotherapy; therefore, the prognostic role of TIL in untreated patients is unknown. This information is essential before defining the role of TIL evaluation in clinical practice. Moreover, only few studies so far have evaluated the value of TIL in predicting sensitivity to specific treatments.
Here, we seek to further explore the clinical utility of TILs in breast cancer. We used archived samples from two large phase III randomized adjuvant trials and evaluated the prognostic and predictive role of TILs in breast cancer patients treated with anthracyclines or no adjuvant chemotherapy.
methods
patients
Two French multicentric phase III trials randomized a total of 1146 breast cancer patients from 1989 to 1995 to receive adjuvant anthracycline-based chemotherapy or not [7]. A total of 935 patients (83% of the trials) were enrolled at the Gustave Roussy. Inclusion criteria and results of these trials have already been reported elsewhere [7].
A total of 688 patients were postmenopausal and presented either with histologically confirmed positive axillary nodes or with negative lymph nodes, but grade 2 or 3. The remaining 247 were premenopausal and node negative, but presented with grade 2 or 3 tumors.
Primary tumor samples from 823 (88%) of the 935 patients enrolled at Institut Gustave Roussy were available for the present analysis. The patients corresponding to the 112 non-retrieved tumors tended to be younger than those corresponding to the 823 retrieved tumors (mean age 54 versus 56 years, P = 0.02) and less postmenopausal (63% versus 75%, P = 0.01). There was no selection bias based on disease stage, tumor grade or ER status.
pathology
Full-face hematoxilin and eosin stained (HES) slides of primary tumors were retrieved and evaluated for the percentage of intratumoral (It) and stromal (Str) TIL, according to predefined criteria [4, 8].
Cases were defined as high-TIL if It-TIL and/or Str-TIL ≥50%, and as low-TIL if It-TIL and Str-TIL <50%, adopting already validated cutoffs [5, 8]. These cutoffs were defined before any statistical analyses.
Details on TIL, ER, HER2 status and grade determination are reported in the supplementary Appendix 1, available at Annals of Oncology online. ER and HER2 status were evaluated by immunohistochemistry on a tissue array containing three spots from each primary tumor, as previously described [9].
treatment
Patients were randomized to receive six courses of 5-fluorouracil 500 mg/mq, doxorubicin or epirubicin 50 mg/mq and cyclophosphamide 500 mg/mq, or no chemotherapy. All postmenopausal patients received adjuvant tamoxifen for at least 2 years and were allowed to participate in a French trial comparing 2 years of tamoxifen with long-term treatment.
statistics
Statistical analysis was carried out using the R project for statistical computing [10].
The primary end point was OS, defined as the time between the date of randomization to the date of death or last follow-up.
The prognostic value of lymphocytic infiltrate was first tested by considering It-TIL and Str-TIL separately as continuous variables, and then accordingly to the high-TIL versus low-TIL categorical definition. Hazard ratios (HR) and 95% confidence interval (95% CI) were calculated with the Cox proportional hazard regression model, adjusted for clinico-pathological prognostic factors. The required assumptions of proportionality in the multivariate survival analysis were checked graphically and by Schoenfeld's test. Kaplan–Meier method was used to estimate OS curves and the log-rank test was used to compare between groups.
Interaction between TIL and chemotherapy was also studied.
The association between TIL status and clinico-pathological variables was calculated using either χ2 tests with continuity correction or Fisher's exact test. All the statistical tests were two-sided, and considered significant when P value ≤0.05.
The REMARK (Reporting Recommendations for Tumor Marker Prognostic studies) criteria were followed in this study [11].
results
clinico-pathological characteristics and association with TIL
HES slides from primary tumor samples were evaluable for 781 of the 816 included cases. No difference was noticed between the study population (781 patients) and the non-assessable patients (supplementary Table S1, available at Annals of Oncology online). The reasons that impaired a correct TIL estimation in the remaining cases were decolored staining and tissue artifacts. The characteristics of the 781 patients are reported in Table 1. The majority of the patients had an ER+/HER2− breast cancer (60%); 238 patients presented with a grade 3 tumor (31%). Forty-three percent of the patients had node-positive disease and 44% patients presented with tumors larger than 2 cm. Median (IQ; interquartile) Str-TIL in the whole population was 10% (15%) (Table 1). Seventy-eight patients (10%) were classified as high-TIL. As reported in Table 1, the presence of a high lymphocytic infiltrate was significantly associated with high-grade and ER-negative tumors (P < 0.001 in both cases) and tended to be more evident in large tumors (P = 0.05). Patients included in the adjuvant anthracycline arm tended to have higher Str-TIL levels (P < 0.001); however, the rate of high-TIL patients was similar in the two treatment arms.
Table 1.
Patients characteristics according to TIL
| Continuous Str-TIL % variable |
Continuous It-TIL % variable |
Categorical TIL variable |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median [range] | Quantiles [Q1–Q3] | IQ | P | Median [range] | Quantiles [Q1–Q3] | IQ | P | Low-TIL, n (%) | High-TIL, n (%) | P | |
| All | 781 (100) | 10 [0–100] | [5–20] | 15 | 5 [0–100] | [0–15] | 15 | 703 (90) | 78 (10) | |||
| Age | ||||||||||||
| <50 | 189 (24) | 10 [0–90] | [5–25] | 20 | 10 [0–100] | [0–15] | 15 | 170 (90) | 19 (10) | |||
| ≥50 | 592 (76) | 10 [0–100] | [5–20] | 15 | 0.02 | 5 [0–100] | [0–15] | 15 | ns | 533 (90) | 59 (10) | ns |
| Grade | ||||||||||||
| 1–2 | 537 (69) | 10 [0–100] | [5–15] | 10 | 5 [0–100] | [0–10] | 10 | 506 (94) | 31 (6) | |||
| 3 | 238 (31) | 20 [0–95] | [10–35] | 25 | 6.0e−15 | 10 [0–95] | [2–25] | 23 | 1.5e−08 | 192 (81) | 46 (19) | 1.3e−08 |
| NA | 6 | 5 | 1 | |||||||||
| N | ||||||||||||
| Neg | 446 (57) | 10 [0–100] | [5–20] | 15 | 5 [0–100] | [0–15] | 15 | 405 (91) | 41 (9) | |||
| Pos | 335 (43) | 10 [0–95] | [5–20] | 15 | ns | 5 [0–100] | [0–15] | 15 | ns | 298 (89) | 37 (11) | ns |
| T size | ||||||||||||
| ≤2 cm | 435 (56) | 10 [0–90] | [5–20] | 15 | 8 [0–100] | [0–15] | 15 | 400 (92) | 35 (8) | |||
| >2 cm | 341 (44) | 10 [0–100] | [5–25] | 20 | ns | 5 [0–100] | [0–15] | 15 | ns | 298 (87) | 43 (13) | 0.05 |
| NA | 5 | 5 | 0 | |||||||||
| ER/HER2 | ||||||||||||
| ER−HER2− | 199 (26) | 15 [0–90] | [10–30] | 20 | 10 [0–90] | [5–25] | 20 | 442 (95) | 21 (5) | |||
| ER+HER2− | 463 (60) | 10 [0–100] | [5–15] | 10 | 5 [0–100] | [0–10] | 10 | 169 (85) | 30 (15) | |||
| HER2+ | 112 (14) | 20 [0–95] | [10–40] | 30 | 1.1e−17 | 10 [0–95] | [5–20] | 15 | 9.7e−13 | 85 (76) | 27 (24) | 1.3e−10 |
| NA | 7 | 7 | 0 | |||||||||
| Arm | ||||||||||||
| No CT | 387 (50) | 10 [0–100] | [5–20] | 15 | 5 [0–100] | [0–10] | 10 | 354 (91) | 33 (8) | |||
| Anthra | 394 (50) | 10 [0–95] | [5–25] | 20 | 1.0e−04 | 10 [0–95] | [2–20] | 18 | 2.9e−06 | 349 (89) | 45 (11) | ns |
N, number; IQ, interquantile; NA, not available; N, node; neg, negative; pos, positive; T, tumor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; CT, chemotherapy; Anthra, anthracyclines; ns, not significant.
association of TILs with prognosis
For the prognostic evaluations, all arms were pooled. At a median follow-up of 12.7 years, both It-TIL and Str-TIL considered as continuous variables (per 10% increase) were significantly associated with OS in multivariate analyses (Table 2). The risk of death was reduced by 15% and 11% for each 10% It-TIL and Str-TIL increment, respectively (HR 0.85, 95% CI 0.77–0.95, P = 0.003 for It-TIL; HR 0.89, 95% CI 0.81–0.96 P = 0.005 for Str-TIL). The other parameters that were independent predictors of outcome were pathological nodal status, tumor grade, ER and HER2 status.
Table 2.
Association of TIL with prognosis (overall survival) in the whole study population
| N | Univariate |
Multivariate |
P | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| It-TIL | ||||||
| 10% increase | 781 | 0.94 | (0.86–1.03) | 0.85 | (0.77–0.95) | 0.003 |
| Age | ||||||
| <50 years | 189 | 1 | ||||
| ≥50 years | 592 | 1.18 | (0.68–2.05) | |||
| Grade | ||||||
| ½ | 537 | 1 | 1 | |||
| 3 | 238 | 1.71 | (1.29–2.25) | 1.53 | (1.12–2.09) | 0.01 |
| LN | ||||||
| Neg | 446 | 1 | 1 | |||
| Pos | 335 | 1.86 | (1.36–2.54) | 1.99 | (1.44–2.74) | <0.0001 |
| pT | ||||||
| ≤2 cm | 435 | 1 | ||||
| >2 cm | 341 | 1.44 | (1.10–1.89) | |||
| ER | ||||||
| Neg | 262 | 1 | 1 | |||
| Pos | 501 | 0.64 | (0.48–0.85) | 0.64 | (0.45–0.90) | 0.01 |
| HER2 | ||||||
| Neg | 664 | 1 | 1 | |||
| Pos | 112 | 1.73 | (1.23–2.43) | 1.41 | (0.96–2.07) | 0.08 |
| Arm | ||||||
| Control | 387 | 1 | ||||
| Anthra | 394 | 0.81 | (0.62–1.05) | |||
| N | Univariate | Multivariate | P | |||
| HR | 95% CI | HR | 95% CI | |||
| Str-TIL | ||||||
| 10% increase | 781 | 0.97 | (0.91–1.05) | 0.89 | (0.81–0.96) | 0.005 |
| Age | ||||||
| <50 years | 189 | 1 | ||||
| >=50 years | 592 | 1.18 | (0.68–2.05) | |||
| Grade | ||||||
| ½ | 537 | 1 | 1 | |||
| 3 | 238 | 1.71 | (1.29–2.25) | 1.61 | (1.17–2.20) | 0.003 |
| LN | ||||||
| Neg | 446 | 1 | 1 | |||
| Pos | 335 | 1.86 | (1.36–2.54) | 2.01 | (1.46–2.77) | <0.0001 |
| pT | ||||||
| ≤2 cm | 435 | 1 | ||||
| >2 cm | 341 | 1.44 | (1.1–1.89) | |||
| ER | ||||||
| Neg | 262 | 1 | 1 | |||
| Pos | 501 | 0.64 | (0.48–0.85) | 0.66 | (0.47–0.92) | 0.02 |
| HER2 | ||||||
| Neg | 664 | 1 | 1 | |||
| Pos | 112 | 1.73 | (1.23–2.43) | 1.51 | (1.03–2.23) | 0.04 |
| Arm | ||||||
| Control | 387 | 1 | ||||
| Anthra | 394 | 0.81 | (0.62–1.05) | |||
N, number, NA, not available; neg, negative; pos, positive; T, tumor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; Anthra, anthracyclines; HR, hazard ratio; CI, confidence interval.
We then explored the association of TIL with prognosis in different breast cancer subtypes.
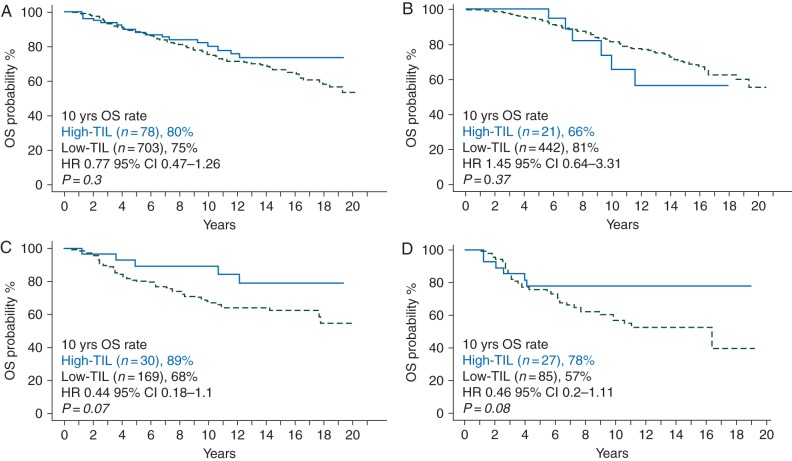
In multivariate analyses, both It-TIL and Str-TIL as continuous variables were significantly associated with OS in the HER2+ and triple-negative subgroups, whereas no effect was observed for ER+/HER2− patients (Table 3). For each 10% Str-TIL increase, a 18% and 15% reduction in death risk was observed for HER2+ and triple-negative patients, respectively (adjusted HR 0.82, 85% CI 0.69–0.96, P = 0.02 for the HER2+ group; adjusted HR 0.85, 95% CI 0.74–0.99, P = 0.04 for the triple-negative group). For each 10% It-TIL increase, a 16% and 18% reduction in the death risk was observed for HER2+ and triple-negative patients, respectively (adjusted HR 0.84, 85% CI 0.70–1.01, P = 0.055 for the HER2+ group; adjusted HR 0.82, 95% CI 0.68–0.99, P = 0.04 for the triple-negative group). Kaplan–Meier survival curves according to TIL as categorical variable in the whole study population and in each breast cancer subtype are represented in Figure 1.
Table 3.
Association of TIL with prognosis (overall survival) in different breast cancer subgroups
| Subgroup | Patients (N) | Events (N) | TIL variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|---|---|
| (10% increase) | HR | 95% CI | P | HR | 95% CI | P | |||
| ER+/HER2− | 463 | 112 | It-TIL | 0.95 | 0.82–1.12 | 0.6 | |||
| Str-TIL | 1.01 | 0.89–1.15 | 0.8 | ||||||
| HER2+ | 112 | 42 | It-TIL | 0.89 | 0.75–1.05 | 0.2 | 0.84 | 0.70–1.01 | 0.055 |
| Str-TIL | 0.88 | 0.76–1.01 | 0.07 | 0.82 | 0.69–0.96 | 0.02 | |||
| ER−/HER2− | 199 | 61 | It-TIL | 0.83 | 0.69–0.99 | 0.04 | 0.82 | 0.68–0.99 | 0.04 |
| Str-TIL | 0.89 | 0.78–1.02 | 0.1 | 0.85 | 0.74–0.99 | 0.04 | |||
Multivariate adjusted on grade, LN and treatment arm for ER−/HER2−, and on pT, LN and treatment arm for HER2+.
N, Number; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; CI, confidence interval.
Figure 1.
Prognostic value of TIL. Estimated Kaplan–Meier curves of disease-free survival for all patients (A) and in different subgroups: ER+/HER2 (B) ER−/HER2− (C) and HER2+ (d).
association of TIL with anthracycline effect
Our next aim was to investigate whether the anthracycline effect was different according to TIL level at baseline and, vice versa, whether TIL had a differential prognostic effect in the treated and untreated population. As reported in Table 4, there was no significant interaction between TIL variables and anthracycline treatment, test for interaction P value was not significant in the whole study population and in different subgroups (ER+/HER2−, HER2+, ER−/HER2−) both for binary and continuous TIL variables. The different distribution of Str-TIL levels in the two arms (as shown in Table 1) might have influenced the test for interaction. However, no difference in the rate of high-TIL patients was observed in the two arms and anthracyclines showed a similar effect in low-TIL and high-TIL patients. For visual purpose, forest plots for the prognostic effect of TIL (continuous variable) in treated and untreated patients and forest plots for the effect of anthracycline treatments in high-TIL and low-TIL categories are reported in supplementary Figures S2 and S3, available at Annals of Oncology online, respectively.
Table 4.
Predictive value of TIL: TIL × anthracycline treatment interaction P tests
| N | OS interaction P | |
|---|---|---|
| Binary TIL variable (high-TIL/low-TIL) | ||
| All patients | 781 | 0.43 |
| ER+/HER2− | 463 | 0.3 |
| ER−/HER2− | 199 | 0.66 |
| HER2+ | 112 | 0.76 |
| It-TIL (per 10% increase) | ||
| All patients | 781 | 0.17 |
| ER+/HER2− | 463 | 0.18 |
| ER−/HER2− | 199 | 0.55 |
| HER2+ | 112 | 0.84 |
| Str-TIL (per 10% increase) | ||
| All patients | 781 | 0.6 |
| ER+/HER2− | 463 | 0.32 |
| ER−/HER2− | 199 | 0.44 |
| HER2+ | 112 | 0.81 |
N, number; OS, overall survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
discussion
In this study, we confirmed the strong prognostic role of TIL in breast cancer. Another aim of our study was to evaluate whether TIL retained any predictive effect for anthracycline benefit. This assumption was based on preclinical findings that cytotoxic chemotherapy, in particular anthracyclines, may induce an immunogenic cell death. Apetoh et al. [1] showed that cancer cell death induced by anthracyclines results in the release of high-mobility-group box 1, which activates antigen-presenting cells through Toll-like receptor-4, finally resulting in the induction of antitumor-specific T lymphocytes. In our study, we did not observe any significant interaction between TIL and anthracyclines.
However, in each breast cancer (BC) subtype, the results varied, emphasizing that immune aspects contribute to the distinct biology of each molecular BC group, which deserve separate discussions.
The absence of prognostic effect of TIL for ER+ disease is in line with previous findings [5, 12] and confirms that immune aspects do not represent the principal hallmark of this cancer type. Given these premises, the results on the absence of a TIL–anthracycline interaction in this ER+HER2− subgroup are not unexpected. Indeed, it is now clear that the role of TIL for this breast cancer subtype is very limited.
In the other hand, we confirmed the strong prognostic role of TIL for triple-negative BC. The HR for the prognostic impact of continuous (10% increase) Str-TIL in TNBC that we reported is highly consistent with previous observations [5, 6, 12]. With regard to the ability of TIL to predict the effect of specific treatments in this patients' population, previous findings have already shown that once an immune response has been generated, the prognosis is improved independently from the type of chemotherapy that is administered. These results come from analysis within the BIG2–98 trial that evaluated whether TIL could predict the benefit from anthracycline-based versus anthracycline and taxane-based adjuvant chemotherapy. No differential effect of the two treatments was observed according to TIL for triple-negative patients [5]. Our study adds to available evidence since it includes a population of untreated TNBC patients, allowing to better establish the clinical implications of the immune infiltrate. There was no heterogeneous effect of anthracyclines according to TIL and, vice versa, no heterogeneous prognostic effect of TIL according to whether anthracyclines were administered or not. Therefore, these data do not allow identifying a specific population of TNBC patients that may particularly benefit from anthracycline, but highlight the need to find out strategies to foster a baseline antitumor immune response. Indeed, the available evidence somehow depict a TIL ‘on/off’ effect on prognosis for TNBC patients: once the recognition of tumor antigens is induced and the immune response is activated, the positive effect on outcome is established. One possible criticism may concern the dose of anthracyclines that were administered in the present study. Although the cumulative dose of Doxorubicin (300 mg/mq; six courses at 50 mg/mq) is the same that was reached in the BIG 2–98 arm that contained the higher dose of anthracycline (arm A1), FE50C does not represent a standard. Indeed, six courses of FE100C have been proven to be superior in terms of efficacy compared with six courses of FE50C [13]. Lower doses of epirubicin might not be sufficient to elicit the immunogenic effect.
As for HER2+ disease, we described in our series of trastuzumab-untreated patients a positive association between TIL and improved outcome, without any interaction with anthracycline administration. However, available results are confounding and suggest that the prognostic effect of TIL in this BC subtype depends on the type of adjuvant treatment that is administered. Due to oncogene addiction, HER2+ cancer cells may interact in a more subtle way than TNBC cells with the immune system. Indeed, oncogene-addicted tumors often activate immune-suppressive pathways that allow escaping the host antitumor immune response [14]. Therefore, the key question for this disease is how to relieve HER-2-mediated immune suppression. In the BIG2-98 trial where no HER2+ patient received adjuvant trastuzumab, there was no TIL prognostic effect reported when all arms were pooled. However, a significant interaction with treatment arm (anthracycline plus taxanes versus higher dose anthracycline-only) was reported. HER2+ patients with high-TIL levels derived higher benefit from an anthracycline-only chemotherapy compared with combined treatment with anthracyclines and taxanes, containing lower anthracyclines doses. At the opposite, patients with low-TIL tumors benefitted from the addition of taxanes to anthracyclines [5]. The authors concluded that a potential explanation for these results may be that higher anthracycline doses are able to induce a higher amount of immunogenic cell death, thus resulting in an enhancement of the antitumor immune response. The design of our study (anthracyclines versus no chemotherapy) allowed for a direct evaluation of anthracyclines effect according to biomarkers than in the BIG 2–98 trial in which all patients received anthracyclines. Moreover, there was no confounding effect of adjuvant anti-HER2 treatment; although some of the HER2-positive patients might have received anti-HER2 drugs at relapse. One hypothesis could be that anthracycline-induced immunogenic cell death might not represent an effective means to relieve HER2-mediated immune suppression and, therefore, that the BIG 2–98 study captured a detrimental immunosuppressive effect of adding taxanes (and premedication with steroids) to anthracyclines for HER2+ tumors presenting an immune infiltrate, rather than a beneficial role of higher anthracycline dosages.
However, we acknowledge that, due to the limited sample size and number of events, the analysis of the interaction between TIL and treatment in the ER−/HER2− and HER2+ subgroups is likely to be underpowered.
To conclude, based on our data, TIL should not be used as a parameter to select patients for adjuvant anthracycline treatment. We add further evidence on the strong prognostic role of TIL in TNBC. The robustness of data across different studies strongly support the adoption of TIL as a stratification parameter in clinical trials for TNBC. We also added evidence in support to the prognostic role of TIL for HER2-positive breast cancer. It is important to underline that there are no data so far that support withholding chemotherapy in patients with high-TIL levels. Further research is needed, in order to better understand immunogenic mechanisms in different breast cancer subtypes and should focus on how to stimulate an efficient antitumor immune response in TNBC and how to modulate HER2-mediated immunosuppression for HER2-positive disease. Agents targeting immune checkpoints certainly hold promises in this context.
funding
This work was supported by TRANSCAN JCT-2011g, French NCI and the Monica Boscolo 2012 Research Grant.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Apetoh L, Ghiringhelli F, Tesniere A et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 2.Demaria S, Volm MD, Shapiro RL et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res 2001; 7: 3025–3030. [PubMed] [Google Scholar]
- 3.Ladoire S, Mignot G, Dabakuyo S et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 2011; 224: 389–400. [DOI] [PubMed] [Google Scholar]
- 4.Denkert C, Loibl S, Noske A et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28: 105–113. [DOI] [PubMed] [Google Scholar]
- 5.Loi S, Sirtaine N, Piette F et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 2013; 31: 860–867. [DOI] [PubMed] [Google Scholar]
- 6.Adams S, Gray RJ, Demaria S et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014; 32: 2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arriagada R, Spielmann M, Koscielny S et al. Results of two randomized trials evaluating adjuvant anthracycline-based chemotherapy in 1146 patients with early breast cancer. Acta Oncol 2005; 44: 458–466. [DOI] [PubMed] [Google Scholar]
- 8.Salgado R, Denkert C, Demaria S et al. Harmonization of the evaluation of tumor infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs-working group 2014. Ann Oncol 2014; 26: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conforti R, Boulet T, Tomasic G et al. Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracyclines-based chemotherapy: a biomarker study from two randomized trials. Ann Oncol 2007; 18: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 10.R Development Core Team. R R: A language and environment for statistical computing. Vienna, Austria: R Foudation for Statistical Computing; http://www.rproject.org/ (20 May 2015, date last accessed). [Google Scholar]
- 11.McShane LM, Altman DG, Sauerbrei W et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97: 1180–1184. [DOI] [PubMed] [Google Scholar]
- 12.Loi S, Michiels S, Salgado R et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014; 25: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 13.French Adjuvant Study Group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 2001; 19: 602–611. [DOI] [PubMed] [Google Scholar]
- 14.Savas P, Caramia F, Teo ZL, Loi S. Oncogene addiction and immunity: clinical implications of tumour infiltrating lymphocytes in breast cancers overexpressing the HER2/neu oncogene. Curr Opin Oncol 2014; 26: 562–567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.