The first St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) Expert Panel identified and reviewed available evidence for the ten most important areas of controversy in advanced prostate cancer management. Recommendations based on expert opinion are presented. Detailed decisions on treatment will involve clinical consideration of disease extent and location, prior treatments, host factors, patient preferences and logistical and economic constraints.
Keywords: advanced prostate cancer, castration-resistant prostate cancer, therapeutics, consensus, castration-naïve prostate cancer
Abstract
The first St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) Expert Panel identified and reviewed the available evidence for the ten most important areas of controversy in advanced prostate cancer (APC) management. The successful registration of several drugs for castration-resistant prostate cancer and the recent studies of chemo-hormonal therapy in men with castration-naïve prostate cancer have led to considerable uncertainty as to the best treatment choices, sequence of treatment options and appropriate patient selection. Management recommendations based on expert opinion, and not based on a critical review of the available evidence, are presented. The various recommendations carried differing degrees of support, as reflected in the wording of the article text and in the detailed voting results recorded in supplementary Material, available at Annals of Oncology online. Detailed decisions on treatment as always will involve consideration of disease extent and location, prior treatments, host factors, patient preferences as well as logistical and economic constraints. Inclusion of men with APC in clinical trials should be encouraged.
introduction
The diagnostic and therapeutic management of men with advanced prostate cancer (APC) has been transformed in recent years. Several new treatments for men with castration-resistant disease have successfully completed phase III trials and have received regulatory approval. The trials have not only shown a significant prolongation of overall survival, but they also reported improved or preserved quality of life (QoL). The latter, however, was less rigorously assessed and documented. Importantly, the currently approved survival-prolonging treatments in metastatic castration-resistant prostate cancer (CRPC) have distinct mechanisms of action. These therapies include unique classes of agents: taxanes, docetaxel and cabazitaxel, an immunotherapeutic agent, sipuleucel-T, novel androgen receptor (AR) pathway inhibitors abiraterone acetate (abiraterone) and enzalutamide as well as a bone targeting alpha-emitting radionuclide, radium-223 chloride (radium-223) [1–7].
Large-scale, prospective randomised trials testing the optimal sequencing of these treatments have not yet been reported. Furthermore, predictive markers to facilitate the selection of patients for a specific therapy or sequence of therapies remain an unmet need. The latter is especially relevant given the increasing evidence that some castration-resistant phenotypes may be more responsive to hormonal strategies, others to cytotoxics and others to biologic approaches. Effective combination therapies may improve outcomes and are under investigation. In addition, trials examining newer agents in the castration-naïve setting are now beginning to emerge, potentially supporting the upfront use of agents such as docetaxel. While addressing one aspect of the sequencing debate, such upfront use clearly then raises a whole raft of new questions about management on development of CRPC.
Novel imaging methods for evaluation of patient selection and response claiming increased sensitivity and specificity have not been adequately tested in prospective clinical trials.
In the absence of evidence, the selection of treatment is based on clinical judgement that includes the experience of the treating physician with the available agents, the status of the disease, when the patient is presenting for treatment, and potential comorbid conditions that might preclude a particular treatment. All treatments can have side-effects, and all of the new treatments are expensive. Of note, global access to the above-mentioned CRPC therapeutics varies across the globe, and the financial burden to an individual patient, but also to the community, must be factored.
Zoledronic acid and denosumab are approved to reduce the risk of skeletal-related events (SREs) based on the results of phase III trials in men with metastatic CRPC [8–10]. Most were approved before the availability of therapies beyond taxanes. The optimal use of these osteoclast-targeted therapies (including sequencing, initiation, frequency and duration of treatment) has not been determined. Moreover, their roles, and in particular their efficacy, in the era of newly approved anticancer-treatment options for men with CRPC [many of which also reduce the risk of SREs or symptomatic skeletal-related events (SSEs)] have not been determined. Studies to address these critical knowledge gaps need to be undertaken.
Physicians may rely on national and international guidelines to base management decisions outside of clinical trials. The level of evidence on which a specific guideline is based varies considerably. Nonetheless, there still remain several topics regarding management decisions where there is either a paucity of level one evidence or conflicting evidence of results. To address this, an international expert consensus, the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC), was organised with the objective of providing the recommendations of experts to complement evidence-based guidelines and to better frame the discussion between men with prostate cancer and physicians when faced with management decisions.
Importantly, several of these recommendations were derived from clinical trial data that have been obtained from trials with specific entry criteria. These criteria will differ from trial to trial. Hence, not all recommendations can be generalised and applied uncritically to every patient, rather they should be tailored to an individual and shared decision making is still required.
methods
The panel included 41 prostate cancer experts from 17 countries, covering different specialties involved in research and treatment of men with APC (Table 1).
Table 1.
Panel members by country and specialty
| Name | Country | Specialty |
|---|---|---|
| Akaza, Hideyuki | Japan | Urology |
| Attard, Gerhardt | UK | Medical Oncology |
| Beer, Tomasz M. | USA | Medical Oncology |
| Beltran, Himisha | USA | Medical Oncology |
| Chinnaiyan, Arul M. | USA | Pathology/Basic research |
| Daugaard, Gedske | Denmark | Medical Oncology |
| Davis, Ian | Australia | Medical Oncology and Palliative Medicine |
| De Bono, Johann | UK | Medical Oncology |
| De Santis, Maria | Austria | Medical Oncology |
| Drake, Charles G. | USA | Medical Oncology |
| Eeles, Rosalind Anne | UK | Oncogenetics and Clinical/Radiation Oncology |
| Efstathiou, Eleni | Greece/USA | Medical Oncology |
| Fanti, Stefano | Italy | Nuclear Medicine |
| Fizazi, Karim | France | Medical Oncology |
| Gillessen, Silke | Switzerland | Medical Oncology |
| Gleave, Martin E. | Canada | Urology |
| Halabi, Susan | USA | Statistics/Epidemiology |
| Heidenreich, Axel | Germany | Urology |
| Hussain, Maha H. A. | USA | Medical Oncology |
| James, Nicholas D. | UK | Clinical/Radiation Oncology |
| Lecouvet, Frédéric | Belgium | Radiology |
| Logothetis, Christopher J. | USA | Medical Oncology |
| Nelson, Peter | USA | Medical Oncology |
| Nilsson, Sten | Sweden | Medical Oncology |
| Oh, William K. | USA | Medical Oncology |
| Olmos, David | Spain | Medical Oncology |
| Padhani, Anwar | UK | Radiology |
| Parker, Chris | UK | Clinical/Radiation Oncology |
| Rubin, Mark A. | USA | Pathology/Basic research |
| Sartor, Oliver A. | USA | Medical Oncology |
| Schalken,Jack A. | Holland | Basic research |
| Scher, Howard I. | USA | Medical Oncology |
| Sella, Avishay | Israel | Medical Oncology |
| Shore, Neal | USA | Urology |
| Small, Eric | USA | Medical Oncology |
| Smith, Matthew R. | USA | Medical Oncology |
| Sternberg, Cora N. | Italy | Medical Oncology |
| Suzuki, Hiroyoshi | Japan | Urology |
| Sweeney, Christopher | USA | Medical Oncology |
| Tannock, Ian | Canada | Medical Oncology |
| Tombal, Bertrand | Belgium | Urology |
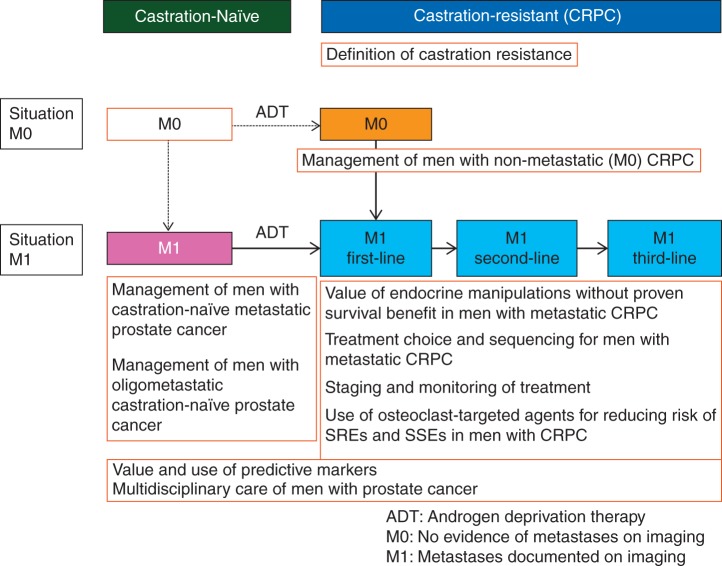
First, the panel members agreed upon the 10 most important areas of controversy relating to the management of men with APC, and these are listed below:
Management of men with castration-naïve metastatic prostate cancer
Management of men with oligometastatic castration-naïve prostate cancer
Definition of castration resistance
Management of men with non-metastatic (M0) CRPC
Value of endocrine manipulations without proven survival benefit in men with metastatic CRPC
Treatment choice and sequencing for men with metastatic CRPC
Staging and monitoring of treatment
Use of osteoclast-targeted agents for reducing risk of SREs and SSEs in men with CRPC
Value and use of predictive markers
Multidisciplinary care of men with prostate cancer
In a modified Delphi process (Figure 1), questions based on the above 10 sections (Figure 2) were created and in the first round sent to all panel members for input. Questions and options for answers were later revised and sent a second time to all panel members, including all inputs received shown in an anonymised fashion, so all the panellists could see every comment from their colleagues [11]. The comments from the second round were included in the third version which was then circulated. After the third round, only important changes were accepted for the fourth and final version of the questions. This process was analogous to the one used for the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer [12]. The conference included presentations and debates from participants (primarily from panellists) who reviewed evidence relevant to the above questions. On the last day of the conference, all questions were presented with options for answers in a multiple-choice format. The questions were voted on publicly but anonymously. In some cases, discussions and re-voting occurred.
Figure 1.
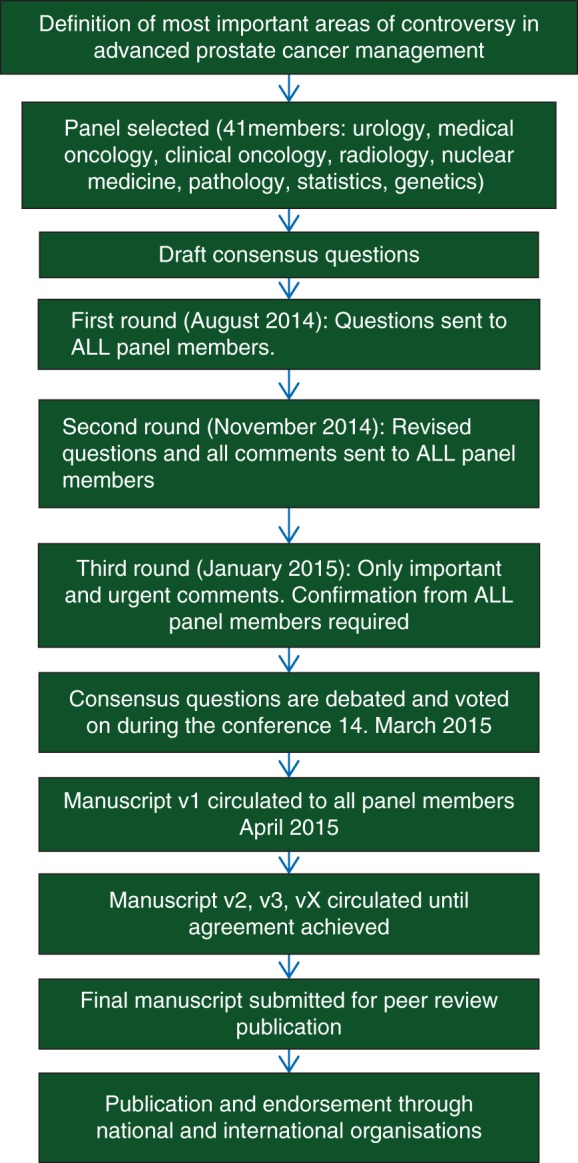
How the Consensus Process works (modified Delphi process).
Figure 2.
Conceptual framework: advanced prostate cancer.
For all questions, if not stated otherwise, it was assumed that any drug recommended must have been approved and was readily available, no treatment contraindications existed and no clinical trial was available. In addition recommendations applied only to non-frail patients [defined as Eastern Cooperative Oncology Group (ECOG) performance status 0–2] and for patients with adenocarcinoma of the prostate (if not stated otherwise). Importantly, in an effort to address questions from an evidenced-based and clinical utility perspective, panellists were specifically instructed not to factor in cost, reimbursement and access as factors in their deliberations, although clearly these are critical factors in decision making for the individual patient.
It was recommended that if a panellist lacked experience or was non-expert for a specific question, the option ‘unqualified to answer’ (short form ‘unqualified’) should be chosen and if a panellist felt unable to vote for a best choice for any reason or had prohibitory conflicts of interest (COI), the option ‘abstain’ should be chosen. The conference also included an explicit approach to management of COI (supplementary Appendix S1, available at Annals of Oncology online).
For the purposes of this article, the term ‘recommend’ is used to reflect the fact that the panellists considered the option as the preferred one, also in the absence of hard clinical trial data. In contrast, the use of the phrase ‘discuss the option’ was used when panellists felt that the option was a consideration, but not necessarily the preferred one.
Detailed voting records for each of the questions brought to the panel are provided in the supplementary Appendix S2, available at Annals of Oncology online. In tabulating the results, the denominator was based on the number of panel members who voted, excluding those that were ‘unqualified to answer’ but including those who chose to ‘abstain’.
If ≥75% of the panellists chose the same option, this was defined as consensus. All panellists have contributed to the editing and approved this final consensus document.
Importantly, this process was also able to emphasize areas of non-consensus where additional data acquisition might be warranted.
management of men with castration-naïve metastatic prostate cancer
The panel members felt that ‘castration-naïve’ is the more appropriate term instead of ‘hormone-sensitive’ or ‘castration sensitive’, as the sensitivity of the cancer to castration is not known before commencement of Androgen deprivation therapy (ADT).
intermittent and combined ADT
ADT by means of orchiectomy, GnRH agonists or antagonists is the standard systemic treatment of metastatic prostate cancer [13–18].
The data supporting equivalence of GnRH analogues and orchiectomy were primarily established by studies using a testosterone suppression end point rather than appropriately sized and powered clinical trials with an end point of clinical efficacy. The majority of patients in economically well-developed countries today are treated with medical castration whereas, in developing countries, surgical castration is a more commonly used option for ADT [19]. Although the majority of the patients experience a profound prostate-specific antigen (PSA) decline with ADT, the median failure-free survival is ∼1 year, yet with a wide range. (11.2 months; interquartile range: 5.1–28.8) [20].
The concept of using ADT intermittently instead of continuously was developed as a consequence of several different hypotheses. These included the theory, based on experimental models [21–23], that intermittent use of ADT might be associated with a delay in development of castration resistance and the expectation that it would lead to less toxicity resulting in improvements in QoL and reduction in costs of treatment during the off phase.
Intermittent ADT (iADT) versus continuous ADT in men with metastatic prostate cancer has been evaluated in several trials, but only two trials have included solely metastatic patients and used the end point of overall survival. One of these trials was small (n = 173) whereas the SWOG 9346 trial randomised more than 1500 patients with initial PSA decline on ADT [24, 25]. The results of the latter trial failed to provide clear evidence for non-inferiority of iADT compared with continuous ADT [hazard ratio (HR) for death 1.1, 95% confidence interval (CI) 0.99–1.23]. The median overall survival (OS) was longer in the continuous ADT arm (5.8 versus 5.1 years) compared with the iADT arm. The findings serve to reject the theory of delay in castration resistance via iADT.
A recent systematic review by Niraula et al. has summarised the results of 9 studies with 5508 patients [24]. This review included men with various stages of disease, including those starting treatment of rising PSA after local treatment and trials with different end points. The authors concluded that there is evidence to recommend use of iADT (combined HR for OS = 1.02). However, meta-analysis does not replace data from large prospective trials. Therefore, the value of iADT in men with metastatic castration-naïve prostate cancer is still controversial since the only study powered and designed to assess non-inferiority, the SWOG 9346 trial, failed to show non-inferiority of iADT compared with continuous ADT.
In patients with metastatic prostate cancer achieving an adequate PSA decline (confirmed PSA fall below 4 ng/ml after ∼6 months of ADT), 71% of the panellists recommended intermittent instead of continuous ADT only for a minority of selected patients.
In a clear consensus, 94% of the panellists would discuss the option of intermittent ADT in metastatic patients, 54% in the majority of patients and 40% in a minority of selected patients who achieved an adequate PSA decline.
For physicians, the interpretation of non-inferiority trials in general and non-positive non-inferiority trials in particular may be challenging especially when applied to the clinical setting and patient management.
Due to the fact that residual androgen production by the adrenals may stimulate prostate cancer growth [25, 26] another attempt to improve the results of ADT treatment alone is combined (or maximal) androgen blockade (CAB), using a permanent combination of ADT and an earlier generation of AR antagonist such as bicalutamide or flutamide. Several phase III trials have evaluated the utility of front-line CAB (generally compared with the late addition of an AR antagonist to gonadal androgen suppression). Of note, most trials did not use the AR antagonist bicalutamide.
Three separate meta-analyses based on the results of these trials have concluded that there is a 3%–5% overall survival advantage of CAB versus ADT alone that is statistically significant when less effective steroidal AR antagonists such as cyproterone acetate are excluded from the analysis [27–29].
Of note, one Japanese trial was positive, testing CAB with bicalutamide, raising the possibility that Asian patients may benefit more than other patients from this treatment [30, 31].
Half of the panel did not recommend CAB whereas 35% recommended it in a minority of selected patients and 15% recommended it in the majority of patients.
Considerations influencing the use of CAB include ethnicity and added toxicity. Data about CAB using a combination of GnRH analogues with newer, more potent AR pathway inhibitors are not yet available.
docetaxel in men with castration-naïve metastatic prostate cancer
ADT alone versus ADT plus docetaxel in patients with metastatic castration-naïve prostate cancer has been tested in two randomised phase III trials, which both reported improved progression-free survival but provided results which differ in respect to overall survival benefit. The French GETUG-15 trial (n = 385) reported no OS benefit [32, 33] whereas, in the US ECOG E3805 (CHAARTED) trial (n = 790), a clinically and statistically significant improvement in OS was demonstrated [34]. Both trials included a high proportion of men (>70%) who presented with de novo metastatic prostate cancer, and this patient population may not be representative of men who develop metastatic disease at some time after diagnosis of localised cancer.
The different OS results between the trials may be due to sample size, a lower percentage of patients with higher tumour volume patients in GETUG-15 (47% versus 65% in CHAARTED), differential use of subsequent life-prolonging treatments (including docetaxel) and/or geographic differences (e.g. prevalence of PSA screening).
Sixty-one percent of the panellists accepted the high-volume definition as used in CHAARTED [visceral (lung or liver) and/or ≥4 bone metastases, at least one beyond pelvis and vertebral column] for use in daily clinical practice, whereas 11% recommended the high-volume definition developed by SWOG [visceral (lung or liver) and/or any appendicular skeletal involvement] [35], and 14% recommended the definition of Glass [diffuse bone disease (chest, head and/or extremities) and/or visceral organ (lung or liver) involvement] [36].
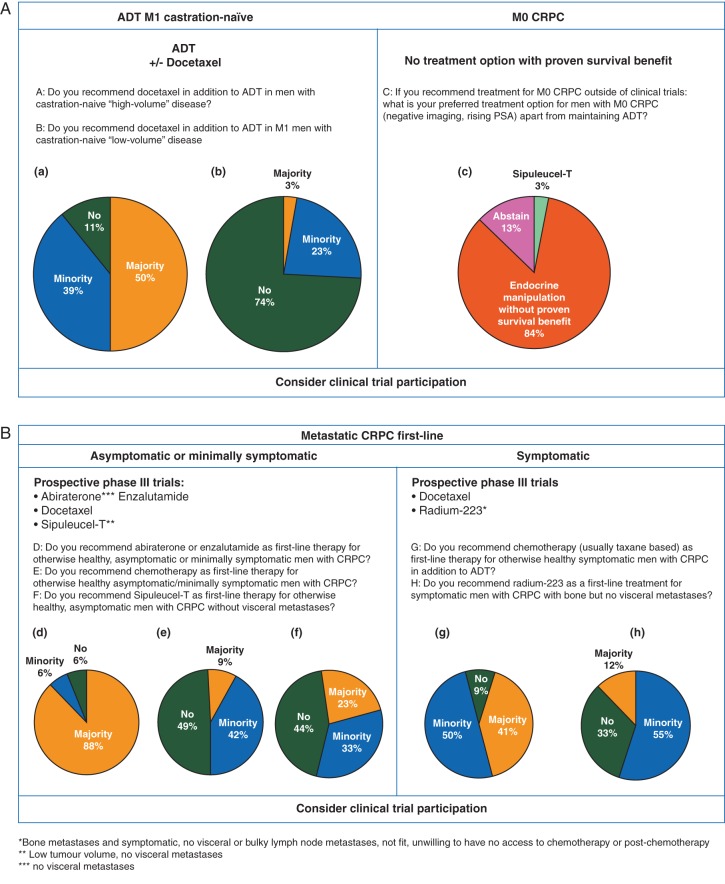
Half of the panel recommended docetaxel with ADT in castration-naïve M1 patients with high-volume disease in the majority of patients, 39% in a minority. Eleven percent did not recommend docetaxel with ADT in these patients at all. In contrast, in patients with low-volume disease, 74% of the panellists did not recommend routine use of docetaxel with ADT (Figure 3A).
Figure 3.
(A, B and C) Summary of treatment options for advanced prostate cancer, March 2015.
Of note at the time of the consensus meeting (March 2015), the votings above were based on the published data of GETUG15 and ASCO 2014 meeting presentation of CHAARTED, knowing the STAMPEDE study would read out in 2015 after the consensus meeting. As such, the consensus votings do not reflect the results of the M1 arm of the STAMPEDE study. The subsequent consensus meeting planned for 2017 will make use of the peer reviewed published data from all three studies and will aim to address issues such as patient profile, performance status, metastatic load, subsequent therapies and other related factors.
An abstract of data of four arms of the STAMPEDE trial (n = 2962 men with high-risk locally advanced or metastatic prostate cancer) was presented at ASCO 2015. Median OS was 67 months in the standard of care (ADT) arm compared with 77 months in the arm in which docetaxel was added to ADT (HR 0.76, 95% CI 0.63–0.91) [37].
osteoclast-targeted therapy in men with M1 castration-naïve prostate cancer
A randomised phase III trial (CALGB 90 202; n = 645) with the bisphosphonate zoledronic acid was conducted in the castration-naïve metastatic bone setting [38]. Compared with placebo, zoledronic acid did not improve time to first SRE, the primary study end point, or overall survival. Denosumab has not been tested for reducing risk of SREs in castration-naïve prostate cancer.
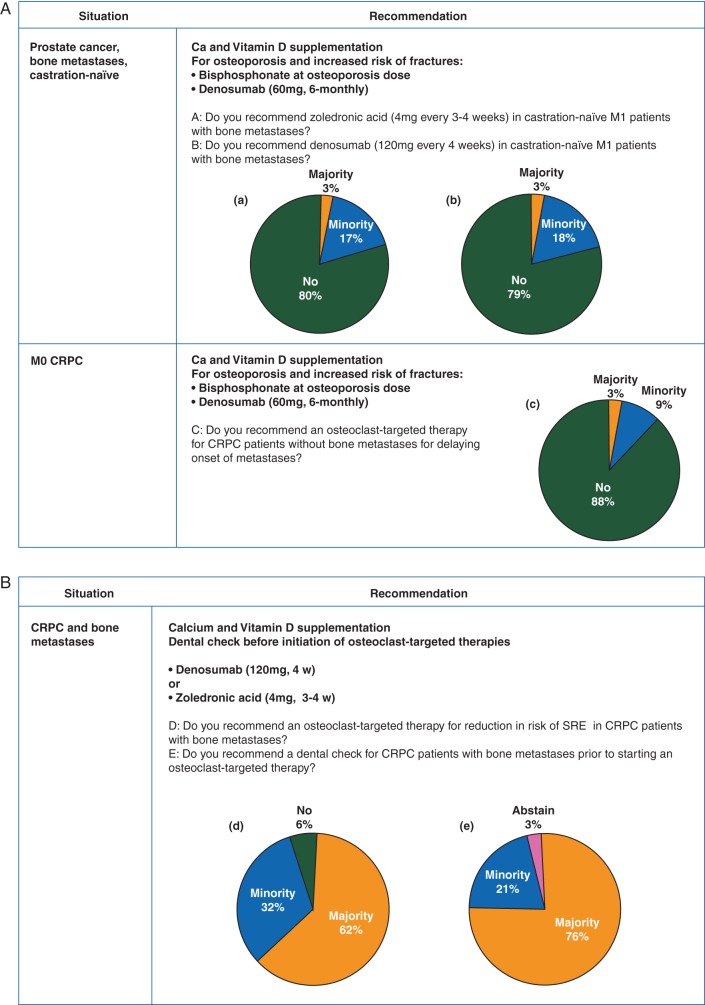
There was consensus among panellists that patients with castration-naïve prostate cancer and bone metastases should not receive zoledronic acid (81% of the panel) or denosumab (79% of the panel) for reducing risk of SREs (Figure 4A).
Figure 4.
(A and B) Osteoclast-targeted therapies for men with prostate cancer, March 2015.
Unfortunately, the approval indications for these drugs oftentimes are not defined clearly for the castration-resistant setting and, hence, may lead to their overuse and increase the incidence of their toxicities including osteonecrosis of the jaw and (potentially life threatening) hypocalcaemia and/or hypophosphataemia.
Notably, denosumab and bisphosphonates (at lower dose or schedule than for reducing risk of SREs) have an established role for the treatment/prevention of osteoporosis/osteoporotic fractures. The panel did not review the use of osteoclast-targeted therapy for treatment/prevention of osteoporosis and osteoporotic fractures.
oligometastatic castration-naïve prostate cancer
As in other tumour types, there is growing evidence that prostate cancer patients diagnosed with a limited number of metastases (oligometastatic) may have a better prognosis compared with those with extensive metastatic recurrence [39]. A recent meta-analysis of 15 single-arm case series in patients with oligometastatic disease concluded that local treatment of metastases in this setting may be promising but that the low level of evidence does not allow its recommendation as standard of care [40].
There was consensus (85% of the panel) that the presence of ≤3 synchronous metastases (bone and/or lymph nodes) is the most meaningful definition of oligometastatic prostate cancer.
The panel addressed whether local treatment of both the primary and all evident metastases was appropriate in patients with oligometastatic disease. Sixty-two percent of the panel did not recommend using this approach instead of systemic treatment (ADT) and 38% recommended it only in a minority of selected patients. When this local therapy was considered in the context of additional short-term ADT, 62% of the panel recommended this treatment in a minority of selected patients and 27% of the panel recommended this treatment in the majority of patients.
Similarly, in the case of relapse with oligometastatic disease after radical local treatment, 58% of the panel did not recommend local treatment of all metastases instead of systemic treatment (ADT), but 39% of the panel would consider it in a minority of selected patients. In the context of additional short-term ADT, 27% of the panel did not recommend local treatment of all metastases, 46% of the panel recommended it in a minority of selected patients and 27% of the panel recommended this treatment in the majority of patients.
The panel recognised that the use of bone scintigraphy and computed tomography (CT), compared with newer magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT imaging modalities, may result in an underestimation of lesion number. This in turn makes recommendations for a specific therapeutic approach of the oligometastatic state even more difficult [41–43].
definition of castration resistance
Over time, castration resistance has been defined in multiple ways, and with the approval of novel agents for the treatment of CRPC it is important to clarify the definition.
There was clear consensus (94% of the panel) that testosterone levels need to be measured and a specific value is required to designate a patient castration-resistant. As a consensus, 82% of the panel recommended a testosterone level <50 ng/dl (<1.7 nmol/l) as an appropriate cut-off value in daily clinical practice, which is in line with the US FDA definition.
Again there was clear consensus (94% of the panel) that a confirmed (by a second value three or more weeks later) rising PSA on ADT in the presence of suppressed testosterone is sufficient for the characterisation of a castration-resistant state in clinical practice.
In case of a rising PSA on ADT, and if testosterone level is not adequately suppressed, luteinizing hormone (LH) can be measured. In case of non-suppressed LH, correct administration of the GnRH analogue should be verified.
If testosterone is not sufficiently suppressed in the presence of suppressed LH, the panel considered several next management options including bilateral orchiectomy (22%), change to alternative GnRH agonist (22%), change to GnRH antagonist (44%) or addition of an AR antagonist (9%).
management of men with non-metastatic (M0) CRPC
The definition of M0 prostate cancer (rising PSA on ADT and no documented metastatic disease with radiographic imaging) is dependent upon the imaging technology chosen. In current clinical practice, the ideal combination of imaging methods to define the M0 state is unclear, as is when to use it. M0 is arguably an artificial disease stage designation, as there is a high likelihood that systemic micro-metastases are missed by commonly used imaging tools (CT and bone scintigraphy). In the absence of data from positive prospective clinical trials concerning overall survival, it is unclear what treatment options should be recommended if no metastases are found radiographically and the PSA continues to rise.
Of note, the randomised trials conducted in this setting with bone-targeted therapies with the objective of delaying the onset of bone metastases were either negative or not convincingly positive [8, 44, 45]. In the placebo arm of the trial testing denosumab in this setting, time to first bone metastasis was 40.8 months in the overall population, and 26 and 18.5 months in the patients with a PSA doubling time (PSA-DT) ≤10 and ≤4 months, respectively [46].
There was clear consensus (91% of the panel) that a PSA-based trigger (level and/or kinetics) should be used for restaging asymptomatic patients with rising PSA on ADT and no known metastases.
The earlier detection of metastases with newer imaging methods (i.e. techniques other than CT and planar bone scintigraphy) and consequent earlier initiation of treatment has not been shown to be associated with a patient benefit.
There was consensus (77% of the panel) that in daily clinical practice a negative CT (thorax and abdomen and pelvis) and a negative bone scintigraphy are sufficient for diagnosis of M0 disease.
With regards to the total PSA cut-off to initiate imaging, the entire panel recommended a PSA below 20 as cut-off, almost equally divided between a PSA of between 2 and 10 (54%) and a PSA between 10 and 20 (46%). The preferred absolute value is influenced by the prior local therapy (radical prostatectomy versus radiation therapy) and PSA-DT. For PSA-DT as a trigger for imaging, 74% of the panel recommended a PSA-DT of ≤6 months, and 9% recommended a PSA-DT of ≤3 months.
A risk-adapted strategy, taking PSA level and kinetics as well as patient preference and characteristics into consideration should be adopted.
Suggestions for when to initiate and repeat imaging in M0 CRPC patients have been recently published by the Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence Group [47].
As a trigger to initiate systemic treatment of M0 CRPC, about half of the panel (52%) recommended a combination of PSA-DT and absolute PSA value whereas 30% of the panel did not recommend treatment of patients with M0 CRPC outside of clinical trials at all, irrespective of PSA level and kinetics. However, most of the panellists acknowledged that withholding additional treatment in a patient who knows that his PSA is rising on ADT can be challenging even without supporting data that any therapy in this stage impacts overall survival.
The treatment option for such men with M0 CRPC, outside of clinical trials, chosen by 84% of the panellists was one of the endocrine manipulations without proven OS benefit (for definition see management of men with non-metastatic (M0) CRPC section; Figure 3A).
There are no data about the benefits of abiraterone or enzalutamide in this situation, but three large randomised phase III clinical trials are ongoing and enrolment of patients with M0 CRPC in such trials is encouraged.
value of endocrine manipulations without proven survival benefit in men with metastatic CRPC
In the era before the newer AR pathway inhibitors, abiraterone and enzalutamide, were shown to improve overall survival, several drugs were used as secondary hormonal manipulation in men progressing on ADT. These drugs are considered as ‘endocrine manipulations without proven OS benefit’.
The drugs most frequently used are AR antagonists (non-steroidal including bicalutamide, flutamide, nilutamide and steroidal cyproterone acetate), oestrogens and estramustine phosphate, ketoconazole and corticosteroids (dexamethasone and prednisone/prednisolone). These agents have been tested in numerous small short-term phase II trials, demonstrating biochemical and/or clinical responses. One phase III trial (n = 260) tested AR antagonist withdrawal and ketoconazole plus hydrocortisone compared with AR antagonist withdrawal alone. The addition of ketoconazole resulted in an improvement in the response rate (PSA and objective), but not OS, although there was considerable crossover [48].
In the absence of large randomised phase III trials, the effect of these drugs on OS remains unknown. The advantages of the above-mentioned drugs are their relatively low cost, widespread access and, for some, their rather favourable safety profile.
For a patient who is not considered a candidate for chemotherapy, 52% of the panel members felt that in the era of AR pathway inhibitors with proven overall survival benefit (abiraterone and enzalutamide), these older agents are not appropriate treatments, if abiraterone and enzalutamide are available. Nevertheless, 32% would use them in a minority of selected patients and 16% in the majority of patients.
If abiraterone and enzalutamide are not available, all panel members considered it appropriate to use these agents (endocrine manipulations without proven OS benefit), 88% in the majority of patients, the remaining panel members in a minority of selected patients.
Even in countries where abiraterone and enzalutamide are officially available, not all patients have access to these agents, either because the ‘out of pocket cost’ that has to be paid by the patient is too high (e.g. United States) or because the drugs are not reimbursed in some countries.
The preferred first treatment option among these alternate endocrine agents was an AR antagonist such as bicalutamide for 63% of the panel members, while another 25% of panel members would use dexamethasone (of note, 0% would use prednisone) in this setting. Six percent of the panel recommended ketoconazole in this situation; however, availability of this agent is limited in many countries.
treatment choice and sequencing for men with metastatic CRPC
To date, six therapies have been shown to prolong survival in men with metastatic CRPC. After docetaxel became the first approved therapy to prolong survival for men with metastatic CRPC in 2004, two registration-driven (i.e. not based on disease biology) treatment ‘spaces’ for patients with CRPC evolved, defined by application of chemotherapy: pre-docetaxel (chemotherapy-naïve) and post-docetaxel. Abiraterone and enzalutamide have both been investigated and shown to prolong overall survival in large phase III trials in both the pre- and post-docetaxel states [3, 4, 6, 7]. Sipuleucel-T was tested predominantly (∼85%) in chemotherapy-naïve men with CRPC [2]. Cabazitaxel was exclusively tested in patients progressing on or after docetaxel [1]. The phase III trial with radium-223 included post-docetaxel patients (57%) or patients who were judged as unfit for chemotherapy, those who declined docetaxel, or had no access to chemotherapy [5]. The trials of abiraterone and enzalutamide excluded patients treated with the other novel AR pathway inhibitor, and abiraterone and enzalutamide were either not available or only available as part of clinical trials when the trials of docetaxel, sipuleucel-T, radium-223 and cabazitaxel were conducted. Evidence from a number of small retrospective cohort studies suggests limited activity from whichever of abiraterone and enzalutamide are used second in a sequential fashion [49–53]. Docetaxel after one or both of newer AR pathway inhibitors may have less activity than in the pivotal trials [54–56]. Cabazitaxel used in the third-line setting post-docetaxel and after one or two lines of newer AR pathway inhibitors abiraterone or enzalutamide seems to have similar anti-tumour activity when compared with that seen in the phase III trial [57–59]. It is of note that all of these studies were not only retrospective but had small patient numbers and heterogeneous patient populations and largely represented mono- or oligocenter experiences. These investigations can be considered hypothesis generating for the development of properly powered randomised, prospective trials.
Prospective phase III trial data of novel agents in the second line are only available in men with CRPC who had been treated with first-line docetaxel.
In daily practice, clinicians often face the difficult task of choosing among treatment options with different mechanisms of action, administration and toxicity profiles. Importantly, these agents have not been compared with one another prospectively. Optimal sequential use of agents with potential for survival prolongation as well as the optimal time point to initiate treatment remains uncertain.
Physicians also are challenged by the problem that the average man with CRPC might not have fulfilled the selection criteria for all of the registration trials. Also patients enrolled in clinical trials were monitored closely with bone scintigraphy and CT scans. Therefore, this raises an important question on whether the trial results may be generalised and extrapolated for a specific patient.
Sixty-three percent of the panel recommended that in patients with metastatic CRPC progressing by PSA without evidence of radiographic progression and in the absence of symptoms and imminent complications, agents with potential for survival prolongation should be initiated within 4–8 weeks. Conversely, 38% of the panel felt that in such patients treatment can be postponed in the presence of adequate disease monitoring.
The most meaningful definition of asymptomatic/mildly symptomatic (related to pain) men with metastatic CRPC was considered by 71% of the panel to be ‘No pain medication or only PRN (as needed) pain medication’. Fatigue and loss of appetite were mentioned as other important symptoms of the disease.
AR pathway inhibitors
There was consensus (88% of the panel) that abiraterone or enzalutamide are recommended as first-line therapy for otherwise healthy, asymptomatic or minimally symptomatic men with CRPC in addition to ADT (Figure 3B).
Both pivotal trials of abiraterone (COU-302) and enzalutamide (PREVAIL) in the pre-chemotherapy setting have only included asymptomatic or minimally symptomatic patients [defined as asymptomatic (score 0–1) or mildly symptomatic (score 2–3) on question 3 of the brief pain inventory short form] [6, 7]. Prior treatment with ketoconazole was not allowed for both trials.
There was consensus (77% of the panel) that it is appropriate to extrapolate the results of the COU-302 and PREVAIL trials to certain symptomatic chemotherapy-naïve men with CRPC; however, 23% did not support this extrapolation. It is important to recognise that symptoms are not the only clinical factor to take into consideration when making a treatment choice (see section on predictive markers and clinically important factors for decision making in daily clinical practice).
In contrast to the PREVAIL trial, the COU-302 trial excluded patients with visceral (lung and liver) metastases.
There was consensus (88% of the panel) that it was appropriate to extrapolate the results of COU-302 to certain chemotherapy-naïve patients with visceral metastases. This may be based on the fact that abiraterone in the post-chemotherapy setting had activity in patients with visceral metastases; 12% of the panel felt that this extrapolation was not appropriate.
With regard to the preferred first-line AR pathway inhibitor, the panel was almost equally divided between abiraterone (39%), enzalutamide (27%) or either one of the two (33%).
Since there are no head-to-head trials comparing these two agents, individual patient factors such as co-medication (several drug interactions have been described for both abiraterone and enzalutamide), co-morbidities as well as patient preference concerning the expected side-effects are important when making a shared choice for either of the AR pathway inhibitors (e.g. abiraterone: low potassium, fluid retention, impaired liver function and cardiac side-effects; enzalutamide: fatigue/asthenia, risk of seizures, QTc prolongation).
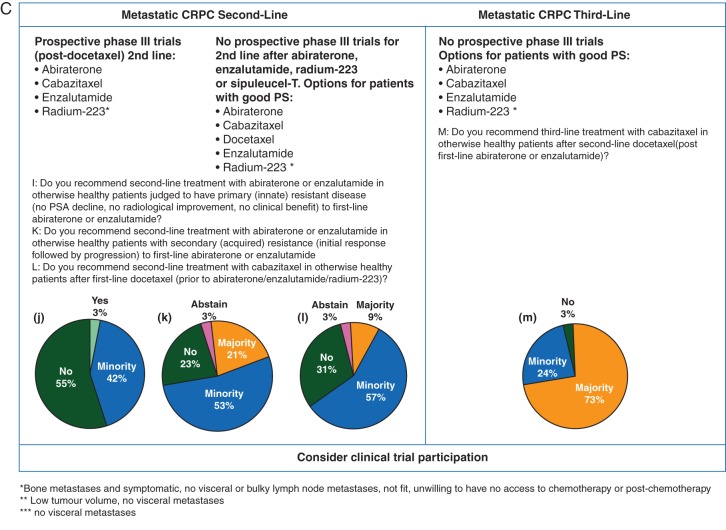
Based on the available retrospective data that indicate impaired activity of the AR pathway inhibitors when used sequentially, the panel did not recommend (55%) or recommended only in a minority of selected patients (42%) second-line treatment with abiraterone or enzalutamide in patients judged to have primary (innate) resistant disease (no PSA decline, no radiological soft-tissue response, no clinical benefit) to first-line enzalutamide or abiraterone (Figure 3C).
In the case of acquired resistance (initial response followed by progression) to first-line abiraterone or enzalutamide, 24% of the panel did not recommend, and 53% recommended only in a minority of selected patients, the other AR pathway inhibitor as immediate next-line treatment.
docetaxel
The panel did not (49%), or only in a minority of selected patients (42%), recommend docetaxel chemotherapy as first-line therapy for otherwise healthy asymptomatic/minimally symptomatic men with CRPC. In symptomatic patients, the panel was divided, with 41% recommending docetaxel as first-line treatment in the majority and 50% in a minority of selected patients (Figure 3B).
When an otherwise healthy symptomatic patient had a short response (≤12 months) to primary ADT, the proportion changed to 56% of the panel recommending docetaxel in the majority of patients and 41% in a minority of selected patients. In contrast, in patients with a short response to primary ADT, but who are asymptomatic or minimally symptomatic, the panel did not (21%), or only in selected patients (49%), recommend docetaxel as first-line treatment.
radium-223
The panel did not recommend (33%) or recommended only in a minority of selected men with CRPC (55%) radium-223 as first-line treatment.
This may reflect the fact that the chemotherapy-naïve patient population included in the ALSYMPCA trial was mixed and not well defined: it is not possible to distinguish the groups of patients who were unfit for chemotherapy, unwilling to have chemotherapy, or who had no access to chemotherapy as these data were not collected. Furthermore, activity of radium-223 is limited to the bone environment and a significant proportion of men with CRPC have soft-tissue (nodal and/or visceral) disease (Figure 3B) [60].
Combination trials of this agent with other agents with a proven survival benefit are ongoing. Routine use of combination therapy should not be undertaken until these data are available.
Sixty-five percent of the panel felt that it was appropriate to extrapolate the results of the ALSYMPCA study to certain symptomatic men with CRPC with bone metastases who qualify as fit for chemotherapy. Furthermore, 56% of the panel felt that it was also appropriate to extrapolate the results of ALSYMPCA to certain asymptomatic patients with bone metastases, whereas 44% felt that this extrapolation was not appropriate.
sipuleucel-T
The panel was divided with regards to recommending sipuleucel-T as first-line therapy for otherwise healthy, asymptomatic men with CRPC without visceral metastases; 23% recommended it in the majority of patients, 33% only in a minority of selected patients, and 43% did not recommend it. This may be due to the fact that experience with the agent is limited to very few countries (and in fact, relatively few physicians), the fact that the agent has no detectable direct antitumor activity, and logistics are challenging. There was consensus (90% of the panel) that it is inappropriate to extrapolate the results of the IMPACT study to patients who are symptomatic and/or have visceral disease (Figure 3B).
Sipuleucel-T is currently only available in the United States and marketing authorisation in Europe was withdrawn recently.
cabazitaxel
In the second-line setting after first-line docetaxel (before abiraterone/enzalutamide/radium-223), 9% of the panel recommended cabazitaxel in a majority of patients, 57% in a minority of selected patients and 31% did not recommend it.
In third-line after second-line docetaxel (post first-line abiraterone or enzalutamide), however, 73% of the panel recommended cabazitaxel in a majority of patients, while 24% recommended it in a minority of selected patients (Figure 3C).
staging and monitoring of treatment
baseline staging
Baseline staging and assessment of effects of anticancer-treatment of patients treated with agents with a proven survival benefit outside of clinical trials in daily clinical practice remains a challenge [61], and most current guidelines (e.g. ESMO, NCCN, EAU) do not provide clear recommendations. The Prostate Cancer Working Group 2 (PCWG2) recommendations provide clear and detailed instructions on baseline staging and treatment monitoring, but PCWG2 focused on clinical trials and was not intended as a guide for routine clinical care [62].
Laboratory and imaging parameters are subject to considerable uncertainty. Disease monitoring in the bone is especially difficult with well-described bone lesion flare phenomena both on CT and bone scans [63–65]. In addition, there are only well-defined criteria for progression on bone scans, with no specific criteria for the positive identification of benefit/response. PSA alone is not reliable enough for monitoring disease activity in advanced CRPC, since visceral metastases may develop in men without rising PSA [60].
PCWG2 recommends a combination of bone scintigraphy and CT scans, PSA measurements and clinical benefit in men with CRPC [62], while NCCN includes also MRI and PET, which are reported as ‘useful’ techniques.
Advanced MRI techniques include endorectal MRI, high magnetic field scanning (3-Tesla), high resolution T2-weighted imaging, contrast enhanced MRI and diffusion-weighted imaging. Multi-parametric MRI (T2-weighted imaging together with one or more of the before mentioned functional techniques) shows great promise for detecting local recurrence [66, 67]. Advanced spinal/whole-body MRI techniques are also better able to identify and gauge the extent of bone disease than planar bone scans [41, 43, 68].
PET/CT can be carried out with different tracers, enabling exploration of different features of the cancer and its interactions with bone. At present, choline PET/CT (either labelled with 11Carbon or 18Fluoride) is the approach for which there is most information, with data suggesting good accuracy for detection of recurrence [69], but validation with randomised prospective trials is still lacking. Also, solid data on new promising tracers (such as PSMA) are still lacking.
There was clear consensus with the panel recommending unanimously (100%) that imaging should be undertaken in men with metastatic CRPC before starting a new line of treatment.
Baseline examinations should include history and clinical examinations as well as baseline blood tests such as blood count (haemoglobin, thrombocytes, total white blood cell count, neutrophil and lymphocyte counts), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH). Some of these variables (Hb, LDH, ALP) are considered established prognostic factors; however, their value in directing treatment decisions is not established. In addition, other assessments such as renal and liver function as well as electrolytes should be carried out.
There was clear consensus that a CT scan of the chest thorax and abdomen-pelvis (91% of the panel) and bone scintigraphy (83% of the panel) should be recommended in the majority of patients before starting a new treatment.
It is recognised that planar bone scintigraphy has short-comings and is less sensitive than other newer imaging technologies (e.g. MRI of the axial skeleton for bone staging, MRI of the whole body and/or PET/CT for concurrent bone and node metastasis screening). But availability of these newer imaging technologies is limited and their added value over bone scintigraphy in the management of APC, i.e. impact of earlier and more reliable detection of metastasis on complications and survival, has not been proven.
Malignant spinal cord compression (MSCC) occurs at a frequency of 4%–8% in men with CRPC and is one of the most devastating complications [5]. The panel stressed the need for a risk-adapted approach to undertaking MRI of the entire spine. Retrospective, small studies have shown occult spinal cord compression or impingement in up to 30% of men with CRPC [70, 71]. Extensive bone metastatic disease on bone scan was shown in both studies to be an independent predictive factor for MSCC. A large randomised phase III clinical trial comparing screening MRI of the spine to standard of care is ongoing (CRUK/11/053).
Seventeen percent of the panel recommended a baseline MRI of the entire spine in the majority of patients and 54% in a minority of selected patients based on extent of spinal metastases on bone scintigraphy or CT scan.
In case of neurologic symptoms possibly indicating MSCC, a MRI of the whole spine should be done immediately.
In routine practice, the panel did not (47%) or only in a minority of selected patients (36%) recommend the use of newer imaging methods beyond bone scintigraphy and CT scan, namely whole-body MRI and/or PET/CT, as a components of baseline prostate cancer staging.
The variability in cost and availability of newer imaging modalities throughout the world, and lack of definitive data concerning their prognostic and/or predictive value makes this area an important unmet medical need for research.
treatment monitoring
There was consensus (83% of the panel) that regular monitoring of treatment (apart from clinical and laboratory assessment) is recommended for men with metastatic CRPC. This reflects the fact that the agents with a proven overall survival benefit all have potential toxicity and considerable costs, and patients with no objective benefit (including disease stabilisation) should not be further exposed to them. It was discussed that, for imaging, generally a risk-adapted approach should be considered depending on response to therapy, extent of disease and clinical situation (e.g. line of therapy, symptoms).
The panel recommended regular measurements of ALP (88%) and LDH (61%). Both markers may be helpful as serial values when it comes to interpretation of discordant results (e.g. PSA rise and clinical improvement).
The panel stressed the need for cautious interpretation of PSA values especially in the first 2–3 months after starting a new treatment. PSA flare has been described following initiation of chemotherapy or newer hormonal therapies with significant PSA falls after initial rise [72–74].
For sipuleucel-T and radium-223 no significant PSA declines have been reported despite an OS benefit. Education of patients and physicians about uncertainties in interpretation of PSA values is critical.
Eighty-three percent of the panellists did not recommend regular monitoring or monitoring at progression with MRI of the entire spine in patients with multiple spine lesions on bone scintigraphy but rather stressed the need for a risk-adapted approach largely dependent on the development of clinical symptoms.
Further studies are necessary to confirm the potential role of systematic disease monitoring using MRI or PET/CT to characterise early response to treatment (i.e. recognition of disease response, stability or progression and not only late confirmation of progression) and its potential impact on the sequential use of agents with a proven overall survival benefits [68, 75–78].
monitoring of men treated with abiraterone or enzalutamide
With regard to PSA measurements 62% of the panel recommended a frequency of every 2–4 months, and 38% recommended measurement every 3–4 weeks. There was consensus (78% of the panel) that regular CT scans even in the absence of clinical indication (e.g. new symptoms or pain) should be carried out. With regards to frequency, 47% of the panel would perform CT scans every 2–4 months and 31% every 6 months. Nineteen percent of the panel recommended CT scans only if clinically indicated.
The panel also recommended regular bone scintigraphy. With regards to frequency 27% of the panel would perform bone scans every 2–4 months and 59% every 6 months.
monitoring of men treated with docetaxel or cabazitaxel
There was consensus (79% of the panel) that PSA measurement should be undertaken every 3–4 weeks while 21% of the panel recommended measurements every 2–4 months. There was consensus (80% of the panel) that regular CT scans even in the absence of clinical indication (e.g. new symptoms or pain) should be carried out. With regard to frequency, 66% of the panel would perform CT scans every 2–4 months and 14% every 6 months Seventeen percent of the panel recommended CT scans only if clinically indicated.
The panel also recommended regular bone scintigraphy. With regard to frequency, 36% of the panel would perform bone scans every 2–4 months and 33% every 6 months. Twenty-four percent of the panel recommended monitoring bone scintigraphy only if clinically indicated.
monitoring of men treated with radium-223 or sipuleucel-T
The panel was split about the recommendation for PSA measurement and recommended every 3–4 weeks (44%), every 2–4 months (34%), or only if clinically indicated (19%). The panel recommended regular CT scans with a frequency of every 2–4 months (42%) or every 6 months (21%). Thirty percent of the panel recommended CT scans only if clinically indicated.
For sipuleucel-T no significant imaging response rates have been shown in the phase III trial, and for radium-223 no imaging was carried out in the phase III trial. Monitoring may therefore primarily be helpful to exclude significant disease progression. For radium-223, the value and interpretation of imaging for monitoring are unclear and should therefore be addressed in clinical trials.
indication to stop treatment
The panel stressed the fact that treatments with a proven survival benefit should in general not be stopped for PSA progression alone (in the absence of radiographic or clinical progression). There was consensus (82% of the panel) that at least two of three criteria (PSA progression, radiographic progression and clinical deterioration) should be fulfilled to stop treatment. In the case of unequivocal progression of visceral disease without clinical deterioration or PSA progression, treatment should be stopped and a biopsy can be considered to rule out secondary malignancy or small-cell histology. In case of significant clinical progression that is very likely related to disease without a rise in PSA or radiographic progression, treatment should be changed. It should be recognised that men with CRPC can often have worsening bone pain related to non-malignant processes such as degenerative disorders or osteoporotic fractures. Similarly, fatigue/asthenia can be a side-effect of treatment and may therefore not be a sign of disease progression.
use of osteoclast-targeted agents for reducing risk of SREs and SSEs in men with CRPC
In men with CRPC and bone metastases, two agents (zoledronic acid and denosumab) have been approved for reducing risk of SREs defined as: radiation to the bone, surgery to the bone, fractures, spinal cord compression and (for zoledronic acid only) change in antineoplastic therapy. In the pivotal trial by Saad et al., zoledronic acid was given at a dose of 4 mg every 3 weeks for a total of 20 cycles (15 months) with an option to continue for an additional 9 months extension (24 months) [9, 10]. It is of note that the trial included a third arm using 8 mg every 3 weeks, which was reduced subsequently to 4 mg because of renal toxicity, and this arm did not show significant reduction of SREs compared with controls.
The dose of denosumab in the pivotal trial by Fizazi et al. was 120 mg s.c. every 4 weeks until discontinuation or until the primary cut-off date, which occurred about 41 months after start of enrolment [79]. Both zoledronic acid and denosumab were developed and investigated before the era of agents with significant anti-tumour effect and survival impact for men with CRPC. For abiraterone post-docetaxel, or enzalutamide both pre- and post-docetaxel, a significant reduction in the time to first SRE in the active treatment arms was reported [7, 80, 81].
For radium-223, also a bone targeting agent, there was also a significant prolongation in time to first SSE in the overall trial population and also in the subgroup of patients receiving bisphosphonates [82].
The optimal timing for starting treatment, optimal treatment intensity (dose and frequency) and optimal duration of osteoclast-targeted agents for men with CRPC is unclear. Also treatment-related toxicity (e.g. osteonecrosis, hypocalcaemia and hypophosphataemia) has to be taken into consideration.
Overall, 62% of the panel recommended that the majority of men with CRPC with bone metastases should receive an osteoclast-targeted agent for prevention of SRE. Thirty-two percent of the panel recommended osteoclast-targeted treatment in a minority of selected patients. If these treatments are planned there was consensus (76% of the panel) that a professional dental check (dentist) should be undertaken at baseline before treatment start in the majority of patients (Figure 4B).
The panel was almost equally divided as to preference between zoledronic acid (30%), denosumab (42%) and either of the two options (27%). This voting result may be influenced by the fact that zoledronic acid has become generic influencing the cost of the treatment.
Regarding the frequency of treatment administration for zoledronic acid or denosumab, 31% of the panel recommended dosing every 3–4 weeks, 34% recommended less frequent administration from the beginning and 28% recommended a 3–4 weekly dosing for ∼2 years and less frequently after that.
This is distinct from the regulatory approvals and likely represents the lack of data supporting a dose response for these agents.
Half of the panel was of the opinion that osteoclast-targeted therapy should be continued indefinitely, whereas 47% of the panel recommended a total duration of ∼2 years for reducing risk of SREs/SSEs.
For men with metastatic CRPC and bone metastases responding to a treatment, 58% of the panel were of the opinion that osteoclast-targeted therapy should be continued at the same schedule, whereas 26% voted for decreased frequency and 16% recommended interruption or discontinuation of the osteoclast-targeted therapy.
Denosumab has been compared with placebo in men with CRPC without bone metastases. Its use led to increased bone metastases-free survival but no overall survival benefit in a large phase III clinical trial [8]. No country has approved denosumab for men without bone metastases.
For men with CRPC without bone metastases, there was consensus (88% of the panel) that an osteoclast-targeted agent for delaying onset of bone metastases is not recommended (Figure 4A).
predictive markers and clinically important factors for decision making in daily clinical practice
The panel stressed the need for predictive markers indicating sensitivity or resistance to a specific therapy.
There was clear consensus (94% of the panel) that, at present, there is no validated and established marker that can be used as a predictive factor in daily clinical practice to inform on treatment choices for men with CRPC.
Factors favouring chemotherapy instead of AR pathway inhibitors with a proven overall survival benefit were discussed. The panel was of the opinion that a Gleason score of ≥8 (88% no) and a circulating tumour cell count of ≥5/7.5 ml (97% no) as single factors should not influence this decision. Also, in patients with extensive disease on imaging, 68% of the panel was of the opinion that this factor alone should not influence treatment choice for men with CRPC.
For several other factors, the panel was divided as to whether these factors would favour use of chemotherapy instead of abiraterone or enzalutamide: expression of AR-V7 splice variants (47% yes, 44% no), presence of visceral metastases (50% yes, 50% no), short response (≤12 months) to primary ADT (53% yes, 47% no) and low PSA (<20 ng/ml) in the setting of high tumour volume (65% yes, 35% no).
In daily clinical practice, decision making is based on many patient-, tumour- and environment-related factors as well as patient and physician preference and the cost and availability of drugs.
tumour biopsy
Fresh tumour biopsies in men with CRPC are increasingly incorporated into clinical trials and advances in imaging and in laboratory technology have made the processing of biopsies from bone metastases feasible [83–87].
It is unclear when a biopsy from a man with CRPC should be considered. Tumour biopsies should only be carried out, if the procedure can be done without unreasonable risks, if adequate handling and analysis of the tissue is ensured, and if the result has potential implications for patient management. In a subset of men with CRPC, an AR signalling-independent phenotype may develop which can be associated with small-cell carcinoma seen on biopsy and clinically with rapidly progressing visceral metastases, predominately lytic bone metastatic disease and stable or low PSA levels. Recommendations from a multidisciplinary committee on aggressive variants of CRPC have been published recently [88]. The optimal treatment in these patients is unclear but platinum-based therapy is often recommended for patients with small-cell carcinoma and sometimes for patients with aggressive clinical features. There are limited data regarding the management of men with CRPC with neuroendocrine differentiation or other atypical morphology.
The panel discussed a number of clinical factors that may indicate that a tumour biopsy should be considered, low PSA (<20 ng/ml) in the setting of high tumour volume (44% yes in the majority, 47% yes in a minority, 9% no); new development of visceral metastases (32% yes in the majority, 56% yes in a minority, 12% no); progressive lesions in case of discordant tumour response to treatment (49% yes in the majority, 46% yes in a minority, 6% no); predominately lytic bone metastatic disease (37% yes in the majority, 37% yes in a minority, 26% no); patients progressing on primary ADT within <6 months (23% yes in the majority, 31% yes in a minority, 46% no).
multidisciplinary care of men with prostate cancer
Multidisciplinary care of men with APC has become much more complex in recent years. The involvement of physicians with expertise in distinct domains of cancer care is crucial in order to achieve the best possible care for men with APC.
Half of the panel members recommended that patients should be discussed in a multidisciplinary team (MDT) before a new line of therapy is started and 44% recommended discussion by the MDT in a minority of selected patients. Although the importance of MDTs is acknowledged, time constraints are the main reason for not discussing all but only complex patient cases in a MDT. There are also clear differences between different health systems, since in some countries discussion in a MDT is a prerequisite for insurance companies to cover the costs of the treatments.
There was strong consensus (94% of the panel) that patients should be informed about the possibility of participating in a clinical trial to improve knowledge of the disease.
Also 64% of the panellists recommended early access of men with CRPC to an expert in symptom palliation or a dedicated palliative care service.
discussion
In the absence of level one evidence or in areas where there are conflicting data or conflicting interpretation of existing data, weighted expert recommendations are helpful for making treatment decisions in daily clinical practice. That was the motivation and goal to initiate the APCCC where prostate cancer experts discussed and voted for different management options for men with APC.
In some areas, there was clear consensus (supplementary Appendix S2–S4, available at Annals of Oncology online) among the prostate cancer experts whereas, in other areas of approaches to patient care, there were divergent opinions. Some of the variation may be due to different patient management philosophies in different geographic regions; other differences may result from lack of data.
Areas of consensus included the definition of castration resistance where there was consensus that rising PSA with a documented testosterone level <50 ng/dl (<1.7 nmol/l) on ADT alone is sufficient and no secondary hormonal manipulations are required. There was also consensus that, in men with metastatic CRPC, staging examinations, including imaging, have to be carried out before each new line of treatment. Regular treatment monitoring with imaging was also recommended for men with CRPC. There was strong consensus that currently there is no validated predictive factor for treatment selection available. In men with castration-naïve prostate cancer, the use of osteoclast-targeted therapy with denosumab or zoledronic acid for reducing risk of SREs or SSEs was not recommended. The same is true for men with CRPC without bone metastases. There was consensus that treatment of CRPC should generally only be stopped if two of three criteria (PSA-, radiographic or clinical progression) are fulfilled. There was strong consensus that patients should be informed about the possibility of joining a clinical trial.
The topics which generated disagreement among the panel should serve as potential areas for research and should be addressed in future clinical trials. Such areas include chemo-hormonal therapy in castration-naïve disease, but lack of consensus may be resolved by the results of the STAMPEDE trial. Another area of discrepant opinions is the treatment of M0 CRPC disease and three large phase III trials in this setting are ongoing.
In the first-line setting for men with CRPC, there seems to be considerable uncertainty. There was no consensus as to whether abiraterone or enzalutamide should be chosen, if the option of a newer AR pathway inhibitor is considered. Also the role of radium-223 in this setting seems controversial. There was not a single factor that the panel recommended to use for choosing chemotherapy first line. Biomarkers capable of informing specific applications and sequencing of treatments are of special interest for future research. Another open question is sequencing of therapies and specifically the use of abiraterone or enzalutamide after progression on the other one of them after initial response.
An important unmet medical need is the assessment of value of the newer imaging modalities in early diagnosis and treatment monitoring and their impact on patient outcome. The variability of cost and availability of these imaging modalities throughout the world are also an issue. Prospective multicentric translational studies embedding these newer modalities are needed to confirm the ability of these techniques to do better than bone scintigraphy and CT in terms of disease detection, demonstration of recurrence and to provide an earlier, more refined approach of response evaluation and defining the optimal timing to switch treatment or type of treatment. Importantly, they need to be assessed in terms of their impact on QoL measures and survival, although the latter is likely to be more challenging given the availability of multiple survival-prolonging treatments.
Furthermore, new ‘registration’ end points are needed in the light of several drugs that have been shown to prolong median OS.
Another area of uncertainty is the ‘best use’ of osteoclast-targeted agents in men with CRPC concerning initiation, dose, frequency and duration. It is also unclear if, in the time of potent new therapies, the effect of these agents is redundant or additive, and that may depend on the new therapy used. The management of men with oligometastatic disease is another area where trial data are lacking and no consensus could be achieved, which makes it an important research question. More data are necessary to address the above topics where consensus is lacking, and this conference pointed to clinical issues where physicians make decisions without certainty of benefit. If the gaps in knowledge are not filled with informative rigorous data, future regulators and payers may decide how a patient will be treated.
We did not capture differences in the opinion of panel members by geographic region, access to drugs or imaging or by different patient ethnic groups or cultures. This will be considered at the next meeting.
Additional important questions remain that have not been addressed in this meeting such as costs and cost-effectiveness of drugs, drug accessibility, ideal clinical settings, health economic issues, patient-reported QoL outcomes, implementation of precision medicine strategies in clinical practice as well as combination strategies.
The panel predicts that by the next APCCC in 2017 some of the unanswered questions will be better understood or hopefully clarified by emerging data. Additional questions will then be addressed to provide further recommendations useful for daily clinical practice: in particular selecting the right treatment, for the right patient and at the right time.
funding
We gratefully acknowledge the financial support of the following non-profit organisations for the Advanced Prostate Cancer Consensus Conference (APCCC): Cantonal Hospital St Gallen, City and Canton of St Gallen, Swiss Cancer Research Organisation, European School of Oncology (ESO), Swiss Cancer League, the Swiss Oncology Research Network SAKK, Swiss Cancer Foundation, Prostate Cancer Foundation (PCF) and the Günter and Regine Kelm Foundation. We thank the Movember Foundation for the sponsoring of the travel grants (no grant number).
disclosure
The full and detailed conflicts of interest statements of all authors are included in supplementary Appendix S1, available at Annals of Oncology online.
Supplementary Material
acknowledgements
We gratefully acknowledge all the participants in the Advanced Prostate Cancer Consensus Conference (APCCC) for their lively, stimulating discussions. In addition to the panel members, we thank Beat Thürlimann for guidance in the development of the questions and for reviewing the manuscript. We also thank Thomas Cerny and the local organising committee, including Hans-Peter Schmid, Arnoud Templeton, Christian Rothermundt, Joachim Müller, Simon Wildermuth, Wolfram Jochum and Ludwig Plasswilm for their support and suggestion of speakers; Carmel Pezaro for reviewing the consensus questions; Lewis Rowett for his editorial assistance in the preparation of this report.
references
- 1.de Bono JS, Oudard S, Ozguroglu M et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Nilsson S, Heinrich D et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MR, Saad F, Coleman R et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012; 379: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad F, Gleason DM, Murray R et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002; 94: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 10.Saad F, Gleason DM, Murray R et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004; 96: 879–882. [DOI] [PubMed] [Google Scholar]
- 11.Hsu C, Sanford B. The Delphi technique: making sense of consensus. Practical Assess Res Eval 2007; 12: 1–9. [Google Scholar]
- 12.Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolis G, Ackman D, Stellos A et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci USA 1982; 79: 1658–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaisary AV, Tyrrell CJ, Peeling WB, Griffiths K. Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol 1991; 67: 502–508. [DOI] [PubMed] [Google Scholar]
- 15.Vogelzang NJ, Chodak GW, Soloway MS et al. Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Zoladex Prostate Study Group. Urology 1995; 46: 220–226. [DOI] [PubMed] [Google Scholar]
- 16.Klotz L, Boccon-Gibod L, Shore ND et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008; 102: 1531–1538. [DOI] [PubMed] [Google Scholar]
- 17.Crawford ED, Tombal B, Miller K et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol 2011; 186: 889–897. [DOI] [PubMed] [Google Scholar]
- 18.Crawford ED, Shore ND, Moul JW et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology 2014; 83: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 19.Akaza H, Procopio G, Pripatnanont C et al. Treatment patterns in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel (DOC)-based chemotherapy (CTX): PROXIMA. Ann Oncol 2014; 25(suppl 4): iv255–iv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James ND, Spears MR, Clarke NW et al. Survival with newly diagnosed metastatic prostate cancer in the “Docetaxel Era”: data from 917 patients in the control arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol 2015; 67: 1028–1038. [DOI] [PubMed] [Google Scholar]
- 21.Gleave M, Bruchovsky N, Goldenberg SL, Rennie P. Intermittent androgen suppression for prostate cancer: rationale and clinical experience. Eur Urol 1998; 34(Suppl 3): 37–41. [DOI] [PubMed] [Google Scholar]
- 22.Akakura K, Bruchovsky N, Goldenberg SL et al. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer 1993; 71: 2782–2790. [DOI] [PubMed] [Google Scholar]
- 23.Klotz LH, Herr HW, Morse MJ, Whitmore WF Jr.. Intermittent endocrine therapy for advanced prostate cancer. Cancer 1986; 58: 2546–2550. [DOI] [PubMed] [Google Scholar]
- 24.Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol 2013; 31: 2029–2036. [DOI] [PubMed] [Google Scholar]
- 25.Huggins C, Scott WW. Bilateral adrenalectomy in prostatic cancer: clinical features and urinary excretion of 17-ketosteroids and estrogen. Ann Surg 1945; 122: 1031–1041. [PMC free article] [PubMed] [Google Scholar]
- 26.Labrie F, Dupont A, Belanger A et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med 1982; 5: 267–275. [PubMed] [Google Scholar]
- 27.Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet 2000; 355: 1491–1498. [PubMed] [Google Scholar]
- 28.Samson DJ, Seidenfeld J, Schmitt B et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002; 95: 361–376. [DOI] [PubMed] [Google Scholar]
- 29.Caubet JF, Tosteson TD, Dong EW et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology 1997; 49: 71–78. [DOI] [PubMed] [Google Scholar]
- 30.Akaza H, Hinotsu S, Usami M et al. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 2009; 115: 3437–3445. [DOI] [PubMed] [Google Scholar]
- 31.Cooperberg MR, Hinotsu S, Namiki M et al. Trans-pacific variation in outcomes for men treated with primary androgen deprivation therapy for prostate cancer. BJU Int 2014. Sep 19 [epub ahead of print], doi: 10.1111/bju.12937. [DOI] [PubMed] [Google Scholar]
- 32.Gravis G, Boher JM, Joly F et al. Androgen deprivation therapy plus docetaxel versus ADT alone for hormone-naive metastatic prostate cancer: long-term analysis of the GETUG-AFU 15 phase III trial. In 2015 Genitourinary Cancers Symposium Presented 26 February 2015. J Clin Oncol 2015; 33(suppl 7): abstr 140. [Google Scholar]
- 33.Gravis G, Fizazi K, Joly F et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14: 149–158. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney C, Chen YH, Carducci MA et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. J Clin Oncol 2014; 32(5s suppl): abstr LBA2. [Google Scholar]
- 35.Eisenberger MA, Blumenstein BA, Crawford ED et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998; 339: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 36.Glass TR, Tangen CM, Crawford ED, Thompson I. Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol 2003; 169: 164–169. [DOI] [PubMed] [Google Scholar]
- 37.James ND, Sydes MR, Mason MD et al. Docetaxel and/or zoledronic acid for hormone-naïve prostate cancer: first overall survival results from STAMPEDE (NCT00268476). J Clin Oncol 2015; 33(suppl): abstr 5001. [Google Scholar]
- 38.Smith MR, Halabi S, Ryan CJ et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol 2014; 32: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweizer MT, Zhou XC, Wang H et al. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol 2013; 24: 2881–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ost P, Bossi A, Decaestecker K et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 2015; 67: 852–863. [DOI] [PubMed] [Google Scholar]
- 41.Lecouvet FE, El Mouedden J, Collette L et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 2012; 62: 68–75. [DOI] [PubMed] [Google Scholar]
- 42.Poulsen MH, Petersen H, Hoilund-Carlsen PF et al. Spine metastases in prostate cancer: comparison of technetium-99m-MDP whole-body bone scintigraphy, [(18) F]choline positron emission tomography(PET)/computed tomography (CT) and [(18) F]NaF PET/CT. BJU Int 2014; 114: 818–823. [DOI] [PubMed] [Google Scholar]
- 43.Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 2014; 43: 1503–1513. [DOI] [PubMed] [Google Scholar]
- 44.Nelson JB, Love W, Chin JL et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 2008; 113: 2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller K, Moul JW, Gleave M et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2013; 16: 187–192. [DOI] [PubMed] [Google Scholar]
- 46.Smith MR, Saad F, Oudard S et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol 2013; 31: 3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford ED, Stone NN, Yu EY et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology 2014; 83: 664–669. [DOI] [PubMed] [Google Scholar]
- 48.Small EJ, Halabi S, Dawson NA et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 2004; 22: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 49.Brasso K, Thomsen FB, Schrader AJ et al. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur Urol 2014. Aug 6 [epub ahead of print], doi: 10.1016/j.eururo.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Petrelli F, Coinu A, Borgonovo K et al. Enzalutamide after docetaxel and abiraterone acetate treatment in prostate cancer: a pooled analysis of 10 case series. Clin Genitourin Cancer 2015; 13: 193–198. [DOI] [PubMed] [Google Scholar]
- 51.Cheng HH, Gulati R, Azad A et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis 2015; 18: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noonan KL, North S, Bitting RL et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 2013; 24: 1802–1807. [DOI] [PubMed] [Google Scholar]
- 53.Loriot Y, Bianchini D, Ileana E et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 2013; 24: 1807–1812. [DOI] [PubMed] [Google Scholar]
- 54.Mezynski J, Pezaro C, Bianchini D et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol 2012; 23: 2943–2947. [DOI] [PubMed] [Google Scholar]
- 55.Suzman DL, Luber B, Schweizer MT et al. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate 2014; 74: 1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aggarwal R, Halabi S, Kelly WK et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance). Cancer 2013; 119: 3636–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al Nakouzi N, Le Moulec S, Albiges L et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol 2014. May 2 [epub ahead of print], doi: 10.1016/j.eururo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Pezaro CJ, Omlin AG, Altavilla A et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol 2014; 66: 459–465. [DOI] [PubMed] [Google Scholar]
- 59.Sella A, Sella T, Peer A et al. Activity of cabazitaxel after docetaxel and abiraterone acetate therapy in patients with castration-resistant prostate cancer. Clin Genitourin Cancer 2014; 12: 428–432. [DOI] [PubMed] [Google Scholar]
- 60.Pezaro CJ, Omlin A, Lorente D et al. Visceral disease in castration-resistant prostate cancer. Eur Urol 2014; 65: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armstrong AJ, Eisenberger MA, Halabi S et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol 2012; 61: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Messiou C, Cook G, Reid AH et al. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiol 2011; 52: 557–561. [DOI] [PubMed] [Google Scholar]
- 64.Ryan CJ, Shah S, Efstathiou E et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res 2011; 17: 4854–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris MJ, Molina A, Small EJ et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol 2015; 33: 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu LM, Xu JR, Gu HY et al. Role of magnetic resonance imaging in the detection of local prostate cancer recurrence after external beam radiotherapy and radical prostatectomy. Clin Oncol (R Coll Radiol) 2013; 25: 252–264. [DOI] [PubMed] [Google Scholar]
- 67.Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and postradiation therapy. Biomed Res Int 2014; 2014: 316272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lecouvet FE, Talbot JN, Messiou C et al. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer 2014; 50: 2519–2531. [DOI] [PubMed] [Google Scholar]
- 69.Evangelista L, Zattoni F, Guttilla A et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med 2013; 38: 305–314. [DOI] [PubMed] [Google Scholar]
- 70.Bayley A, Milosevic M, Blend R et al. A prospective study of factors predicting clinically occult spinal cord compression in patients with metastatic prostate carcinoma. Cancer 2001; 92: 303–310. [DOI] [PubMed] [Google Scholar]
- 71.Venkitaraman R, Sohaib SA, Barbachano Y et al. Detection of occult spinal cord compression with magnetic resonance imaging of the spine. Clin Oncol (R Coll Radiol) 2007; 19: 528–531. [DOI] [PubMed] [Google Scholar]
- 72.Thuret R, Massard C, Gross-Goupil M et al. The postchemotherapy PSA surge syndrome. Ann Oncol 2008; 19: 1308–1311. [DOI] [PubMed] [Google Scholar]
- 73.Angelergues A, Maillet D, Flechon A et al. Prostate-specific antigen flare induced by cabazitaxel-based chemotherapy in patients with metastatic castration-resistant prostate cancer. Eur J Cancer 2014; 50: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 74.Burgio SL, Conteduca V, Rudnas B et al. PSA flare with abiraterone in patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 2015; 13: 39–43. [DOI] [PubMed] [Google Scholar]
- 75.Tombal B, Rezazadeh A, Therasse P et al. Magnetic resonance imaging of the axial skeleton enables objective measurement of tumor response on prostate cancer bone metastases. Prostate 2005; 65: 178–187. [DOI] [PubMed] [Google Scholar]
- 76.Lecouvet FE, Larbi A, Pasoglou V et al. MRI for response assessment in metastatic bone disease. Eur Radiol 2013; 23: 1986–1997. [DOI] [PubMed] [Google Scholar]
- 77.Blackledge MD, Collins DJ, Tunariu N et al. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: a feasibility study. PLoS One 2014; 9: e91779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Padhani AR, Makris A, Gall P et al. Therapy monitoring of skeletal metastases with whole-body diffusion MRI. J Magn Reson Imaging 2014; 39: 1049–1078. [DOI] [PubMed] [Google Scholar]
- 79.Fizazi K, Carducci M, Smith M et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011; 377: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Logothetis CJ, Basch E, Molina A et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol 2012; 13: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 81.Fizazi K, Scher HI, Miller K et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 2014; 15: 1147–1156. [DOI] [PubMed] [Google Scholar]
- 82.Sartor O, Coleman R, Nilsson S et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15: 738–746. [DOI] [PubMed] [Google Scholar]
- 83.Spritzer CE, Afonso PD, Vinson EN et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology 2013; 269: 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Efstathiou E, Titus M, Tsavachidou D et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol 2012; 30: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Efstathiou E, Titus M, Wen S et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferraldeschi R, Nava Rodrigues D, Riisnaes R et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol 2015; 67: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Allen EM, Foye A, Wagle N et al. Successful whole-exome sequencing from a prostate cancer bone metastasis biopsy. Prostate Cancer Prostatic Dis 2014; 17: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beltran H, Tomlins S, Aparicio A et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 2014; 20: 2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.