The prevalence of type 2 diabetes is soaring worldwide and is now recognized as one of the main threats to human health being associated with comorbidities, such as cardiovascular disease. Type 2 diabetes is a condition characterized by abnormalities in carbohydrate, lipid and protein metabolism, with the most characteristic features being hyperglycemia and dyslipidemia. The underlying pathological aberrations comprise insulin resistance and bihormonal dysfunction of the pancreatic α- and β-cells.
As aforementioned, high-protein diets are associated with impaired glucose tolerance, insulin resistance and an increased incidence of type 2 diabetes1. Protein consists of amino acids (AAs). AAs were traditionally classified as essential or non-essential for humans and animals. Essential AAs cannot be synthesized from other compounds in the body at the level required for normal growth, so they must be obtained from food. Leucine, isoleucine and valine are named as branched-chain amino acids (BCAAs). BCAAs are the most abundant of the essential AAs. Leucine is the most abundant BCAA in many dietary proteins, accounting for over 20% of total dietary protein obtained from the human diet. Of the AAs studied, the BCAAs have generated the most research interest, as they have emerged as potential biomarkers of metabolic disease. Circulating levels of BCAAs are elevated in individuals with obesity, impaired fasting glucose and type 2 diabetes2. Furthermore, circulating levels of BCAAs have the potential to predict development of type 2 diabetes3.
Not only carbohydrate and fat metabolism, but also protein metabolism is related to insulin resistance. Interestingly, in insulin-resistant states of obesity, plasma concentrations of AAs are elevated, particularly BCAAs. These findings are in agreement with studies showing that the infusion of AAs induces insulin resistance in experimental settings, similar to what is observed with lipid administration4. The addition of BCAAs to a high-fat diet contributes to the development of insulin resistance, impaired glucose homeostasis that can occur independent of body weight1. Individuals with morbid obesity have raised levels of BCAAs that normalize after gastric bypass surgery. Recently, growing evidence has shown an interaction between excess fat and BCAAs; that impaired BCAAs catabolism, especially in adipose tissue, contributes to the rise in BCAAs in obesity and insulin resistant states. Abnormal BCAAs metabolism in obesity results in an accumulation of toxic BCAAs metabolites that in turn trigger the mitochondrial dysfunction and stress signaling associated with insulin resistance5. BCAAs and in particular leucine interfere with insulin signaling through stimulation of mammalian target of rapamycin and its downstream effector, S6 kinase, and phosphorylation of insulin receptor substrate-1 (IRS-1) on serine residues4, and might thereby interfere with insulin signaling (Figure1). The insulin resistance provoked by the addition of BCAAs to a high-fat diet is reversed with administration of the mammalian target of rapamycin inhibitor, rapamycin1. However, it is noteworthy that some conflicting results have been reported regarding the role of BCAAs in the regulation of insulin resistance. For instance, the elevation of BCAAs was accompanied with increased energy expenditure and better insulin sensitivity in global knock-out of mitochondrial branched-chain aminotransferase in mice6. Increasing dietary leucine intake reduces diet-induced obesity, and improves glucose and cholesterol metabolism in mice7. It raises the possibility that the effects of BCAAs on insulin resistance might be a result of species difference.
Figure 1.
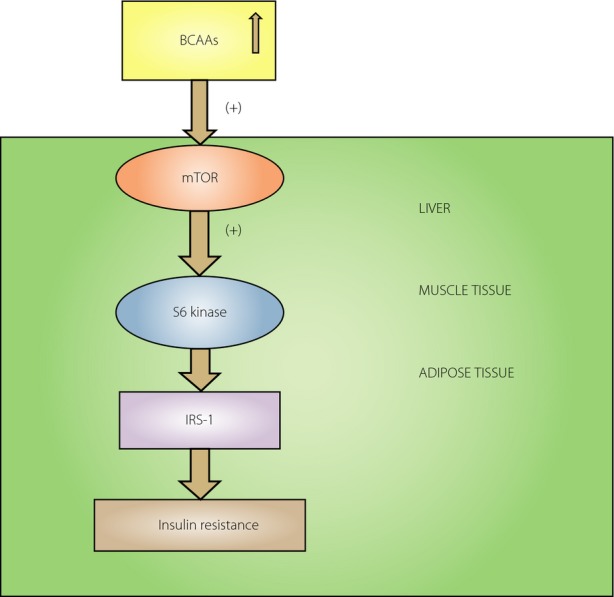
Branched-chain amino acids (BCAAs) could be involved in the development of insulin resistance. The elevated BCAAs are able to activate mammalian target of rapamycin (mTOR) and its downstream effector S6 kinase in the liver, muscle and adipose tissue. Persistent S6 kinase activation leads to serine phosphorylation of insulin receptor substrate-1 (IRS–1) and therefore the inhibition of IRS-1. This could result in insulin resistance.
It is widely recognized that diabetic patients show elevated fasting and postprandial glucagon levels relative to their high glucose levels. AAs exert multiple actions on pancreatic islet cells. It is well known that arginine acutely stimulates glucagon secretion. However, much less information exists about the influence of BCAAs on α-cell function. Leucine was reported to affect glucagon and insulin secretion in the pancreas8. Leucine is a positive stimulus for glucagon release, especially in the absence of another amino acid. The mechanisms by which leucine affects glucagon secretion are speculated to involve membrane receptors, carrier systems, modification of adenylate cyclase activity, altered ionic fluxes, reciprocal effects on amino acid transport and paracrine processes8. Our results showed that chronic exposure to leucine led to hyperglucagonemia from α-TC1-6 cells and mice islets, and concomitantly suppressed glucose stimulated insulin secretion in mice islets. Long-term exposure to leucine increased gene expression involved in the glucagon synthesis, stimulated cholesterol and triglyceride accumulation, and enhanced α-cell proliferation9. However, knowledge about the potential of BCAAs and their pathways of metabolism affecting α-cell function is lacking. It needs to be clarified whether BCAAs could over time contribute to the progressive α-cell dysfunction, and play a pathogenic role in the formation and progressive development of type 2 diabetes – like lipo- and glucotoxicity.
In summary, not only hyperglycemia and dyslipidemia, but also AAs derangement is a characteristic part of the diabetic state that might play a pathogenic role. The underlying mechanisms of the relationship of BCAAs to type 2 diabetes severity, progression or prediction remain to be defined. BCAAs metabolic pathways could also serve as potential targets for the treatment of type 2 diabetes.
References
- 1.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu F, Tavintharan S, Sum CF, et al. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metabol. 2013;98:E1060–E1065. doi: 10.1210/jc.2012-4132. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebs M, Brunmair B, Brehm A, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 5.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.She P, Reid TM, Bronson SK, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Guo K, LeBlanc RE, et al. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multi-mechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 8.Leclercq-Meyer V, Marchand J, Woussen-Colle MC, et al. Multiple effects of leucine on glucagon, insulin, and somatostatin secretion from the perfused rat pancreas. Endocrinology. 1985;116:1168–1174. doi: 10.1210/endo-116-3-1168. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Hermansen K, Jeppesen PB. Impact of glucagon-like peptide-1(7-36) amide, isosteviol and 5-aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside on leucine-mediated alpha-cell dysfunction. Diabetes Obes Metab. 2012;14:1020–1031. doi: 10.1111/j.1463-1326.2012.01633.x. [DOI] [PubMed] [Google Scholar]


